Abstract
Isoflurane is an inhalational anesthetic that is widely used in medical procedures or biomedical research. The duration of anesthesia administration varies from minutes to hours. It is known that isoflurane has dose-dependent effects on brain functionality and physiology, and long-duration anesthesia administration could cause neurocognitive decline in animals and humans. However, the duration effect of isoflurane on the brain physiology and functionality still remains poorly understood. In the present study, cerebral blood flow (CBF) and functional connectivity of adult rhesus monkeys (maintained with 1% isoflurane for 4 h) were examined by using magnetic resonance imaging. The results demonstrate that long-duration isoflurane exposure could result in CBF reduction in most brain areas and functional connectivity decrease in the dominant default-mode network. This study reveals the anesthetic duration effects in the central nervous system of anesthetized subjects and suggests that such duration effects should be considered in examining the brain function of anesthetized animals or humans with contemporary neuroimaging approaches.
Keywords: : anesthesia, cerebral blood flow, default-mode network, non-human primate, pseudo continuous arterial spin-labeling
Introduction
Isoflurane is an inhalational anesthetic that is widely used for general anesthesia in medical procedures and biomedical studies. In particular, it is commonly utilized for sedation purpose in various in vivo neuroimaging (such as magnetic resonance imaging [MRI] and positron emission tomography [PET]) examinations of animals or uncooperative patients. The duration of anesthesia administration varies from minutes to hours, depending on the requirement of each specific surgical procedure or experimental design. It can last up to 24 h in some clinical cases (Bomberg et al., 2016).
Previous studies have reported that repeated exposure to anesthesia in children is an important factor of learning disabilities (Flick et al., 2011). Also, it has been demonstrated that the duration of anesthesia is one of the risk factors for early postoperative cognitive dysfunction (Moller et al., 1998), and the duration of general anesthesia is associated with the risk of cell death in the developing brains (McCann and Soriano, 2012). Meanwhile, it has been reported that an increased risk for neurobehavioral disturbances correlates positively with the duration of anesthesia exposure (Block et al., 2012; Wilder et al., 2009). Animal studies have further demonstrated that spatial memory was impaired for 2 weeks after long-duration administration of isoflurane in aged rats (Culley et al., 2004) and isoflurane might cause brain cell death, neurocognitive decline in immature rats (Stratmann et al., 2010).
Cerebral blood flow (CBF) quantifies the blood supply to the brain, is highly auto-regulated to maintain normal brain functionality, and closely coupled to brain metabolism. CBF decrease has been seen in normal aging (Chen et al., 2011) and is associated with cognitive decline (Birdsill et al., 2013; Hirsch et al., 1997; Jagust et al., 1992; Poels et al., 2008). CBF and cerebral metabolism can be altered by sedatives, analgesics, and anesthetics dramatically and the effects vary substantially from one drug to another (Van Aken and Van Hemelrijck, 1991; Werner, 1995). Isoflurane has strong dose-dependent effects on CBF as demonstrated in prior studies of anesthetized macaques or baboons (Li et al., 2013, 2014; Van Aken et al., 1986). The duration effects of isoflurane on CBF have also been previously examined in anesthetized dogs (McPherson and Traystman, 1988) and goats (Albrecht et al., 1983), and monkeys (McPherson et al., 1994), but the conclusions remain controversial.
Resting-state functional MRI (rsfMRI) is a robust tool that is used to examine the intrinsic functional connectivity, including the default mode network (DMN), in the brains of awake human subjects or anesthetized animals (Biswal et al., 1997; Deshpande et al., 2011; Keilholz et al., 2013; Koch et al., 2012; Liu et al., 2011; Meng et al., 2016; Murnane et al., 2015; Santhanam et al., 2011; Vincent et al., 2007; Wu et al., 2015; Zhao et al., 2008), and it is associated with CBF (Jann et al., 2015; Li et al., 2012). Therefore, we hypothesized that the CBF and brain connectivity might be affected by the duration of anesthesia administration in subjects maintained with isoflurane.
Non-human primates (NHPs) resemble most aspects of humans and are widely used in preclinical studies and various neuroscience investigations. Furthermore, previous studies have confirmed the existence of a DMN in monkeys and demonstrated the topological similarities of the monkey's DMN with the human's DMN (Mantini et al., 2011). One to 3% isoflurane (up to 5% for induction) is usually used for general anesthesia of NHPs or other animals. As isoflurane has a strong dose-dependent suppression effect on neural activation in the brain, a lower dosage of isoflurane is usually applied for light anesthesia in various neuroimaging studies of NHPs. In the present study, adult rhesus monkeys were used to examine the long-duration effects of 1% isoflurane on CBF and DMN by using the continuous arterial spin-labeling perfusion MRI and rsfMRI techniques.
Materials and Methods
Animal preparation
Adult female rhesus monkeys (n = 5, 7–11 years old) were employed in the present study. The animals were initially anesthetized with Telazol (5 mg/kg, i.m.), and then switched to ∼1% isoflurane mixed with 100% oxygen by using an isoflurane vaporizer (Patterson Veterinary, Devens, MA). The anesthetized and spontaneously breathing animals were intubated and immobilized with a home-made head holder and placed in the “supine” position during MRI scanning for about 4 h.
Respiration rate, isoflurane concentration (end-tidal), and Et-CO2 were continuously monitored with a PROCARE Monitor B40 anesthesia machine (GE Healthcare, Milwaukee, WI); heart rate and O2 saturation with a Nonin pulse oximeter (Nonin medical, Plymouth, MN); blood pressure by a Surgivet V6000 (Smiths Medical PM, Waukesha, WI); and body temperature with a Digi-Sense Temperature controller (Cole-Parmer, IL), respectively. Lactated ringer's solution was administered intravenously to prevent dehydration during scanning. The physiological parameters were recorded and maintained in normal ranges (Li et al., 2013). All procedures followed the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Data acquisition
MRI data collection was conducted by using a Siemens 3T Trio scanner (Siemens Medical Solutions, Malvern, PA) with an 8-channel phased-array volume coil (Invivo, Inc., FL). rsfMRI was conducted by using a gradient-echo Echo Planar Imaging (EPI) sequence (time of repetition [TR]/time of echo [TE] = 2060/25 ms, 34 contiguous slices to cover the whole brain, 430 volumes, field of view [FOV] = 96 × 96 mm, spatial resolution = 1.5 × 1.5 × 1.5 mm3). The single-shot EPI was applied for CBF measurement with the pseudo-continuous arterial spin-labeling MRI technique (Li et al., 2014; Wu et al., 2007). The MRI parameters were as follows: TR/TE = 3830/21 ms, FOV = 96 × 96 mm, data matrix = 64 × 64, 16 slices with slice thickness = 1.5 mm, labeling offset = 55 mm, post-labeling delay = 0.8 sec, and labeling duration = 2.0 sec. Eighty pairs of control and labeling images with six repetitions were acquired. CBF and rsfMRI data collection started ∼15 min after the animal was moved into the scanner and then repeated ∼3.5 h later.
T2-weighted images were acquired by using fast spin-echo sequences with TR/TE = 5040/125 ms, FOV = 96 × 96 mm, matrix = 128 × 128, slice thickness = 1.5 mm, 16 slices, and 2 averages. High-resolution structural T1-weighed images were acquired by using a 3D magnetization-prepared rapid gradient echo sequence with the generalized autocalibrating partial parallel acquisition (R = 2) (TR/TE = 3000/3.51 ms, FOV = 96 × 96 mm, spatial resolution = 0.5 × 0.5 × 0.5 mm3). The field map (TR/TE1/TE2 = 1100/5.36/7.82 ms, FOV = 96 × 96 mm) was obtained for each animal. The T1, T2-weighted MRI and field map and Diffusion Tensor Imaging (not reported in the present study) scans were conducted after the first set of rsfMRI, and CBF data were collected. Each scanning session lasted about 4 h.
Image data processing and analysis
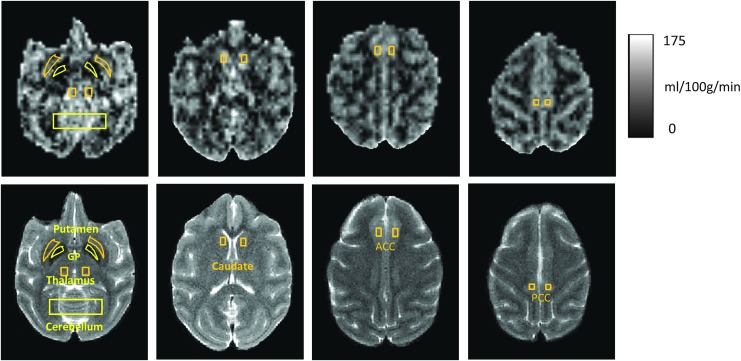
CBF data analyses were performed by using home-built Matlab scripts (MathWorks, MA) and Stimulate software (www.cmrr.umn.edu/stimulate) (Li et al., 2013). The bilateral caudate, putamen, globus pallidus, anterior cingulated cortex (ACC), posterior cingulate cortex (PCC), thalamus, cerebellum, white matter (WM), grey matter (GM), and cortical and subcortical cortex were selected as regions of interest (ROIs) to acquire averaged CBF values (Fig. 1). Paired t-test was performed to analyze the CBF differences statistically.
FIG. 1.
CBF maps of an adult macaque monkey acquired with pCASL at 3T. ROIs are illustrated on the CBF maps (top) and corresponding T2-weighted structural images (bottom). ACC, anterior cingulated cortex; CBF, cerebral blood flow; GP, globus pallidus; PCC, posterior cingulate cortex; pCASL, pseudo-continuous arterial spin-labeling; ROIs, regions of interest.
The rsfMRI data were preprocessed for image distortion correction by using the FSL software (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FUGUE). Slice timing correction, rigid body registration, regressing out of WM and cerebrospinal fluid time series, temporal filtering with 0.009–0.0237 Hz band-pass, and spatial smoothing with 2.5-mm full width at half maximum Gaussian blur were performed by using a script of AFNI (http://afni.nimh.nih.gov) (Li et al., 2008). Anatomical ROI for the whole PCC, ACC, and dorsal/media prefrontal cortex (DMPFC) were selected by using the graphical user interface of AFNI software (https://afni.nimh.nih.gov) with the monkey brain atlas (Saleem and Logothetis, 2007) and anatomical T1-weighted images as references. PCC was used as the seed region for the seed-based functional connectivity data analysis.
Z transformation was applied to the individual correlation maps to show normalized correlation maps. The averaged z values of connectivity between PCC and ACC or DMPFC were examined for statistical differences.
All statistical analyses were conducted in SPSS 21.0. p Values less than 0.05 were considered statistically significant.
Results
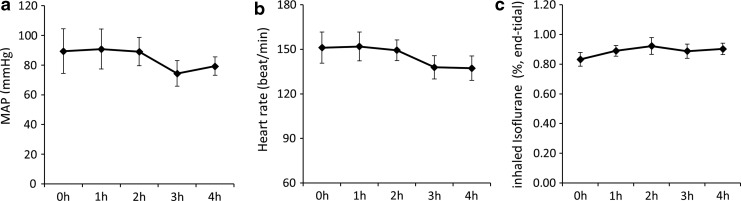
Mean arterial pressure (MAP) and heart rates were not showing significant changes during the 4-h anesthesia, even though decreasing trends were seen at 3 h post isoflurane administration (Fig. 2a, b). Isoflurane dosage was kept stable during each 4 h scanning session, and no significant changes of end-tidal concentration were seen (Fig. 2c).
FIG. 2.
MAP (a), heart rate (b) and end-tidal concentration changes of isoflurane (c) of rhesus monkeys during 4-h 1% isoflurane administration. Data are reported as mean ± SEM, *p < 0.05 versus 0.5 h isoflurane (baseline). MAP, mean arterial pressure.
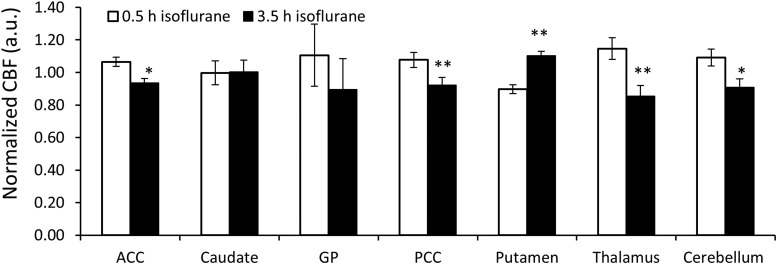
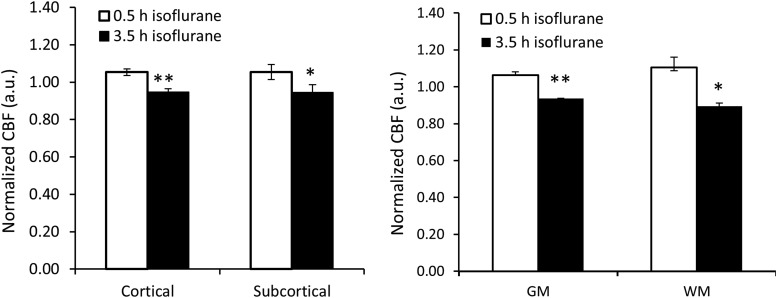
The CBF changes in different brain regions during the 4-h isoflurane administration are illustrated in Figure 3. CBF of each ROI at the end time point was normalized to that at the starting time point in each scan to minimize the inter-subject variation. CBF decreased significantly in thalamus (25.6% ± 8.9%, p < 0.01), ACC (9.5% ± 7.3%, p < 0.05), PCC (12.5% ± 7.7%, p < 0.01), and cerebellum (16.2% ± 7.5%, p < 0.05) (Fig. 3). In contrast, CBF significantly increased in putamen (15.1% ± 25.9%, p < 0.05) (Fig. 3). A significant CBF decrease was seen in both cortical (10.1% ± 3.2%, p < 0.01) and subcortical regions (14.2% ± 6.9%, p < 0.05) (Fig. 4 left). Also, CBF in both GM (10.8% ± 3.5%, p < 0.01) and WM (16.1% ± 9.7%, p < 0.05) (calculated with five consecutive slices) reduced significantly (Fig. 4 right).
FIG. 3.
CBF changes in selected ROIs of normal macaque monkeys maintained with 1% isoflurane administration (n = 5); error bar indicate standard deviation. **p < 0.01; *p < 0.05 versus 0.5 h. h, hour.
FIG. 4.
CBF changes in selected cortical and subcortical regions of macaque monkeys (n = 5) maintained with 1% isoflurane administration; error bar indicates standard deviation. **p < 0.01; *p < 0.05 versus 0.5 h.
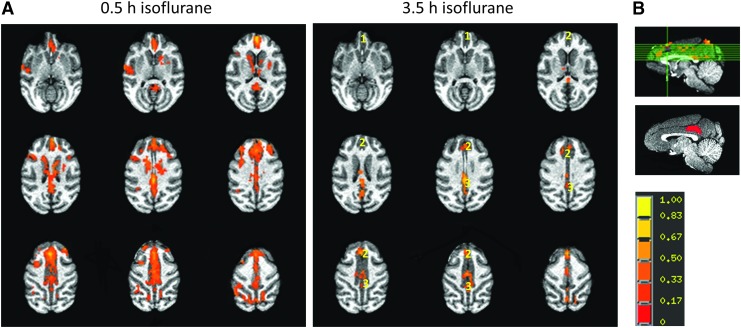
The duration effect of isoflurane on the default mode network of an adult monkey is illustrated in Figure 5. The rsfMRI results demonstrate that the correlation degree (z score) of PCC with either DMPFC or ACC obviously decreased after 3.5-h isoflurane administration. The decrease of the PCC-DMPFC connectivity was nearly significant statistically (p = 0.08), and that in PCC-ACC was significant (p < 0.01) (Table 1).
FIG. 5.
The representative changes of DMN in an adult rhesus monkey maintained with 1% isoflurane exposure. (A) The activation axial maps were generated with PCC as a seed. The color bar represents the magnitude of the regression coefficient (z score threshold p < 1 × 10−33, cluster threshold = 376 mm3/overall). (B) The slice locations are shown on a sagittal view of the monkey brain. The seed with PCC is highlighted as red. ROIs: 1, mPFC; 2, ACC; 3, PCC. DMN, default mode network; mPFC, medial prefrontal cortex.
Table 1.
The z Score Changes in the Network PCC-ACC and PCC-DMPFC of Adult Monkeys Maintained with 1% Isoflurane
| PCC-ACC | PCC-DMPFC | |
|---|---|---|
| 0.5 h isoflurane (baseline) | 0.67 ± 0.11 | 0.66 ± 0.10 |
| 3.5 h isoflurane | 0.36 ± 0.13a | 0.33 ± 0.15b |
Data are reported as means ± SEM.
p < 0.01.
p = 0.08 versus baseline isoflurane.
ACC, anterior cingulated cortex; DMPFC, dorsal/medial prefrontal cortex; PCC, posterior cingulate cortex.
Discussion
This study examined the changes of CBF and DMN in the adult macaque brain during long-duration administration of isoflurane under 1% maintenance dosage. The results demonstrate significant CBF reduction in most brain regions after 3-h isoflurane exposure. In addition, evident deactivation in the PCC dominant DMN was observed. These findings reveal the evident duration effect of isoflurane administration on the brain functionality of anesthetized macaques.
The administration duration of anesthetics in general anesthesia varies from minutes to hours, and long duration is usually applied in invasive surgical operation or non-surgical procedure in patients and animals by using intravenous sedatives or inhaled anesthetics such as isoflurane. Also, it is a popular procedure in neuroimaging studies of animals or children, uncooperative patients to minimize the motion artifacts. In fact, 1.0% isoflurane is usually applied for sedation purpose in non-surgical procedures such as neuroimaging examinations of animals or patients for a few hours and beyond (Satoh et al., 2002; Stevens et al., 1993; Zhang et al., 2015).
It has been demonstrated that isoflurane has dose-dependent effects on physiology (such as CBF, blood pressure, heart rate, cerebral metabolism, systemic vascular resistance) of monkeys and humans (Kato et al., 1992; Li et al., 2013, 2014; Lorenz et al., 2001; Reinstrup et al., 1995). In addition, neurobehavioral disturbances and cognitive decline have been reported in patients post-anesthesia (Block et al., 2012; Flick et al., 2011; Sprung et al., 2012; Wilder et al., 2009). As the cerebral physiology closely correlates with the neural activation and cognitive performance in the brain, it is important to examine the duration effects of isoflurane on CBF and brain functionality when subjects are anesthetized.
Long-duration effect of isoflurane on CBF
Isoflurane increases CBF in a dose-dependent manner by producing vasodilation through the ATP-sensitive K+ channel activation (Fujita et al., 2006; Iida et al., 1998). The administration of isoflurane can last for hours in animals and patients. However, the effect of long-duration administration of isoflurane on CBF remains controversial. Our current CBF results are in agreement with prior preclinical studies in anesthetized dogs (McPherson and Traystman, 1988) and goats (Albrecht et al., 1983). Also, the MAP and heart rates of monkeys in the present study were maintained in the normal ranges during the entire study of each session (although a decreasing tendency in MAP or heart rate was seen after 2 h of isoflurane exposure) (Fig. 2), in agreement with those seen in a previous study in rats under 1 minimum alveolar concentration (MAC) isoflurane for 4 h (Stratmann et al., 2010) and dogs (1.4% isoflurane from 2 to 6 h) (Brian et al., 1990).
Isoflurane duration-induced CBF decrease was not observed in prior studies of adults with 1.5 MAC (Kuroda et al., 1996, 1997), children with 1 MAC (Bisonnette and Leon, 1992) during prolonged administration of isoflurane (over 3 h), and dogs with 1% isoflurane for 3–4 h (Roald et al., 1989). In particular, increased CBF was seen in the forebrain and hindbrain of cynomolgus monkeys with 1 MAC isoflurane for 4 h reported by McPherson et al. (1994), different from the present findings. By comparing the experimental methods and materials between these primate and adult studies, such differences in the CBF findings are very likely due to the different experimental setting and procedures. (1) Mechanical ventilation (with pancuronium bromide administered) was applied in McPherson et al.'s (1994) primate study and Kuroda et al.'s (1996, 1997) studies in surgical patients. In addition, Phenylephrine was used in those patients to maintain cerebral perfusion pressure.
Previous reports also have indicated that mechanical ventilation might alter CBF (Baenziger et al., 1994; Raichle et al., 1970). In contrast, our monkeys were breathing spontaneously during each scanning session. (2) Initial CBF baseline might be different due to the usage of different induction drugs. Thiopental could result in lower CBF in comparison with Telazol (Joshi et al., 2005). Therefore, the baseline CBF measured in the present study could be different from Mcpherson et al.'s study due to the difference of the induction drugs. (3) The isoflurane dosage in McPherson's study was 1 MAC, slightly higher than that used in the present study. The dose-dependent effects of isoflurane on regional CBF have been previously reported (Bisonnette and Leon, 1992; Li et al., 2013, 2014; Van Aken et al., 1986). Therefore, the duration effect of isoflurane on CBF may be dose dependent as well.
In consideration of all the approach differences in the two experiments, it is not unexpected to have different findings between the two studies. In particular, these inconsistent findings suggest CBF's sensitivity to the experimental setting and procedures, including induction drugs, anesthetic dosages and durations, ventilation status, and maintenance of physiology of the subjects.
Interestingly, CBF in putamen was found to be significantly increased in our study, different from those in cortical and other subcortical structures such as thalamus (Fig. 3). The opposite changes in fMRI and perfusion measures in putamen were also previously reported in a rat study (Mishra et al., 2011). More investigation is needed to address such neuronal and vascular activity alteration in putamen after long-duration exposure of isoflurane.
The suppression effect of isoflurane on neuronal activation might be a major contribution to the CBF reduction caused by the long-duration administration. It is known that isoflurane induces suppression effect to neuronal activities via exerting antagonistic actions on N-methyl-d-aspartate receptors and enhancing GABAA receptor-mediated functions (Brosnan, 2011; Dong et al., 2013; Harrison et al., 1993; Shelton and Nicholson, 2010). The suppression effect is dose dependent, as seen in the isoflurane-induced burst suppression pattern (Ferron et al., 2009). The anesthetic potency of isoflurane is highly correlated with the lipid bilayer partition (Smith et al., 1981). As a longer duration of anesthesia allows a higher concentration of isoflurane in the lipid tissue of the nervous system, the neuronal activity might be decreasing progressively over the duration of anesthesia. Accordingly, CBF is reduced because of its close coupling with the brain metabolism in a normally functioning brain.
Long-duration effect of isoflurane on DMN of the brain
Previous studies have shown that the deactivation of DMN is related with cognitive decline (Hansen et al., 2014; Nelson et al., 2016; Onoda et al., 2012; Vidal-Pineiro et al., 2014). Vincent et al. (2010) demonstrated that DMN in human also exists in macaques under isoflurane (0.8–1.5%) in which PCC is shown as a dominant region in DMN. The resemblance of PCC correlation maps between the monkey and human suggests that many elements of the DMN system may be conserved across primate species.
In the present study, the network of PCC to its association areas was examined to investigate how DMN in monkeys would be affected during long-term isoflurane exposure. ACC is often considered a sub-section of the medial PFC (mPFC) in monkeys (Bissonette et al., 2013; Gabbott and Bacon, 1996). Dorsal PFC in rhesus macaques is comparable to the area nine in Buckner's report and a part of mPFC (Buckner et al., 2008). Therefore, both ACC and DMPFC were selected as hubs of PCC-dominated DMN in data analysis of the present study. Our result indicated that PCC-ACC associations decreased significantly (p < 0.01), and PCC-DMPFC associations decreased nearly significantly (p = 0.08, most likely due to the sample size) after 3.5 h of isoflurane exposure (Table 1 and Fig. 5). Probably, this is because anesthetic results in breakdown of large-scale synchronization between brain regions (Lu et al., 2007; Vincent et al., 2007).
Also, due to the fact that anesthetic causes a global loss of functional segregation/specialization, regional specificity is decreased or becoming more homogeneously connected to each other (Vincent et al., 2007). Meanwhile, because of the progressive increase of isoflurane concentration in the lipid tissue of the nervous system, longer silent periods or decreased neuronal activity could result in the suppress-burst neuronal activity pattern of isoflurane (Ferron et al., 2009). Consequently, the low-frequency fluctuation (the basis of connectivity analysis) can decrease, resulting in reduced functional connectivity after prolonged administration of isoflurane.
CBF is tightly coupled with neuronal activity and brain metabolism, and resting-state neuronal activity and energetics uses about 80% of brain energy (Shulman et al., 2004). Positive correlation between resting CBF (or brain metabolism) and brain connectivity in several regions (including PCC) was previously reported in arterial spin-labeling-perfusion (Zou et al., 2009) and fluorodeoxyglucose positron emission tomography studies (Di and Biswal, 2012). In addition, a previous study (Li et al., 2012) showed that the resting spontaneous brain activity varied with regional CBF in different structures and positive correlations were demonstrated between the findings with CBF and PCC functional connectivity in DMN.
A similar finding was also reported in another study of humans (Tak et al., 2015). Our finding of decrease in both DMN and CBF of PCC is consistent with that reported in prior studies. In addition, CBF and brain connectivity decrease is usually seen in subjects with cognitive decline (Onoda et al., 2012; Alosco et al., 2013). Our results suggest that the duration effects of isoflurane administration might be associated with the post-anesthesia cognitive decline seen in preclinical and clinical studies. Further behavior studies are needed to validate the relationship.
Isoflurane is a commonly used anesthetic in neuroimaging studies of animals such as rodents and NHPs, serving to eliminate motion artifacts, physiological stress, and training requirements (Hutchison et al., 2014). The animals can be maintained under anesthesia for 3–7 h or even longer. Our results suggest that the acquired neural activation information from anesthetized animals can be biased due to the duration effect of anesthesia. Therefore, CBF or functional MRI measures should be acquired sooner for better sensitivity in anesthetized subjects or the duration-dependent bias should be considered in data collection to avoid or minimize the long-duration effects. In addition, the duration effect of isoflurane on spontaneous functional connectivity can be varying substantially in different networks comprising PCC, ACC, and motor/visual cortex (Supplementary Data; Supplementary Data are available online at www.liebertpub.com/brain), suggesting that such an effect is heterogeneous across different brain networks.
In conclusion, the duration-dependent effect of isoflurane on CBF and brain functional connectivity can cause an unneglectable impact on the brain functionality. In addition, the findings suggest that such an effect can exist in other anesthetics as well and should be considered in various neuroimaging studies of anesthetized subjects.
Supplementary Material
Acknowledgments
The authors are grateful to Sudeep Patel, Ruth Connelly, and Doty Kempt (DVM) for assistance in data acquisition and animal handling. The project was funded by the National Center for Research Resources (P51RR000165) and is currently supported by the Office of Research Infrastructure Programs (OD P51OD011132).
Author Disclosure Statement
No competing financial interests exist.
References
- Albrecht RF, Miletich DJ, Madala LR. 1983. Normalization of cerebral blood flow during prolonged halothane anesthesia. Anesthesiology 58:26–31 [DOI] [PubMed] [Google Scholar]
- Alosco ML, Gunstad J, Jerskey BA, Xu X, Clark US, Hassenstab J, et al. . 2013. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav 3:626–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger O, Jaggi JL, Mueller AC, Morales CG, Lipp HP, Lipp AE, et al. . 1994. Cerebral blood flow in preterm infants affected by sex, mechanical ventilation, and intrauterine growth. Pediatr Neurol 11:319–324 [DOI] [PubMed] [Google Scholar]
- Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, et al. . 2013. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 21:1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisonnette B, Leon JE. 1992. Cerebrovascular stability during isoflurane anaesthesia in children. Can J Anaesth 39:128–134 [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. 2013. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res 250:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. 1997. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 10:165–170 [DOI] [PubMed] [Google Scholar]
- Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. 2012. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology 117:494–503 [DOI] [PubMed] [Google Scholar]
- Bomberg H, Groesdonk HV, Bellgardt M, Volk T, Meiser A. 2016. AnaConDa and Mirus for intensive care sedation, 24 h desflurane versus isoflurane in one patient. Springerplus 5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian JE, Jr., Traystman RJ, McPherson RW. 1990. Changes in cerebral blood flow over time during isoflurane anesthesia in dogs. J Neurosurg Anesthesiol 2:122–130 [DOI] [PubMed] [Google Scholar]
- Brosnan RJ. 2011. GABA(A) receptor antagonism increases NMDA receptor inhibition by isoflurane at a minimum alveolar concentration. Vet Anaesth Analg 38:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. 2011. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. 2004. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100:309–314 [DOI] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X. 2011. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage 54:1043–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Biswal BB. 2012. Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain Connect 2:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Wu X, Zhang G, Xu Z, Zhang Y, Gautam V, et al. . 2013. Isoflurane facilitates synaptic NMDA receptor endocytosis in mice primary neurons. Curr Mol Med 13:488–498 [DOI] [PubMed] [Google Scholar]
- Ferron JF, Kroeger D, Chever O, Amzica F. 2009. Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neurosci 29:9850–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. . 2011. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Ogura T, Tamagawa M, Uemura H, Sato T, Ishida A, et al. . 2006. A key role for the subunit SUR2B in the preferential activation of vascular KATP channels by isoflurane. Br J Pharmacol 149:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. 1996. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol 364:567–608 [DOI] [PubMed] [Google Scholar]
- Hansen NL, Lauritzen M, Mortensen EL, Osler M, Avlund K, Fagerlund B, et al. . 2014. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain's default mode network. Hum Brain Mapp 35:4488–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. 1993. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol 44:628–632 [PubMed] [Google Scholar]
- Hirsch C, Bartenstein P, Minoshima S, Mosch D, Willoch F, Buch K, et al. . 1997. Reduction of regional cerebral blood flow and cognitive impairment in patients with Alzheimer's disease: evaluation of an observer-independent analytic approach. Dement Geriatr Cogn Disord 8:98–104 [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. 2014. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain's functional architecture. Hum Brain Mapp 35:5754–5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. 1998. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology 89:954–960 [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Reed BR, Martin EM, Eberling JL, Nelson-Abbott RA. 1992. Cognitive function and regional cerebral blood flow in Parkinson's disease. Brain 115 (Pt 2):521–537 [DOI] [PubMed] [Google Scholar]
- Jann K, Gee DG, Kilroy E, Schwab S, Smith RX, Cannon TD, et al. . 2015. Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage 106:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Wang M, Etu JJ, Pile-Spellman J. 2005. Reducing cerebral blood flow increases the duration of electroencephalographic silence by intracarotid thiopental. Anesth Analg 101:851–858, table of contents [DOI] [PubMed] [Google Scholar]
- Kato M, Komatsu T, Kimura T, Sugiyama F, Nakashima K, Shimada Y. 1992. Spectral analysis of heart rate variability during isoflurane anesthesia. Anesthesiology 77:669–674 [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Magnuson ME, Pan WJ, Willis M, Thompson GJ. 2013. Dynamic properties of functional connectivity in the rodent. Brain Connect 3:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde AL, et al. . 2012. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer's disease. Neurobiol Aging 33:466–478 [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Murakami M, Tsuruta J, Murakawa T, Sakabe T. 1996. Preservation of the ration of cerebral blood flow/metabolic rate for oxygen during prolonged anesthesia with isoflurane, sevoflurane, and halothane in humans. Anesthesiology 84:555–561 [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Murakami M, Tsuruta J, Murakawa T, Sakabe T. 1997. Blood flow velocity of middle cerebral artery during prolonged anesthesia with halothane, isoflurane, and sevoflurane in humans. Anesthesiology 87:527–532 [DOI] [PubMed] [Google Scholar]
- Li CX, Patel S, Auerbach EJ, Zhang X. 2013. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 541:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Patel S, Wang DJ, Zhang X. 2014. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging 32:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. 2008. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behav 2:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu Y, Childress AR, Detre JA, Wang Z. 2012. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PLoS One 7:e44556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. 2011. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex 21:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz IH, Kolbitsch C, Hormann C, Schocke M, Felber S, Zschiegner F, et al. . 2001. Subanesthetic concentration of sevoflurane increases regional cerebral blood flow more, but regional cerebral blood volume less, than subanesthetic concentration of isoflurane in human volunteers. J Neurosurg Anesthesiol 13:288–295 [DOI] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, et al. . 2007. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A 104:18265–18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, et al. . 2011. Default mode of brain function in monkeys. J Neurosci 31:12954–12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann ME, Soriano SG. 2012. General anesthetics in pediatric anesthesia: influences on the developing brain. Curr Drug Targets 13:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RW, Kirsch JR, Tobin JR, Ghaly RF, Traystman RJ. 1994. Cerebral blood flow in primates is increased by isoflurane over time and is decreased by nitric oxide synthase inhibition. Anesthesiology 80:1320–1327 [DOI] [PubMed] [Google Scholar]
- McPherson RW, Traystman RJ. 1988. Effects of isoflurane on cerebral autoregulation in dogs. Anesthesiology 69:493–499 [DOI] [PubMed] [Google Scholar]
- Meng Y, Hu X, Bachevalier J, Zhang X. 2016. Decreased functional connectivity in dorsolateral prefrontal cortical networks in adult macaques with neonatal hippocampal lesions: relations to visual working memory deficits. Neurobiol Learn Mem 134 Pt A:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, et al. . 2011. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci 31:15053–15064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. . 1998. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351:857–861 [DOI] [PubMed] [Google Scholar]
- Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL. 2015. Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology (Berl) 232:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Savalia NK, Fishell AK, Gilmore AW, Zou F, Balota DA, et al. . 2016. Default mode network activity predicts early memory decline in healthy young adults aged 18–31. Cereb Cortex 26:3379–3389 [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. 2012. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci 24:2186–2198 [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, Vernooij MW, Krestin GP, Hofman A, Niessen WJ, et al. . 2008. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab 28:1652–1655 [DOI] [PubMed] [Google Scholar]
- Raichle ME, Posner JB, Plum F. 1970. Cerebral blood flow during and after hyperventilation. Arch Neurol 23:394–403 [DOI] [PubMed] [Google Scholar]
- Reinstrup P, Ryding E, Algotsson L, Messeter K, Asgeirsson B, Uski T. 1995. Distribution of cerebral blood flow during anesthesia with isoflurane or halothane in humans. Anesthesiology 82:359–366 [DOI] [PubMed] [Google Scholar]
- Roald OK, Forsman M, Steen PA. 1989. The effects of prolonged isoflurane anaesthesia on cerebral blood flow and metabolism in the dog. Acta Anaesthesiol Scand 33:210–213 [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. 2007. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press, London [Google Scholar]
- Santhanam P, Coles CD, Li Z, Li L, Lynch ME, Hu X. 2011. Default mode network dysfunction in adults with prenatal alcohol exposure. Psychiatry Res 194:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Abe M, Inoue S, Hirabayashi Y, Seo N. 2002. [Drug-induced side effects in a patient with status asthmaticus treated with long-term isoflurane inhalation]. Masui 51:418–421 [PubMed] [Google Scholar]
- Shelton KL, Nicholson KL. 2010. GABA(A) positive modulator and NMDA antagonist-like discriminative stimulus effects of isoflurane vapor in mice. Psychopharmacology (Berl) 212:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. 2004. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci 27:489–495 [DOI] [PubMed] [Google Scholar]
- Smith RA, Porter EG, Miller KW. 1981. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta 645:327–338 [DOI] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, et al. . 2012. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JJ, Griffin RM, Stow PJ. 1993. Prolonged use of isoflurane in a patient with tetanus. Br J Anaesth 70:107–109 [DOI] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. 2010. Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg 110:431–437 [DOI] [PubMed] [Google Scholar]
- Tak S, Polimeni JR, Wang DJ, Yan L, Chen JJ. 2015. Associations of resting-state fMRI functional connectivity with flow-BOLD coupling and regional vasculature. Brain Connect 5:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken H, Fitch W, Graham DI, Brussel T, Themann H. 1986. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg 65:565–574 [PubMed] [Google Scholar]
- Van Aken H, Van Hemelrijck J. 1991. Influence of anesthesia on cerebral blood flow and cerebral metabolism: an overview. Agressologie 32:303–306 [PubMed] [Google Scholar]
- Vidal-Pineiro D, Valls-Pedret C, Fernandez-Cabello S, Arenaza-Urquijo EM, Sala-Llonch R, Solana E, et al. . 2014. Decreased default node network connectivity correlates with age-associated structural and cognitive changes. Front Aging Neurosci 6:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Van Essen DC, Buckner RL. 2010. Functional connectivity of the macaque posterior parahippocampal cortex. J Neurophysiol 103:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. . 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86 [DOI] [PubMed] [Google Scholar]
- Werner C. 1995. [Effects of analgesia and sedation on cerebrovascular circulation, cerebral blood volume, cerebral metabolism and intracranial pressure]. Anaesthesist 44 Suppl 3:S566–S572 [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. . 2009. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. 2007. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 58:1020–1027 [DOI] [PubMed] [Google Scholar]
- Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, et al. . 2015. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci 35:12932–12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tong F, Li CX, Yan Y, Kempf D, Nair G, et al. . 2015. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS one 10:e0117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. 2008. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage 39:248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. 2009. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 48:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.