Abstract
High-intensity locomotor exercise is suggested to contribute to improved recovery of locomotor function after neurological injury. This may be secondary to exercise-intensity–dependent increases in neurotrophin expression demonstrated previously in control subjects. However, rigorous examination of intensity-dependent changes in neurotrophin levels is lacking in individuals with motor incomplete spinal cord injury (SCI). Therefore, the primary aim of this study was to evaluate the effect of locomotor exercise intensity on peripheral levels of brain-derived neurotrophic factor (BDNF) in individuals with incomplete SCI. We also explored the impact of the Val66Met single-nucleotide polymorphism (SNP) on the BDNF gene on intensity-dependent changes. Serum concentrations of BDNF and insulin-like growth factor-1 (IGF-1), as well as measures of cardiorespiratory dynamics, were evaluated across different levels of exercise intensity achieved during a graded-intensity, locomotor exercise paradigm in 11 individuals with incomplete SCI. Our results demonstrate a significant increase in serum BDNF at high, as compared to moderate, exercise intensities (p = 0.01) and 15 and 30 min post-exercise (p < 0.01 for both), with comparison to changes at low intensity approaching significance (p = 0.05). Serum IGF-1 demonstrated no intensity-dependent changes. Significant correlations were observed between changes in BDNF and specific indicators of exercise intensity (e.g., rating of perceived exertion; R = 0.43; p = 0.02). Additionally, the data suggest that Val66Met SNP carriers may not exhibit intensity-dependent changes in serum BDNF concentration. Given the known role of BDNF in experience-dependent neuroplasticity, these preliminary results suggest that exercise intensity modulates serum BDNF concentrations and may be an important parameter of physical rehabilitation interventions after neurological injury.
Key words: : brain-derived neurotrophic factor, high-intensity exercise, locomotion, spinal cord injury, Val66Met polymorphism
Introduction
Spinal cord injury (SCI) results in sensory and motor deficits that can lead to loss of functional walking capacity. In individuals with motor incomplete SCI, the extent of residual sensorimotor function can contribute substantially to the recovery of locomotor ability. Significant research efforts have identified two specific intervention parameters that promote locomotor recovery after neurological injury: task specificity and amount of practice.1,2 Selected studies also suggest that intensity of practice (modulated by gait speed and indirectly estimated by cardiovascular measures) may be another critical training parameter to improve locomotor function. Studies in subjects post-stroke demonstrate that locomotor training at high speeds or aerobic intensities (commonly manipulated by alterations in gait speed3–5) elicit greater improvements in locomotor function and increased neural activity in selected supraspinal centers,3 as compared to lower-speed or lower-intensity interventions.4,6,7 Importantly, participation in high-intensity motor activities is accomplished through increased neural and muscular activity, which can lead to improved long-term synaptic potentiation as demonstrated in basic8 and applied9 research studies. Together, these findings indicate that intensity of practice may be an important factor in potentiating experience-dependent neuroplasticity.
Direct evidence for the role of exercise intensity on motor recovery after neurological injury in humans is lacking. However, previous data in animal models suggest that brain-derived neurotrophic factor (BDNF) has a critical role in exercise-induced neuroplasticity10,11 that may contribute to improvements in locomotor function. Studies have demonstrated that BDNF, unlike most other neurotrophic factors, is released in an activity-dependent manner12 and is involved in short- and long-term plasticity throughout the central nervous system (CNS).13–15 Within the motor system, BDNF is known to influence motor neuron excitability12,16 and survival,17 remodeling of injured axons,17 and training-induced cortical excitability changes.18,19 In animal models of SCI, there is evidence of a positive correlation between exercise intensity and amount of spinal BDNF and related markers of synaptic plasticity.20–22 Critically, increased spinal BDNF and downstream effectors below the level of the lesion have been linked with the recovery of stepping after cervical hemisection.23
Given the beneficial actions of BDNF and the demonstrated ability to regulate its expression with neural activity, the effect of exercise on BDNF in humans has become an area of significant interest. Though the exact cellular mechanisms remain unclear, acute bouts of high-intensity aerobic exercise in healthy adults have consistently been shown to lead to transient increases in peripheral BDNF.24,25 Exercise is also suggested to regulate expression of other molecules, such as insulin-like growth factor-1 (IGF-1).26–29 IGF-1 is not only known to modulate BDNF expression, but also has overlapping signaling cascades with BDNF that similarly effect synaptic protein synthesis.26,30 As such, IGF-1 has also been suggested to contribute to exercise-induced BDNF synthesis.31,32 Yet, the impact of aerobic, as compared to resistance, exercise on serum IGF-1 may be more variable.33,34
In general, the effect of exercise intensity on growth factor concentrations in humans with incomplete SCI has not been thoroughly examined. One potential barrier to facilitating increased BDNF levels in individuals with incomplete SCI during exercise may be the reduced ability to achieve high absolute powers or cardiovascular intensities. Patients with incomplete SCI present, by definition, with decreased volitional descending drive, and this diminished capacity to engage the neuromuscular system can result in lower absolute capacity for high-intensity exercise relative to individuals without neurological injury.35 To date, only one study has evaluated the impact of exercise on peripheral BDNF in humans with motor complete SCI,36 with no studies in ambulatory individuals with motor incomplete SCI.
In addition to exercise intensity, genetic factors also impact activity-dependent BDNF release and influence neuroplastic changes that occur with exercise. Namely, a single-nucleotide polymorphism (SNP) on the BDNF gene (Val66Met) is known to decrease the activity-dependent release of BDNF.37 Present in ∼30% of the population,38 the Val66Met SNP has been linked to a reduction in short-term motor cortical plasticity19,39,40 and poorer error-based motor learning41,42 in healthy control subjects, as well as worse outcomes post-SCI.43 Therefore, this genetic variation, combined with the potential inability to fully activate the neuromuscular system post-SCI, may significantly mitigate the effect of exercise on peripheral BDNF levels.
The primary aim of this study was to determine the effect of different levels of locomotor exercise intensity on peripheral concentrations of selected neurotrophins in humans with motor incomplete SCI. We hypothesized that the peripheral concentration of BDNF would significantly increase as the intensity of exercise increased and that these changes would be positively correlated with different metrics of exercise intensity. Given the prevalence and potential impact of the Val66Met SNP on this exercise-dependent response, this factor was also assessed. We further hypothesized that subjects who possess the Val66Met SNP would exhibit minimal intensity-dependent changes in BDNF concentration.
Methods
Nineteen subjects met the following inclusion and exclusion criteria and were enrolled in this study. Selected measures were collected on all enrolled subjects; however, data from 8 subjects are not included in the analysis secondary to an inability to obtain blood samples (procedures described below) from these subjects. Inclusion criteria for participation were a history of motor incomplete SCI (>6 months) with spinal lesion above neurological level T10, between 18 and 75 years old, and an ability to independently complete at least three speeds during the graded-intensity treadmill test (described below). Exclusion criteria consisted of concurrent illness that could limit safety or walking performance, including: anemia (defined as hemoglobin levels of <13 g/dL for men and <12 g/dL for women); clotting disorders; unhealed decubiti; uncontrolled cardiopulmonary disease, including orthostatic hypotension and recurrent autonomic dysreflexia; active heterotopic ossification; and other peripheral or central neurological injury. Use of antispastic agents, antidepressants, or other serotonergic agents within the past 2 weeks also excluded patients from participation secondary to the known effects of these medications on motor output,44 locomotor function,45 and BDNF expression.46 All subjects obtained medical clearance and provided written informed consent before participation. All study procedures were conducted in accord with the Declaration of Helsinki and approved by the local institutional review board.
Data collection and analysis
Before exercise testing, clinical measures of spastic motor activity, strength, and overground gait speed were assessed by a licensed physical therapist. Spastic motor activity was evaluated using the modified Ashworth (mAsh) scores47 for bilateral knee flexors and extensors and the Spinal Cord Assessment Tool for Spastic Reflexes (SCATS).48 Raw scores for the mAsh were converted to an ordinal scale to allow for the calculation of a composite score. Scores for both measures of spastic motor activity were summed within and between legs to obtain a composite score44,49 (mAsh range, 0–20; SCATS range, 0–18). Strength was assessed with the International Standards for the Neurological Classification of SCI Lower Extremity Motor Scores (LEMS)50 for specific lower-extremity muscles. Raw scores for strength were summed within and between limbs to generate a composite score (range, 0–50). Overground gait speeds were collected over a 3.85-m instrumented walkway (GaitMat II; Equitest, Chalfont, PA) with 1.8 m on each end to allow for acceleration and deceleration. For collection of self-selected speed (SSS), subjects were instructed to walk at their normal, comfortable pace. Two trials were collected and averaged to determine overground walking speeds.
Subjects subsequently participated in a graded-intensity locomotor exercise paradigm on a treadmill. For this test, subjects were fitted with a harness for safety without body-weight support and instructed to walk on a motorized, instrumented treadmill (Bertec Corp, Colombus, OH) starting at 0.1 m/s for 2 min, with 0.1-m/s increases in speed every 2 min until the subject required support from the safety harness or voluntarily stopped the test. All subjects enrolled had performed graded exercise testing previously and were familiar with the testing conditions. To evaluate the effect of manipulating locomotor exercise intensity and to normalize across subjects, measures of interest were evaluated at three levels of intensity defined as a percentage of peak gait speed; low intensity was defined as 33%, moderate intensity as 66%, and high intensity as 100% of peak gait speed achieved during the exercise paradigm.
Indirect measures of exercise intensity were also determined at each speed during testing. Specifically, cardiorespiratory measures were collected continuously, and the subject's rating of perceived exertion (RPE)51 was collected during the last 30 sec of each speed increment. In cases where RPE was not successfully collected at the time point of interest (n = 5), the RPE reported during the preceding speed was utilized for analysis. Heart rate (HR; beats/min) and rate of oxygen consumption (VO2; mL/kg/min) were determined using a portable metabolic system (CosMed USA, Inc., Chicago, IL) calibrated before each testing with room air and a reference gas mixture (16% O2 and 5% CO2). Cardiorespiratory data were collected on a breath-by-breath basis and stored for subsequent analysis. HR and VO2 values collected in the last 30 sec of each speed increment were averaged. NetVO2 was calculated as the difference between the average VO2 at a particular speed and the average VO2 measured during 2 min of quiet sitting before exercise testing. VO2Peak was defined as the maximum NetVO2 collected during the exercise paradigm. To normalize measures across subjects, percentages of age predicted heart rate maximum (%PredHRmax) was determined using the formula: 208-0.7*age,52 and percentages of VO2Peak (%VO2Peak) were also calculated.
Serial venous blood draws were also collected before, during, and after exercise testing. Before testing, an intravenous (i.v.) catheter was placed in the subject's upper extremity by a registered nurse. Upon insertion, the i.v. was flushed with 10 mL of 0.9% normal saline (0.9%NS) to ensure patency. After initial placement, at least 30 min passed before blood samples were collected. Immediately before exercise testing, three blood samples were collected at 2-min intervals while the subject was sitting at rest. During testing, blood samples were collected during the last 30 sec of each speed increment. Finally, three blood samples were taken immediately after testing and at 15 and 30 min post-exercise. All samples were collected using an IV Leur Lock Collection set into a 10-mL serum separator vacutainer tube (BD; Franklin Lakes, NJ). When a 0.9%NS flush was used, an equivalent volume was drawn to waste before collection of the blood sample used for analysis to prevent dilution. Blood samples were kept at room temperature for 30 min to allow for clotting and then placed on ice. After clotting, all samples were centrifuged at 1000g for 15 min at 4°C (Thermo Fisher Scientific; Waltham, MA). Separated serum was divided into several aliquots in microcentrifuge tubes and stored at −80°C until analysis (Thermo Fisher Scientific).
Serum samples were analyzed for peripheral levels of BDNF, IGF-1, and lactate using commercially available kits. Concentrations of BDNF and IGF-1 were determined using Multiplex Assays (intra-assay precision of <10%; MILLIPLEX MAP Human Pituitary Magnetic Bead Panel 2 Kit and Human IGF-I, II Magnetic Bead Panel Kit; EMD Millipore; Darmstadt, Germany). Lactate concentrations were assessed using colormetric assays (Lactate Colormetric Assay Kit II; Biovision Incorporated, Milpitas, CA). All samples were analyzed in duplicate and mean concentrations were calculated; only duplicates with a coefficient of variation of less than 10% were accepted for analysis. Resting concentrations were determined as an average of the concentration found in the three serum samples taken before exercise. Changes in BDNF, IGF-1, and blood lactate concentration from resting levels at time points of interest were calculated as the absolute difference between the concentration at that time point and the average resting concentration as well as the percent change from average resting levels. In cases where a sample was not successfully obtained during exercise (n = 4), the group mean change for that level was imputed. Further, when timing of testing termination did not allow for sample collection at the subject's peak speed (n = 8), the sample collected immediately post-exercise was used for analysis. In instances when a sample was successfully collected both at peak speed and immediately post-exercise, an average at these time points was calculated and considered as high intensity.
Each subject also provided a 2-mL saliva sample in a DNA Self-Collection Kit (DNA Genotek, Kanata, Ontario, Canada) for processing and analysis. Samples were then sent to Rutgers Biomedical and Health Science for processing and analysis. Primers were created to amplify the region surrounding the SNP (Val66Met: rs6265) of the BDNF gene, and then each sample was examined for the presence or absence of the Val66Met SNP using a TaqMan genotyping assay. Subjects without the SNP are indicated as Val/Val, and subjects who were hetero- or homozygous for the SNP are indicated as Val/Met or Met/Met, respectively.
Statistical analysis
To determine the effect of exercise intensity on serum concentrations of BDNF and IGF-1 (s[BDNF] and s[IGF-1]) a repeated-measures analysis of variance (ANOVA) with five levels based on level of intensity or time post-exercise (low, moderate, high intensity, 15 min post-exercise, and 30 minutes post-exercise) was used. When significant differences were observed, planned comparisons with Bonferroni-corrected paired t-tests were performed. Values at high intensity were compared to those at low, moderate, and post-exercise time points, with α adjusted to 0.0125 for multiple comparisons. Similarly, a one-factor, repeated-measures ANOVA with three levels (low, moderate, and high intensity) was utilized to assess differences in indirect indicators of exercise intensity. When significant differences were observed, post-hoc paired t-tests were performed with α = 0.017 for the three comparisons. Correlations between changes in s[BDNF] during exercise and indirect measures of exercise intensity (Pearson's correlation coefficient) were also tested. Finally, group data were separated into two groups based on subject's BDNF genotype (Val/Val or Val/Met). A Freidman test was utilized to assess intensity-dependent changes in s[BDNF] in each group with α = 0.05. Relationships between changes in BDNF and indirect measures of exercise intensity in Val/Val subjects were also explored with Pearson's correlation coefficient. All data presented in text and tables are presented as mean ± standard deviation, and data presented in figures are presented as means ± standard error. All statistical analyses were performed with SPSS software (version 21; SPSS, Inc., Chicago, IL).
Results
Eleven subjects (2 female) with chronic motor incomplete SCI completed the present study. Subjects had an average age of 41 ± 14 years and duration of injury of 103 ± 85 months. Participants were independent ambulators, with an average SSS of 0.80 ± 0.32 m/s and Walking Index for Spinal Cord Injury-II (WISCI II) score of 19 ± 2.8. Consistent with population estimates, 36% of the subjects (n = 4) were found to have the Val66Met SNP on one allele (indicated as Met/Val vs Val/Val in Table 1). Other demographic and clinical characteristics of these subjects are displayed in Table 1.
Table 1.
Subjects' Demographic and Clinical Characteristics
| Subject | Age | Sex | DOI (mos) | LOI | AIS | LEMS | SCATS | mAsh | WISCI II | SSS (m/s) | Peak TM speed (m/s) | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | M | 65 | C6 | D | 39 | 14 | 12 | 20 | 0.6 | 1.2 | Met/Val |
| 2 | 63 | M | 91 | C4 | D | 43 | 11 | 2 | 19 | 0.6 | 1.0 | Val/Val |
| 3 | 49 | M | 299 | C5 | D | 40 | 9 | 10 | 19 | 0.8 | 0.8 | Val/Val |
| 4 | 49 | M | 188 | C5 | D | 49 | 9 | 12 | 20 | 0.7 | 1.0 | Met/Val |
| 5 | 30 | M | 80 | C3 | C | 44 | 10 | 5 | 13 | 0.2 | 0.4 | Val/Val |
| 6 | 53 | F | 109 | C4 | D | 48 | 6 | 0 | 20 | 1.1 | 1.4 | Met/Val |
| 7 | 40 | M | 168 | C5 | D | 37 | 12 | 13 | 13 | 0.5 | 0.8 | Val/Val |
| 8 | 46 | M | 54 | C7 | D | 50 | 5 | 0 | 20 | 0.9 | 1.5 | Val/Val |
| 9 | 23 | F | 23 | T4 | D | 41 | 6 | 4 | 20 | 1.1 | 1.2 | Val/Val |
| 10 | 26 | M | 19 | C5 | D | 50 | 10 | 3 | 20 | 1.2 | 1.6 | Met/Val |
| 11 | 22 | M | 39 | C4 | D | 41 | 6 | 5 | 20 | 1.1 | 1.2 | Val/Val |
Measures that capture scores for individual muscle strength or spastic motor activity are provided as the summation of scores from each tested muscle on bilateral lower extremities. For all measures, larger scores indicate increased strength, spastic motor behavior, or independence with ambulation.
M, male; F, female; DOI, duration of injury; mos, months; LOI, neurological level of injury; AIS, American Spinal Injury Association Impairment Scale Classification; LEMS, International Standards for the Neurological Classification of SCI Lower Extremity Motor Score (range, 0–50); mAsh, modified Ashworth (range, 0–10); SCATS, Spinal Cord Assessment Tool for Spastic Reflexes (range, 0–18); WISCI II, Walking Index for Spinal Cord Injury-II (range, 0–20); SSS, self-selected gait speed; TM, treadmill.
Manipulation of exercise intensity
In the present study, locomotor exercise intensity was manipulated by increasing gait speed, and levels of exercise intensity were normalized to the subject's peak gait speed reached (see Table 1) to allow for group mean comparisons. Subjects demonstrated a wide range of peak gait speeds reached during testing (0.4–1.6 m/s) and similarly varied total durations of exercise (8–32 min). The average speed reached at low, moderate, and high exercise intensities were 0.36 ± 0.12 (33% of peak speed), 0.75 ± 0.23 (66% of peak speed), and 1.1 ± 0.35 (100% of peak speed) m/s, respectively. Group mean comparisons across levels of exercise intensity demonstrated significant increases in absolute and relative cardiorespiratory and subjective measures of exercise intensity from low to moderate to high intensities (Table 2). Despite peak VO2 levels remaining well below normative values for individuals with poor fitness,53 subjects' HR and RPEs were consistent with normative data at low, moderate, and vigorous intensities.54 Of note, RPE data from 2 subjects were not collected.
Table 2.
Indirect Measures of Exercise Intensity Across Level of Exercise Intensity
| Rest | Low | Moderate | High | p value | Differences | |
|---|---|---|---|---|---|---|
| Heart rate (bpm) | 72 ± 15 | 95 ± 21 | 119 ± 23 | 152 ± 25 | <0.01 | L<M<H |
| Predicted HR max (%) | 40.0 ± 7.7 | 53 ± 11 | 67 ± 13 | 85 ± 15 | <0.01 | L<M<H |
| NetVO2 (mL/kg/min) | — | 7.0 ± 2.2 | 12.0 ± 3.2 | 16.0 ± 4.9 | <0.01 | L<M<H |
| Percentage peak VO2 (%) | 18.0 ± 5.0 | 53 ± 10 | 79 ± 12 | 96.0 ± 7.2 | <0.01 | L<M<H |
| RPE (a.u.) | — | 9.3 ± 2.5 | 12.0 ± 1.9 | 16.0 ± 1.8 | <0.01 | L<M<H |
| Blood lactate (mM) | 1.4 ± 0.5 | 1.6 ± 0.6 | 2.3 ± 1.1 | 3.4 ± 0.9 | <0.01 | L<M<H |
Comparisons made only between levels of intensity, not resting values. Resting values included for reference. Values are represented as means ± standard deviation.
HR, heart rate; max, maximum; VO2, oxygen consumption; RPE, rating of perceived exertion; a.u., arbitrary units.
Intensity-dependent changes in serum concentrations of brain-derived neurotrophic factor and insulin-like growth factor-1
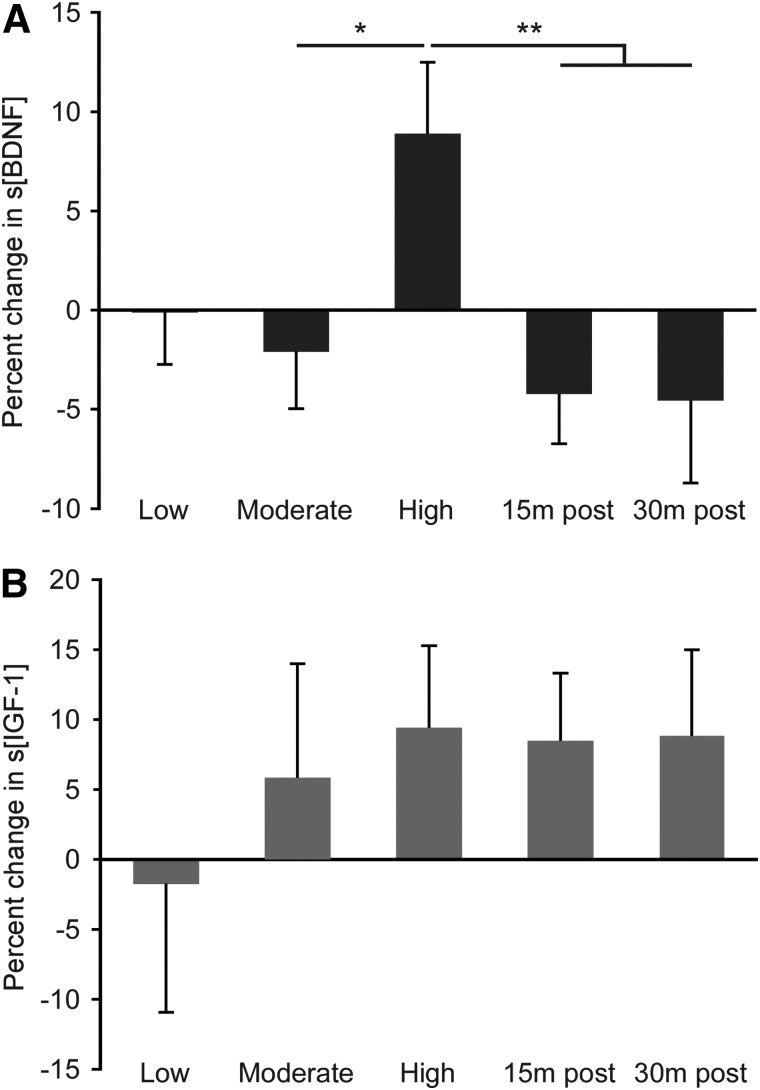
Evaluation of the percent change in s[BDNF] from resting levels demonstrated significant changes with exercise and recovery (Fig. 1A; p < 0.01). Specific differences include greater increases in s[BDNF] during high-intensity locomotor exercise, as compared to moderate intensity (p = 0.01), with comparison to low intensity approaching significance (p = 0.05). Further, s[BDNF] decreased significantly at 15 and 30 min post-exercise, as compared to high-intensity conditions (p < 0.01 for both comparisons). Similar intensity-dependent changes in s[IGF-1] were not found (Fig. 1B; p = 0.56).
FIG. 1.
Changes in s[BDNF] and s[IGF-1] with locomotor exercise. Percent change in s[BDNF] (A) and s[IGF-1] (B) during and post-locomotor exercise at different intensities. High levels of exercise intensity led to a significantly larger percent change in s[BDNF] from rest, as compared to that elicited at moderate intensity or post-exercise, with the difference between the change in BDNF at low and high intensities approaching significance. No significant intensity-dependent changes were found in s[IGF-1]. Error bars reflect standard error. BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth factor-1; m, minutes; s[BDNF], serum concentration of BDNF; s[IGF-1], serum concentration of IGF-1.
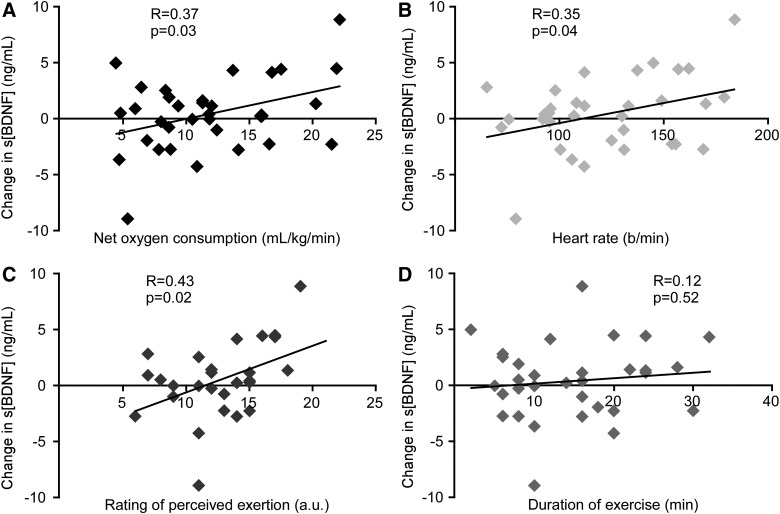
Low, but significant, correlations between changes in s[BDNF] and multiple measures of exercise intensity were observed, including NetVO2, HR, RPE (Fig. 2A–C), and percentage of VO2 peak (R = 0.38; p = 0.03, not pictured). Changes in s[BDNF] were also positively correlated to blood lactate levels (R = 0.30; p = 0.09) and %PredHRMax (R = 0.33; p = 0.06), though these relationships only approached significance. In addition, no relationship was found between changes in s[BDNF] and duration of the exercise (Fig. 2D), supporting the primary role of intensity in augmentation of s[BDNF] with exercise, as opposed to exercise duration.
FIG. 2.
Relationships between changes in s[BDNF] and indirect measures of exercise intensity and duration of exercise. Changes in s[BDNF] are significantly and positively correlated to Net VO2 (A), HR (B), and RPE (C; n = 9), but not duration of exercise (D). a.u., arbitrary units; BDNF, brain-derived neurotrophic factor; HR, heart rate; s[BDNF], serum concentration of BDNF; VO2, oxygen consumption.
Potentially blunted response of serum concentration of brain-derived neurotrophic factor to exercise in Val66Met single-nucleotide polymorphism carriers
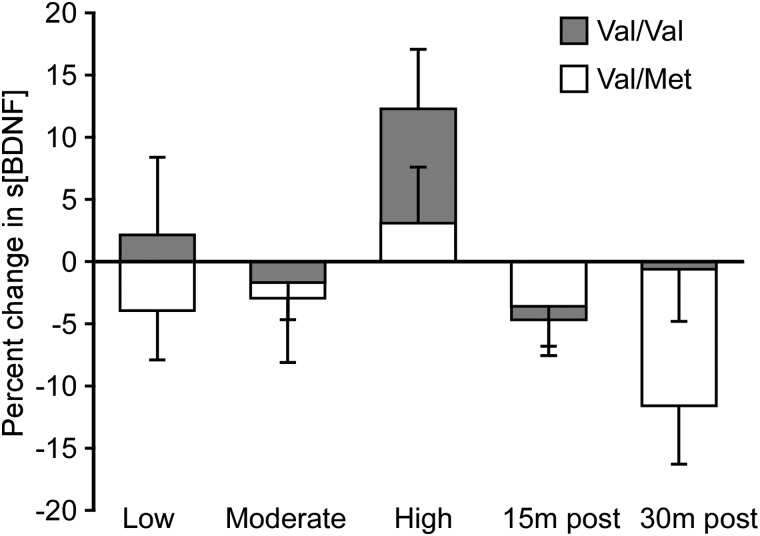
Val/Val subjects demonstrated a nearly significant trend of increased s[BDNF] with high-intensity exercise (p = 0.05), similar to that observed in the group data. In contrast, subjects who are Val/Met demonstrated no effect of exercise intensity on s[BDNF] (p = 0.56). Data are represented in Figure 3. Correlation analysis performed in Val/Val subjects indicate stronger positive relationships between changes in s[BDNF] and measures of exercise intensity, as compared to group data (e.g., NetVO2 [R = 0.47; p = 0.03] and RPE [R = 0.54; p = 0.02]).
FIG. 3.
Potential impact of Val66Met SNP on intensity-dependent changes in s[BDNF]. Percent change in s[BDNF] during and post-exercise from resting levels in Val/Val (gray; n = 7) and Val/Met (white; n = 4) subjects. Error bars reflect standard error. BDNF, brain-derived neurotrophic factor; m, minutes; s[BDNF], serum concentration of BDNF; SNP, single-nucleotide polymorphism.
Discussion
The present study examined the effect of a graded-intensity locomotor exercise paradigm on peripheral concentrations of specific neurotrophic factors in humans with motor incomplete SCI. To our knowledge, this is the first study to evaluate the effect of exercise on BDNF expression in humans with motor incomplete SCI and only the second of its kind in humans with SCI.36 We found that subjects with incomplete SCI were able to achieve relatively high levels of exercise intensity, with certain indirect indicators of exercise intensity reaching values similar to those in healthy adults. The data demonstrate an intensity-dependent change in the concentration of peripheral BDNF, with the largest increase in BDNF elicited at the highest exercise intensity. Further, changes in BDNF were positively correlated with cardiorespiratory and subjective measures of exercise intensity. The results also provide preliminary evidence for a potential genetic influence on intensity-dependent changes in BDNF concentrations.
Augmentation of peripheral BDNF with single bouts of aerobic exercise is well established in healthy control subjects.24,25 Consistent with previous reports in animal and human models, our data support that aerobic exercise is an effective means to manipulate serum levels of BDNF.24,55 Similar to other studies that suggest that resistance exercise may have a stronger influence on IGF-1 expression, we found no change in serum IGF-1 after aerobic exercise.56 Given the role of BDNF in the functional plasticity of the healthy and injured spinal cord,57–60 this result may be particularly important in the patient population studied here. Studies have demonstrated that increased BDNF in the spinal cord protects motor neurons from degeneration,17 increases axonal61 and collateral sprouting,62 and upregulates markers of synaptic plasticity.10,20,63 Importantly, increased spinal BDNF through training or exogenous application has also been linked to reflex normalization64 and recovery of locomotor function in cat and rodent models of SCI.23,62,65,66 These findings, together with our results, suggest that locomotor exercise may be an effective means of potentiating BDNF-mediated spinal plasticity and related improvements in motor function after incomplete SCI.
The results not only demonstrate a general effect of exercise on BDNF expression, but also indicate that increases in peripheral BDNF are related, in part, to the intensity of exercise. This finding is consistent with previous evaluations of the effect of exercise intensity on BDNF levels in control subjects.67–71 For example, data from Ferris and colleagues67 demonstrate that 30 min of exercise at a work rate 10% above versus 20% below ventilatory thresholds elicited a larger percent change in peripheral BDNF. The average percentages of HR maximum elicited by these work rates (∼86 and 70%, respectively) were comparable to those recorded in this study at high and moderate levels of intensity (∼85 and 67%, respectively). Similar to the results of Ferris and colleagues, these intensities resulted in significantly different changes in peripheral BDNF. The relationship between exercise intensity and levels of BDNF in humans with incomplete SCI demonstrated here may serve to further delineate parameters of physical interventions that potentiate neuroplasticity after neurological injury.
These results, however, are inconsistent with the outcome of the only other investigation into the effect of exercise on BDNF levels in individuals with SCI. Previously, Rojas-Vega and colleagues69 used two separate hand-cycling paradigms that differed in duration and relative intensity in 11 subjects with motor complete SCI. Subjects performed a 10-min warm-up exercise at ∼54% maximal HR followed by a time trial over a marathon distance at ∼89% maximal HR (average duration, ∼84 minutes). In contrast to the results of the current study, the lower-intensity exercise led to significantly increased BDNF when compared to resting levels, whereas the high-intensity exercise did not. Rojas Vega and colleagues69 suggest that the extended duration of the high-intensity exercise may explain this effect, discussing the possibility of increased degradation or uptake of BDNF during long-lasting, high-intensity exercise. Yet, the underlying mechanism for this outcome remains unclear. The discrepancy between the results from Rojas-Vega and colleagues and those presented here highlights the need for further research into the effect of exercise intensity on BDNF expression in individuals with SCI.
Notably, we examined the effects of a single exercise bout on serum levels of BDNF, but did not evaluate its potential relation to behavioral changes. Previously, exercise-induced increases in BDNF have been linked to improved performance on measures of cognitive function.67,68 Investigation into the effects of aerobic exercise on the motor system has recently been initiated to expand upon the current understanding of the impact of exercise on cognitive function and overall brain health.31,55,72 Studies have reported increased cerebellar excitability and cortical plasticity73–75 as well as improved motor skill acquisition76 and retention73,77 when motor learning was paired with a single bout of high-intensity aerobic exercise in healthy control subjects. One of these studies73 demonstrated that the high-intensity exercise was able to facilitate changes in motor output and also induced increases in circulating BDNF. Taken together, these studies suggest that high-intensity exercise may be an effective means of potentiating neuroplasticity to improve motor learning in humans. However, continued research is necessary to more clearly delineate the role of exercise-induced increases in BDNF on changes in motor output.
Interestingly, similar to previous reports,24,25 our data demonstrate that exercise-dependent changes in peripheral BDNF levels are fairly transient and return to baseline or below baseline levels less than 15 min post-exercise. One interpretation of this result could be that any central effect of increasing BDNF with exercise would be similarly transient. Yet, given the slow, amplified metabotropic signaling cascade that results from the BDNF/tropomyosin receptor kinase B interaction,13,15 it is highly likely that any effect from an increase in central BDNF signaling would outlast the changes in serum BDNF used as a biomarker. Data to support this contention are delineated above (please see previous paragraph). These findings suggest that central or behavioral effects of high-intensity exercise continue long after exercise-dependent changes in peripheral BDNF return to baseline.
The present study also provides a preliminarily estimate of the effect of the Val66Met SNP on exercise-dependent changes in circulating BDNF. Although there are currently no comparable investigations in human incomplete SCI, studies utilizing electrophysiological probes to evaluate plasticity of the motor system have elucidated some consequences of this SNP in control subjects. Previous research has suggested decreased use-dependent neuroplasticity39 and susceptibility to plasticity-inducing stimulation protocols in the motor cortex19 of individuals who carry the SNP, although findings from other studies are inconsistent.78 In addition, one study recently demonstrated similar effects of the Val66Met SNP on stimulation-induced spinal plasticity.40 Assuming a relationship between exercise-dependent increases in circulating BDNF and capacity for plasticity in the nervous system, these findings are consistent with our results. Yet, further investigation is necessary to substantiate these potential relationships and evaluate the role of other genetic factors that may modulate neuroplasticity.39,79
One limitation of this study is the small sample size (n = 11). A larger sample size could improve the generalizability or strengthen the results of the study; however, multiple factors contributed to the number of subjects that participated in this study. For example, the experimental protocol necessitated enrollment of subjects who could independently ambulate and modulate their walking speed. In addition, data from 8 subjects were not included secondary to the inability to consistently sample blood from the i.v. at rest. Both of these factors made it difficult to increase the sample size. Another potential limitation to consider is that the use of peripheral BDNF levels as a biomarker for central BDNF as the source of circulating BDNF remains debated. Neurons are the primary source of BDNF within the CNS, but there are several non-neuronal sites of BDNF synthesis and storage in the periphery.80–82 However, BDNF is known to bidirectionally cross the blood–brain-barrier,83,84 and studies have estimated that 75% of circulating BDNF comes from the CNS.85,86 Although these studies support the utility of peripheral BDNF as a biomarker for central levels, we can only speculate on the location within the CNS that may be the source of these intensity-dependent increases in BDNF, as based on extensive work in animal models thus far. Similarly, there is evidence to suggest that specific subject characteristics, such as fitness level87 and body composition,88 influence resting levels of peripheral BDNF, though the effect of these factors on exercise-dependent changes in BDNF has yet to be determined and should be considered in future work. Finally, we measured changes in BDNF in response to exercise intensities that were introduced in a continuous, ordered pattern. Although the graded-intensity exercise paradigm allowed for subjects with SCI to more easily accommodate to rapid changes in treadmill speeds, this design limits our ability to definitively determine the relative effects of exercise intensity, duration of exercise, or previous exercise. Future studies should utilize a randomized intensity interval exercise paradigm to focus on determining the effects of these other factors.
Conclusions/Implications
In summary, this study demonstrates that single-session, exercise-dependent changes in peripheral BDNF are related to the relative intensity of locomotor exercise in individuals with motor incomplete SCI. These results suggest that high-intensity exercise may promote neuroplastic changes, consistent with previous animal and human studies. This indicates that intensity may be an important parameter of physical rehabilitation interventions after neurological injury. Yet, substantial future research is necessary to determine the long-term effects of high-intensity training. This preliminary work provides a potential mechanistic basis and foundational evidence for a randomized, controlled trial to evaluate the efficacy of a high-intensity locomotor training paradigm that elicits repeated increases of BDNF expression on recovery of motor function. Additionally, these preliminary data suggest that acute high-intensity exercise may not augment BDNF levels in Val66Met carriers. This highlights the potential for a genetic contribution to efficacy of high-intensity rehabilitation interventions post-SCI, although more work is needed to further explore the impact of this genetic variation.
Acknowledgments
This work was funded, in part, by the Foundation for Physical Therapy, the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust, the Chicago Blackhawks Community Fund, and the NIH Ruth L. Kirschstein National Research Service Award (1F31NS084723). We thank Rossana Bonfonti, RN, and Rowena Dapitan, RN, for their assistance with the collection of blood samples.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Barbeau H., Nadeau S., and Garneau C. (2006). Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J. Neurotrauma 23, 571–585 [DOI] [PubMed] [Google Scholar]
- 2.Hornby T.G., Straube D.S., Kinnaird C.R., Holleran C.L., Echauz A.J., Rodriguez K.S., Wagner E.J., and Narducci E.A. (2011). Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Topics Stroke Rehabil. 18, 293–307 [DOI] [PubMed] [Google Scholar]
- 3.Luft A., Macko R., Forrester L., Goldberg A., and Hanley D.F. (2008). Post-stroke exercise rehabilitation: what we know about retraining the motor system and how it may apply to retraining the heart. Cleve Clin J Med 75, Suppl. 2, S83–S86 [DOI] [PubMed] [Google Scholar]
- 4.Macko R.F., Ivey F.M., and Forrester L.W. (2005). Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Topics Stroke Rehabil. 12, 45–57 [DOI] [PubMed] [Google Scholar]
- 5.Moore J.L., Roth E.J., Killian C., and Hornby T.G. (2010). Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke 41, 129–135 [DOI] [PubMed] [Google Scholar]
- 6.Sullivan K.J., Knowlton B.J., and Dobkin B.H. (2002). Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch. Phys. Med. Rehabil. 83, 683–691 [DOI] [PubMed] [Google Scholar]
- 7.Pohl M., Mehrholz J., Ritschel C., and Ruckriem S. (2002). Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke 33, 553–558 [DOI] [PubMed] [Google Scholar]
- 8.Lisman J., and Spruston N. (2005). Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat. Neurosci. 8, 839–841 [DOI] [PubMed] [Google Scholar]
- 9.Peinemann A., Reimer B., Loer C., Quartarone A., Munchau A., Conrad B. and Siebner H.R. (2004). Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 115, 1519–1526 [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Pinilla F., Ying Z., Roy R.R., Molteni R., and Edgerton V.R. (2002). Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 88, 2187–2195 [DOI] [PubMed] [Google Scholar]
- 11.Vaynman S., Ying Z., and Gomez-Pinilla F. (2003). Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 122, 647–657 [DOI] [PubMed] [Google Scholar]
- 12.Lu B. (2003). BDNF and activity-dependent synaptic modulation. Learn. Mem. 10, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshii A., and Constantine-Paton M. (2010). Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 70, 304–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottmann K., Mittmann T., and Lessmann V. (2009). BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp. Brain Res.. 199, 203–234 [DOI] [PubMed] [Google Scholar]
- 15.Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., and Kunugi H. (2010). BDNF function and intracellular signaling in neurons. Histol. Histopathol. 25, 237–258 [DOI] [PubMed] [Google Scholar]
- 16.Kovalchuk Y., Holthoff K., and Konnerth A. (2004). Neurotrophin action on a rapid timescale. Curr. Opin. Neurobiol. 14, 558–563 [DOI] [PubMed] [Google Scholar]
- 17.Jakeman L.B., Wei P., Guan Z., and Stokes B.T. (1998). Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 154, 170–184 [DOI] [PubMed] [Google Scholar]
- 18.Antal A., Chaieb L., Moliadze V., Monte-Silva K., Poreisz C., Thirugnanasambandam N., Nitsche M.A., Shoukier M., Ludwig H., and Paulus W. (2010). Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 3, 230–237 [DOI] [PubMed] [Google Scholar]
- 19.Cheeran B., Talelli P., Mori F., Koch G., Suppa A., Edwards M., Houlden H., Bhatia K., Greenwood R,. and Rothwell J.C. (2008). A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 586, 5717–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying Z., Roy R.R., Edgerton V.R., and Gomez-Pinilla F. (2005). Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 193, 411–419 [DOI] [PubMed] [Google Scholar]
- 21.Oliff H.S., Berchtold N.C., Isackson P., and Cotman C.W. (1998). Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res. Mol. Brain Res. 61, 147–153 [DOI] [PubMed] [Google Scholar]
- 22.Neeper S.A., Gomez-Pinilla F., Choi J., and Cotman C.W. (1996). Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726, 49–56 [PubMed] [Google Scholar]
- 23.Ying Z., Roy R.R., Zhong H., Zdunowski S., Edgerton V.R., and Gomez-Pinilla F. (2008). BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155, 1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knaepen K., Goekint M., Heyman E.M., and Meeusen R. (2010). Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 40, 765–801 [DOI] [PubMed] [Google Scholar]
- 25.Huang T., Larsen K.T., Ried-Larsen M., Moller N.C., and Andersen L.B. (2014). The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand. J. Med. Sci. Sports 24, 1–10 [DOI] [PubMed] [Google Scholar]
- 26.Carro E., Nunez A., Busiguina S., and Torres-Aleman I. (2000). Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trejo J.L., Carro E., and Torres-Aleman I. (2001). Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang P., Brandt J., Degerblad M., Enberg G., Kaijser L., Thoren M., and Hall K. (1990). Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur. J. Clin. Invest. 20, 285–292 [DOI] [PubMed] [Google Scholar]
- 29.Schwarz A.J., Brasel J.A., Hintz R.L., Mohan S., and Cooper D.M. (1996). Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 81, 3492–3497 [DOI] [PubMed] [Google Scholar]
- 30.Ding Q., Vaynman S., Akhavan M., Ying Z., and Gomez-Pinilla F. (2006). Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833 [DOI] [PubMed] [Google Scholar]
- 31.Cotman C.W., and Berchtold N.C. (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 [DOI] [PubMed] [Google Scholar]
- 32.Voss M.W., Erickson K.I., Prakash R.S., Chaddock L., Kim J.S., Alves H., Szabo A., Phillips S.M., Wojcicki T.R., Mailey E.L., Olson E.A., Gothe N., Vieira-Potter V.J., Martin S.A., Pence B.D., Cook M.D., Woods J.A., McAuley E., Kramer A.F. (2013). Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 28, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin E.W., Mullally S., Foley C., Warmington S.A., O'Mara S.M. and Kelly A.M. (2011). Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 104, 934–941 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen U.N., Mougin F., Simon-Rigaud M.L., Rouillon J.D., Marguet P. and Regnard J. (1998). Influence of exercise duration on serum insulin-like growth factor and its binding proteins in athletes. Eur. J. Appl. Physiol. Occup. Physiol. 78, 533–537 [DOI] [PubMed] [Google Scholar]
- 35.Gorman P.H., Scott W., York H., Theyagaraj M., Price-Miller N., McQuaid J., Eyvazzadeh M., Ivey F.M., and Macko R.F. (2016). Robotically assisted treadmill exercise training for improving peak fitness in chronic motor incomplete spinal cord injury: a randomized controlled trial. J. Spinal Cord Med. 39, 32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas Vega S., Abel T., Lindschulten R., Hollmann W., Bloch W., and Struder H.K. (2008). Impact of exercise on neuroplasticity-related proteins in spinal cord injured humans. Neuroscience 153, 1064–1070 [DOI] [PubMed] [Google Scholar]
- 37.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., and Weinberger D.R. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 [DOI] [PubMed] [Google Scholar]
- 38.Shimizu E., Hashimoto K., and Iyo M. (2004). Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 126B, 122–123 [DOI] [PubMed] [Google Scholar]
- 39.Kleim J.A., Chan S., Pringle E., Schallert K., Procaccio V., Jimenez R., and Cramer S.C. (2006). BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 9, 735–737 [DOI] [PubMed] [Google Scholar]
- 40.Lamy J.C., and Boakye M. (2013). BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J. Neurophysiol. 110, 109–116 [DOI] [PubMed] [Google Scholar]
- 41.McHughen S.A., Rodriguez P.F., Kleim J.A., Kleim E.D., Marchal Crespo L., Procaccio V., and Cramer S.C. (2010). BDNF val66met polymorphism influences motor system function in the human brain. Cereb. Cortex 20, 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joundi R.A., Lopez-Alonso V., Lago A., Brittain J.S., Fernandez-Del-Olmo M., Gomez-Garre P., Mir P., Jenkinson N., Cheeran B., and Brown P. (2012). The effect of BDNF val66met polymorphism on visuomotor adaptation. Exp. Brain Res. 223, 43–50 [DOI] [PubMed] [Google Scholar]
- 43.Abode-Iyamah K.O., Stoner K.E., Grossbach A.J., Viljoen S.V., McHenry C.L., Petrie M.A., Dahdaleh N.S., Grosland N.M., Shields R.K., and Howard M.A., 3rd (2016). Effects of brain derived neurotrophic factor Val66Met polymorphism in patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 24, 117–121 [DOI] [PubMed] [Google Scholar]
- 44.Thompson C.K., and Hornby T.G. (2013). Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medication in human incomplete spinal cord injury. J. Neurotrauma 30, 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leech K.A., Kinnaird C.R., and Hornby T.G. (2014). Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J. Neurotrauma 31, 1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinowich K., and Lu B. (2008). Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83 [DOI] [PubMed] [Google Scholar]
- 47.Bohannon R.W., and Smith M.B. (1987). Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 67, 206–207 [DOI] [PubMed] [Google Scholar]
- 48.Benz E.N., Hornby T.G., Bode R.K., Scheidt R.A., and Schmit B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59 [DOI] [PubMed] [Google Scholar]
- 49.Saraf P., Rafferty M.R., Moore J.L., Kahn J.H., Hendron K., Leech K., and Hornby T.G. (2010). Daily stepping in individuals with motor incomplete spinal cord injury. Phys. Ther. 90, 224–235 [DOI] [PubMed] [Google Scholar]
- 50.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borg G. (1970). Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 2, 92–98 [PubMed] [Google Scholar]
- 52.Tanaka H., Monahan K.D., and Seals D.R. (2001). Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 37, 153–156 [DOI] [PubMed] [Google Scholar]
- 53.Heywood V. (2006). Advanced Fitness Assessment and Exercise Prescription, 5th ed. Human Kinetics: Champaign, IL [Google Scholar]
- 54.Norton K., Norton L., and Sadgrove D. (2010). Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 13, 496–502 [DOI] [PubMed] [Google Scholar]
- 55.Vaynman S., and Gomez-Pinilla F. (2005). License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair 19, 283–295 [DOI] [PubMed] [Google Scholar]
- 56.Schiffer T., Schulte S., Hollmann W., Bloch W., and Strüder H.K. (2009). Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm. Metab. Res. 41, 250–254 [DOI] [PubMed] [Google Scholar]
- 57.Boyce V.S. and Mendell L.M. (2014). Neurotrophic factors in spinal cord injury. Handb. Exp. Pharmacol. 220, 443–460 [DOI] [PubMed] [Google Scholar]
- 58.Boyce V.S., and Mendell L.M. (2014). Neurotrophins and spinal circuit function. Front. Neural Circuits 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arvanian V. (2013). Role of neurotrophins in spinal plasticity and locomotion. Curr. Pharm. Des. 19, 4509–4516 [DOI] [PubMed] [Google Scholar]
- 60.Fouad K., and Tse A. (2008). Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol. Res. 30, 17–27 [DOI] [PubMed] [Google Scholar]
- 61.Lu P., Jones L.L., and Tuszynski M.H. (2005). BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 191, 344–360 [DOI] [PubMed] [Google Scholar]
- 62.Vavrek R., Girgis J., Tetzlaff W., Hiebert G.W., and Fouad K. (2006). BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain 129, 1534–1545 [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Pinilla F., Ying Z., Roy R.R., Hodgson J., and Edgerton V.R. (2004). Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J. Neurophysiol. 92, 3423–3432 [DOI] [PubMed] [Google Scholar]
- 64.Cote M.P., Azzam G.A., Lemay M.A., Zhukareva V., and Houle J.D. (2011). Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma 28, 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyce V.S., Tumolo M., Fischer I., Murray M., and Lemay M.A. (2007). Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J. Neurophysiol. 98, 1988–1996 [DOI] [PubMed] [Google Scholar]
- 66.Macias M., Nowicka D., Czupryn A., Sulejczak D., Skup M., Skangiel-Kramska J., and Czarkowska-Bauch J. (2009). Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 10, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferris L.T., Williams J.S., and Shen C.L. (2007). The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 39, 728–734 [DOI] [PubMed] [Google Scholar]
- 68.Winter B., Breitenstein C., Mooren F.C., Voelker K., Fobker M., Lechtermann A., Krueger K., Fromme A., Korsukewitz C., Floel A., and Knecht S. (2007). High impact running improves learning. Neurobiol. Learn Mem. 87, 597–609 [DOI] [PubMed] [Google Scholar]
- 69.Rojas Vega S., Struder H.K., Vera Wahrmann B., Schmidt A., Bloch W., and Hollmann W. (2006). Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 1121, 59–65 [DOI] [PubMed] [Google Scholar]
- 70.Goekint M., Heyman E., Roelands B., Njemini R., Bautmans I., Mets T., and Meeusen R. (2008). No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Med. Sci. Sports Exerc. 40, 1990–1996 [DOI] [PubMed] [Google Scholar]
- 71.Schmidt-Kassow M., Schadle S., Otterbein S., Thiel C., Doehring A., Lotsch J., and Kaiser J. (2012). Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport 23, 889–893 [DOI] [PubMed] [Google Scholar]
- 72.Cotman C.W., Berchtold N.C., and Christie L.A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472 [DOI] [PubMed] [Google Scholar]
- 73.Mang C.S., Snow N.J., Campbell K.L., Ross C.J., and Boyd L.A. (2014). A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J. Appl. Physiol. (1985) 117, 1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mang C.S., Brown K.E., Neva J.L., Snow N.J., Campbell K.L., and Boyd L.A. (2016). Promoting motor cortical plasticity with acute aerobic exercise: a role for cerebellar circuits. Neural Plast. 2016, 6797928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh A.M., Neva J.L., and Staines W.R. (2014). Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp. Brain Res. 232, 3675–3685 [DOI] [PubMed] [Google Scholar]
- 76.Statton M.A., Encarnacion M., Celnik P., and Bastian A.J. (2015). A single bout of moderate aerobic exercise improves motor skill acquisition. PLoS One 10, e0141393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roig M., Skriver K., Lundbye-Jensen J., Kiens B., and Nielsen J.B. (2012). A single bout of exercise improves motor memory. PLoS One 7, e44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaieb L., Antal A., Ambrus G.G., and Paulus W. (2014). Brain-derived neurotrophic factor: its impact upon neuroplasticity and neuroplasticity inducing transcranial brain stimulation protocols. Neurogenetics 15, 1–11 [DOI] [PubMed] [Google Scholar]
- 79.Witte A.V., Kurten J., Jansen S., Schirmacher A., Brand E., Sommer J., and Floel A. (2012). Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J. Neurosci. 32, 4553–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E., Kolbeck R., Hoppe E., Oropeza-Wekerle R.L., Bartke I., Stadelmann C., Lassmann H., Wekerle H., and Hohlfeld R. (1999). Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 189, 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakahashi T., Fujimura H., Altar C.A., Li J., Kambayashi J., Tandon N.N., and Sun B. (2000). Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 470, 113–117 [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto H., and Gurney M.E. (1990). Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 10, 3469–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan W., Banks W.A., Fasold M.B., Bluth J., and Kastin A.J. (1998). Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 84.Radka S.F., Holst P.A., Fritsche M., and Altar C.A. (1996). Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 709, 122–301 [DOI] [PubMed] [Google Scholar]
- 85.Rasmussen P., Brassard P., Adser H., Pedersen M.V., Leick L., Hart E., Secher N.H., Pedersen B.K., and Pilegaard H. (2009). Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 94, 1062–1069 [DOI] [PubMed] [Google Scholar]
- 86.Seifert T., Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B., Adser H., Jakobsen A.H., Pilegaard H., Nielsen H.B., and Secher N.H. (2010). Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R372–R377 [DOI] [PubMed] [Google Scholar]
- 87.Jung S.H., Kim J., Davis J.M., Blair S.N., and Cho H.C. (2011). Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur. J. Appl. Physiol. 111, 303–311 [DOI] [PubMed] [Google Scholar]
- 88.Wisse B.E., and Schwartz M.W. (2003). The skinny on neurotrophins. Nat. Neurosci. 6, 655–656 [DOI] [PubMed] [Google Scholar]