Abstract
Introduction: Diabetic retinopathy (DR) is the leading cause of new-onset blindness in adults. Telemedicine is a validated, cost-effective method to improve monitoring. However, little is known of patients’ attitudes toward telemedicine for DR. Our study explores factors that influence patients’ attitudes toward participating in telemedicine. Materials and Methods: Ninety seven participants in a university and the Veterans Administration setting completed a survey. Only people with diabetes mellitus (DM) were included. The main outcome was willingness to participate in telemedicine. The other outcomes were perceived convenience and impact on the patient–physician relationship. Participants reported demographic information, comorbidities, and access to healthcare. Analysis was performed with t-tests and multivariable logistic regression. Results: Demographic factors were not associated with the outcomes (all p > 0.05). Patients had decreased odds of willingness if they valued the patient–physician relationship (adjusted odds ratio [OR] = 0.08, confidence interval [CI] = 0.02–0.35, p = 0.001) or had a longer duration of diabetes (adjusted OR = 0.93, CI = 0.88–0.99, p = 0.02). Patients had increased odds of willingness if they perceived increased convenience (adjusted OR = 8.10, CI = 1.77–36.97, p = 0.01) or had more systemic comorbidities (adjusted OR = 1.85, CI = 1.10–3.11, p = 0.02). Discussion: It is critical to understand the attitudes of people with DM where telemedicine shows promise for disease management and end-organ damage prevention. Patients’ attitudes are influenced by their health and perceptions, but not by their demographics. Receptive patients focus on convenience, whereas unreceptive patients strongly value their patient–physician relationships or have long-standing DM. Telemedicine monitoring should be designed for people who are in need and receptive to telemedicine.
Keywords: : e-health, ophthalmology, telehealth, telemedicine
Introduction
By 2030, diabetes mellitus (DM) will affect an estimated 336 million people worldwide.1 Approximately 35% of individuals with DM have diabetic retinopathy (DR), which is the leading cause of new-onset blindness globally as well as in the United States among adults.2,3 The American Academy of Ophthalmology and the American Diabetes Association generally recommend an annual eye examination for people with diabetes.4,5 Less than 65% of people with diabetes receive these screenings and in underserved populations, the rates can be as low as 10–20%.6–8
Poor health status, legal blindness, younger age, and short duration of diabetes are associated with lower rates of follow-up of eye examinations.6,9,10 Barriers to care include lack of access to care, high costs, limited insurance, distance, time away from work, and difficulty with transportation.9–15 Low comprehension of risk for DR, even after discussions with the provider, also leads to lack of adherence to eye screening recommendations.6,10 In one study, 91% of patients cited “the doctor's recommendation” as a key reason to follow up with eye examinations.16 DR telemedicine programs address some of these barriers through point-of-care eye imaging at primary care offices, and they are cost-effective, efficient, and accurate with high sensitivity rates (up to 95%).17–21 A randomized control trial showed that screening rates were 56% for traditional, in-person visits compared with 94% in the telemedicine group.22 In England and Wales, DR is no longer the leading cause of blindness, which is attributed to a national DR telemedicine screening program.23 Telemedicine for DR screening has the potential to grow rapidly as a result of these successes.24–26
Despite the promise of telemedicine for DR, we do not understand patients’ perceptions of telemedicine for eye disease management. Patient satisfaction with DR telemedicine varies widely from 35% to 99%.27–31 But patient satisfaction does not measure patients’ underlying beliefs. It is essential to engage patients to understand their attitudes, concerns, and perceptions before wide-scale implementation. This is especially critical in the United States, where a nationally sponsored program is less likely to be implemented. Telemedicine programs should reflect patients’ beliefs and be tailored toward those most in need and those most likely to utilize this type of service. We performed a study of patients, largely unfamiliar with telemedicine, to explore their attitudes toward telemedicine and their willingness to participate in a telemedicine program for DR screening.
Materials and Methods
The Institutional Review Board at Duke University and the Durham Veterans Administration approved this study. Participants were recruited from the Duke University Medical Center and from the Durham Veterans Affairs primary care and endocrine clinics, and all eligible patients received informed consent. Consecutive adults with diabetes from a convenience sample of patients were recruited from these centers. The study coordinator identified individuals who had DM in coordination with the treating physician and clinic. Then, the study coordinator approached the participants in the waiting room or after the examination by the physician and took the patients to a private interview space to conduct the study. There, the study coordinator re-confirmed the diagnosis of diabetes before survey administration.
Survey content
The survey included questions on demographics (patient age, sex, race, education level), duration of DM, ocular and systemic comorbidities, access to medical care, and attitudes toward telemedicine for DR (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tmj). Patients reported ocular comorbidities that included: cataracts, glaucoma, macular degeneration, lazy eye, dry eyes, diabetic eye disease, or other eye problems. The number of reported ocular comorbidities was summed for analysis. Participants reported systemic comorbidities using ten items from a modified Charlson Comorbidity Index (Supplementary Table S1).32 The number of reported systemic comorbidities was summed for analysis. The RAND tele-ophthalmology questionnaire was used to assess information that was specific to people with DM, including duration of diabetes.33 The survey utilized validated instruments to assess access to medical care.34 This was then scored on a 5-point scale based on the patient's level of agreement with five different statements. Scores for each question were summed (range 5 to 25). Higher scores represented greater access to medical care (Supplementary Table S2).
During the survey, the interviewer asked, “Have you heard of telemedicine?” Then, the interviewer read a one-paragraph explanation of telemedicine for DR screening followed by the opportunity to ask questions.
The statements to measure patients’ attitudes were developed through cognitive interviewing. First, the instrument was assessed for face validity by advisors, members of the study team, and medical colleagues. Then, 42 patients completed open-ended cognitive interviews for further feedback (not included in final analysis). These patients were asked what they understood from the questions, and their feedback was used to refine the statements until thematic saturation was achieved.
The three measures of patients’ attitudes were: (1) willingness to participate in telemedicine (Willingness) stated as “I would be willing to receive my exams this way.”; (2) perception of the convenience of telemedicine (Convenience) stated as “I believe this method of eye exam would be more convenient than going to a separate eye appointment.”; and (3) the perceived impact of telemedicine on the patient–physician relationship (Relationship) stated as “I would miss interacting with my eye doctor in person.” Responses were recorded on a five-point Likert scale and then converted to binary outcomes by combining “strongly agree” with “agree,” and combining “uncertain,” “disagree,” and “strongly disagree.”
Survey administration
Two research assistants conducted all interviews in person with the participants. Interviews were standardized by protocol. To test the protocol for inter-interviewer reliability, both research assistants interviewed the same three participants. Responses to the telemedicine attitude scale were compared after each interview, and reliability was confirmed.
Statistical analysis
Participant characteristics were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Our primary outcome of interest was participants’ willingness to participate in telemedicine. Predictor variables included participant demographic factors (age, sex, race, and education level), duration of DM, number of ocular and systemic comorbidities, and access to care. We included patients’ perception of convenience and patient–physician relationship to assess their impact on willingness to participate in DR screening via telemedicine. Our secondary outcome measures were patient perception of convenience and the perception of the patient–physician relationship. We analyzed outcomes using univariable and multivariable logistic regression models. The model generated odds ratios with 95% confidence intervals (95% confidence interval). Regression diagnostics were performed, including checks for multicollinearity. We considered p-values less than 0.05 to be statistically significant. All statistical analysis was performed using Stata 11.0 (Statacorp, College Station, TX).
Results
Ninety-seven participants completed the survey (Table 1). The median age of participants was 57 years (interquartile range = 52–70 years). Thirty (31%) of 97 participants were women. The median duration of diabetes was 10 years (interquartile range = 5–20 years). Of 97 participants, only 3 (3%) had heard of telemedicine before the interview.
Table 1.
Characteristics of Survey Participants (n = 97)
| MEDIAN (RANGE) | INTERQUARTILE RANGE | |
|---|---|---|
| Age (years) | 57 (20–84) | 52–70 |
| Diabetes duration (years) | 10 (0.02–48) | 5–20 |
| Number of systemic comorbidities | 2 (0–8) | 1–3 |
| Number of ophthalmologic comorbidities | 1 (0–3) | 0–1 |
| NUMBER | PERCENTAGE | |
|---|---|---|
| Female | 30 | 30.9 |
| Race | ||
| Caucasian | 63 | 65.0% |
| Black | 30 | 30.9% |
| Native American/American Indian | 2 | 2.1% |
| Other | 2 | 2.1% |
| Education Level | ||
| Less than 8th grade | 10 | 10.3% |
| Some high school | 5 | 5.2% |
| High school graduate or GED | 29 | 3.0% |
| Some college or 2-year degree | 28 | 28.9% |
| 4-year college graduate | 15 | 15.5% |
| Postgraduate | 10 | 10.3% |
| Diabetes therapy | ||
| No medications | 9 | 9.3% |
| Oral medications only | 35 | 36.1% |
| Oral medications and insulin | 17 | 16.5% |
| Insulin only | 36 | 37.1% |
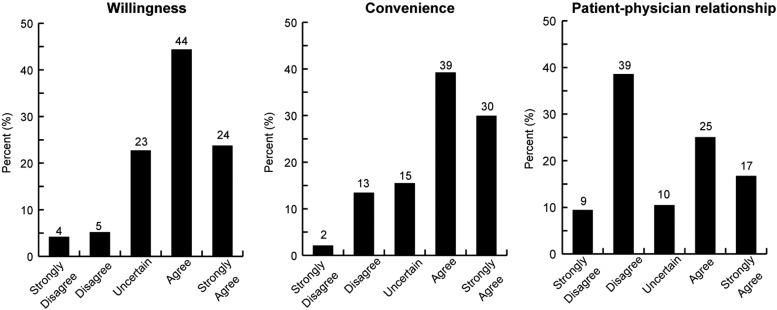
Thirty-one (32%) of the 97 participants were either not willing or uncertain of participating in telemedicine. Of the 97 participants, 67 (69%) believed that telemedicine would be more convenient than an in-person eye exam. Of the 96 participants, 50 (48%) reported that they would not miss their relationship with the eye physician (Fig. 1-Distribution of responses).
Fig. 1.
Attitudes toward telemedicine for diabetic retinopathy for patients with diabetes mellitus. Distribution of participant responses for survey questions regarding telemedicine for diabetic retinopathy is displayed in the figure. Patients answered strongly disagree, disagree, uncertain, agree, or strongly agree for the following questions: “I would be willing to receive my eye exams this way” (left), “I believe this method of eye exam would be more convenient than going to a separate eye appointment” (center), and “I would miss interacting with my eye doctor in person” (right).
Participant demographic factors, including age, sex, race, and education level, were not associated with willingness, convenience, or the patient–physician relationship (all p > 0.05) (Tables 2–4).
Table 2.
Logistic Regression Models for Willingness to Accept Telemedicine for Diabetic Retinopathy Examinations
| UNIVARIABLEa | MULTIVARIABLEb | |||
|---|---|---|---|---|
| OR (95% CI) | P VALUE | OR (95% CI) | P VALUE | |
| Age (per year increase) | 0.98 (0.95–1.02) | 0.27 | 1.00 (0.95–1.06) | 0.96 |
| Female (Ref = Male) | 0.48 (0.19–1.18) | 0.11 | 0.27 (0.06–1.28) | 0.10 |
| Educationc | ||||
| Some high school | 0.44 (0.05–3.98) | 0.47 | 0.65 (0.26–16.24) | 0.80 |
| High school graduate or GED | 1.27 (0.29–5.56) | 0.75 | 1.63 (0.18–14.76) | 0.67 |
| Some college or 2-year degree | 2.44 (0.52–11.57) | 0.26 | 1.87 (0.18–18.92) | 0.60 |
| 4-year college graduate | 1.33 (0.25–7.01) | 0.73 | 2.22 (0.17–29.45) | 0.55 |
| Postgraduate | 1.56 (0.24–9.91) | 0.64 | 2.13 (0.10–43.09) | 0.62 |
| Raced | ||||
| Black | 0.55 (0.22–1.39) | 0.21 | 0.63 (0.16–2.52) | 0.16 |
| Native American/American Indian | 0.37 (0.02–6.24) | 0.49 | 0.13 (0.00–3.61) | 0.23 |
| Duration of diabetes mellitus (per year increase) | 0.96 (0.93–0.99) | 0.02 | 0.93 (0.88–0.99) | 0.02 |
| Number of systemic comorbidities | 1.12 (0.87–1.43) | 0.38 | 1.85 (1.10–3.12) | 0.02 |
| Number of ophthalmologic comorbidities | 0.68 (0.42–1.08) | 0.10 | 1.21 (0.55–2.67) | 0.63 |
| Access to care scale | 1.00 (0.92–1.08) | 0.98 | 1.06 (0.94–1.20) | 0.34 |
| Convenience | 6.23 (2.41–16.10) | 0.0002 | 8.10 (1.77–36.97) | 0.007 |
| Patient–physician relationship | 0.14 (0.05–0.36) | 0.00006 | 0.08 (0.02–0.35) | 0.001 |
Univariable logistic regression.
Multivariable logistic regression adjusted for all other variables in the table.
The referent category is “less than 8th grade.”
The referent category is “Caucasian.”
OR, odds ratio; CI, confidence interval.
Table 3.
Logistic Regression Models for Perception of Convenience of Telemedicine for Diabetic Retinopathy Examinations
| UNIVARIABLEa | MULTIVARIABLEb | |||
|---|---|---|---|---|
| OR (95% CI) | P VALUE | OR (95% CI) | P VALUE | |
| Age (per year increase) | 0.99 (0.96–1.03) | 0.63 | 0.99 (0.95–1.02) | 0.47 |
| Female (Ref = Male) | 1.07 (0.42–2.72) | 0.90 | 1.88 (0.57–6.25) | 0.30 |
| Educationc | ||||
| Some high school | 1.00 (0.11–8.95) | 1.00 | 0.17 (0.01–2.86) | 0.22 |
| High school graduate or GED | 1.09 (0.25–4.75) | 0.91 | 0.20 (0.02–1.63) | 0.13 |
| Some college or 2-year degree | 2.44 (0.52–11.57) | 0.26 | 1.07 (0.16–7.34) | 0.95 |
| 4-year college graduate | 1.33 (0.25–7.01) | 0.73 | 0.18 (0.02–1.88) | 0.15 |
| Postgraduate | 2.67 (0.36–19.71) | 0.34 | 0.31 (0.02–4.54) | 0.39 |
| Raced | ||||
| Black | 0.86 (0.34–2.19) | 0.76 | 0.72 (0.22–2.34) | 0.59 |
| Native American/American Indian | 0.43 (0.03–7.27) | 0.56 | 0.88 (0.03–27.15) | 0.94 |
| Duration of diabetes mellitus (per year increase) | 0.98 (0.95–1.01) | 0.26 | 0.98 (0.94–1.03) | 0.38 |
| Number of systemic comorbidities | 0.92 (0.73–1.16) | 0.47 | 1.03 (0.73–1.45) | 0.85 |
| Number of ophthalmologic comorbidities | 0.57 (0.35–0.92) | 0.02 | 0.32 (0.15–0.71) | 0.005 |
| Access to care scale | 0.90 (0.81–1.00) | 0.06 | 0.80 (0.68–0.93) | 0.004 |
Univariable logistic regression.
Multivariable logistic regression adjusted for all other variables in the table.
The referent category is “less than 8th grade.”
The referent category is “Caucasian.”
OR, odds ratio; CI, confidence interval.
Table 4.
Logistic Regression Models for Missing the Patient–Physician Relationship in Telemedicine for Diabetic Retinopathy Examinations
| UNIVARIABLEa | MULTIVARIABLEb | |||
|---|---|---|---|---|
| OR (95% CI) | P VALUE | OR (95% CI) | P VALUE | |
| Age (per year increase) | 0.99 (0.96–1.03) | 0.73 | 0.99 (0.95–1.03) | 0.52 |
| Female | 1.10 (0.46–2.64) | 0.82 | 1.37 (0.45–4.13) | 0.58 |
| Educationc | ||||
| Some high school | 0.64 (0.07–6.06) | 0.70 | 1.38 (0.08–23.97) | 0.82 |
| High school graduate or GED | 0.30 (0.06–1.41) | 0.13 | 0.54 (0.10–2.96) | 0.48 |
| Some college or 2-year degree | 0.15 (0.03–0.75) | 0.02 | 0.20 (0.03–1.18) | 0.08 |
| 4-year college graduate | 0.38 (0.07–2.03) | 0.26 | 0.87 (1.13–6.01) | 0.89 |
| Postgraduate | 0.29 (0.04–1.82) | 0.19 | 0.40 (0.05–3.34) | 0.40 |
| Raced | ||||
| Black | 1.42 (0.58–3.44) | 0.44 | 1.71 (0.58–5.02) | 0.33 |
| Native American/American Indian | 1.52 (0.09–25.43) | 0.77 | 0.92 (0.02–36.41) | 0.96 |
| Duration of diabetes mellitus (per year increase) | 1.02 (0.99–1.06) | 0.22 | 1.01 (0.97–1.05) | 0.62 |
| Number of systemic comorbidities | 1.33 (1.05–1.70) | 0.02 | 1.36 (0.98–1.90) | 0.07 |
| Number of ophthalmologic comorbidities | 1.88 (1.16–3.05) | 0.01 | 1.53 (0.82–2.84) | 0.18 |
| Access to care scale | 0.94 (0.87–1.01) | 0.12 | 0.96 (0.88–1.05) | 0.39 |
Univariable logistic regression.
Multivariable logistic regression adjusted for all other variables in the table.
The referent category is “less than 8th grade.”
The referent category is “Caucasian.”
OR, Odds ratio; CI, Confidence interval.
Willingness
In univariable analyses, the patient's duration of DM (unadjusted odds ratio (OR) = 0.96, 95% confidence interval (CI) = 0.93–0.99, p = 0.02) and the perception that they would miss the patient–physician relationship (unadjusted OR = 0.136, 95% CI = 0.05–0.36, p < 0.001) significantly decreased a participant's likelihood of being willing to participate in telemedicine (Table 2). Conversely, if patients perceived the program to be convenient, that significantly increased their likelihood of being willing to participate in the program (unadjusted OR = 6.23, 95% CI = 2.41–16.10, p < 0.001) (Table 2).
In multivariable analysis, the patient–physician relationship and the longer duration of diabetes decreased the willingness to participate in telemedicine compared with an in-person examination. Patients who valued the patient–physician relationship had 92% decreased odds of being willing to participate in telemedicine (adjusted OR = 0.08, 95% CI = 0.02–0.35, p = 0.001). For each additional year of having DM, patients had 7% decreased odds of being willing to participate in telemedicine (adjusted OR = 0.93, 95% CI = 0.88–0.99, p = 0.02). The perceived convenience and the number of systemic comorbidities increased willingness. Patients who believed telemedicine to be more convenient had eight times higher odds of being willing to participate in telemedicine (adjusted OR = 8.10, 95% CI = 1.77–36.97, p = 0.01). For each additional systemic comorbidity, patients had 85% higher odds of being willing to participate in telemedicine (adjusted OR = 1.85, 95% CI = 1.10–3.11, p = 0.02).
Convenience and patient–physician relationship
Perception of convenience, when analyzed as an outcome independent of willingness, was influenced by patients’ number of ocular comorbidities and their access to care (Tables 3 and 4). In multivariable analysis, for each additional ocular comorbidity, the patient had 68% decreased odds of considering telemedicine to be more convenient (adjusted OR = 0.32, 95% CI = 0.15–0.71, p = 0.005). For each unit increase in access to care, participants had 20% decreased odds of perceiving telemedicine to be more convenient (adjusted OR = 0.80, 95% CI = 0.68–0.93, p = 0.004) (Table 3).
In univariable analyses, patients with greater ocular and systemic comorbidities had higher odds of missing the patient–physician relationship through telemedicine (unadjusted OR = 1.88, 95% CI = 1.16–3.05, p = 0.01 and unadjusted OR = 1.33, 95% CI = 1.05–1.70, p = 0.02, respectively). These associations were not significant in multivariable models (p = 0.18 and p = 0.07, respectively) (Table 4).
Discussion
Telemedicine is a care delivery method proven to deliver high-quality screening for DR. However, whether patients will accept this new model of care is unknown. We explored the willingness to accept telemedicine by focusing on individuals’ beliefs about telemedicine, demographics, and health characteristics. In this study, 97% of the study population had not heard of “telemedicine,” which is reflective of the majority of U.S. citizens. Only pockets of the U.S. population (e.g., Veterans, Native Americans, indigent populations in specific regions) currently use telemedicine. The clinic for our recruitment had not instituted their DR telemedicine program at the time of enrollment. In our study, 32% of participants were either unwilling or unsure about participating in telemedicine for DR screening. It is important to take these perceptions into account when designing telemedicine programs. We found that patients’ age, gender, race, and education level did not influence their attitudes toward telemedicine. We were surprised that age did not influence attitudes toward telemedicine, as it is well known that older adults are less likely to use technology.35 We hypothesize that older participants believed that the burden of technology in a telemedicine encounter is placed on the provider, and not placed on the patient.
Patients’ willingness to participate in telemedicine was influenced by how much they value the relationship they have with their physician, their perceived convenience of telemedicine, and their current health status. Patients who highly valued the patient–physician relationship had 92% lower odds of being willing to participate in telemedicine. Patients with diabetes see the same physician frequently (often more than four times a year), leading to close patient–physician relationships. A strong patient–physician relationship is often among the highest priorities for patients.36 Patients with a longer duration of diabetes were less willing to participate in telemedicine. “Willingness” to try new technologies is closely tied to the concepts of trust and openness.37,38 Patients with long-standing disease are less likely to trust a new, less-personal delivery model. In our study, the highest impact on willingness was convenience. Patients who believed telemedicine to be more convenient had eight times (or 800%) higher odds of being willing to participate in telemedicine. Patients with multiple systemic comorbidities had 85% higher odds of being willing to participate in telemedicine. Though this result seems to contradict the findings that those who highly valued their patient–physician relationship and had a longer duration of diabetes were less willing to use telemedicine for DR screening, perhaps patients with multiple chronic diseases value simpler screening methods for conditions such as eye disease, which may not be their highest medical priority.39–41 Our results indicate that willingness to participate in telemedicine for DR screening reflects how patients perceive convenience, the patient–physician relationship, and their health.
We evaluated the perceived convenience of telemedicine independent of willingness. Two key factors influenced perceived convenience: access to care and number of ocular comorbidities. Patients with better access to care did not believe telemedicine to be more convenient than an in-person eye examination. Therefore, telemedicine programs should focus on individuals who have limited access to care.24, 42 Programs could also highlight the shortened travel distances and decreased time away from work for those who already have good access to care.39 Not surprisingly, patients with ocular comorbidities did not believe telemedicine to be more convenient than an in-person examination. However, in the multivariable model, having ocular comorbidities did not impact overall willingness to participate in telemedicine for DR. The results indicate that a person's general health and beliefs outweigh ocular health in terms of willingness to participate in telemedicine for DR.
Our study has limitations. All participants were patients in an endocrinology or primary care clinic. We did not include patients outside of the healthcare system. We used a convenience sample approach for patient recruitment, which introduces some potential biases. Also, a low percentage of women were enrolled in the study, largely due to recruitment at the Veterans Affairs clinics. This limits generalizability of our results. Self-reported ocular and systemic comorbidities may differ from clinical diagnoses, which could potentially impact their association with the measured outcomes. Interviewers provided information about telemedicine, whereas patient responses were based on hypothetical extrapolation, not on practical experience with telemedicine services. We find lack of telemedicine exposure both a weakness and a strength of the study, because lack of experience also more closely reflects the experience of the general U.S. population. Additional research will need to be conducted to better understand attitudes toward telemedicine for those already familiar with the approach or those in remote areas.
Telemedicine programs should be designed based on consumer needs. Patients’ attitudes toward telemedicine eye care are influenced by their health and beliefs, not by their demographics. Patients who valued convenience or had more systemic health problems were more willing to participate in telemedicine for diabetic eye care. Patients who valued their relationship with their physician or had a longer duration of DM were less willing to participate. Access to care did not influence patients’ willingness, but it did influence their perceived convenience of telemedicine. Patients with more ocular or systemic health issues were more likely to miss the patient–physician interactions in a telemedicine care model. Telemedicine programs should focus on patients with the greatest need for services and those who would be the most receptive to telemedicine. Understanding patients’ attitudes toward telemedicine is key to developing, improving, and maintaining patient-centered telemedicine programs.
Supplementary Material
Acknowledgments
This work was supported by the National Eye Institute, Bethesda, MD [grant numbers K23EY023596-01 (Maria A Woodward), K12EY022299 (Paula Anne Newman-Casey)]; Research to Prevent Blindness, New York, NY (Paul Lee as unrestricted grant to the Department of Ophthalmology, University of Michigan); and the National Center for Advancing Translational Sciences (NCATS), Bethesda, MD [grant number UL1TR000433 (Nita Valikodath)]. The funding organizations had no role in the design or conduct of this research.
Disclosure Statement
M.W: Intelligent Retinal Imaging Systems (Scientific Advisory Board), 418 West Garden St., Suite 209, Pensacola, Florida 32502
P.L: Centers for Disease Control (Consulting), 1600 Clifton Rd, Atlanta, GA 30333
The following authors have no financial disclosures: Nita Valikodath, Thellea Leveque, Sophia Wang, Sean Hansen, and Paula Anne Newman-Casey.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: Department of Health and Human Services, 2011 [Google Scholar]
- 4.AAO Quality of Care Secretariat, Hoskins Center for Quality Eye Care. Screening for diabetic retinopathy; San Francisco, CA: American Academy of Ophthalmology, 2014 [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes. J Clin Appl Res Educ 2015;38:1–94 [Google Scholar]
- 6.Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: Baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology 2001;108:563–571 [DOI] [PubMed] [Google Scholar]
- 7.Abramoff MD, Niemeijer M, Suttorp-Schulten MS, Viergever MA, Russell SR, van Ginneken B. Evaluation of a system for automatic detection of diabetic retinopathy from color fundus photographs in a large population of patients with diabetes. Diabetes Care 2008;31:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazin R, Barazi MK, Summerfield M. Challenges to establishing nationwide diabetic retinopathy screening programs. Curr Opin Ophthalmol 2011;22:174–179 [DOI] [PubMed] [Google Scholar]
- 9.Whiting PS, Greenberg SE, Thakore RV, et al. What factors influence follow-up in orthopedic trauma surgery? Arch Orthop Trauma Surg 2015;135:321–327 [DOI] [PubMed] [Google Scholar]
- 10.Thompson AC, Thompson MO, Young DL, et al. Barriers to follow-up and strategies to improve adherence to appointments for care of chronic eye diseases. Invest Ophthalmol Vis Sci 2015;56:4324–4331 [DOI] [PubMed] [Google Scholar]
- 11.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: Transportation barriers to health care access. J Community Health 2013;38:976–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DJ, Kumar N, Feuer WJ, et al. Dilated eye examination screening guideline compliance among patients with diabetes without a diabetic retinopathy diagnosis: The role of geographic access. BMJ Open Diabetes Res Care 2014;2:e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellish NJ, Royak-Schaler R, Passmore SR, Higginbotham EJ. Knowledge, attitudes, and beliefs about dilated eye examinations among African-Americans. Invest Ophthalmol Vis Sci 2007;48:1989–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheppler CR, Lambert WE, Gardiner SK, Becker TM, Mansberger SL. Predicting adherence to diabetic eye examinations: Development of the compliance with Annual Diabetic Eye Exams Survey. Ophthalmology 2014;121:1212–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Improving the Nation's Vision Health: A Coordinated Public Health Approach. Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- 16.Walker EA, Basch CE, Howard CJ, Zybert PA, Kromholz WN, Shamoon H. Incentives and barriers to retinopathy screening among African-Americans with diabetes. J Diabetes Complicat 1997;11:298–306 [DOI] [PubMed] [Google Scholar]
- 17.Choremis J, Chow DR. Use of telemedicine in screening for diabetic retinopathy. Can J Ophthalmol 2003;38:575–579 [DOI] [PubMed] [Google Scholar]
- 18.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology 2007;114:1748–1754 [DOI] [PubMed] [Google Scholar]
- 19.Vujosevic S, Benetti E, Massignan F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol 2009;148:111–118 [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Wu H, Dong J, Jiang K, Lu X, Shi J. Telemedicine for detecting diabetic retinopathy: A systematic review and meta-analysis. Br J Ophthalmol 2015;99:823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bursell SE, Cavallerano JD, Cavallerano AA, et al. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology 2001;108:572–585 [DOI] [PubMed] [Google Scholar]
- 22.Mansberger SL, Gleitsmann K, Gardiner S, et al. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: A randomized controlled trial. Telemed J E Health 2013;19:942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014;4:e004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HK, Horton M, Bursell SE, et al. Telehealth practice recommendations for diabetic retinopathy, second edition. Telemed J E Health 2011;17:814–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das T, Raman R, Ramasamy K, Rani PK. Telemedicine in diabetic retinopathy: Current status and future directions. Middle East Afr J Ophthalmol 2015;22:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bashshur RL, Shannon GW, Smith BR, Woodward MA. The empirical evidence for the telemedicine intervention in diabetes management. Telemed J E Health 2015;21:321–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzio S, Hatcher S, Zahlmann G, et al. Feasibility of using the TOSCA telescreening procedures for diabetic retinopathy. Diabet Med 2004;21:1121–1128 [DOI] [PubMed] [Google Scholar]
- 28.Surendran TS, Raman R. Teleophthalmology in diabetic retinopathy. J Diabetes Sci Technol 2014;8:262–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurji K, Kiage D, Rudnisky CJ, Damji KF. Improving diabetic retinopathy screening in Africa: Patient satisfaction with teleophthalmology versus ophthalmologist-based screening. Middle East Afr J Ophthalmol 2013;20:56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul PG, Raman R, Rani PK, Deshmukh H, Sharma T. Patient satisfaction levels during teleophthalmology consultation in rural South India. Telemed J E Health 2006; 12:571–578 [DOI] [PubMed] [Google Scholar]
- 31.Kumari Rani P, Raman R, Manikandan M, Mahajan S, Paul PG, Sharma T. Patient satisfaction with tele-ophthalmology versus ophthalmologist-based screening in diabetic retinopathy. J Telemed Telecare 2006;12:159–160 [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 33.Beecher J, Briggs RP, Chiesa J, et al. Investigations of sufficiency in telemedicine applications: Standards in context of populations and technologies. The Advanced Biotechnical Consortium of the University of Southern California; National Library of Medicine. 2000. September 15 139 p. Report No.: RFP-NLM-96–105/MV A Proposal ID No. 41 [Google Scholar]
- 34.Cunningham WE, Hays RD, Williams KW, Beck KC, Dixon WJ, Shapiro MF. Access to medical care and health-related quality of life for low-income persons with symptomatic human immunodeficiency virus. Med Care 1995;33:739–754 [DOI] [PubMed] [Google Scholar]
- 35.Czaja SJ, Charness N, Fisk AD, et al. Factors predicting the use of technology: Findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE). Psychol Aging 2006;21:333–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Detsky AS. What patients really want from health care. JAMA 2011;306:2500–2501 [DOI] [PubMed] [Google Scholar]
- 37.Hoy WK, Tschannen-Moran M. Five faces of trust: An empirical confirmation in urban elementary schools. J Sch Leadersh 1999;9:184–208 [Google Scholar]
- 38.Arora S, Kurji AK, Tennant MT. Dismantling sociocultural barriers to eye care with tele-ophthalmology: Lessons from an Alberta Cree community. Clin Invest Med 2013;36:E57-63 [DOI] [PubMed] [Google Scholar]
- 39.National Conference of State Legislatures. Telehealth policy trends and considerations. NCSL Foundation for State Legislatures, Washington, DC, 2015: 38 [Google Scholar]
- 40.Robinson JD, Turner JW, Wood KS. Patient perceptions of acute care telemedicine: A pilot investigation. Health Commun 2015;30:1269–1276 [DOI] [PubMed] [Google Scholar]
- 41.Miller EA. Telemedicine and doctor-patient communication: An analytical survey of the literature. J Telemed Telecare 2001;7:1–17 [DOI] [PubMed] [Google Scholar]
- 42.Hiratsuka V, Delafield R, Starks H, Ambrose AJ, Mau MM. Patient and provider perspectives on using telemedicine for chronic disease management among Native Hawaiian and Alaska Native people. Int J Circumpolar Health 2013;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.