Abstract
More than 90% of spinal cord injuries are caused by traumatic accidents and are often associated with other tissue damage (polytrauma) that can provide a source of continued pain input during recovery. In a clinically relevant spinal cord contusion injury model, prior work has shown that noxious stimulation at an intensity that engages pain (C) fibers soon after injury augments secondary injury and impairs functional recovery. Noxious input increases the expression of pro-inflammatory cytokines (interleukin 1β and 18), cellular signals associated with cell death (caspase 3 and 8), and physiological signs of hemorrhage. Here, it is shown that reducing neural excitability after spinal cord injury (SCI) with the local anesthetic lidocaine (micro-injected by means of a lumbar puncture) blocks these adverse cellular effects. In contrast, treatment with an analgesic dose of morphine had no effect. Contused rats that received nociceptive stimulation soon after injury exhibited poor locomotor recovery, less weight gain, and greater tissue loss at the site of injury. Prophylactic application of lidocaine blocked the adverse effect of nociceptive stimulation on behavioral recovery and reduced tissue loss from secondary injury. The results suggest that quieting neural excitability using lidocaine can reduce the adverse effect of pain input (from polytrauma or surgery) after SCI.
Keywords: : cell death, pain, polytrauma, secondary injury, spinal cord injury
Introduction
The majority of spinal cord injuries are accompanied by additional peripheral tissue damage (e.g., fractures, lacerations, and abrasions) that will engage pain (C) fibers. Pain (nociceptive) pathways also are engaged during surgical interventions. Using an animal (rat) model of spinal cord injury (SCI), we have shown that nociceptive input soon (1–4 days) after SCI impairs functional recovery.1–3 Just 6 min of nociceptive stimulation a day after injury undermines locomotor recovery, slows the recovery of bladder function, and increases spasticity, mortality, and tissue loss. These adverse effects have been linked to a down-regulation of brain-derived neurotrophic factor and an up-regulation of tumor necrosis factor, as well as increased cellular signals (e.g., caspase 3 and 8) associated with programmed cell death (apoptosis).4,5 Noxious stimulation soon after injury also enhances reactivity to tactile stimulation (allodynia) weeks later, a behavioral sign of chronic pain.5 Recent work suggests that noxious input increases the extent of secondary injury because it leads to a breakdown of the blood–spinal cord barrier and an increase in hemorrhage.6
In a prior report, we evaluated whether pre-treatment with the opiate analgesic morphine would block the adverse effect noxious input has on functional recovery.7 While systemic morphine treatment completely blocked behavioral reactivity to noxious shocks, the drug had no effect on the shock-induced impairment in behavioral recovery and tissue loss after SCI. Further, morphine treatment enhanced mortality and subsequent work has shown that morphine per se adversely affects behavioral recovery.8 The present paper reinforces these observations, demonstrating that morphine does not affect the induction of pro-inflammatory cytokines, signals associated with cell death, or shock-induced hemorrhage in response to noxious stimulation soon after SCI.
Because nociceptive input enhances secondary injury and impairs functional recovery and because current treatments that rely on opiate analgesics are not only ineffective but counter-indicated, we have sought alternative treatments that could be safely applied within a clinical setting.7 The present article examines whether prophylactic treatment with the local anesthetic lidocaine, administered by means of a lumbar puncture, attenuates the adverse effect of nociceptive stimulation. Lidocaine was the first sodium channel blocker discovered and is still used clinically.9 Over 60% of laboring patients in the United States receive some form of epidural analgesia and local or regional anesthesia has been successfully used for anesthesia in lower limb surgical procedures and to control post-operative pain after abdominal surgeries.10–12 We show that pre-treatment with lidocaine blocks behavioral reactivity to acute pain and prevents the detrimental behavioral and cellular effects of nociceptive input. Spinal anesthesia should be considered as an alternative analgesic therapy for patients with acute pain following SCI.
Methods
Subjects
Adult male Sprague-Dawley rats (100–120 days old) were obtained from Envigo (Houston, TX) and acclimated to handling and the open enclosures used for locomotor assessment for at least 7 days prior to experimentation. Prior to contusion, subjects were pair housed with water and food ad libitum and maintained on a 12-h light-dark cycle. Behavioral testing and surgeries were performed during the light portion of the cycle. All experiments were carried out in accordance with National Institutes of Health (NIH) standards for the care and use of laboratory animals (NIH publication No. 80-23), and were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used to that which was scientifically necessary.
Surgery
All subjects received a moderate contusion injury at the T10-11 vertebral level using the MASCIS device.13 Anesthesia was induced using a mixture of 5% isoflurane in medical oxygen and maintained at a concentration of 2–3% during surgery. Two longitudinal incisions were made on either side of the vertebral column extending approximately 2 cm rostral and caudal to the injury site. The T10-11 vertebrae were located by palpation and exposed, and a laminectomy was performed. The dura remained intact. The MASCIS device was then secured around the vertebral column and the 10-g impactor was centered on the lesion site. The drop height was set at 12.5 mm. After surgery, the wound was closed using Michel clips. To prevent urinary tract infection and to compensate for fluid loss, subjects received 100,000 units/kg of penicillin and 3 mL of saline after surgery.
After surgery, subjects were singly housed and allowed to recover overnight (18–24 h) in a temperature-controlled room (25°C) with water and food ad libitum. Subjects were transferred back to standard single housing on the first day after injury.
Nociceptive stimulation
Subjects were loosely restrained in opaque Plexiglas tubes and placed in an acoustic isolation chamber. An electrode was applied to the tail with electrode gel. Shocks were administered on a variable spaced schedule (0.2–3.8 sec interstimulus interval [ISI]). Shock intensity was set to 1.5 mA and shock duration was 100 msec. Subjects in groups treated with electrical stimulation received 180 shocks administered over approximately 6 min. This shock schedule and mode of application has been shown to impact spinal cord plasticity, behavioral recovery, and tissue sparing.1,2,4,5,7 Controls were loosely restrained in the Plexiglas tubes for an equivalent duration, but no shocks were administered.
Drug treatment
Morphine was administered using a dose and injection protocol that has been previously shown to induce a robust anti-nociception that blocks spinal and brain-dependent responses to noxious stimulation.7 Morphine sulfate was dissolved in sterile normal saline and administered by intraperitoneal (i.p.) injection at a dose of 20 mg/kg. Drug was administered 30 min prior to shock treatment.
Because systemic lidocaine treatment would be toxic at a dose that would impact spinal neurons and because we sought to impact spinal function using a clinically approved procedure, lidocaine was administered by means of a lumbar puncture. To minimize movement and stress during drug infusion, subjects were anesthetized with isoflurane at a concentration of 2–3% in medical oxygen. While in a flaccid state, the rat was supported on the edge of a surgical table so that the hindquarters hung freely, which increased the separation between the vertebrae along the dorsal surface. After palpation of the fifth lumbar (L5) vertebrae, a 1-inch, 25-gauge needle was inserted just caudal to the vertebra until the injection flowed with no resistance. Twenty-five μL of 15% lidocaine in normal saline was slowly infused into the space between the L5 and L6 vertebrae.14 Complete spinal block was verified by lack of behavioral response to tail pinch.
Behavioral verification of drug effectiveness
Contused rats (N = 18) that had exhibited some locomotor function prior to lidocaine treatment appeared totally paralyzed after drug administration. As with morphine, none (0 out of 9) of the subjects treated with lidocaine exhibited a spinal (e.g., tail withdrawal) or brain-dependent (e.g., vocalization) response to noxious stimulation. In contrast, all of the vehicle-treated rats exhibited a behavioral response (9/9). As expected, subjects did not exhibit a tail-withdrawal or vocalization in the absence of stimulation (0/18). A chi-square test revealed a statistically significant increase in motor and vocal reactivity thresholds in subjects that received electrical stimulation and vehicle treatment (χ2 = 27.00, p < 0.05).
Assessment of recovery
Health checks were performed daily throughout the recovery period. Subjects were examined for signs of self-mutilation, stress, and infection. Weight was assessed daily as a measure of general health. Bladders were expressed manually twice per day until voluntary control was established (six consecutive expressions with no urine).
Locomotor function was assessed every day following injury for the first week and again on Day 10. From Day 14 to 42, behavioral function was assessed weekly. A blinded observer used the commonly used Basso, Beattie, Bresnahan (BBB) scoring system to examine locomotor function.15 BBB scores were converted as recommended by Ferguson and colleagues, yielding scores that are more amenable to parametric analyses.16
In subjects that had regained stepping behavior (raw BBB score ≥9), the beam and ladder tests were used to better characterize locomotor function. Beam width at first misstep, number of ladder missteps, and number of ladder slips were recorded for each subject.7
Tactile and thermal reactivity were assessed using von Frey filament testing and tail flick, respectively. A blinded observer examined mechanical threshold using the up-down method with von Frey filaments.17 The tail flick test was conducted using the IITC tail flick device (Envigo) as a measure of thermal pain sensitivity. Heat exposure was limited to 8 sec to prevent tissue damage.7
At-level pain was assessed using a 26-g von Frey filament applied on a 4 × 11 grid across the girdle region of each subject.8 Vocalization to the stimulus was recorded as a positive response and the total number of responses was calculated for each subject.
Tissue collection and immunoblotting
Subjects used to assess the impact of our experimental treatments on cellular indices of cell death, cytokine expression, and hemorrhage were euthanized with 100 mg/kg of pentobarbital 3 h following electrical stimulation. One centimeter of spinal tissue centered at the lesion was dissected and flash frozen in liquid nitrogen. Protein was extracted using the Qiagen RNeasy Lipid Tissue Mini kit according to Qiagen manufacturer instructions. A Bradford assay was performed to determine the concentration of protein extracts. Protein samples were diluted in 4 × Laemmli buffer to a final concentration of 3 mg/mL. Spectral analysis was performed using a Nanodrop spectrophotometer (Thermo Scientific).
Thirty μL of protein was loaded into each well of a 15% Tris-HCl Criterion precast gel and run according to Bio-Rad manufacturer's instructions. Proteins were transferred onto a polyvinylidene fluoride membrane and blocked with 5% milk for 1 h at room temperature prior to incubation with primary antibody overnight at 4°C (interleukin [IL]-1β: Santa Cruz Biotechnologies sc-7884; IL-18: Santa Cruz Biotechnologies sc-7954; Caspase 3: Novus NB100-56113; Caspase 8: Novus NB100-56116; Lamin B: Abcam ab16048; hemoglobin α: Abcam ab92492).
Lesion reconstruction
Rats used to assess the impact of lidocaine treatment on recovery after a contusion injury were euthanized with 100 mg/kg of pentobarbital at the completion of behavioral testing on Day 42. Subjects were flushed transcardially with phosphate-buffered saline prior to perfusion with 4% paraformaldehyde. One centimeter of spinal tissue centered at the lesion was dissected and post-fixed in 4% paraformaldehyde overnight. After a rinse with phosphate-buffered saline, spinal cords were cryoprotected in 30% sucrose for at least 1 week prior to sectioning.
Twenty-micron sections of tissue were collected and every 10th section was mounted on a slide. Sections were stained with cresyl violet/luxol fast blue and traced by blinded observers to reconstruct the lesion.1
Experimental designs
Subjects received a moderate spinal cord injury and 24 h later, locomotor function was assessed using the BBB locomotor scale. Subjects were randomly assigned to one of four groups (lidocaine or vehicle crossed with shocked or unshocked). The lidocaine dose used was based on prior work.18,19 Converted BBB scores (mean ± standard error of the mean [SEM) prior to drug treatment ranged from 3.2 (± 0.7) to 3.7 (± 1.3) and did not differ (all Fs <1.00; p > 0.05). All subjects were then lightly anesthetized with isoflurane and an acute injection of lidocaine or vehicle was administered. Thirty minutes later, all subjects were restrained for 6 min in an opaque Plexiglas tube and had an electrode fastened to the tail. Subjects in shocked groups received 6 min of intermittent uncontrollable electrical stimulation while the subjects in unshocked groups received identical treatment without electrical stimulation. After treatment, all subjects were removed from the Plexiglas tube and kept in a temperature-controlled room until sacrifice. Three hours after stimulation, subjects were sacrificed and tissue was processed for immunoblotting as described above.
The impact of systemic morphine on cellular indices was examined using an analogous experimental design. Prior to drug treatment, BBB scores ranged from 3.7 (± 0.3) to 4.2 (± 1.2) and did not differ across groups (all Fs <1.00; p > 0.05). Subjects then received morphine (20 mg/kg, i.p.) or its vehicle, followed 30 min later by shock or an equivalent period of restraint (Unshocked). The dosage used was based on past work demonstrating that it induces a robust anti-nociception and blocks reactivity to shock stimulation.7 Three hours later, tissue was collected as described above.
To assess the impact of lidocaine treatment on the recovery of locomotor function, contused rats were evaluated and randomly assigned to one of four conditions a day after injury. Baseline BBB scores ranged from 2.8 (± 0.5) to 3.4 (± 0.6) and did not differ (all Fs <1.00; p > 0.05). Subjects were lightly anesthetized and received lidocaine or its vehicle by means of a lumbar puncture as described above. Thirty minutes later, subjects received shock or remained unshocked. All subjects were then returned to the colony room and behavioral recovery was monitored over the next 6 weeks. Finally, after additional behavioral tests were conducted on Day 42, subjects were sacrificed and injury site was collected to assess tissue loss. One lidocaine-treated shocked subject exhibited autophagia and was sacrificed at Day 24. Because we were able to score this subject through Day 21 (10 of 13 scores), and because behavioral recovery asymptotes approximately 3 weeks after injury, we used this subject's BBB score from Day 21 to fill in the three missing values.20 This represents a conservative approach because BBB performance exhibited some improvement from Day 21 to 42. Further, to assure our assessment of tissue loss was not biased, we excluded this subject from those analyses.
Statistical analysis
All data were analyzed using chi-square tests, analysis of variance (ANOVA), or analysis of covariance (ANCOVA). When necessary, post hoc comparisons of the group means were performed using Duncan's New Multiple Range test. In all cases, a criterion of p < 0.05 was set as the threshold for statistical significance.
Results
Lidocaine blocks the effect of nociceptive stimulation on cellular indices of inflammation, cell death, and hemorrhage
We have shown that nociceptive input within 4 days of SCI impairs behavioral recovery and enhances tissue loss at the site of injury.1 Tissue loss has been linked to cellular processes related to cell death and hemorrhage.5,6 Here, we examine whether lidocaine, administered by means of a lumbar puncture, blocks these cellular effects.
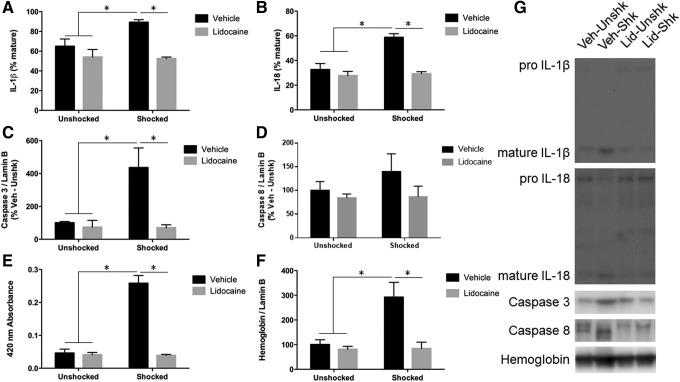
Nociceptive stimulation has been shown to engage pro-inflammatory cytokines (IL-1β and IL-18) linked to the development of pyroptosis.6,21–24 Immunoblotting for IL-1β revealed that electrical stimulation produced increased processing of the pro-inflammatory cytokine in shocked subjects, and that lidocaine treatment blocked this increase (Fig. 1A). An ANOVA yielded a significant main effect of drug, as well as a significant interaction between drug and stimulation (all Fs >5.43; p < 005). Post hoc comparisons between groups revealed a significant difference between the Vehicle-Shocked group and all other groups. Immunoblotting for IL-18 showed similar results to those for IL-1β (Fig. 1B). An ANOVA yielded significant main effects of drug and stimulation, as well as the drug with stimulation interaction (all Fs >12.81; p < 0.005). Again, post hoc comparisons between groups revealed a significant difference between the Vehicle-Shocked group and all other groups.
FIG. 1.
Inflammation, apoptosis, and hemorrhage 3 h after electrical stimulation and complete spinal block. (A) Subjects receiving electrical stimulation show increased interleukin (IL)-1β processing that is blocked by lidocaine treatment. (B) Subjects receiving electrical stimulation show increased IL-18 processing that is blocked by lidocaine treatment. (C) Subjects receiving electrical stimulation show increased levels of caspase after electrical stimulation. This increase is blocked by lidocaine treatment. (D) Caspase 8 levels were unchanged after electrical stimulation. (E) Subjects that received electrical stimulation showed increased absorbance at 420 nm that was blocked by pre-treatment with lidocaine. (F) Subjects that received electrical stimulation show increased levels of hemoglobin α after stimulation that was blocked by pre-treatment with lidocaine. (G) Representative blots for each target. * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 3).
Noxious input appears to increase cell loss at the site of injury by inducing apoptotic cell death, which is associated with expression of caspases 3 and 8.5 Immunoblotting for caspase 3 revealed that subjects that received electrical stimulation showed increased levels of the active form of the apoptotic protease and that lidocaine treatment blocked this increase (Fig. 1C). An ANOVA yielded significant main effects of drug and stimulation and a significant interaction between drug and stimulation (all Fs >6.58; p < 0.05). Post hoc comparisons between groups revealed a significant difference between the Vehicle-Shocked group and all other groups. While vehicle-treated shocked rats exhibited greater caspase 8 expression (Fig. 1D), this effect was not statistically significant (all Fs <2.00; p > 0.05).
We have noted that nociceptive stimulation leads to a breakdown of the blood spinal cord barrier and the infiltration of red blood cells.6 This effect is evident from the reddish tint of our samples and an increase in hemoglobin protein. Verifying these observations, we found that the samples from shocked rats exhibited increased absorbance at the wavelength (420 nm) associated with hemoglobin (Fig. 1E). This effect was blocked by lidocaine treatment. An ANOVA revealed significant main effects of stimulation and drug treatment, as well as a significant interaction between stimulation and drug treatment (all Fs >57.82; p < 0.0001). Post hoc comparisons between groups revealed a significant difference between the Vehicle-Shocked group and all other groups. Immunoblotting for hemoglobin was performed to confirm the source of coloration. Subjects that received electrical stimulation showed increased levels of hemoglobin α within the tissue, and this increase was blocked by pre-treatment with lidocaine (Fig. 1F). An ANOVA revealed significant main effects of stimulation and drug treatment, as well as a significant interaction between stimulation and drug treatment (all Fs >7.42; p < 0.05). Post hoc comparisons between groups revealed a significant difference between the Vehicle-Shocked group and all other groups.
Morphine has no effect on cellular indices of nociceptive stimulation
We have previously shown that an analgesic dose of morphine does not block the adverse effect nociceptive stimulation has on behavioral recovery.7 We have not, however, tested whether this treatment impacts cellular processes engaged by shock treatment. This is an important issue because it provides a clinically relevant comparison for the effect of lidocaine.
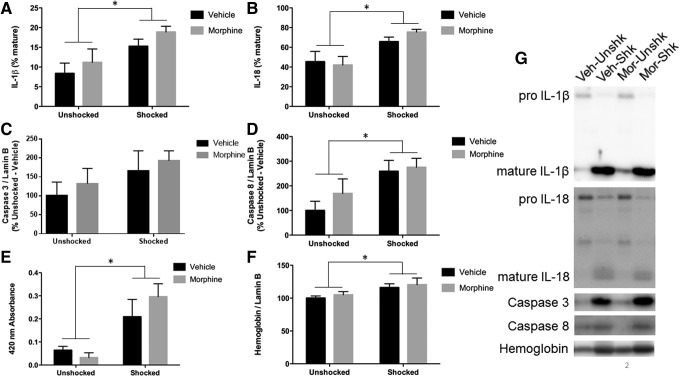
As reported above, shock treatment increased the expression of IL-1β and IL-18 (Fig. 2A, 2B). In no case did morphine attenuate the effect of shock. If anything, it tended to have the opposite effect. ANOVAs confirmed that the main effect of shock treatment was statistically significant (both Fs >9.80; p < 0.01). In both cases, the interaction between shock and drug treatments did not approach significance (Fs <1.0; p > 0.05). This indicates that morphine did not affect the shock-induced expression of IL-1β or IL-18.
FIG. 2.
Inflammation, apoptosis, and hemorrhage 3 h after electrical stimulation and systemic morphine. (A) Subjects receiving electrical stimulation show increased interleukin (IL)-1β processing that is not blocked by morphine treatment. (B) Subjects receiving electrical stimulation show increased IL-1β processing that is not blocked by morphine treatment. (C) Caspase 3 levels were unchanged after electrical stimulation. (D) Subjects receiving electrical stimulation show increased levels of caspase 8 that is not reversed by morphine treatment. (E) Subjects that received electrical stimulation showed increased absorbance at 420 nm irrespective of morphine treatment. (F) Subjects that received electrical stimulation show increased levels of hemoglobin α after stimulation that was not reversed by morphine treatment. (G) Representative blots for each target. * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 6).
A similar pattern was observed for markers of apoptosis. Again, shock generally enhanced caspase 3 and 8 expression (Fig. 2C, 2D). Here, the effect on caspase 8 was somewhat more robust (F = 7.34; p < 0.05), relative to caspase 3 (all Fs <2.80; p > 0.05). Most importantly, the interaction between morphine and shock treatment did not approach statistical significance (all Fs <1.0; p > 0.05).
Markers of hemorrhage indicated that shock increased absorbance at 420 nm (Fig. 2E) and hemoglobin protein (Fig. 2F). Again, independent ANOVAs verified that shock treatment had a significant effect (both Fs >10.46; p < 0.005). In neither case did the drug by shock treatment interaction approach significance (both Fs <1.51; p > 0.05). The results indicate that morphine treatment has no effect on cellular processes engaged by nociceptive stimulation.
Lidocaine blocks the effect of nociceptive stimulation on behavioral recovery and tissue loss
While morphine was unsuccessful at blocking the cellular effects of acute pain (Fig. 2), lidocaine blocked the increased expression of markers associated with inflammation, apoptosis, and hemorrhage (Fig. 1). For this reason, we examined whether lidocaine also blocks the adverse effects of acute pain on long-term recovery.
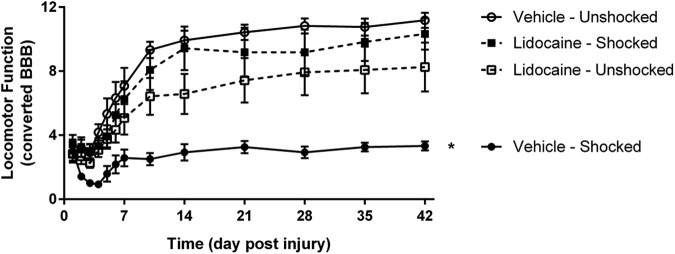
Vehicle-treated contused rats that received noxious shocks exhibited poor recovery, relative to the unshocked controls. This effect was blocked by pre-treatment with lidocaine (Fig. 3; see Supplementary Data for representative videos). An ANCOVA, using the pre-stimulation locomotor score as the covariate, yielded main effects of stimulation and time, as well as significant interactions of drug and stimulation, time and stimulation, and time by drug by stimulation (all Fs >3.50; p < 0.01). These effects emerged because subjects receiving electrical stimulation without lidocaine showed impaired locomotor recovery, compared with all other groups.
FIG. 3.
Locomotor function after electrical stimulation and complete spinal block. Subjects receiving electrical stimulation show impaired locomotor recovery that is blocked by lidocaine treatment. * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 6).
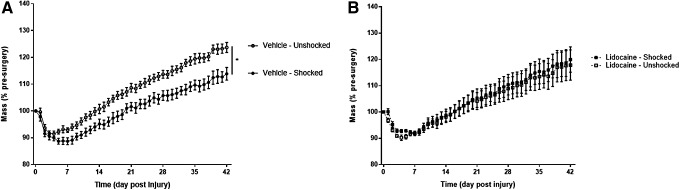
Noxious stimulation also impaired weight gain in vehicle-treated contused rats (Fig. 4A) and this effect was blocked by lidocaine (Fig. 4B). An ANCOVA, using the pre-surgery mass as a covariate examining the percent change in weight across the recovery period, revealed significant main effects of initial mass and time and significant interactions between drug and stimulation, time and initial mass, and time by drug by stimulation (all Fs >3.61; p < 0.05). These effects emerged because subjects receiving electrical stimulation without lidocaine showed impaired weight regain, compared with subjects receiving neither electrical stimulation nor lidocaine. However, no difference in weight regain was seen between subjects receiving lidocaine treatment.
FIG. 4.
Weight gain after electrical stimulation and complete spinal block. Electrical stimulation impaired weight gain (A) and this effect was blocked by lidocaine treatment (B). * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 6).
Further examination of locomotor function was performed for subjects capable of stepping (defined as a BBB score ≥9) using the beam and ladder tests. No subjects that received shock without lidocaine met the minimum locomotor requirements necessary to complete these tests. A chi-square test examining the fraction of subjects within each group that were capable of completing the beam and ladder assessments revealed significance (χ2 = 11.00; p < 0.05), indicating that subjects that received electrical stimulation without lidocaine had significantly worse locomotor function, compared with all other groups. Importantly, an ANOVA performed on the behavioral scores for the remaining three groups (see Supplementary Data) showed that they did not differ (all Fs <1.0; p > 0.05).
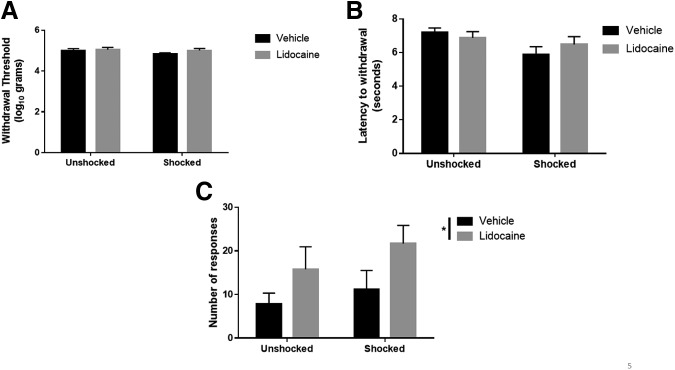
Rats responded similarly to tactile stimulation of the plantar surface of the hind paw (Fig. 5A), regardless of treatment condition (all Fs <1.56; p > 0.05). Shocked rats were generally more responsive to thermal stimulation (Fig. 5B), and this effect approached statistical significance (F = 4.22; p = 0.054). Lidocaine had no effect on thermal reactivity (all Fs <1.21; p > 0.05). Assessment of at-level pain using a girdle tactile test for vocalizations revealed a statistically significant increase in the number of vocalizations in subjects receiving lidocaine (Fig. 5C). An ANOVA revealed a main effect of drug (F = 5.17; p < 0.05) but not shock treatment (both Fs <1.28; p > 0.05).
FIG. 5.
Pain sensitivity after electrical stimulation and complete spinal block. Rats responded similarly to tactile (A) and thermal (B) stimulation, regardless of treatment group. (C) Subjects receiving complete spinal block with lidocaine show increased vocalizations to girdle stimulation. * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 6 for Vehicle-Unshocked, Vehicle-Shocked, Lidocaine-Shocked; n = 5 for Lidocaine-Unshocked).
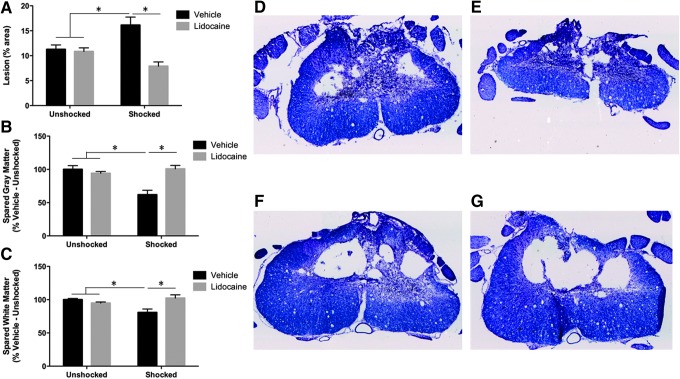
Analyses of tissue sparing showed that vehicle-treated shocked rats exhibited larger lesions (Fig. 6A) and greater loss of gray matter (Fig. 6B) and white matter (Fig. 6C). These effects were complete blocked by pre-treatment with lidocaine. Independent ANOVAs confirmed that in all three cases, there was a significant effect of shock treatment, and a shock by drug treatment interaction (all Fs >4.51; p < 0.05).
FIG. 6.
Lesion volume after electrical stimulation and complete spinal block. Subjects receiving electrical stimulation show increased lesion volume (A), reduced spared gray matter (B), and reduced spared white matter (C) that is blocked by lidocaine treatment. Representative stained sections from Vehicle-Unshocked (D), Vehicle-Shocked (E), Lidocaine-Unshocked (F), and Lidocaine-Shocked (G) groups. * indicates statistical significance (p < 0.05). Error bars represent standard error of the mean (n = 6 for Vehicle-Unshocked, Vehicle-Shocked, Lidocaine-Shocked; n = 5 for Lidocaine-Unshocked).
Discussion
Prior work has shown that nociceptive stimulation soon after injury impairs recovery and increases tissue loss at the site of injury. These adverse effects have been linked to signal processes related to the induction of apoptosis (caspase 3 and 8), pyroptosis (IL-1β and 18), and hemorrhage (increased hemoglobin protein within the injured tissue).6 Here, we showed that pre-treatment with the Na+ channel blocker lidocaine blocks all of these cellular effects. Shock treatment also disrupted locomotor recovery, undermined weight gain, and increased tissue loss at the site of injury. Again, all of these effects were blocked by pre-treatment with lidocaine.
Locomotor testing at the end of the recovery period provided further evidence that lidocaine blocked the adverse effect of nociceptive stimulation. Vehicle-treated shocked rats exhibited poor locomotor function 6 weeks after treatment and were unable to perform on either the beam or ladder walk tasks. Lidocaine shocked rats were able to perform these tasks and were indistinguishable from the unshocked subjects.
Prior work has shown that injured rats, relative to sham controls, exhibit enhanced motor reactivity to tactile stimulation and that early exposure to nociceptive stimulation fosters the development of this effect.5 While vehicle-treated shocked rats were more responsive to tactile and thermal stimulation applied to the paw or tail, this effect did not reach statistical significance. A less robust effect was likely observed because the present study assessed nociceptive reactivity just once at the end of the recovery period, whereas prior work employed multiple tests (which would increase statistical power).
When vocalization to tactile stimulation applied to the girdle region was assessed at the end of the recovery period, we found that lidocaine-treated rats were generally more responsive. This effect could arise because lidocaine-treated rats retained greater sensory/nociceptive function or because drug treatment per se fosters chronic pain. If pain was enhanced, the effect seems unrelated to a general, spinally-mediated sensitization of nociceptive processing because there was no evidence lidocaine affected motor reactivity to tactile or thermal stimulation. Further work is needed to determine whether the heightened responsiveness is related to at-level pain or a general increase in behavioral reactivity, potentially related to the preservation of neural function.
Prior work had shown that an injection of morphine at a dose that blocks behavioral reactivity to nociceptive stimulation does not attenuate the adverse effect of shock on long-term recovery.7 The present study reinforced these observations, demonstrating that an analgesic dose of morphine does not block the shock-induced enhancement in IL-1, IL-18, caspase-8, or hemorrhage. In contrast, lidocaine blocked the expression of pro-inflammatory cytokines, indices of cell death, hemorrhage, and the adverse effect nociceptive stimulation has on long-term recovery. Moreover, while morphine treatment has been associated with increased mortality and tissue loss, there was little evidence that lidocaine per se adversely affects physiological function.7,8 Further work is needed to determine the optimal dose range for lidocaine and whether early, prolonged treatment reduces the development of secondary injury independent of nociceptive input.
Acute pain undermines recovery after neural injury
Other laboratories have begun to explore the effect of polytrauma in animal models of central nervous system damage. In a model of multi-trauma associated with traumatic brain injury (TBI), tibial fracture significantly impaired functional outcomes and increased cell loss. Mice that received a mild TBI showed no impairment in behavior 5 weeks after injury. In contrast, subjects that received a mild TBI in conjunction with a unilateral tibial fracture showed behavioral abnormalities and increased ventricular volume.25
The results presented here, coupled with work from other laboratories, suggest that polytrauma may impair patient outcomes, especially in situations with damage to nervous tissue. Because the cause of most spinal cord injuries is traumatic, the prevalence of associated injuries is assumed to be high. However, no published studies have examined the prevalence and severity of associated injuries in patients with spinal cord injury. Additional studies are needed to evaluate whether an inhibition of neural excitability with a local anesthetic has a protective effect in other models of neural injury.
Likewise, further work is needed to assess current treatment protocols, which rely heavily upon the use of opiate analgesics. While opiates are effective at reducing psychological pain, their use soon after injury may synergistically interact with processes engaged by neural injury to promote tissue loss. For example, the current study showed that nociceptive stimulation up-regulates the expression of pro-inflammatory cytokines (IL-1β and 18) related to pyroptotic cell death. Local application of an opiate also increases IL-1β expression and this effect has been related to the loss of neural function.7 In contrast, micro-injecting lidocaine by means of a lumbar puncture can effectively block nociceptive transmission and the secondary activation of signal pathways tied to cell death and hemorrhage. In many cases of SCI, lidocaine may provide an effective treatment for pain and reduce secondary injury.
Clinical application
The use of sodium channel blockers for the control of pain is common. In dentistry, sodium channel blockers are routinely used for the management of pain during surgical procedures.26 Sodium channel blockers also are routinely used for pain control during child birth, to provide anesthesia during surgical procedures and to manage post-operative pain.10–12
In the present experiments, lidocaine was chosen as a sodium channel blocker due to its widespread availability and use. However, recent evidence suggests that lidocaine also has anti-inflammatory properties that may contribute to its efficacy.27–29 The relative importance of the anti-inflammatory and sodium channel blocking properties of lidocaine were not examined. However, because lidocaine had little effect in unshocked subjects, we expect that inhibition of action potentials is the primary mechanism of lidocaine.
Another treatment that has had success at improving functional outcomes after SCI is therapeutic hypothermia. The effects of this treatment in improving functional outcomes has been linked to a number of different mechanisms, including reducing metabolic demands, blood–brain barrier permeability, inflammatory signaling, neuronal cell death, and edema. Importantly, therapeutic hypothermia also has been tied to the reduction of neural excitability and excitotoxicity.30,31 We would anticipate that cooling spinal tissue during the application of nociceptive stimulation would have a protective effect. Indeed, clinical studies may have yielded mixed results, in part because there was variation in the extent of polytrauma. Lidocaine's effect on acute pain may be thought of in a similar fashion to hypothermia's effect on general neural excitability. Because pharmacological inactivation with lidocaine relies upon procedures that are well-known and easily controlled, this procedure may be preferred in many clinical settings.
We propose that therapeutic strategies aimed at interrupting C fiber signaling distal to the spinal cord injury may successfully treat both the conscious perception of pain and the detrimental impacts of pain on the injury site. We showed that complete spinal block caudal to the injury site with lidocaine was successful at reversing cellular and behavioral impacts of acute pain. Thus, lidocaine should be considered as an alternative analgesic therapy in patients with acute pain after spinal cord injury. Additionally, if peripheral tissue damage is limited to the innervation of a single nerve, a peripheral nerve block would be expected to adequately treat both conscious perception of pain and the negative sequelae associated with it.
Supplementary Material
Acknowledgments
The authors would like to thank Mabel Terminel, Melissa Brumley, Ashley DeLeon, and Julia Forsberg for their help with this work. This work was supported by grants from the Craig H. Neilsen Foundation and NIH (NINDS NS091723) to JWG.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grau J.W., Washburn S.N., Hook M.A., Ferguson A.R., Crown E.D., Garcia G., Bolding K.A., and Miranda R.C. (2004). Uncontrollable stimulation undermines recovery after spinal cord injury. J. Neurotrauma 21, 1795–1817 [DOI] [PubMed] [Google Scholar]
- 2.Grau J.W., Huie J.R., Garraway S.M., Hook M.A., Crown E.D., Baumbauer K.M., Lee K.H., Hoy K.C., and Ferguson A.R. (2012). Impact of behavioral control on the processing of nociceptive stimulation. Front. Physiol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grau J.W., Huie J.R., Lee K.H., Hoy K.C., Huang Y.J., Turtle J.D., Strain M.M., Baumbauer K.M., Miranda R.M., Hook M.A., Ferguson A.R., and Garraway S.M. (2014). Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front. Neural Circuits 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garraway S.M., Turtle J.D., Huie J.R., Lee K.H., Hook M.A., Woller S.A., and Grau J.W. (2011). Intermittent noxious stimulation following spinal cord contusion injury impairs locomotor recovery and reduces spinal brain-derived neurotrophic factor–tropomyosin-receptor kinase signaling in adult rats. Neuroscience 199, 86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garraway S.M., Woller S.A., Huie J.R., Hartman J.J., Hook M.A., Miranda R.C., Huang Y.J., Ferguson A.R., and Grau J.W. (2014). Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain 155, 2344–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtle J., Strain M.M., Reynolds J.A., Huang Y.J., Garraway S.M., and Grau J.W. (2015). Abstracts from the 33rd Annual National Neurotrauma Symposium. J. Neurotrauma 32, A–91 [Google Scholar]
- 7.Hook M.A., Liu G.T., Washburn S.N., Ferguson A.R., Bopp A.C., Huie J.R., and Grau J.W. (2007). The impact of morphine after a spinal cord injury. Behav. Brain Res. 179, 281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hook M.A., Moreno G., Woller S., Puga D., Hoy K., Jr, Balden R., and Grau J.W. (2009). Intrathecal morphine attenuates recovery of function after a spinal cord injury. J. Neurotrauma 26, 741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildsmith J.A.W. (2011). Lidocaine: a more complex story than simple chemistry suggests. Proc. Hist. Anaesth. Soc. 43, 9–16 [Google Scholar]
- 10.Michelle J.K., Osterman M.H.S., and Martin J.A. (2011). Epidural and spinal anesthesia use during labor: 27-state reporting area, 2008. Natl. Vital Stat. Rep. 59. [PubMed] [Google Scholar]
- 11.Burke D., Kennedy S., and Bannister J. (1999). Spinal anesthesia with 0.5% S(−)-bupivacaine for elective lower limb surgery. Reg. Anesth. Pain Med. 24, 519–523 [DOI] [PubMed] [Google Scholar]
- 12.McDonnell J.G., O Donnell B., Curley G., Heffernan A., Power C., and Laffey J.G. (2007). The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial: Anesth. Analg. 104, 193–197 [DOI] [PubMed] [Google Scholar]
- 13.Gruner J.A. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–128 [DOI] [PubMed] [Google Scholar]
- 14.Mestre C., Pélissier T., Fialip J., Wilcox G., and Eschalier A. (1994). A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods 32, 197–200 [DOI] [PubMed] [Google Scholar]
- 15.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open rield testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 16.Ferguson A.R., Hook M.A., Garcia G., Bresnahan J.C., Beattie M.S., and Grau J.W. (2004). A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma 21, 1601–1613 [DOI] [PubMed] [Google Scholar]
- 17.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., and Yaksh T.L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 18.Crown E.D., Ferguson A.R., Joynes R.L., and Grau J.W. (2002). Instrumental learning within the spinal cord: II. Evidence for central mediation. Physiol. Behav. 77, 259–267 [DOI] [PubMed] [Google Scholar]
- 19.Joynes R.L., Ferguson A.R., Crown E.D., Patton B.C., and Grau J.W. (2003). Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behav. Brain Res. 141, 159–170 [DOI] [PubMed] [Google Scholar]
- 20.Hook M.A., Ferguson A.R., Garcia G., Washburn S.N., Koehly L.M., and Grau J.W. (2004). Monitoring recovery after injury: procedures for deriving the optimal test window. J. Neurotrauma 21, 109–118 [DOI] [PubMed] [Google Scholar]
- 21.Fink S.L. and Cookson B.T. (2005). Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsbaken T., Fink S.L., and Cookson B.T. (2009). Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Rivero Vaccari J.P., Dietrich W.D., and Keane R.W. (2014). Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 34, 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccari J.P. de R., Lotocki G., Marcillo A.E., Dietrich W.D., and Keane R.W. (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz S.R., Sun M., Wright D.K., Brady R.D., Liu S., Beynon S., Schmidt S.F., Kaye A.H., Hamilton J.A., O'Brien T.J., Grills B.L., and McDonald S.J. (2015). Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 35, 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas D.A. (2002). An update on local anesthetics in dentistry. J. Can. Dent. Assoc. 68, 546–552 [PubMed] [Google Scholar]
- 27.Caracas H.C.P.M., Maciel J.V.B., de Souza M.M.G., and Maia L.C. (2009). The use of lidocaine as an anti-inflammatory substance: a systematic review. J. Dent. 37, 93–97 [DOI] [PubMed] [Google Scholar]
- 28.Hollmann M.W., and Durieux M.E. (2000). Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93, 858–875 [DOI] [PubMed] [Google Scholar]
- 29.Van Der Wal S., Vaneker M., Steegers M., Van Berkum B., Kox M., Van Der Laak J., Van Der Hoeven J., Vissers K., and Scheffer G.J. (2015). Lidocaine increases the anti-inflammatory cytokine IL-10 following mechanical ventilation in healthy mice. Acta Anaesthesiol. Scand. 59, 47–55 [DOI] [PubMed] [Google Scholar]
- 30.Dietrich W.D., Atkins C.M., and Bramlett H.M. (2009). Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 26, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich W.D., Levi A.D., Wang M., and Green B.A. (2011). Hypothermic treatment for acute spinal cord injury. Neurotherapeutics 8, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.