Abstract
Spinal cord injury (SCI) results in devastating changes to almost all aspects of a patient's life. In addition to a permanent loss of sensory and motor function, males also will frequently exhibit a profound loss of fertility through poorly understood mechanisms. We demonstrate that SCI causes measureable pathology in the testis both acutely (24 h) and chronically up to 1.5 years post-injury, leading to loss in sperm motility and viability. SCI has been shown in humans and rats to induce leukocytospermia, with the presence of inflammatory cytokines, anti-sperm antibodies, and reactive oxygen species found within the ejaculate. Using messenger RNA and metabolomic assessments, we describe molecular and cellular changes that occur within the testis of adult rats over an acute to chronic time period. From 24 h, 72 h, 28 days, and 90 days post-SCI, the testis reveal a distinct time course of pathological events. The testis show an acute drop in normal sexual organ processes, including testosterone production, and establishment of a pro-inflammatory environment. This is followed by a subacute initiation of an innate immune response and loss of cell cycle regulation, possibly due to apoptosis within the seminiferous tubules. At 1.5 years post-SCI, there is a chronic low level immune response as evidenced by an elevation in T cells. These data suggest that SCI elicits a wide range of pathological processes within the testes, the actions of which are not restricted to the acute phase of injury but rather extend chronically, potentially through the lifetime of the subject. The multiplicity of these pathological events suggest a single therapeutic intervention is unlikely to be successful.
Keywords: : blood–testes barrier, gene expression, metabolomics, microarray, spinal cord injury
Introduction
Spinal cord injury (SCI) affects up to 12,000 new patients in the United States every year.1,2 Although sensory and motor loss are the signature symptom of this injury, these represent only a fraction of the negative consequences of SCI. SCI elicits pathological changes to the gastrointestinal and urinary tracts, loss of bone density, muscle atrophy, neuropathic pain, autonomic dysfunction, sleep disturbances, and increased pulmonary complications.3–9 In addition to the physical ailments, patients with SCI commonly experience marital stress, job loss, depression, and other psychological and social issues semi-unique to each patient. One physical symptom that causes male patients much psychological stress, yet remains largely understudied, is the development of infertility.
More than 80% of patients with SCI are male, and the majority of those are young men of reproductive age who may not have had the opportunity to have a family yet, causing even more psychological stress.1,10 Reproductive assistance techniques have greatly improved in recent history: fewer than 1% of men with SCI in 1960 became fathers via intercourse. This has improved in 2003 to an approximately 51% pregnancy rate in those whi seek to reproduce including extensive medical assistance. However, such assistance is prohibitively expensive and unsuccessful in half of patients.10,11 Contrary to popular belief, sensory loss does not eliminate sexual capacity. Motor function loss can be circumvented and erectile dysfunction from neurological damage can be treatable via oral medication or mechanically in the majority of patients.1,2,12–15 Depending on the level of the lesion, even anejaculation can be treated with mechanical devices in the majority of patients.15–18 However, infertility due to unfavorable sperm and ejaculate parameters are not so easily treated. Although a portion of SCI patient's sperm immotility appears to be from seminal plasma influence, a portion is intrinsic to the sperm itself.19 This problem has remained largely unexplored.
Human and animal data show that SCI elicits whole–body, systemic changes that may contribute to the disturbances described above.6,20–22 These changes consist of both local and systemic inflammatory events, as well as altered immune function.6,22,23 In addition, it has long been established that SCI causes a breakdown of the blood–spinal cord barrier, which results in the influx of foreign substances and activated immune cells into traumatized spinal tissues.24 SCI also has been shown to cause inflammation, loss of structural integrity, and immune cell activation/infiltration in tissues such as the uroepithelium of the bladder and the tissues of the lung.6,25,26 In an earlier study, we showed that a clinically-relevant spinal contusion injury produced an early but sustained long-term disruption of the blood–testes barrier (BTB) in the testes of adult male Sprague-Dawley rats.20 SCI-induced early/chronic BTB failure was shown by dynamic contrast enhanced MRI, with subsequent tissue analysis demonstrating a loss of tight junction protein expression, immune cell infiltration, and the presence of normally-excluded serum proteins.20 BTB leakage was sustained up to 10 months post-SCI, suggesting a long-term, potentially permanent functional deficit in testicular function following SCI.20 The mechanism(s) underlying this sustained BTB pathology are unknown and are the focus of this present study. We performed a temporal metabolomic and microarray analysis of SCI-induced changes within the testis of male rats following spinal contusion injury from early acute (24 h) to chronic (3 months) post-SCI. We demonstrate that spinal contusion injury, the most common form of SCI, results in early but sustained biochemical, molecular, and cellular events as detected by metabolomics and gene expression analysis that may contribute to the condition of SCI-induced infertility in males.27,28
Methods
Animal groups
Metabolomics/microarrays
Eighty-eight male Sprague Dawley rats (200–250 g) were used for metabolomic and 69 were used for gene expression microarray studies. The difference between these studies is that eight naïve rats were used for the acute time-points in the metabolomics experiments and 16 in the messenger RNA (mRNA) experiments. They were divided into groups as indicated in Table 1. Naïve cohorts from the 24 h and 72 h groups were determined to be age-equivalent and were used as one group in the metabolomic studies.
Table 1.
Animal Groups for Metabolomics and Array Experiments
One animal was excluded due to a surgical error.
One animal died during sham operation.
Two animals in the same cage died post-operative day 4, two animals died post-operative day 7.
One animal was excluded due a BBB score of 3 on day 2, and one animal died during surgery.
In the metabolomics experiments, 4 naïve animals at 24 hours and 4 naïve animals at 72 hours were used and these data were comined for analysis.
SCI, spinal cord injury.
Immune cell infiltrate analysis
An additional 30 animals (n = 6/group) were used for immune cell identification and quantification via flow cytometry in three groups at 72 h (naïve, SCI, and sham) and in two groups at 1.5 years (naïve, SCI) after SCI at Baylor College of Medicine.
Spinal cord injury
All animals were handled in accordance with our Institutional Animal Care and Use Committee approved protocol. SCI rats received a spinal cord contusion injury at thoracic level 10 (T10) using the Infinite Horizons Spinal Impactor Device (Precision Systems Instrumentation) with 150 kdynes of force delivered over a 1-sec dwell time period. Sham-injured (sham) subjects were anesthetized and received a spinal laminectomy at T10, but did not receive a spinal cord contusion injury. All SCI and sham subjects received the following treatment during the post-operative period: 1) antibiotic (2.5 mg/kg Baytril) for a period of 10 days; 2) the opiate buprenorphine (0.025 mg/kg, twice daily for a period of 5 days, then as needed); and 3) 1.5 cc of 0.9% saline, twice daily, for a period of 3 days to ensure hydration. SCI animals received twice-daily, manual bladder evacuations using the method of Crede as modified for rats for an anticipated period of 10–14 days, or until neurogenic bladder evacuation was established. Beginning on Day 1 post-surgery, SCI, and sham subjects were examined using the non-invasive, Basso, Beattie, Bresnahan open field locomotor test.29 Subjects that scored more than 2 on either Day 1 or 2 of the study were excluded as such a score indicates an insufficient level of spinal damage. All animals were fed ad libitum. Naïve animals received no other manipulations.
End-stage tissue collection
At each of the time-points described above, animals were euthanized with 75 mg/kg of Beuthanasia (390 mg/mL pentobarbital, 50 mg/mL phenytoin). Subjects were quickly decapitated and the testes were collected, snap frozen in liquid nitrogen, and stored at −80°C. The epididymides were not collected. A different set of SCI animals were injured and euthanized using the same methods but were transcardially perfused with ice-cold saline prior to perfusion; blood was collected transcardially into BD Microtainer tubes, allowed to coagulate for 30 min, spun at 14,000 rpm for 10 min to separate out the serum supernatant. Collected serum was used to determine serum levels of bile acids.
Flow cytometry
A single-cell suspension was prepared from rat testes using a 70-μm cell strainer (BD) as described.30 Isolated cells were washed with ice-cold flow cytometry wash solution (phosphate-buffered saline [PBS] + 2% goat serum +2% bovine serum albumin), stained with fluorophore-conjugated antibodies (Table 2) or with ShK-F6CA, a fluorophore-conjugated peptide selective for Kv1.3, a marker of activated effector-memory T cells.
Table 2.
Antibodies Used for Flow Cytometry to Identify Immune Cell Populations
| Markers | Conjugation | Vendor | Catalog number |
|---|---|---|---|
| CD3 | APC | BD Pharmingen | 557030 |
| CD3 | Brilliant Violet 605 | BD Pharmingen | 563949 |
| B220 | PE | eBioscience | 12-0460-82 |
| CD11b | V450 | BD Pharmingen | 562108 |
| CD103 | Alexa Fluor 647 | Biolegend | 205509 |
| CD161a | PE | BD Pharmingen | 555009 |
| Ly6G | FITC | Abcam | ab25024 |
| CD4 | V450 | BD Pharmingen | 561579 |
| CD8 | PE | BD Pharmingen | 554857 |
| CD62L | APC | Biolegend | 202916 |
Selection of antibodies used for flow experiments
CD3 is part of the T cell receptor complex, expressed at the surface of only T lymphocytes. B220 is a 220 kDa isoform of CD45 expressed mainly at the surface of B cells in mouse and rat.31 CD11b is a surface marker for monocytes, macrophages and granulocytes.32 CD103 is expressed on the surface of most dendritic cells.33 Ly6G is a surface protein predominantly expressed on neutrophils.34 CD161a (NK1.1) is a surface marker for natural killer lymphocytes.35 CD4 and CD8 are surface markers of helper T cells and cytotoxic T cells, respectively.36 Kv1.3 is a voltage-gated potassium channel whose expression on the cell surface is upregulated in C-C chemokine receptor type 7 effector memory T cells upon activation.37 CD62L (L-selectin) is expressed at the surface of naïve but not memory T cells.36 CD4, CD8, Kv1.3, and CD62L also are expressed by other cells; thus, the use of double staining with CD3 to ensure their expression was only studied on T cells.38
Cells were washed and fixed in cold PBS + 1% paraformaldehyde. FACSCanto II or LSRFortessa flow cytometers (Becton Dickinson) with the FACSDiva software Data were used to acquire sample data within the Cytometry and Cell Sorting facility at Baylor College of Medicine, and analyzed using FlowJo software (Treestar). For each sample, doublet discrimination was performed on 30,000 acquired events.39
Metabolomic analysis
After collection at each of the time-points listed above, the right testes were homogenized under liquid nitrogen. Samples from 88 animals were processed and metabolomics profiling was performed by Metabolon Inc. (Durham, NC). Briefly, an unbiased metabolomic profile of testis from SCI, sham, and age-matched control rats was performed using gas chromatography coupled to mass spectrometry (gas chromatography [GC/mass spectrometry [MS, Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization) and ultra–high performance liquid chromatography coupled to mass spectroscopy (ultra-performance liquid chromatography [UPLC/MS, Waters ACQUITY ultra-performance liquid chromatography and a Thermo-Finnigan LTQ mass spectrometer, which consisted of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer). At the time of this experiment, Metabolon's metabolomics platform was capable of detecting more than 3,500 substances. Compounds found in the sample were cross-referenced to their library of compounds for identification. Analysis of variance (ANOVA) contrasts were used to identify biochemicals that differed significantly between experimental groups following log transformation and imputation of missing values, if any, with the minimum observed value for each compound. Metabolite outliers of more than 2 standard deviations away from the group mean were similarly excluded from analysis. Metabolites were filtered out of the dataset if they were not detectable in >60% of animals in the majority of groups or where the entire difference between sham and SCI group means was due to changes in 25% of animals or less, and subsequently excluded from analysis. Values were converted to a ratio to the mean of the sham group for graphing purposes.
Serum bile acid
Serum bile acids were measured using a method adapted from Woolbright and colleagues.40 Rat serum samples were prepared using a methanol extraction procedure to facilitate the removal of serum proteins by centrifugation. This was done by mixing 30 μL of serum with 80 μL of methanol spiked with internal standards and briefly vortexing, then centrifuging at 15 000 × g for 20 min. The supernatant extracts were injected (5 μL) into a HPLC system (Agilent Technologies, Santa Clara, CA). Bile acid separation was achieved using a 1260 Infinity Binary LC System equipped with a 100 mm × 2.1 mm (C-18 BEH, Waters) column. The column temperature was maintained at 45°C and the flow rate was 0.3 mL/min with a gradient in a 25-min run. Gradients were run starting from 95% buffer A containing ammonia acetate (water/methanol, 80:20 v/v, pH = 8.4) and 5% buffer B containing ammonia acetate (acetonitrile/water 90:10 v/v, pH = 8.4) and to 75% A from 0–5.0 min; 75% A to 60% A from 5.0–10.5 min; 65% A to 5% A from 10.5 to 18 min; 5% A to 0% A from 18–22 min; 0% A was held for 1.0 min; 0% A to 95% A from 23–23.5 min; 95% A was held from 23.5–25 min to re-equilibrate the column. The HPLC eluate was analyzed in negative mode with electrospray ionization on a 6490 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) using the multiple reaction monitoring method. The drying gas temperature was set up at 250°C for positive mode and 290°C for negative mode, correspondingly. Drying and sheath gas flow maintained at 14 l/min and 12 l/min for both modes, respectively. Capillary voltage was set at 3500 V for positive mode and 3200 V for negative mode. The relative abundance of bile acids was calculated based on the peak area.
Microarray analysis
Microarray studies were performed in the UTHealth Microarray Core Facility under the direction of Dr. David Loose. The Microarray Core Facility provided experimental handling and support for all aspects of the Agilent microarray platform used in this study. The specific arrays used were the Agilent SurePrint G3 Rat GE 8x60K Microarrays. These arrays have 60,000 probes and a number of control probes for quality control assessment. We performed a total of 96 arrays on 12 groups as indicated in Table 1 with n = 8 for all groups. Following treatment, total RNA was extracted from the testis (Qiagen, Hilden, Germany) and gene expression profiles examined by array. The arrays were pre-processed and captured image data for microarray features was accomplished with Agilent's SureScan and Feature Extraction software. Data were background corrected with the local background algorithms in the Feature Extractor software. The main software used for subsequent analysis was BRB Array tools (v 4.4). Data were normalized using quantile normalization, and transcripts that were differentially expressed identified by univariate analysis at p < 0.01 corrected for multiple testing (Bonferroni).
Caspase-3 assay
Caspase activity was performed on testis protein extract using the EnzChek caspase-3 assay kit #1, following the manufacturers protocol (Molecular Probes). This assay measures the increase in fluorescence generated by the cleavage of the aminomethylcoumarin (AMC)-labelled caspase-3 substrate Z-DEVD-AMC. The production of fluorescent substrate was monitored continuously every 5 min for approximately 2 h in a 96-well dish using an automated fluorescent plate reader with data acquisition software (LS55 luminescence spectrometer, PerkinElmer Instruments, Shelton, CT). The slope of the linear regression drawn through each time-point was used to determine change in fluorescence over time for each sample. A standard curve using known amounts of AMC was used to convert fluorescent values to specific catalytic activity.41
All assays were run under blinded conditions and all samples were randomized prior to analysis.
Results
SCI-dependent cellular immune components
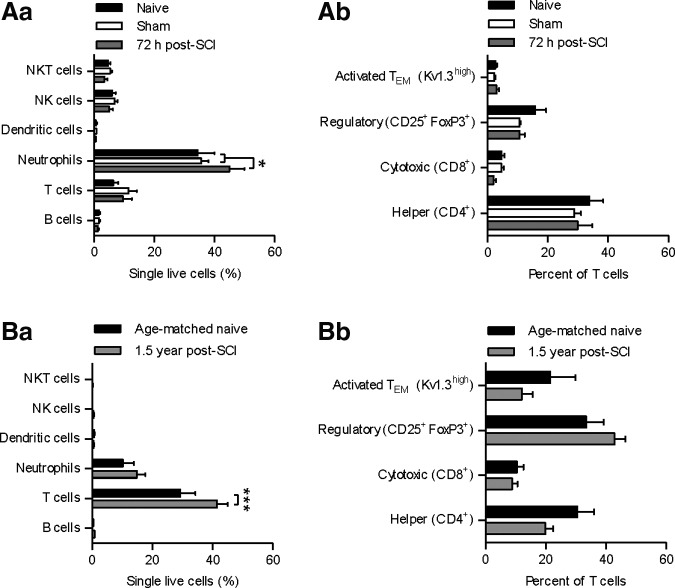
Immune cells were identified and quantified by flow cytometry using cell surface specific markers in the testis. These included neutrophils, dendritic cells, and lymphocytes (T, B, NK, and NKT), as well as differentiating between subsets of T cells. Flow cytometric analysis of testicular tissue (Fig. 1) showed that neutrophil counts were significantly increased at 72 h in the acute SCI animals versus both sham and naïve animals (26.4% and 30.8% increase, respectively; p < 0.05), but were not elevated in the chronic 1.5 year post-surgery SCI animals. In the 1.5 year chronic SCI animals, we observed an elevation in T cells in the testes (41.6% increase; p < 0.001) that was not seen at 72 h. There was no significant change in subsets of T-cell phenotypes at either time-point.
FIG. 1.
Flow cytometric analysis of testes tissue for immune cell phenotypes: *p < 0.05, ***p < 0.001. (Aa) Neutrophils were elevated 72 h post–spinal cord injury (SCI), compared with sham and naïve (26.4% and 30.8%, respectively; p < 0.05). (Ab) T-cell phenotypes were unchanged after injury. (Ba) T-cells were elevated 1.5 years post-SCI (41.6%; p < 0.001). (Bb) T-cell phenotypes were unchanged after injury.
Metabolomics
A total of 369 metabolites were detected in the testes by UPLC/MS and GC/MS. These metabolites were clustered by biochemical function into amino acid, peptide, carbohydrate, energy, lipid, nucleotide, cofactors and vitamins, and xenobiotic “super pathways” and clustered again into smaller “sub-pathways” such as eicosanoids, lysolipids, glycolysis, bile acids, and 70 others. We used Metabolon's initial ANOVA analysis as a screening tool, and further scrutinized the data to find sub-pathways where over 30% of metabolites were significantly changed and subjected them to further analysis as described in the Methods section.
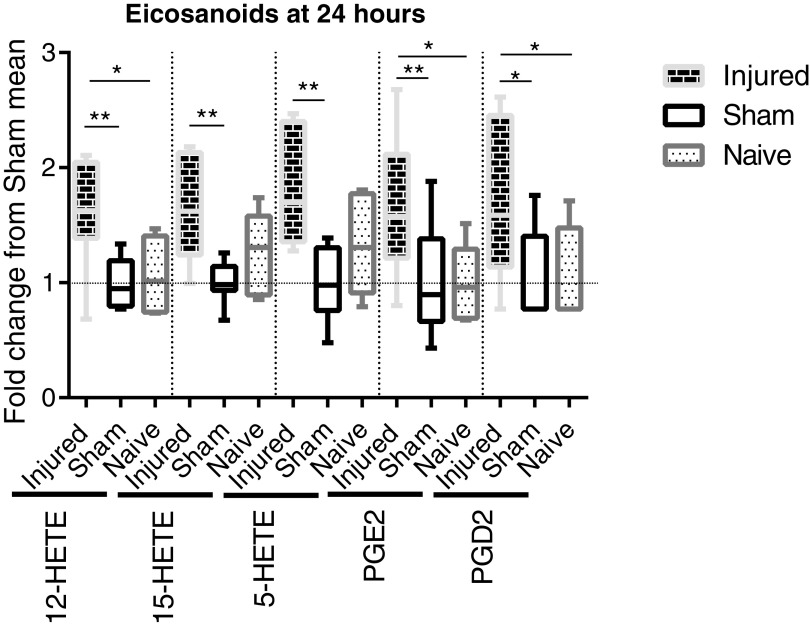
Eicosanoid metabolites exhibited a significant difference between groups (Fig. 2). Five eicosanoids were found via metabolomics analysis within the testes; these include prostaglandin E2 (PGE2), prostaglandin D2 (PGD, 5-hydroxyicosatetraenoic acid (HETE), 12-HETE, and 15-HETE. All five were significantly increased at 24 h after SCI, compared with sham, as analyzed by ANOVA with Bonferroni correction for multiple comparisons. Three of the five metabolites were significantly increased after SCI, compared with naïve; namely, PGE2, PGD2, and 12-HETE. The SCI mean fold change, compared with sham (SCI/Sham), ranged from 1.6 to 1.828. PGD2 remained significantly elevated at 72 h; at no other time-points were there significant differences in eicosanoid metabolites.
FIG. 2.
Metabolomic assessment of eicosanoids in the teste at 24 h post-surgery. Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01. 12- Hydroxyicosatetraenoic acid (HETE) in spinal cord injury (SCI) animals is elevated (analysis of variance [ANOVA: p = 0.0044), compared with both sham (p ≤ 0.01) and naïve (p ≤ 0.05), with an SCI mean fold change from sham mean of 1.6. 15-HETE in SCI rats is elevated (ANOVA: p = 0.0050), compared with sham (p ≤ 0.01), with an SCI mean fold change from sham mean of 1.655. 5-HETE in SCI rats is elevated (ANOVA: p = 0.0023), compared with sham (p ≤ 0.01), with an SCI mean fold change from sham mean of 1.828. Prostaglandin E2 (PGE2) in SCI rats in elevated (ANOVA: p = 0.0147), compared with sham (p ≤ 0.01) and naïve (p ≤ 0.05), with an SCI mean fold change from sham mean of 1.661. Prostaglandin E2 (PGD2) in SCI rats is elevated (ANOVA: p = 0.0198), compared with sham and naïve (p ≤ 0.05), with an SCI mean fold change from sham mean of 1.703.
Testicular lysolipids, phospholipids that by metabolism have lost one acyl chain, were also significantly different after SCI. After filtering as described in the Methods section, 38 lysolipids were detected in the testes: 11 of phosphocholine (PC) origin, 14 of phosphoethanolamine (PE) origin, six of phosphoinositol (PI) origin, four of phosphoserine origin, and three of phosphoglycerol origin. Nine of 11 lysolipids of PC origin (Fig. 3) were significantly elevated by ANOVA with Bonferroni correction for multiple comparisons in SCI rats, compared with sham, at 72 h post-surgery (the remaining two were significantly increased if analyzed by Student's t-test). The increase in lysolipid levels at 72 h post-SCI ranged from 3.587 to 10.04. Remarkably, none of the lysolipids of any other lipid class were significantly changed 72 h after SCI. Lysolipid changes at other time-points were not significant.
FIG. 3.
Metabolomic assessment of lysolipids at 72 h post-surgery. Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01. Compared with sham, lysolipids that were elevated after spinal cord injury (SCI) were:
1: 1-palmitoylglycerophosphocholine (p ≤ 0.01; analysis of variance [ANOVA: p = 0.0055; mean fold change: 8.118);
2: 2-palmitoylglyerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0113; mean fold change: 5.021);
3: 1-stearoylglycerophosphocholine (p ≤ 0.01; ANOVA p = 0.0067; mean fold change: 7.889);
4: 1-oleoylglycerophosphocholine (p ≤ 0.01; ANOVA: p = 0.0043; mean fold change: 5.929);
5: 2-oleoylglycerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0092; mean fold change: 7.841);
6: 1-linoleoylglycerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0316; mean fold change: 3.587);
7: 2-linoleoylglycerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0362; mean fold change: 6.869);
8: 1-arachidonoylglycerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0388; mean fold change: 4.694); and
9: 2-arachidonoylglycerophosphocholine (p ≤ 0.05; ANOVA: p = 0.0207; mean fold change: 10.04).
Compared with naïve, lysolipids elevated after SCI were:
3: 1-stearoylglycerophosphocholine (p ≤ 0.05);
4: 1-oleoylglycerophosphocholine (p ≤ 0.05); and
5: 2-oleoylglycerophosphocholine (p ≤ 0.05).
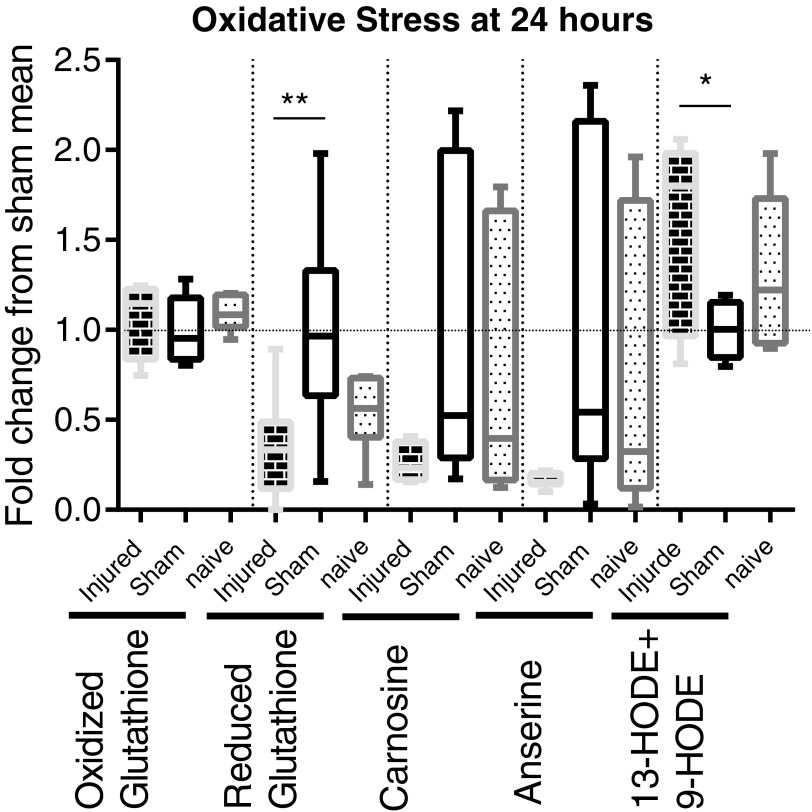
Five selected metabolites that are indicative of a state of oxidative stress also were measured. (Fig. 4). Oxidized glutathione is unchanged at all time-points, but the protective reduced form of glutathione is decreased at 24 h, compared with sham animals, which reduces the overall ratio of oxidized to reduced glutathione. Carnosine and anserine are unchanged as analyzed by ANOVA but are significantly different between SCI and sham as analyzed by the less stringent Student's t-test at 24 h (p = 0.033729 and 0.026128), 72 h (p = 0.024144 and 0.047352), and 28 days (p = 0.006919 and 0.026611) post-surgery. This trend of an ANOVA 0.05 < p < 0.1 and t-test of p < 0.05 for carnosine and anserine continues at 72 h and 28 days post-SCI (data not shown). 13-hydroxyoctadecadienoic acid (HODE) +9-HODE, two similar linoleic acid metabolites often lumped together that are elevated in oxidative stress, is elevated in SCI rats, compared with sham, at 24 h.
FIG. 4.
Metabolomic assessment of oxidative stress markers at 24 h post-surgery. Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01. Oxidized glutathione was not changed at 24 h. Reduced glutathione was suppressed after spinal cord injury (SCI), compared with sham (p ≤ 0.01; analysis of variance [ANOVA: p = 0.0064; mean fold change: 0.3376). Carnosine and anserine were unchanged at 24 h (ANOVA: p = 0.0966 and p = 0.0786, respectively). 13-Hydroxyoctadecadienoic acid (HODE) +9-HODE was elevated after SCI, compared with sham (p ≤ 0.05; ANOVA: p = 0.0337; mean fold change: 1.550).
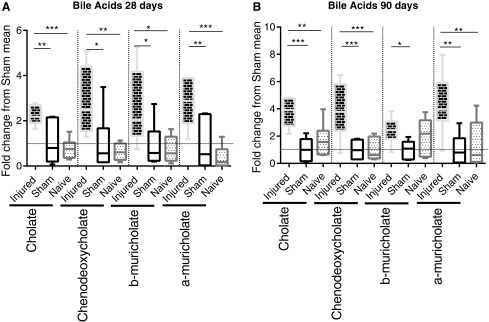
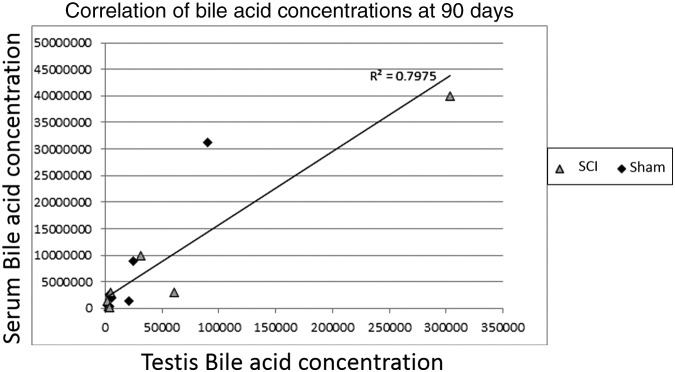
Four unconjugated bile acids were detected in the metabolomics profiling (Fig. 5) with all four elevated in the SCI animals, compared with shams (three of four, compared with naïve) at 28 and 90 days post-injury. The SCI mean fold change from sham mean ranged from 2.238 to 4.352. Bile acid changes at earlier time-points were negligible. In addition, 21 bile acids were measured in the serum of 90 day post-surgery rats by a separate mass spectrometric analysis, but only 1 (GLCA) was found to be statistically changed (elevated) from sham as analyzed by ANOVA (data not shown). Serum levels of bile acids showed a significant correlation (Fig. 6) to testis levels of bile acids (R2 = 0.7975), which is even more pronounced when separated into SCI and sham groups (R2 = 0.9506 and 0.9567, respectively). Interestingly, the difference between sham and SCI bile acid concentrations in the serum versus the difference between sham and SCI bile acid concentrations in the teste had an even higher correlation (R2 = 0.9845). There was a correlation (R2) value of 0.7975 between plasma levels and testes levels, but no statistical differences in unconjugated bile acids between SCI and sham in the serum.
FIG. 5.
Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. (A) Metabolomic assessment of unconjugated bile acids in the teste 28 days post-surgery Cholate in spinal cord injury (SCI) rats was elevated (analysis of variance [ANOVA: p = 0.0003), compared with sham (p ≤ 0.01; mean fold change: 2.238) and naïve (p ≤ 0.001). Chenodeoxycholate in SCI rats was elevated (ANOVA: p = 0.0016), compared with sham (p ≤ 0.05; mean fold change: 2.844) and naïve (p ≤ 0.01). b-Muricholate in SCI rats was elevated (ANOVA: p = 0.0096), compared with sham (p ≤ 0.05; mean fold change: 2.534) and naïve (p ≤ 0.05). a-Muricholate in SCI rats was elevated (ANOVA: p = 0.0001), compared with sham (p ≤ 0.01; mean fold change: 2.815) and naïve (p ≤ 0.001). (B) Metabolomic assessment of unconjugated bile acids in the teste 90 days post-surgery. Cholate in SCI rats was elevated (analysis of variance [ANOVA: p = 0.0001), compared with sham (p ≤ 0.001; mean fold change: 3.760) and naïve (p ≤ 0.01). Chenodeoxycholate in SCI rats was elevated (ANOVA: p = 0.0002), compared with sham (p ≤ 0.001; mean fold change: 3.836) and naïve (p ≤ 0.001). b-Muricholate in SCI rats was elevated (ANOVA: p = 0.0303), compared with sham (p ≤ 0.05; mean fold change: 2.420). a-Muricholate in SCI rats was elevated (ANOVA: p = 0.0010), compared with sham (p ≤ 0.01; mean fold change: 4.352) and naïve (p ≤ 0.01).
FIG. 6.
Correlation between levels of bile acids in the testes and levels of bile acids in the serum at 90 days post-surgery for both spinal cord injury (SCI) and sham animals combined (R2 = 0.7975). Separating the measurements into SCI and sham groups increased the correlation (R2 = 0.9506 and 0.9567, respectively). Taking the difference between sham and SCI bile acid concentrations in the serum vs. the difference between sham and SCI bile acid concentrations in the teste had an even higher correlation (R2 = 0.9845).
mRNA
We were interested in determining what changes occurred within the testis at the RNA level after SCI and therefore performed microarray experiments at the same time-points that were done in the metabolomic experiments. After analyzing these data for significantly changed transcripts in BRB Array tools, Ingenuity IPA software was used to cluster mRNA into pathways. Some pathways were changed in a rather incoherent pattern with conflicting activation and suppression of several transcripts, such as immune function genes at all time-points, while others were single gene changes whose significance was suspect. Overall, there were 267 transcripts significantly changed at 24 h after SCI, compared with sham, 252 transcripts at 72 h after SCI, 507 transcripts 28 days after SCI, and 392 transcripts 90 days after SCI. Here we present the major pathways affected by SCI.
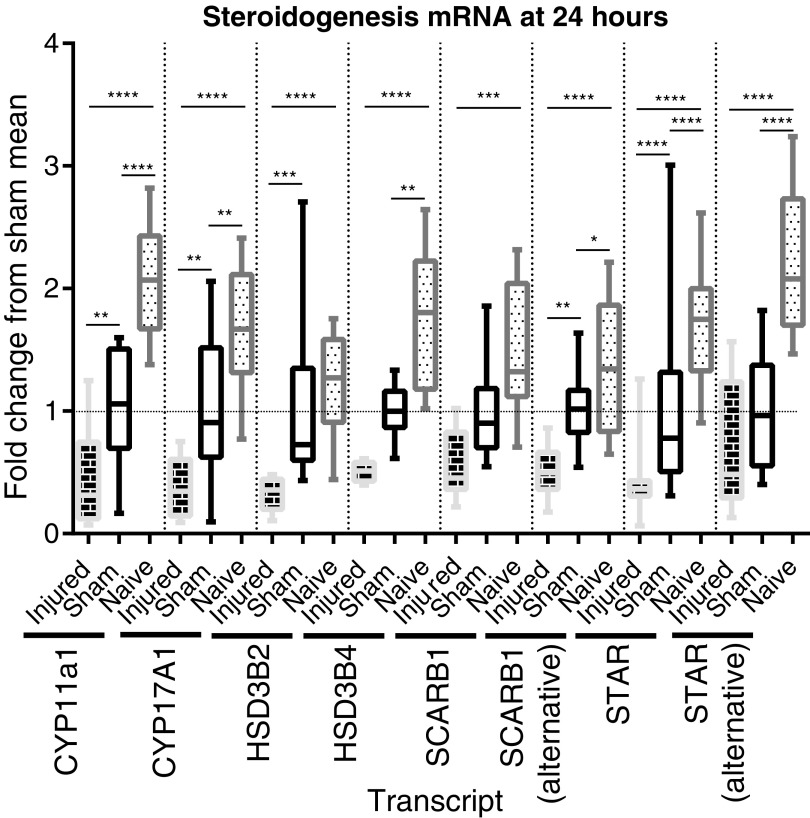
The 24 h post-SCI animals had decreased expression of eight transcripts directly involved in the testosterone production pathway (Fig. 8), including the rate limiting step of cholesterol transport into mitochondria (STAR) and the rate limiting enzyme (CYP11A1).42-44 The SCI mean fold change from sham mean ranged from 0.2882 to 0.5190.
FIG. 8.
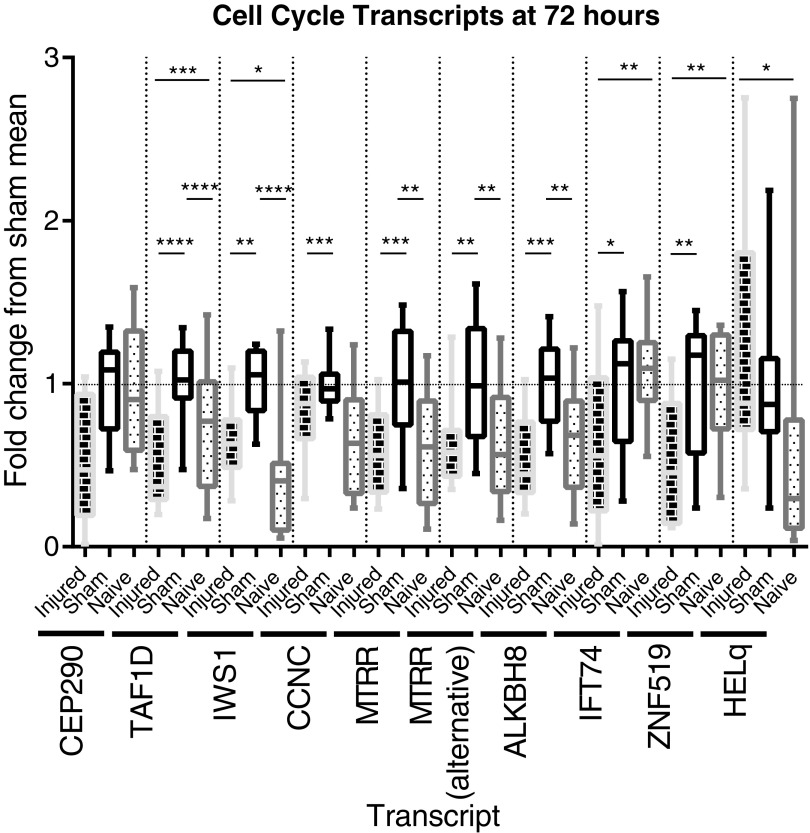
Array assessment of the expression of cell cycle and DNA function genes' messenger RNA (mRNA) transcripts at 72 h post-surgery. Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. CEP290 mRNA expression is altered between groups (analysis of variance [ANOVA: p = 0.0341) but the multiple comparison test failed to differentiate between groups. TAF1D mRNA expression is elevated in sham animals (sham vs. naïve: p ≤ 0.0001), and suppressed in spinal cord injury (SCI) animals (SCI vs. sham: p ≤ 0.0001; SCI vs. naïve: p ≤ 0.001), with a mean fold change vs. sham of 0.5214 (ANOVA: p < 0.0001). IWS1 mRNA expression is elevated in sham animals (sham vs. naïve: p ≤ 0.0001), and less elevated in SCI animals (SCI vs. sham: p ≤ 0.01; SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.6554 (ANOVA: p < 0.0001).CCNC mRNA expression is suppressed in SCI animals, compared with sham (sham vs. naïve: p ≤ 0.001), with a mean fold change vs. sham of 0.8242 (ANOVA: p = 0.0014). MTRR mRNA expression is elevated in sham animals (sham vs. naïve: p ≤ 0.01), but is not in SCI animals (SCI vs. sham: p ≤ 0.001), with a mean fold change vs. sham of 0.5730 (ANOVA: p = 0.0003). MTRR alt mRNA expression is elevated in sham animals (sham vs. naïve: p ≤ 0.01), but is not in SCI animals (SCI vs. sham: p ≤ 0.01), with a mean fold change vs. sham of 0.6093 (ANOVA: p = 0.0022). ALKBH8 mRNA expression is elevated in sham animals (sham vs. naïve: p ≤ 0.01) but not in SCI animals (SCI vs. sham: p ≤ 0.001), with a mean fold change vs. sham of 0.5452 (ANOVA: p = 0.0002). IFT74 mRNA expression is suppressed in SCI animals (SCI vs. sham: p ≤ 0.05, SCI vs. naïve: p ≤ 0.01), with a mean fold change vs. sham of 0.6086 (ANOVA: p = 0.0009). ZNF519 mRNA expression is suppressed in SCI animals (SCI vs. sham: p ≤ 0.01, SCI vs. naïve: p ≤ 0.01), with a mean fold change vs. sham of 0.5451 (ANOVA: p = 0.0014). HELQ mRNA expression is elevated in SCI animals (SCI vs. naïve: p ≤ 0.05), with a mean fold change vs. naive of 2.169 (ANOVA: p = 0.0182).
The mRNA array at 72 h shows that eight different transcripts involved in DNA function and cell cycle were decreased post-SCI (Fig. 7). The SCI mean fold change from sham mean ranged from 0.5214 to 0.8242.
FIG. 7.
Array assessment of the expression of steroidogenesis genes' messenger RNA (mRNA) transcripts at 24 h post-surgery. Whisker plots illustrate the median (horizontal bar), the 25th to 75th percentile (box) and the minimum to maximum value (whiskers). All values were scaled to set the sham mean equal to 1 (horizontal dotted line). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. CYP11a1 mRNA expression is suppressed in sham animals (sham vs. naïve: p ≤ 0.0001), and even more suppressed in spinal cord injury (SCI) animals (SCI vs. sham: p ≤ 0.01; SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.4486 (analysis of variance [ANOVA: p < 0.0001). CYP17a1 mRNA expression is suppressed in sham animals (sham vs. naïve: p ≤ 0.01), and even more suppressed in SCI animals (SCI vs. sham: p ≤ 0.01, SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.3878 (ANOVA: p < 0.0001). HSD3B2 mRNA expression is not suppressed in sham animals but is suppressed in SCI animals (SCI vs. sham: p ≤ 0.001; SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.3214 (ANOVA: p < 0.0001). HSD3B4 mRNA expression is suppressed in both sham animals (sham vs. naïve: p ≤ 0.01) and SCI animals (SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. naive of .2882 (ANOVA: p < 0.0001).SCARB1 mRNA expression is not suppressed in sham animals but is suppressed in SCI animals (SCI vs. naïve: p ≤ 0.001), with a mean fold change vs. naive of 0.3653 (ANOVA: p = 0.0012).SCARB1 alt (alternative transcript) mRNA expression is suppressed in sham animals (sham vs. naïve: p ≤ 0.05), and even more suppressed in SCI animals (SCI vs. sham: p ≤ 0.01; SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.5190 (ANOVA: p < 0.0001). STAR alt mRNA expression is suppressed in sham animals (sham vs. naïve: p ≤ 0.0001), and even more suppressed in SCI animals (SCI vs. sham: p ≤ 0.0001, SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. sham of 0.4382 (ANOVA: p < 0.0001). STAR mRNA expression is suppressed in both sham animals (sham vs. naïve: p ≤ 0.0001) and SCI animals (SCI vs. naïve: p ≤ 0.0001), with a mean fold change vs. naive of 0.3082 (ANOVA: p < 0.0001).
In addition to the mixed suppression and induction of immune function genes seen at all time-points, the mRNA array data from 28 days and 90 days show a similar mix of transcripts involved in chemotaxis.
Discussion
Little is known regarding the cascade of pathological events initiated in the testes following SCI. In order to begin deriving a greater understanding of these acute and long-term pathological changes that negatively impact fertility, we elected to take a broad, multifaceted approach examining immunological, metabolomics, and mRNA expression studies. These studies were driven by prior studies that demonstrated that SCI caused significant activation of the immune system,22,45 changes in hepatic function21 and globally altered gene expression.46–48 The amount of data generated via these methods is immense, encompassing multiple pathways and systems at multiple time-points.
Metabolomics is a recent tool that permits the quantitative assessment of large numbers of biochemicals or metabolites from many different pathways in order to get an unbiased snapshot of a tissue's metabolic profile. We analyzed our results focusing on multi-metabolite networks that could identify broad shifts in the metabolic state in the testis after SCI.
The greatest changes in the testicular metabolome observed at 24 h post-SCI were: 1) the establishment of an environment favoring both inflammatory and pro-oxidative conditions, and 2) a significant reduction in testosterone production. Inflammatory conditions appear to be favored via activation of two canonical pathways for arachidonic acid metabolism (cyclooxygenase [COX and lipoxygenase), which produce prostaglandins (PGE2/D2) and leukotriene precursors (some HETEs).49 Oxidative stress is evident by a decrease in reduced glutathione, which is protective. An increase in the ratio of reduced glutathione to oxidized glutathione is recognized as a well-established marker of oxidative stress.50 13-HODE +9-HODE, which together are markers for oxidative stress of free radical-mediated oxidation, is also increased at 24 h, although these can also be made by COX.51 These metabolites, and the consistent decrease in antioxidants anserine and carnosine, point toward at least a mild oxidative insult in the testes at 24 h. Both oxidative stress and inflammation have been shown to be associated with SCI-dependent male infertility.52
The decrease in the mRNA of the enzymes responsible for testosterone production shows that low testosterone in acute SCI patients is at least partly due to changes at the transcriptional level. This could indicate that Leydig cells are under stress and are unresponsive, but we think it is equally likely that SCI causes a shock to the entire central nervous system (CNS), reducing the amount of leutenizing hormone or gonadotropin-releasing hormone released into the system at the level of the CNS, which would lead to less stimulation of the testosterone production pathway. Because previous studies have been contradictory, endocrine profiles after SCI are poorly understood; however, any disrupted testosterone production would reduce fertility.2,49,53 These acute changes likely further develop or lay the groundwork for the alterations that follow.
At 72 h post-SCI, our data indicate increased cell death, debris, and catabolism within the testes. In the healthy teste, apoptosis occurs continuously at higher rates than most of the body as part of normal spermatogenesis.54 These apoptotic sperm cells are quickly absorbed by the supportive sustentacular Sertoli cells in a non-pathologic manner. Our lab has previously shown extensive terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and disorganization in segments of the seminiferous tubules 72 h after SCI.20 In such an environment, sperm production is not expected to continue at normal rates, if at all. Increased cell death within the seminiferous tubules, which would coincide with a cessation or slowing of spermatogenesis, would account for the coherent but small in magnitude decrease of so many mRNAs related to cell cycle and DNA function as seen in Figure 6. In support of this, at 72 h we also see an increase in nine lysophosphatidylcholines (LPCs). In apoptotic cells, caspase-3 activates calcium independent phospholipase A2, which catalyzes PCs into LPCs.55–57 LPC has been shown to be released by apoptotic cells to attract phagocytic cells and to induce pro-apoptotic pathways.58,59 This leads us to speculate that the increase in neutrophils at this same time-point may be caused by this increase in LPC acting as a chemo attractant.
All these data point to an SCI-induced enhanced apoptotic environment within the testes. However, direct caspase activity assays (data not shown) show no change in caspase 3 activity in the testes 24 or 72 h after injury, indicating that overall apoptosis rates in the whole testis is not significantly elevated. However, previous data shows significant TUNEL-positive staining in a portion of the seminiferous tubules in a cross-section of the testes at 72 h after SCI.20 This leads us to believe that a global insult to the testes occurs after SCI, but that only the seminiferous tubules in the developmental stages most sensitive to damage are affected enough to induce apoptosis, similar to what has been described in testis heat stress injury.58,60,61 Heat injury induces apoptosis in seminiferous tubules at early (I–IV) and late (XII–XIV) developmental stages of spermatogenesis, while segments of seminiferous tubule at other developmental stages showed little damage.60 Further study into which seminiferous tubule and spermatogenesis developmental stages are most affected is needed. These pathologic processes are further compounded by an immune response, including a neutrophilic infiltrate. Because the testis is a uniquely immune privileged environment, many cell types are attenuated or suppressed when they would be highly activated and increased in other tissue.62 Any change in immune cells in the testis first must overcome the suppressive effect of the Sertoli cells, which modulate immune function in order to prevent autoimmunity against the antigens in sperm cells that appear after the process of tolerance.62 Whether these neutrophils were present earlier and contributed to the stronger inflammatory and oxidative environment seen at 24 h or whether they were recruited via chemotaxis to the testes by the increase in lysolipids seen at 72 h is a question that requires further study. In summary, we postulate that SCI causes cell death in localized segments of the seminiferous tubules and a decrease in the overall rate of spermatogenesis throughout the testis.
There is strong evidence of bile acid metabolism changes in SCI. Regardless of SCI lesion level, patients with SCI have a greatly increased risk of developing biliary sludge as early as 3 months and gallstones as a late secondary complication, despite some studies showing voiding time and contractility in the gall bladder being within normal levels in late injury. This, together with our data, suggests a more fundamental change in the bile acid metabolism than just decreased mechanical function. We found that all four unconjugated bile acids detected above background in the testes are elevated at 28 and 90 days after injury. Because the enzymes needed to create bile acids are not present in the testis, this suggests a hepatic change in bile acid metabolism and provides a strong case to further investigate into bile acid metabolism changes after SCI. Indeed, bile acid levels in the serum correlated (Fig. 7) with bile acid levels in the testis. Serum bile acids were increased in the serum of SCI rats but these changes did not reach statistical significance as occurred in the testis. This suggests the sequestration and trapping of bile acids in the damaged testis after SCI. Bile acids have direct cytotoxic activity due to detergent action and elevated bile acids have been shown to reduce male fertility via farnesoid X receptor alpha (FXR-α) and TGR5 (G-protein-coupled bile acid receptor 1; GPBAR1) receptor signaling in mice, causing sloughing of sperm cells, spermatid apoptosis, and a breakdown of the BTB, which suggests another mechanism by which the testis is unable to heal the BTB in chronic SCI.63,64,67,68 Of note, tauroursodeoxycholic acid, recently shown to be protective against apoptosis in the cord in SCI, was only detected at low levels in two of the 88 animals.69,70 Further study is needed to determine the complete etiology of bile acid alterations both systemically and within the testes to determine how altered bile acid metabolism shapes male fertility throughout the chronic phase of SCI.
A previous study at 72 h and 10 months post-SCI showed a permeable BTB and presence of immune cells, but overall normal histology in the testes at 10 months post-SCI.20 These acute immune cell populations were not shown to be elevated in our data, suggesting that the histological changes were not changes in overall testes immune cell population, but rather were localized changes due to localized damage as discussed above. Either there is a similar localized change in our 1.5 year animals, or there is an evolving immune cell population that changed in the 8 months between those two time-points. This slowly and subtly changing pathology could explain the uncoordinated mRNA data we have seen in the 28 day and 90 day time-points. Additionally, the testes, particularly the seminiferous tubules in the presence of a failed blood–testes barrier would be a target for immune activity/auto-antibody production in response to highly antigenic sperm and sperm progenitor cells.20
In summary, male infertility caused by SCI is a multifaceted problem. At 24 h post-SCI, we observed increased inflammation, oxidative stress, and reduced steroidogenesis, which have all been shown to cause infertility. At 72 h post-SCI, we see evidence of a pathologic apoptotic environment that affects localized segments of the seminiferous tubules, similar to that seen with increased scrotal temperature, and a neutrophilic infiltrate, which is necessary for ischemia reperfusion induced apoptosis, both of which can be expected to contribute to reduced fertility.71 Increased levels of bile acids, such as seen at 28 and 90 days, have been shown to interfere with spermatogenesis. Finally, chronic immune responses in the testes, like the increased T-cell numbers observed at 1.5 years post-SCI, have also been shown to be detrimental to male fertility. Interestingly, an increase in T lymphocyte numbers points to a long-term activation of the adaptive immune response, suggesting the possibility of an autoimmune response to testis components secondary to SCI. All six of these processes can cause infertility. What remains to be seen are which ones, if prevented or treated, can preserve or restore male fertility.
Acknowledgments
We are grateful for the U.S. Department of Defense's generous funding and support (Award Number: W81XWH-12-1-0481). Thanks to Feng Li, PhD, the Metabolomics core, Advance Technology Cores of Baylor College of Medicine, for his proficient analysis of bile acids in the serum. We would also like to thank Darren Boehning, PhD, UTHealth, for his generosity in teaching and helping perform caspase assays on our samples; and Redwan Huq, Baylor College of Medicine, for his proficiency in flow cytometry, as well as his willingness to troubleshoot and dedication to proper science. We thank Ashley Hood and the rest of the Center for Clinical and Translational Sciences TL1 program for generous funding and support. We would like to thank Jeff Frost, PhD, UTHealth, for helping to teach proper lab techniques. Thanks to Rebecca Berdeaux, PhD, UTHealth, for generous use of lab equipment. Finally, thanks to Sarah Riosa and Alissa Poteete, MS, for their patience, support, and technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Devivo M.J. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim E., Lynne C.M., and Brackett N.L. (2016). Male fertility following spinal cord injury: an update. Andrology 4, 13–26 [DOI] [PubMed] [Google Scholar]
- 3.Sankari A., Bascom A., Oomman S., and Badr M.S. (2014). Sleep disordered breathing in chronic spinal cord injury. J. Clin. Sleep Med. 10, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karapolat I., Karapolat H.U., Kirazli Y., Capaci K., Akkoc Y., and Kumanlioglu K. (2015). Longitudinal study of bone loss in chronic spinal cord injury patients. J. Phys. Ther. Sci. 27, 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebert E. (2012). Gastrointestinal involvement in spinal cord injury: a clinical perspective. J. Gastrointestin. Liver Dis. 21, 75–82 [PubMed] [Google Scholar]
- 6.Herrera J.J., Haywood-Watson R.J.L., and Grill R.J. (2010). Acute and chronic deficits in the urinary bladder after spinal contusion injury in the adult rat. J. Neurotrauma 27, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore C.D., Craven B.C., Thabane L., Laing A.C., Frank-Wilson A.W., Kontulainen S.A., Papaioannou A., Adachi J.D., and Giangregorio L.M. (2015). Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 15, 32–41 [PMC free article] [PubMed] [Google Scholar]
- 8.Finnerup N.B. (2013). Pain in patients with spinal cord injury. Pain 154 Suppl 1, S71–S76 [DOI] [PubMed] [Google Scholar]
- 9.Cowan H. (2015). Autonomic dysreflexia in spinal cord injury. Nurs. Times 111, 22–24 [PubMed] [Google Scholar]
- 10.Deforge D., Blackmer J., Garritty C., Yazdi F., Cronin V., Barrowman N., Fang M., Mamaladze V., Zhang L., Sampson M., and Moher D. (2005). Fertility following spinal cord injury: a systematic review. Spinal Cord 43, 693–703 [DOI] [PubMed] [Google Scholar]
- 11.Bors E., Engle E.T., Rosenquist R.C., and Holliger V.H. (1950). Fertility in paraplegic males; a preliminary report of endocrine studies. J. Clin. Endocrinol. Metab. 10, 381–398 [DOI] [PubMed] [Google Scholar]
- 12.Derry F., Hultling C., Seftel A.D., and Sipski M.L. (2002). Efficacy and safety of sildenafil citrate (Viagra) in men with erectile dysfunction and spinal cord injury: a review. Urology 60, 49–57 [DOI] [PubMed] [Google Scholar]
- 13.Lombardi G., Macchiarella A., Cecconi F., and Del P.G. (2009). Ten-year follow-up of sildenafil use in spinal cord-injured patients with erectile dysfunction. J. Sex. Med. 6, 3449–3457 [DOI] [PubMed] [Google Scholar]
- 14.Brackett N.L., Lynne C.M., Ibrahim E., Ohl D.A., and Sonksen J. (2010). Treatment of infertility in men with spinal cord injury. Nat. Rev. Urol. 7, 162–172 [DOI] [PubMed] [Google Scholar]
- 15.Brackett N.L., Ibrahim E., Iremashvili V., Aballa T.C., and Lynne C.M. (2010). Treatment for ejaculatory dysfunction in men with spinal cord injury: an 18-year single center experience. J. Urol. 183, 2304–2308 [DOI] [PubMed] [Google Scholar]
- 16.Brackett N.L., Ferrell S.M., Aballa T.C., Amador M.J., Padron O.F., Sonksen J., and Lynne C.M. (1998). An analysis of 653 trials of penile vibratory stimulation in men with spinal cord injury. J. Urol. 159, 1931–1934 [DOI] [PubMed] [Google Scholar]
- 17.Ohl D.A., Sonksen J., Menge A.C., McCabe M., and Keller L.M. (1997). Electroejaculation versus vibratory stimulation in spinal cord injured men: sperm quality and patient preference. J. Urol. 157, 2147–2149 [PubMed] [Google Scholar]
- 18.Ohl D.A., Menge A.C., and Sonksen J. (1996). Penile vibratory stimulation in spinal cord injured men: optimized vibration parameters and prognostic factors. Arch. Phys. Med. Rehabil. 77, 903–905 [DOI] [PubMed] [Google Scholar]
- 19.Brackett N.L., Davi R.C., Padron O.F., and Lynne C.M. (1996). Seminal plasma of spinal cord injured men inhibits sperm motility of normal men. J. Urol. 155, 1632–1635 [PubMed] [Google Scholar]
- 20.Dulin J.N., Moore M.L., Gates K.W., Queen J.H., and Grill R.J. (2011). Spinal cord injury causes sustained disruption of the blood-testis barrier in the rat. PLoS One 6, e16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauerbeck A.D., Laws J.L., Bandaru V.V., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popovich P.G. (2014). Neuroimmunology of traumatic spinal cord injury: a brief history and overview. Exp. Neurol. 258, 1–4 [DOI] [PubMed] [Google Scholar]
- 23.Popovich P.G. (2000). Immunological regulation of neuronal degeneration and regeneration in the injured spinal cord. Prog. Brain. Res. 128, 43–58 [DOI] [PubMed] [Google Scholar]
- 24.Whetstone W.D., Hsu J.Y., Eisenberg M., Werb Z., and Noble-Haeusslein L.J. (2003). Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 74, 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gris D., Hamilton E.F., and Weaver L.C. (2008). The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp. Neurol. 211, 259–270 [DOI] [PubMed] [Google Scholar]
- 26.Kabatas S., Yu D., He X.D., Thatte H.S., Benedict D., Hepgul K.T., Black P.M., Sabharwal S., and Teng Y.D. (2008). Neural and anatomical abnormalities of the gastrointestinal system resulting from contusion spinal cord injury. Neuroscience 154, 1627–1638 [DOI] [PubMed] [Google Scholar]
- 27.Young W. (2002). Spinal cord contusion models. Prog. Brain Res. 137, 231–255 [DOI] [PubMed] [Google Scholar]
- 28.Grill R.J. (2005). User-defined variables that affect outcome in spinal cord contusion/compression models. Exp. Neurol. 196, 1–5 [DOI] [PubMed] [Google Scholar]
- 29.Basso D.M., Beattie M.S., Bresnahan J.C., Anderson D.K., Faden A.I., Gruner J.A., Holford T.R., Hsu C.Y., Noble L.J., Nockels R., Perot P.L., Salzman S.K., and Young W. (1996). MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 13, 343–359 [DOI] [PubMed] [Google Scholar]
- 30.Beeton C. and Chandy K.G. (2007). Preparing T cell growth factor from rat splenocytes. J. Vis. Exp. e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welinder E., Ahsberg J., and Sigvardsson M. (2011). B-lymphocyte commitment: identifying the point of no return. Semin. Immunol. 23, 335–340 [DOI] [PubMed] [Google Scholar]
- 32.Gorczyca W., Sun Z.Y., Cronin W., Li X., Mau S., and Tugulea S. (2011). Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell. Biol. 103, 221–266 [DOI] [PubMed] [Google Scholar]
- 33.Liu K. and Nussenzweig M.C. (2010). Origin and development of dendritic cells. Immunol. Rev. 234, 45–54 [DOI] [PubMed] [Google Scholar]
- 34.Lee P.Y., Wang J.X., Parisini E., Dascher C.C., and Nigrovic P.A. (2013). Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 94, 585–594 [DOI] [PubMed] [Google Scholar]
- 35.Seillet C., Belz G.T., and Huntington N.D. (2016). Development, homeostasis, and heterogeneity of NK Cells and ILC1. Curr. Top. Microbiol. Immunol. 395, 37–61 [DOI] [PubMed] [Google Scholar]
- 36.Farber D.L. and Ahmadzadeh M. (2002). Dissecting the complexity of the memory T cell response. Immunol. Res. 25, 247–259 [DOI] [PubMed] [Google Scholar]
- 37.Wulff H., Calabresi P.A., Allie R., Yun S., Pennington M., Beeton C., and Chandy K.G. (2003). The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Invest. 111, 1703–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huq R., Samuel E.L., Sikkema W.K., et al. (2016). Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation. Sci. Rep. 6, 33808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beeton C., Wulff H., Singh S., Botsko S., Crossley G., Gutman G.A., Cahalan M.D., Pennington M., and Chandy K.G. (2003). A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes. J. Biol. Chem. 278, 9928–9937 [DOI] [PubMed] [Google Scholar]
- 40.Woolbright B.L., McGill M.R., Staggs V.S., Winefield R.D., Gholami P., Olyaee M., Sharpe M.R., Curry S.C., Lee W.M., Jaeschke H.; Acute Liver Failure Study Group. (2014). Glycodeoxycholic acid levels as prognostic biomarker in acetaminophen-induced acute liver failure patients. Toxicol. Sci. 142, 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehning D., Patterson R.L., Sedaghat L., Glebova N.O., Kurosaki T., and Snyder S.H. (2003). Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell. Biol. 5, 1051–1061 [DOI] [PubMed] [Google Scholar]
- 42.Rossier M.F. (2006). T channels and steroid biosynthesis: in search of a link with mitochondria. Cell. Calcium. 40, 155–164 [DOI] [PubMed] [Google Scholar]
- 43.Shirakawa H., Ohsaki Y., Minegishi Y., Takumi N., Ohinata K., Furukawa Y., Mizutani T., and Komai M. (2006). Vitamin K deficiency reduces testosterone production in the testis through down-regulation of the Cyp11a a cholesterol side chain cleavage enzyme in rats. Biochim. Biophys. Acta 1760, 1482–1488 [DOI] [PubMed] [Google Scholar]
- 44.Midzak A.S., Chen H., Papadopoulos V., and Zirkin B.R. (2009). 23Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 299, 23–31 [DOI] [PubMed] [Google Scholar]
- 45.Popovich P.G., Stuckman S., Gienapp I.E., and Whitacre C.C. (2001). Alterations in immune cell phenotype and function after experimental spinal cord injury. J. Neurotrauma 18, 957–966 [DOI] [PubMed] [Google Scholar]
- 46.Duan H., Ge W., Zhang A., Chen Z., Luo D., Cheng Y., Fan K.S., Horvath. S., Sofroniew M.V., Cheng L., Yang Z., Sun Y.E., and Li X. (2015). Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A. 112, 13360–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H. and Wang Y. (2016). Identification of molecular pathway changes after spinal cord injury by microarray analysis. J. Orthop. Surg. Res. 11, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallejo R., Tilley D.M., Cedeno D.L., Kelley C.A., DeMaegd M., and Benyamin R. (2016). Genomics of the effect of spinal cord stimulation on an animal model of neuropathic pain. Neuromodulation 19, 576–586 [DOI] [PubMed] [Google Scholar]
- 49.Huang H.F.S., Linsenmeyer T.A., Li M.T., Giglio W., Anesetti R., von Hagen J., Ottenweller J.E., Serenas C., and Pogach L. (1995). Acute effects of spinal cord injury on the pituitary-testicular hormone axis and sertoli cell functions: a time course study. J. Androl. 16, 148–157 [PubMed] [Google Scholar]
- 50.Halprin K.M. and Ohkawara A. (1967). The measurement of glutathione in human epidermis using glutathione reductase. J. Invest. Dermatol. 48, 149–152 [DOI] [PubMed] [Google Scholar]
- 51.Yoshida Y., Itoh N., Hayakawa M., Piga R., Cynshi O., Jishage K., and Niki E. (2005). Lipid peroxidation induced by carbon tetrachloride and its inhibition by antioxidant as evaluated by an oxidative stress marker, HODE. Toxicol. Appl. Pharmacol. 208, 87–97 [DOI] [PubMed] [Google Scholar]
- 52.Tremellen K. (2008). Oxidative stress and male infertility—a clinical perspective. Hum. Reprod. Update 14, 243–258 [DOI] [PubMed] [Google Scholar]
- 53.Brackett N.L. (2012). Infertility in men with spinal cord injury: research and treatment. Scientifica 2012, 578257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaha C., Tripathi R., and Mishra D.P. (2010). Male germ cell apoptosis: regulation and biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1501–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalo H., Brieva L., Tatzber F., Jové M., Cacabelos D., Cassanyé A., Lanau-Angulo L., Boada J., Serrano J.C., González C., Hernández L., Peralta S., Pamplona R., and Portero-Otin M. (2012). Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J. Neurochem. 123, 622–634 [DOI] [PubMed] [Google Scholar]
- 56.Chimenti S., Tucci P., Candi E., Perricone R., Melino G., and Willis A.E. (2013). Metabolic profiling of human CD4+ cells following treatment with methotrexate and anti-TNF-a infliximab. Cell Cycle 12, 3025–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokumura A. (2004). Metabolic pathways and physiological and pathological significances of lysolipid phosphate mediators. J. Cell. Biochem. 92, 869–881 [DOI] [PubMed] [Google Scholar]
- 58.Lauber K., Bohn E., Krober S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., Marini P., Wiedig C., Zobywalski A., Baksh S., Xu Y., Autenrieth I.B., Schulze-Osthoff K., Belka C., Stuhler G., and Wesselborg S. (2003). Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 [DOI] [PubMed] [Google Scholar]
- 59.Han M.S., Park S.Y., Shinzawa K., Kim S., Chung K.W., Lee J.H., Kwon C.H., Lee K.W., Lee J.H., Park C.K., Chung W.J., Hwang J.S., Yan J.J., Song D.K., and Tsujimoto Y., (2008). Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 49, 84–97 [DOI] [PubMed] [Google Scholar]
- 60.Lue Y.H., Hikim A.P., Swerdloff R.S., Im P., Taing K.S., Bui T., Leung A., and Wang C. (1999). Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology 140, 1709–1717 [DOI] [PubMed] [Google Scholar]
- 61.Durairajanayagam D., Agarwal A., and Ong C. (2015). Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 30, 14–27 [DOI] [PubMed] [Google Scholar]
- 62.Kaur G., Thompson L.A., and Dufour J.M. (2014). Sertoli cells—immunological sentinels of spermatogenesis. Semin. Cell. Dev. Biol. 30, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rotter K.P. and Larrain C.G. (2003). Gallstones in spinal cord injury (SCI): A late medical complication? Spinal Cord 41, 105–108 [DOI] [PubMed] [Google Scholar]
- 64.Xia C.S., Han Y.Q., Yang X.Y., and Hong G.X. (2004). Spinal cord injury cholelithiasis. Hepatobiliary Pancreat. Dis. Int. 3, 595–598 [PubMed] [Google Scholar]
- 65.Baltas C.S., Balanika A.P., Sgantzos M.N., Papakonstantinou O., Bizimi V., Tsouroulas M., and Guglielmi G. Gallstones and biliary sludge in Greek patients with complete high spinal cord injury: An ultrasonographical evaluation. Singapore Med. J. 50, 889–893 [PubMed] [Google Scholar]
- 66.Fong Y.C., Hsu H.C., Sun S.S., Kao A., Lin C.C., and Lee C.C. (2003). Impaired gallbladder function in spinal cord injury on quantitative Tc-99m DISIDA cholescintigraphy. Abdom. Imaging. 28, 87–91 [DOI] [PubMed] [Google Scholar]
- 67.Hofmann A.F. (1999). The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 159, 2647–2658 [DOI] [PubMed] [Google Scholar]
- 68.Baptissart M., Vega A., Martinot E., Pommier A.J., Houten S.M., Marceau G., de Haze A., Baron S., Schoonjans K., Lobaccaro J.M., and Volle D.H. (2014). Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 60, 1054–1065 [DOI] [PubMed] [Google Scholar]
- 69.Dong Y., Miao L., Hei L., Lin L., and Ding H. (2015). Neuroprotective effects and impact on caspase-12 expression of tauroursodeoxycholic acid after acute spinal cord injury in rats. Int. J. Clin. Exp. Pathol. 8, 15871–15878 [PMC free article] [PubMed] [Google Scholar]
- 70.Colak A., Kelten B., Sagmanligil A., Akdemir O., Karaoğlan A., Sahan E., Celik O., and Barut S. (2008). Tauroursodeoxycholic acid and secondary damage after spinal cord injury in rats. J. Clin. Neurosci. 15, 665–671 [DOI] [PubMed] [Google Scholar]
- 71.Celebi M. and Paul A.G. (2008). Blockade of p-selectin reduces neutrophil infiltration into the murine testis after ischemia-reperfusion-injury. Dtsch. Tierarztl. Wochenschr. 115, 457–460 [PubMed] [Google Scholar]