The paradoxical growth (PG) to echinocandins has been observed in vitro in Candida and Aspergillus species.1 Its mechanisms are incompletely understood, although activation of calcineurin and chitin biosynthesis pathways have been implicated.2-4 Importantly, the in vivo relevance of PG is not well defined as there is paucity of both animal model and clinical data on the implications of this phenomenon.5
As PG in vivo has been studied in the context of echinocandin exposure, it remains unknown whether there are differences in virulence of Candida strains showing or not showing PG when they infect the host in the absence of echinocandin pressure. To that end, we examined differences in virulence of Candida isolates showing or not PG in our Toll-deficient fly model of candidiasis, a model we have shown good concordance with mouse models of candidiasis.6 We used 20 clinical bloodstream isolates: 10 Candida albicans (5 with PG to caspofungin, 5 without PG) and 10 C. tropicalis (5 with PG, 5 without PG).1 All isolates had comparable growth in vitro in the absence of caspofungin (data not shown). We used female OregonR Toll-deficient flies and followed standard procedures for feeding, housing and manipulation of flies.6 The flies were exposed to a 12-h light/dark cycle. All Candida strains were grown on yeast-peptone-dextrose (YPD) agar medium. For the infection experiments, cultures of each strain were grown overnight at 30 °C on YPD liquid medium before collection of yeast-cells, which were counted using a hemocytometer and then suspended in sterile phosphate buffered saline. The injection method was used for fly infection. Specifically, we injected flies (n = 23 per experimental group) in the thorax with a 0.1 μm thin needle that had previously been dipped in a concentrated solution of 5 × 105 Candida yeast cells/ml (~1 × 102 yeast cells per fly), as previously described.6 After infection, the flies were maintained at 29 °C and were transferred to fresh vials every 2 d. Survival was assessed daily up until day 7 after infection. Flies that died within 3 h post-injection were excluded from the survival analysis. Each experiment was performed in triplicate on different days. Survival curves were plotted using Kaplan-Meier analysis and differences in survival rates were analyzed using the log-rank test in the GraphPad Prism software (version 5.0; GraphPad Software, Inc.). A P value of ≤0.05 was considered statistically significant.
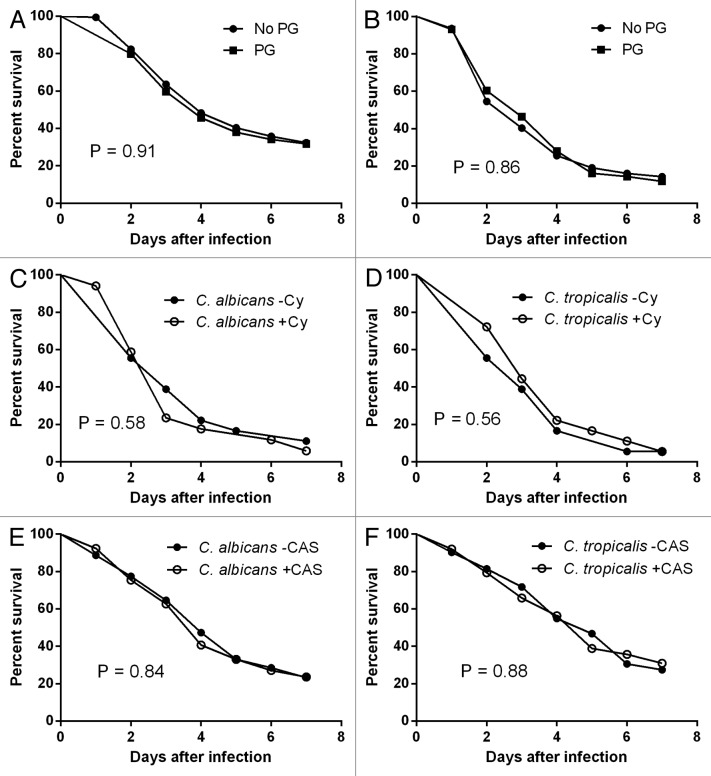
We observed no differences in fly mortality between C. albicans and C. tropicalis isolates irrespective of PG (Fig. 1A and B). As hyperacute infection caused by a high inoculum of fungi could mask subtle differences in strain virulence, we repeated the experiments using a 10-fold lower inoculum, but, again, no differences in survival were detected between strains showing or not PG (data not shown). Next, as PG seems to be modulated by the calcineurin pathway,2-4 and stimulation of chitin synthesis may represent a rescue mechanism against caspofungin activity,3 we asked whether pre-exposure of Candida to the calcineurin inhibitor cyclosporine or to a sub-inhibitory caspofungin concentration uncovers subtle differences in virulence among Candida strains showing or not PG. To that end, we inoculated C. albicans and C. tropicalis (one strain each) showing PG onto YPD liquid medium with or without cyclosporine (1 μg/ml) (Fig. 1C and D) and with or without caspofungin (MIC/2) (Fig. 1E and F), and incubated overnight at 30 °C. Thereafter, Candida yeast cells were washed three times with PBS and injected onto Toll-deficient flies. Survival was assessed as previously described. Again, no mortality differences were appreciated. In conclusion, using multiple, yet genetically unmatched C. albicans and C. tropicalis strains having comparable growth rates in a defined mini-host model of experimental candidiasis, our data imply that PG to caspofungin is unlikely to be linked to virulence differences, even in the presence of echinocandin pre-exposure.
Figure 1. Survival rates of Toll mutant flies injected with a needle previously dipped in a solution containing 5 × 105C. albicans (A) and C. tropicalis (B) yeast cells/ml each showing (n = 5) or not showing (n = 5) paradoxical growth (PG) against caspofungin. Survival rates of Toll mutant flies injected with a needle previously dipped in a solution containing 5 × 105 yeast cells/ml which were grown overnight at 30 °C in the presence or not of 1 µg/ml cyclosporine (C and D) and in the presence or not of caspofungin (MIC/2) (E and F). Data shown are the means of 3 independent experiments (n = 23 flies/group).
Submitted
05/14/13
Revised
06/24/13
Accepted
06/24/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
DPK acknowledges the MD Anderson Cancer Center core grant (CA 16672) and The Frances King Black Endowment. We thank Nathaniel D Albert for invaluable technical support. This study was supported in part by the National Council of Scientific and Technological Development (CNPq—Brazil).
References
- 1.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. . Paradoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother 2007; 51:2257 - 9; http://dx.doi.org/ 10.1128/AAC.00095-07; PMID: 17438060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields RK, Nguyen MH, Du C, Press E, Cheng S, Clancy CJ. . Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob Agents Chemother 2011; 55:2641 - 7; http://dx.doi.org/ 10.1128/AAC.00999-10; PMID: 21422223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizerra FC, Melo ASA, Katchburian E, Freymüller E, Straus AH, Takahashi HK, et al. . Changes in cell wall synthesis and ultrastructure during paradoxical growth effect of caspofungin on four different Candida species. Antimicrob Agents Chemother 2011; 55:302 - 10; http://dx.doi.org/ 10.1128/AAC.00633-10; PMID: 21060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. . Attenuation of the activity of caspofungin at high concentrations against candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother 2005; 49:5146 - 8; http://dx.doi.org/ 10.1128/AAC.49.12.5146-5148.2005; PMID: 16304189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, et al. , Caspofungin High-Dose Study Group. . A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 2009; 48:1676 - 84; http://dx.doi.org/ 10.1086/598933; PMID: 19419331 [DOI] [PubMed] [Google Scholar]
- 6.Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, et al. . Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis 2006; 193:1014 - 22; http://dx.doi.org/ 10.1086/500950; PMID: 16518764 [DOI] [PubMed] [Google Scholar]