1. Introduction
According to the International Diabetes Federation, diabetes mellitus (DM) is estimated to affect around 415 million adults worldwide, roughly 8.8% of the adult population, with the figure projected to rise to over 600 million by 2040.20 Regional prevalence varies from 3.2% in Africa to 12.9% in North America. Diabetes mellitus is associated with a number of chronic sequelae and around 50% of people with DM go on to develop polyneuropathy.35 This condition has a variety of clinical manifestations, which are grouped into positive symptoms including dysesthesia (abnormal sense of touch), tingling and itching, and negative symptoms including numbness, muscle weakness, and trouble with balance. Up to 25% of people with diabetic neuropathy (DN) also develop neuropathic pain (NP).39 Neuropathic pain is defined by the International Association for The Study of Pain as “pain directly caused by a lesion or disease affecting the somatosensory system.”12,22 Symptoms of painful diabetic neuropathy (PDN) include those described above for nonpainful DN with additional “burning,” “electric shocks,” “stabbing,” and “pins and needles” symptoms all being described. Painful diabetic neuropathy is associated with increased distress and poor quality of life compared with nonpainful DN, DM, and the general population38 including depression, anxiety, and sleep disturbance.15 In addition, an association has been described with reduced productivity and employability at work compared with nonpainful DN.37 The combination of these factors places a large economic burden on patients and health care services,10 a situation likely to grow steadily worse with the aforementioned projected rise in DM prevalence. This situation is further exacerbated by the fact that 13% of patients with PDN do not report their symptoms to primary care, and 39% of patients with PDN have never received treatment.8 Even for those patients who do attend primary and secondary care for their diabetes, pain is not a symptom that is always included in clinical assessments. Furthermore, not all patients with DN develop PDN, and the reasons for this are unclear.
Understanding the risk factors for PDN will go some way to resolving this and will also help to inform management and prevention of this painful condition by health care services. Any factor that increases the risk of DM or DN is likely to be a risk factor for PDN. However, it is the specific nature and magnitude of the risk that remains unclear and is the focus of this topical review.
2. Risk factors
There have been relatively few published studies examining risk factors specifically for PDN in DM. Clinical, environmental, and genetic factors have been shown to be predictive of developing DM and some of these have also been implicated in the development of DN, including age,11 body mass index,25,28 hypertension,13 smoking, and waist circumference28 (Fig. 1). Given the likely overlap of risk factors between DM and DN, it seems reasonable to hypothesize that some of these factors will also influence the development of PDN.
Figure 1.
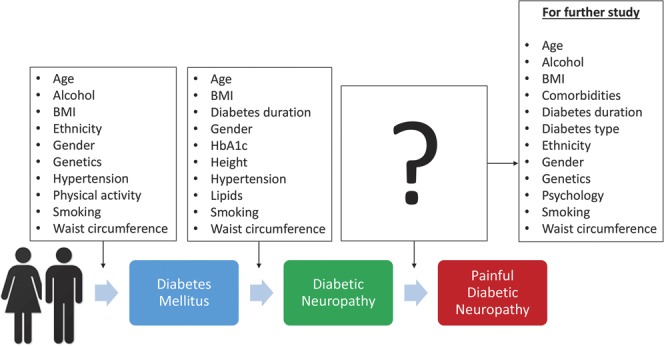
Schematic of the process from diabetes mellitus to diabetic neuropathy and finally painful diabetic neuropathy. Both diabetes mellitus and diabetic neuropathy have their own set of risk factors, both of which could provide important clues as to the risk factors that contribute to painful diabetic neuropathy.
We conducted a literature search using relevant key words and terms aiming to identify a wide range of studies that investigated risk factors for PDN and to include all the important studies (Table 1). A number of limitations can be identified with these studies as a whole. Most of these studies are cross sectional in nature and therefore unable to establish temporal relationship between patient characteristics/factors and PDN. Some studies report only univariate analysis and are therefore unable to assess intervariable relationships and to identify confounding between variables.2,6,8,9,17,23,30 In addition, it is not always clear in the methods and statistical analyses whether PDN or nonpainful DN is being analysed and what control group the PDN subjects are being compared with. In some studies, those in the control group are diabetic participants with nonpainful neuropathy30,38,41 and in others they are diabetic participants without neuropathy of any form.1,2,6,8,11,17 In other studies, it is not possible to determine the nature of the control group from the description of the methods. There was considerable heterogeneity in PDN case ascertainment, with only 6 studies using a validated NP screening questionnaire (the DN46,7,17,21,38 or the Leeds assessment of neuropathic symptoms and signs11) with the remainder using nonvalidated questionnaires or clinical examinations. This makes it difficult to assess the sensitivity and specificity of each study to identify PDN cases and to make direct comparisons between studies as effect size estimations and associations are likely to be different. Despite these limitations, some potential risk factors have emerged, including environment, clinical, lifestyle, and genetic factors.
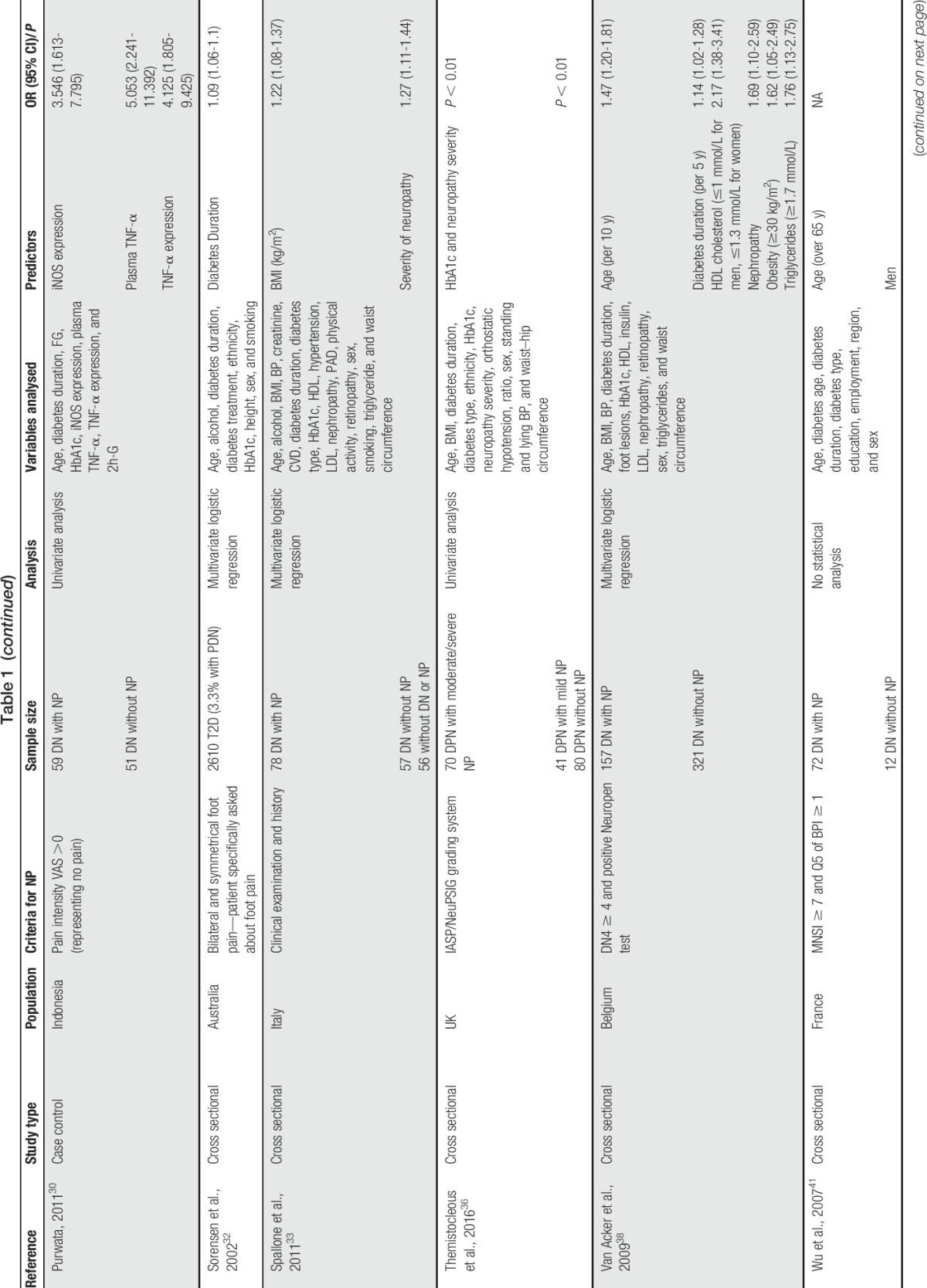
Table 1.
List of studies conducted on predictors of painful diabetic neuropathy and their characteristics.
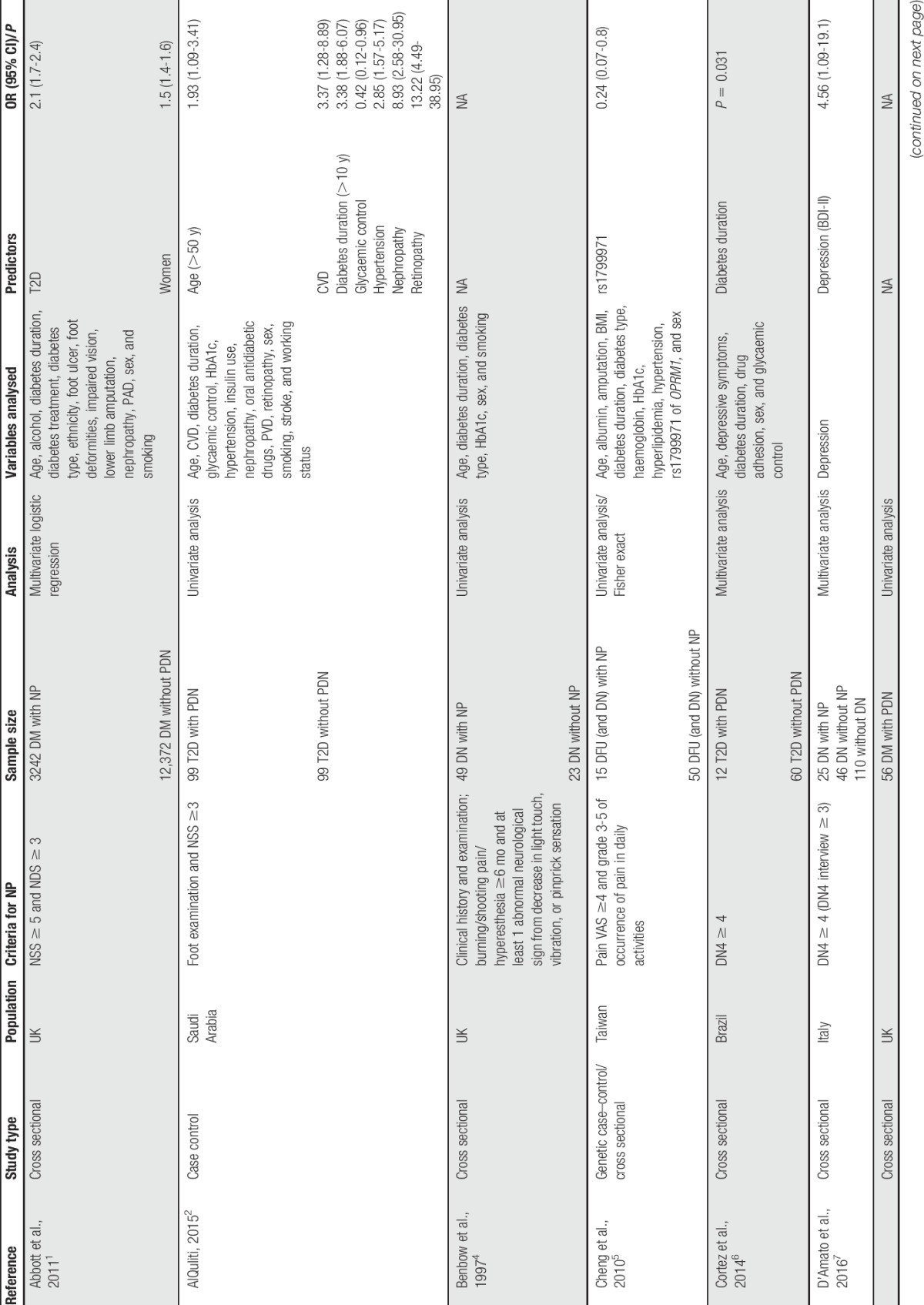
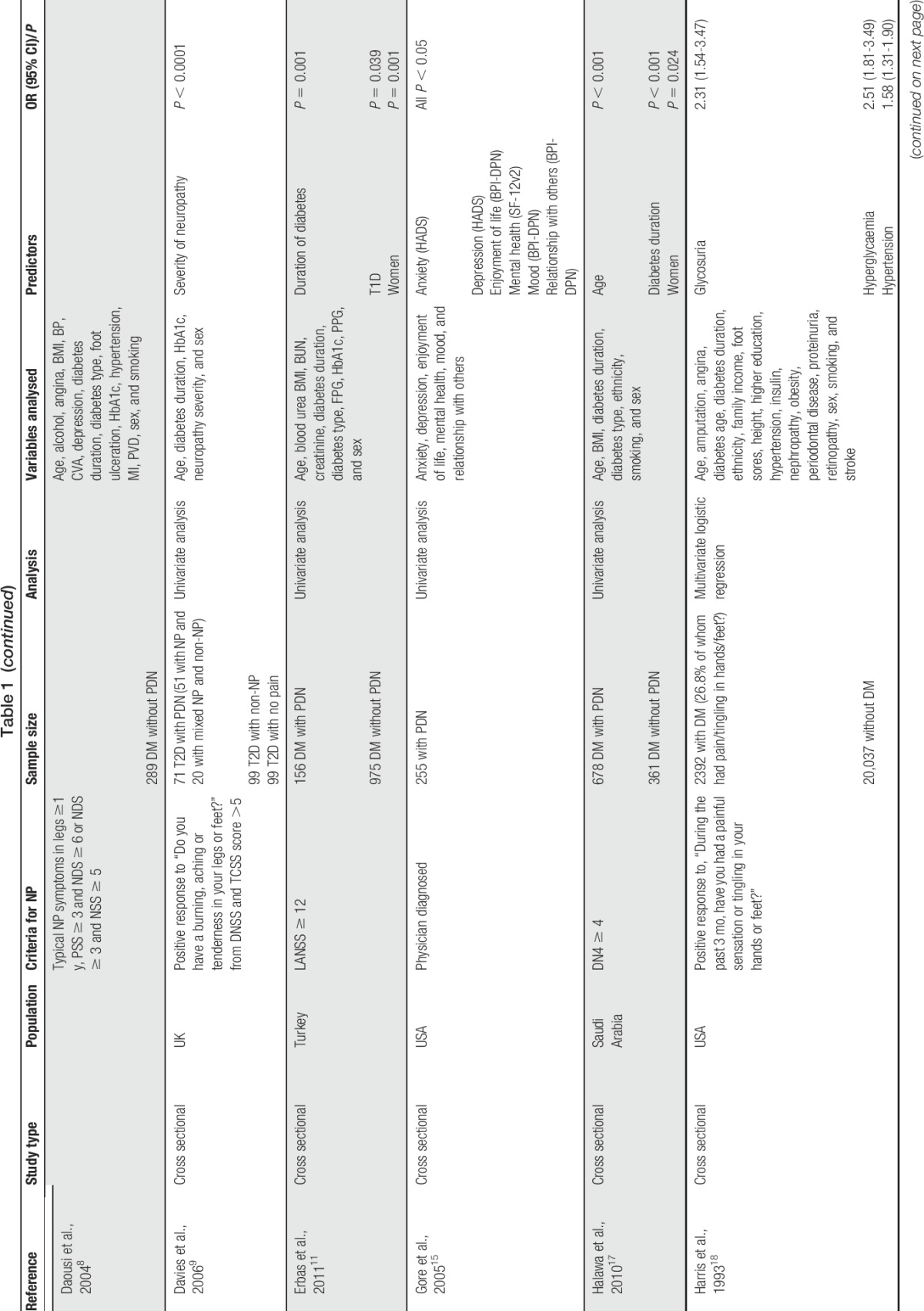
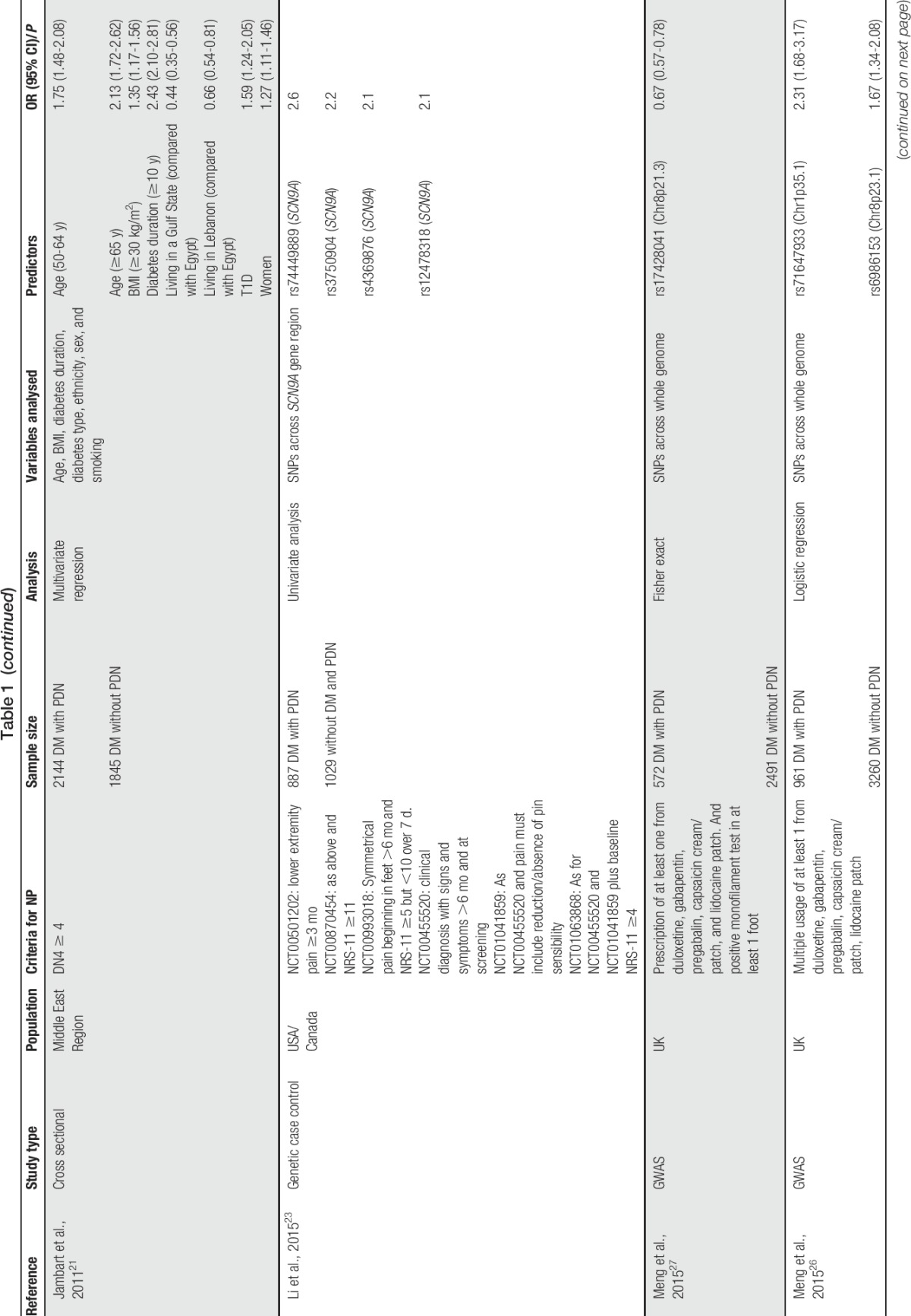

2.1. Demographic
Two nonmodifiable factors—age2,17,21,38,42 and sex1,11,17,21,41—have been specifically associated with PDN, in addition to their known roles as risk factors for DM. Although these are of limited use to clinicians in terms of intervention, they could provide useful clues as to the underlying biological pathways involved and increased awareness of at-risk patients. In particular, the association of PDN with older age (>50 years) is likely to be related to the time it takes for nerve damage and painful symptoms to develop after the onset of DM and the decreased ability of the body to deal with this. Similarly, gender associations may indicate possible subtle differences in biology and psychosocial factors that affect the risk of PDN, something that requires further investigation. It is interesting to note that while 4 of the studies report greater risk in women,1,11,17,21 1 study reports greater risk in men.41 This discrepancy in the latter study could be related to the limited statistical analysis, which did not adjust for potential confounding factors. Despite the prevalence of DM varying according to ethnicity, this has not been found as a risk factor so far for PDN.1,17,18,21,32,36 One study reported that South Asians were more likely to report painful symptoms than people in other ethnic groups in the absence of clinical neuropathy.1 Another found an association with pain among people with DM residing in a Gulf state and Lebanon compared with Egypt, but did not analyse ethnic origin.21
2.2. Clinical
Clinical and physiological factors associated with PDN are important for clinicians and primary care as they may indicate possibilities for targeted treatment or primary prevention strategies. The clinical diagnosis of the type of DM and the duration since onset of the disease may be particularly relevant. Two studies found an association with DM type in multivariate analysis, with 1 identifying type 1 diabetes (T1D)21 and the other type 2 diabetes (T2D)1 as conferring greater risk of PDN. Differences in case definition and study populations could have contributed to the heterogeneity in these results. A clearer consensus is apparent for DM duration with risk increasing over time since diagnosis.2,6,17,21,32,38 Severity of preceding neuropathy has been found to be associated with PDN, but associations with neuropathy duration and comparison with type of (peripheral or sensory) neuropathy have not been found.9,11,33,36 Most studies included only 1 type of neuropathy in their analysis. A number of clinical factors and comorbidities have been found to be associated with PDN. These include poor glycaemic control and high HbA1c levels,2,18,36 hypertension,2,18 retinopathy,2 nephropathy,2,38 cardiovascular disease,2,42,43 and glycosuria.18 However, as these conditions are all known complications of DM, it is uncertain from cross-sectional analysis whether these factors are contributing to PDN risk and onset, or simply coexisting factors, perhaps confounded by other factors or with shared aetiology. Biomarkers for the development of PDN can be exploited by providing preventative or diagnostic tests. In this respect, tumour necrosis factor alpha and inducible nitric oxide synthase expression,30 triglycerides, and low high-density lipoprotein cholesterol38 all show promising associations but require replication to be confident in their role in disease pathogenesis.
2.3. Lifestyle
Behavioural and social circumstances are important lifestyle aspects that patients can theoretically influence and act on, with greater or less practical difficulty. In particular, some physical characteristics known to be associated with DM and DN are also implicated in PDN. Body mass index has been clearly linked to PDN, particularly in the form of obesity (≥30 kg/m2),21,33,38 while in another study, weight was reported independently of height and found to be significantly associated with PDN, although this was attenuated in multivariate analysis.42 A related study also found a positive correlation with increased waist circumference and high levels of physical activity and risk of PDN.43 Despite being included in the analyses in most of the studies,1,2,4,8,17,18,21,32,33,38,42,43 smoking and alcohol consumption have not been specifically associated with PDN. Psychological factors have also been widely reported in the context of general chronic pain, but its relationship with PDN is less clear. Increased depression, anxiety, enjoyment of life, and social relationships are associated with PDN, but without prospective studies and longitudinal analysis, the temporal relationship cannot be established.7,15
2.4. Genetics
Numerous published studies have found that both T1D and T2D have a heritable component3,19,29,31 and genetic studies have been conducted in DN,24,34 although heritability studies have yet to be conducted. A heritable component to PDN has been hinted at in 1 study, which found that 56% of participants also reported a family member with the condition.14 Two PDN genome-wide association studies have been conducted, both in the same Scottish diabetic cohort, but with slightly differing phenotype definitions. The first used a positive monofilament test combined with a prescription history of at least 1 from 5 recommended NP medicines. This yielded an association at chromosome 8p21.3 near the gene GFRA2 and estimated the narrow-sense heritability (proportion of phenotypic variance explained by additive genetic variance) to be 11%.27 The second used the same definition but without the monofilament test and aimed to capture associations that may have been missed in the previous study, using a less specific cohort. This found sex-specific associations at chromosome 1p35.1 in the ZSCAN20/TLR12P gene regions in females and chromosome 8p23.1 next to HMGB1P46 in males.26 The narrow-sense heritability was 30% in males and 14.7% in females. In both of these studies, controls were defined as patients who had not previously been prescribed any of the 5 NP medicines or other medicines which are predominantly prescribed for other conditions but are also known to be prescribed for NP. Two separate candidate-gene studies have been conducted and have reported associations with PDN. The first was in the sodium channel gene SCN9A, which is expressed in dorsal root ganglia, using a combination of numerical rating scales and clinical examinations to compare PDN with healthy controls.23 The second was in the opioid receptor gene OPRM1 using a visual analogue scale for pain intensity and a grading system for pain occurrence during daily activities.5 All 4 of these studies require independent replication, and further studies are required to establish the extent to which genetics contribute specific risk of PDN and the mechanism of this contribution.
3. Conclusions
Despite the limited number of studies reporting specific predictors for PDN, clear similarities are emerging with the known general risk factors for both DM and DN. These include clinical factors such as diabetes type and duration and lifestyle factors such as body mass index and waist circumference, some of which are not easily or not at all modifiable. Although further work is needed, this suggests that PDN is a manifestation of longer-lasting and more severe diabetes and certainly that it requires specific testing and diagnosis in routine diabetic care. However, there are likely to be factors, among those with DM (with and without DN) that confer a greater risk of PDN, and these require further exploration. It should be noted that while the published literature (and this topical review) has mainly explored NP arising from DN, NP can also arise in the diabetic population through other causes, for example, sciatic neuralgia and carpal tunnel syndrome. The influence of diabetes on the development of NP in these conditions is an area that requires further investigation. There is clear evidence to suggest PDN has a negative impact on quality of life; however, the extent to which the reverse is true—bidirectional aetiology—is currently unknown. Future studies need to be conducted in a longitudinal manner or as clinical trials to establish the temporal relationship between variable and disease, particularly with respect to identifying specific PDN risk factors in T1D and T2D patients. The previous point can be further strengthened by running Mendelian randomization studies, something that has been used in DM.16 Mendelian randomization studies establish causal relationship by comparing 2 groups of individuals with and without a genetic marker known to influence the variable being studied. As genotype assignment is random and not subject to confounding typically found in epidemiological studies, a higher prevalence of disease in the group with the marker implies causality. However, we would first need clearer evidence to identify genetic factors associated with PDN. Finally, greater clarity is needed in specifying whether painful or nonpainful DN is being analysed. This can be enhanced by forming a consensus on PDN phenotype definition, to enable studies to be more comparable with one another. This is something that has been addressed for NP generally and could also be applied to PDN.40 This would make replication of results more likely and brings the added potential of being able to perform meta-analyses in the future. All these limitations will be addressed in the DOLORisk study (http://dolorisk.eu/), a European consortium which aims to identify risk factors for NP.
Conflict of interest statement
B. H. Smith is a member of the DOLORisk consortium which is funded by the European Commission Horizon 2020 (ID: 633491) and is partly supported by this grant. He has received, on behalf of his institution, occasional lecture and consultancy fees from Pfizer Ltd, Napp Pharmaceuticals, Grunenthal and Eli Lilly. H. L. Hébert and A. Veluchamy are supported by DOLORisk. N. Torrance has no conflicts of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].AlQuliti K. Predictors of painful diabetic neuropathy in Saudi patients with type 2 diabetes. J Pain Relief 2015;4:181. [Google Scholar]
- [3].Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benbow SJ, Williams G, MacFarlane IA. Smoking habits and painful diabetic neuropathy. J Diabetes Complications 1997;11:334–7. [DOI] [PubMed] [Google Scholar]
- [5].Cheng KI, Lin SR, Chang LL, Wang JY, Lai CS. Association of the functional A118G polymorphism of OPRM1 in diabetic patients with foot ulcer pain. J Diabetes Complications 2010;24:102–8. [DOI] [PubMed] [Google Scholar]
- [6].Cortez J, Reis C, Cardoso Y, Onofre A, Piovezan AP. Prevalence of neuropathic pain and associated factors in diabetes mellitus type 2 patients seen in outpatient setting. Revista Dor 2014;15:256–9. [Google Scholar]
- [7].D'Amato C, Morganti R, Greco C, Di Gennaro F, Cacciotti L, Longo S, Mataluni G, Lauro D, Marfia GA, Spallone V. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diab Vasc Dis Res 2016;13:418–28. [DOI] [PubMed] [Google Scholar]
- [8].Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004;21:976–82. [DOI] [PubMed] [Google Scholar]
- [9].Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–22. [DOI] [PubMed] [Google Scholar]
- [10].DiBonaventura MD, Cappelleri JC, Joshi AV. Association between pain severity and health care resource use, health status, productivity and related costs in painful diabetic peripheral neuropathy patients. Pain Med 2011;12:799–807. [DOI] [PubMed] [Google Scholar]
- [11].Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M, Group TS. Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol 2011;28:51–5. [DOI] [PubMed] [Google Scholar]
- [12].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 1997;46:665–70. [DOI] [PubMed] [Google Scholar]
- [14].Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000;47:123–8. [DOI] [PubMed] [Google Scholar]
- [15].Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374–85. [DOI] [PubMed] [Google Scholar]
- [16].Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL cholesterol and risk of type 2 diabetes: a Mendelian Randomization Study. Diabetes 2015;64:3328–33. [DOI] [PubMed] [Google Scholar]
- [17].Halawa MR, Karawagh A, Zeidan A, Mahmoud AE, Sakr M, Hegazy A. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010;26:337–43. [DOI] [PubMed] [Google Scholar]
- [18].Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the U.S. population. Diabetes Care 1993;16:1446–52. [DOI] [PubMed] [Google Scholar]
- [19].Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a Nationwide Follow-Up Study. Diabetes 2003;52:1052–5. [DOI] [PubMed] [Google Scholar]
- [20].International Diabetes Federation. IDF Diabetes Atlas, 7th ed Brussels: International Diabetes Federation, 2015. Available at: http://www.diabetesatlas.org. Accessed August 9, 2016. [Google Scholar]
- [21].Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, Selwan CA, Sunna N, Wajsbrot D, Youseif E. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res 2011;39:366–77. [DOI] [PubMed] [Google Scholar]
- [22].Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. PAIN 2011;152:2204–5. [DOI] [PubMed] [Google Scholar]
- [23].Li QS, Cheng P, Favis R, Wickenden A, Romano G, Wang H. SCN9A variants may be implicated in neuropathic pain associated with diabetic peripheral neuropathy and pain severity. Clin J Pain 2015;31:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Tong N. Angiotensin-converting enzyme I/D polymorphism and diabetic peripheral neuropathy in type 2 diabetes mellitus: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2015;16:787–92. [DOI] [PubMed] [Google Scholar]
- [25].Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr 2006;84:483–9. [DOI] [PubMed] [Google Scholar]
- [26].Meng W, Deshmukh HA, Donnelly LA, Wellcome Trust Case Control Consortium 2 (WTCCC2), Surrogate markers for Micro-and Macro-vascular hard endpoints for Innovative diabetes Tools (SUMMIT) study group, Torrance N, Colhoun HM, Palmer CN, Smith BH. A genome-wide association study provides evidence of sex-specific involvement of chr1p35.1 (ZSCAN20-TLR12P) and chr8p23.1 (HMGB1P46) with diabetic neuropathic pain. EBioMedicine 2015;2:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meng W, Deshmukh HA, van Zuydam NR, Liu Y, Donnelly LA, Zhou K, Wellcome Trust Case Control Control Consortium 2, Surrogate Markers for Micro and Macro-Vascular Hard Endpoints for Innovative Diabetes Tools Study Group, Morris AD, Colhoun HM, Palmer CN, Smith BH. A genome-wide association study suggests an association of Chr8p21.3 (GFRA2) with diabetic neuropathic pain. Eur J Pain 2015;19:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miralles-Garcia JM, de Pablos-Velasco P, Cabrerizo L, Perez M, Lopez-Gomez V. Prevalence of distal diabetic polyneuropathy using quantitative sensory methods in a population with diabetes of more than 10 years' disease duration. Endocrinol Nutr 2010;57:414–20. [DOI] [PubMed] [Google Scholar]
- [29].Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 1999;42:139–45. [DOI] [PubMed] [Google Scholar]
- [30].Purwata TE. High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res 2011;4:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–5. [DOI] [PubMed] [Google Scholar]
- [32].Sorensen L, Molyneaux L, Yue DK. Insensate versus painful diabetic neuropathy: the effects of height, gender, ethnicity and glycaemic control. Diabetes Res Clin Pract 2002;57:45–51. [DOI] [PubMed] [Google Scholar]
- [33].Spallone V, Morganti R, D'Amato C, Cacciotti L, Fedele T, Maiello MR, Marfia G. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain 2011;15:153–60. [DOI] [PubMed] [Google Scholar]
- [34].Tang TS, Prior SL, Li KW, Ireland HA, Bain SC, Hurel SJ, Cooper JA, Humphries SE, Stephens JW. Association between the rs1050450 glutathione peroxidase-1 (C > T) gene variant and peripheral neuropathy in two independent samples of subjects with diabetes mellitus. Nutr Metab Cardiovasc Dis 2012;22:417–25. [DOI] [PubMed] [Google Scholar]
- [35].Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2011;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN 2016;157:1132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications 2006;20:26–33. [DOI] [PubMed] [Google Scholar]
- [38].Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, Mathieu C, Colin IM. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–13. [DOI] [PubMed] [Google Scholar]
- [39].van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. PAIN 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- [40].van Hecke O, Kamerman PR, Attal N, Baron R, Bjornsdottir G, Bennett DL, Bennett MI, Bouhassira D, Diatchenko L, Freeman R, Freynhagen R, Haanpaa M, Jensen TS, Raja SN, Rice AS, Seltzer Z, Thorgeirsson TE, Yarnitsky D, Smith BH. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: a NeuPSIG systematic review, Delphi survey, and expert panel recommendations. PAIN 2015;156:2337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu EQ, Borton J, Said G, Le TK, Monz B, Rosilio M, Avoinet S. Estimated prevalence of peripheral neuropathy and associated pain in adults with diabetes in France. Curr Med Res Opin 2007;23:2035–42. [DOI] [PubMed] [Google Scholar]
- [42].Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400. [DOI] [PubMed] [Google Scholar]
- [43].Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009;13:582–7. [DOI] [PubMed] [Google Scholar]


