Abstract
Calcitonin gene–related peptide (CGRP) is a 37-amino acid peptide found primarily in the C and Aδ sensory fibers arising from the dorsal root and trigeminal ganglia, as well as the central nervous system. Calcitonin gene–related peptide was found to play important roles in cardiovascular, digestive, and sensory functions. Although the vasodilatory properties of CGRP are well documented, its somatosensory function regarding modulation of neuronal sensitization and of enhanced pain has received considerable attention recently. Growing evidence indicates that CGRP plays a key role in the development of peripheral sensitization and the associated enhanced pain. Calcitonin gene–related peptide is implicated in the development of neurogenic inflammation and it is upregulated in conditions of inflammatory and neuropathic pain. It is most likely that CGRP facilitates nociceptive transmission and contributes to the development and maintenance of a sensitized, hyperresponsive state not only of the primary afferent sensory neurons but also of the second-order pain transmission neurons within the central nervous system, thus contributing to central sensitization as well. The maintenance of a sensitized neuronal condition is believed to be an important factor underlying migraine. Recent successful clinical studies have shown that blocking the function of CGRP can alleviate migraine. However, the mechanisms through which CGRP may contribute to migraine are still not fully understood. We reviewed the role of CGRP in primary afferents, the dorsal root ganglion, and in the trigeminal system as well as its role in peripheral and central sensitization and its potential contribution to pain processing and to migraine.
Keywords: Calcitonin gene–related peptide, Peripheral sensitization, Central sensitization, Primary afferent neurons, Dorsal root ganglion, Trigeminal system, Migraine, Pain
1. Introduction
Recent developments in the treatment of migraine, along with advances in the understanding of the structure and function of the calcitonin gene–related peptide (CGRP) receptor have spurred an increased interest in the role of this neuropeptide. Although CGRP was identified over 30 years ago, its precise role in the transmission and modulation of nociceptive signals, especially regarding migraine, is not fully understood. The receptors that bind CGRP have only recently been fully characterized. In contrast to many G-protein–coupled receptors, the CGRP receptor requires the heterodimerization of 2 components, the receptor activity–modifying protein 1 (RAMP1) accessory protein and the calcitonin receptor–like receptor (CLR) protein, to efficiently couple to the Gs subunit.152,159,160,190 Moreover, CGRP has affinity to other receptors, including the amylin 1 (AMY1) receptor, which is also a heterodimer containing RAMP1 but with the calcitonin receptor (CT) protein.159,160,190 Calcitonin gene–related peptide's affinity for AMY1 is comparable with the CGRP receptor, and has been proposed as a second CGRP receptor site.185 In addition to its role in the somatosensory system, CGRP is also an extremely potent vasodilator, and vascular beds, including the mesenteric and the meningeal vasculatures, richly express CGRP receptors.59,136 In the meninges, the middle meningeal and the cerebral arteries are innervated by primary afferent trigeminal fibers that express CGRP.59,136 Evolving evidence of CGRP's role in the trigeminal system and the recent success of clinical trials with monoclonal antibodies directed at CGRP or its receptor, coupled with the clinical efficacy of CGRP receptor antagonists, have provided certain evidence that CGRP is a critical component for the pathogenesis of migraine. However, the mechanism by which CGRP plays a role in migraine is unclear. Moreover, although there is certainly overlap between the function of CGRP at somatic and cephalic sites, there are also some important mechanistic differences. In light of these developments, we have undertaken a review of the literature to better understand the state of the art regarding CGRP in pain-processing pathways, and contrast this with what is understood about migraine pain.
2. Calcitonin gene–related peptide and the calcitonin gene–related peptide receptor
Calcitonin gene–related peptide and its receptor are widely expressed in somatosensory and autonomic peripheral nerves, the cardiovascular and the enteric systems, and are increasingly described in neuroanatomical tracts of the central nervous system. The prominent localization of CGRP in Aδ- and C-fiber peripheral nerves has elicited considerable attention to this peptide, but its functional role in pain transmission is still unclear.
2.1. Pharmacology
This 37-amino acid neuropeptide was discovered serendipitously when Amara et al.6 found that alternate splicing of the gene for CT produced a very different peptide, which was eventually designated as α-CGRP. Shortly thereafter, a novel gene coding a homologous peptide was discovered.5 This peptide, β-CGRP, differs from α-CGRP by a single amino acid in the rat, and 3 amino acids in humans.5,56,138,157 Based on receptor-binding assays and in vitro bioassays, the biological activity of these 2 forms of CGRP is nearly identical, although there are small differences in potency.5,56,138,145 α-CGRP and β-CGRP, along with the homologous proteins pro-CT, CT, adrenomedullin (AM), amylin (AMY), and intermedin (AM 2), as well as the C-terminal fragments, CGRP(18-37) and CGRP(19-37), form the CT receptor family of peptides.11,23,117,152 In addition, these CGRP fragments have been found in the rat spinal cord, and the CGRP(19-37) fragment is an antagonist of the receptor.7
Until fairly recently, the CGRP antagonists available for the examination of CGRP receptors were peptidic fragments and analogs based on the CGRP molecule. These early pharmacologic studies, using isolated tissue preparations, suggested the existence of a CGRP1 receptor, based on a greater sensitivity to the peptidic antagonist CGRP8-37, and a CGRP2 receptor that was more sensitive to the CGRP analog antagonists [Cys(ACM)2,7]- and [Cys(Et)2,7]hCGRP.159 The development of selective, nonpeptidic antagonists has allowed more detailed investigations of the CGRP receptors. The CGRP receptor is considered to be atypical among G-protein–coupled receptors, as the functional receptor is composed from the heterodimerization of 2 different peptides.159 This construct provided challenges in its discovery and characterization.159 Consequently, there is overlap among activities of CGRP, AMY, and AM in this family of receptors.159,160,190 The proposed CGRP2 receptor has never been identified, and it is believed that this was an artifact because of the ability of CGRP to bind to the AM and AMY receptors. Expression cloning strategies, which successfully cloned the CT receptor, were applied to the search for the CGRP receptor. Although a CLR (formerly CRLR) was identified, it did not show any binding or activity in response to CGRP and was long considered to be an orphan receptor. However, expression studies performed with isolated cell cultures revealed that the combined expression of CLR with a RAMP was required to express the pharmacologic phenotype of the CGRP receptor. At least 3 RAMPs have been identified (RAMP1, RAMP2, and RAMP3). The dimerization of CLR with RAMP1 shows the highest affinity for CGRP and corresponds to the previously characterized CGRP1 receptor in pharmacologic activity (Fig. 1). Dimerizations of CLR with RAMP2 or RAMP3 produce the AM1 and AM2 receptors. Moreover, RAMPs can also combine with the CT receptor to form the AMY receptors AMY1–3.42,152,159 Importantly, as CGRP, AMY, and AM have considerable homology, they also have differing degrees of binding affinity and function for each of these receptors. For example, CGRP binds to the AMY1 receptor (ie, CT/RAMP1), although at one-tenth the affinity of AMY itself, and this affinity may account for the reported displacement of AMY by CGRP in the nucleus accumbens.42,160 Thus, although CGRP acts most prominently via the CGRP receptor, it is nonetheless capable of exerting functional activity through these related receptors as well. Notably, however, a recent study using TaqMan G protein–coupled receptor arrays and immunohistochemistry found that the AMY1 receptor has high affinity for CGRP that is comparable with the CGRP receptor, and proposed that it be considered as a dual CGRP/AMY receptor.185 It is present in the human trigeminal ganglion (TG), and may represent a second CGRP receptor site pertinent to migraine.185 In addition to CLR and the RAMPs, a third protein, the receptor component protein, is important for the full expression of the CGRP receptor activity.159,160,190 This protein is responsible for effective coupling of the CLR to the Gαs subunit, allowing activation of adenylate cyclase.159
Figure 1.
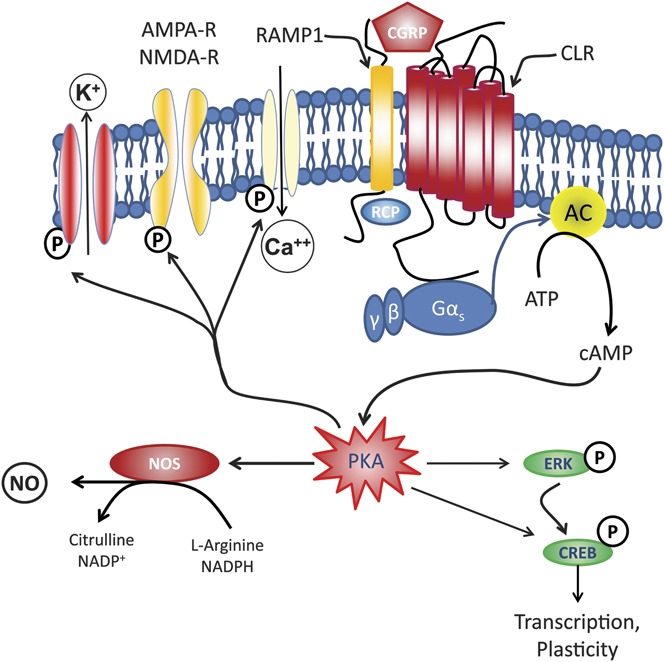
Schematic representation of a CGRP receptor. A functional calcitonin gene–related peptide (CGRP) receptor requires 3 components. The calcitonin receptor–like receptor (CLR) which consists of 7 transmembrane domains, the receptor activity–modifying protein 1 (RAMP1), which confers specificity for binding with CGRP, and the RCP, which seems to be critical for signal transduction by promoting effective coupling to the Gs protein. Gs activates adenylyl cyclase (AC), which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which in turn activates protein kinase A (PKA). Activated PKA can then regulate the activity of numerous intracellular processes, including the K+ channels, L-type Ca2+ channels, and cAMP response element binding (CREB) protein. Thus, the downstream effects of activation of the CGRP receptor include neuronal excitability, neurotransmitter release, nitric oxide (NO) production, and vasodilation. AMPA-R, amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor; ERK, extracellular receptor–activated kinase; NMDA-R, N-methyl-D-aspartate receptor; NOS, nitric oxide synthase; P, phosphorylation sites; RCP, receptor component protein.
2.2. Distribution
The distribution of CGRP and the CGRP receptor is largely conserved across mammalian species.190 The CGRP is almost exclusively found in neurons and is most abundantly expressed in sensory nerves.152,159 Immunohistochemical and in situ hybridization studies revealed that α-CGRP predominates in sensory nerves with cell bodies in the dorsal root ganglion (DRG) and TG, whereas β-CGRP predominates in motor neurons and is almost exclusively expressed in the enteric nervous system.138,145 In the TG, labeling for α-CGRP was 10-fold greater than for β-CGRP.5 Messages for both α-CGRP and β-CGRP are found in small- and medium-diameter DRG neurons, whereas large-diameter DRG neurons predominantly express mRNA for α-CGRP, suggesting that the expression of the protein is likely regulated at the transcriptional level.145
The highest concentrations of CGRP have been found in the outer laminae of the spinal cord dorsal horn and in the trigeminal nucleus caudalis (TNC), which correspond to the central terminals of primary afferent neurons with soma in the DRG and TG, respectively.63,64,66,152,159,160 Importantly, in these regions, CGRP is localized within the primary afferent terminals, and there is little evidence of CGRP in cell bodies of second-order neurons. This distribution is consistent with the role of CGRP as a primary afferent transmitter.64,68,69,119 Calcitonin gene-related peptide is also found in peripheral fibers innervating the heart, coronary arteries, vascular beds, and myenteric system.157,159,160,190 The CGRP receptor is highly concentrated in the spinal cord, particularly the dorsal horns of the spinal cord and cerebral gray matter, and overlaps with the distribution of CGRP protein.145 Of relevance to migraine, in the TG, CGRP is primarily found in the small- and medium-diameter neurons, and the CGRP receptors, as defined by CLR and RAMP1 immunoreactivity, are found on large-diameter TG neurons that do not express CGRP and on satellite glial cells.63,66 Other studies also found very little expression of CGRP receptors on neurons that also express the CGRP peptide.136 The CGRP receptor is also highly expressed in the meningeal vasculature, which is innervated by primary afferent fibers from the TG that express CGRP.63,66 Recent studies using antibodies that specifically recognize the CGRP-binding site, which was discovered by using a fusion protein of the extracellular domains of RAMP1 and CLR, showed binding to human TG neurons and human vascular smooth muscle cells of the meningeal vasculature.136 More detailed examination of tissues obtained from cynomolgus monkeys confirmed the presence of the CGRP receptor on dural vascular smooth muscle cell, neurons, and satellite glial cells of the TG and TNC neurons.136 In that study, binding to both postsynaptic and presynaptic sites were observed, but the CGRP receptor was predominantly expressed by second-order neurons of the TNC.136
There is a growing understanding of the neuroanatomical tracts that include CGRP-expressing neurons in addition to the primary afferent fibers (Fig. 2). Immunohistochemical, autoradiographic, and in situ hybridization studies performed with rat or human brains have provided evidence for the presence of CGRP or for mRNA for CGRP in the cerebellum, hippocampus, hypothalamus, amygdala, basal ganglia, parabrachial nucleus, Kölliker-Fuse nucleus, striatum, colliculi, TNC, medullary cranial motor nuclei, nucleus ambiguous, peripeduncular, posterior, centromedial thalamic nuclei, central gray, and the inferior colliculus.67,109,110,157,159,160,180,190 Growing evidence shows that the parabrachial region is an important component of ascending transmission of nociceptive signaling, and there are significant projections from the parabrachial region to the central nucleus of amygdala (CeA) that express CGRP.45,88,156 The projections from the parabrachial region to the rostral ventromedial medulla, which are likely pronociceptive and engage descending pain facilitatory systems from the rostral ventromedial medulla, were found recently to not express CGRP.156
Figure 2.
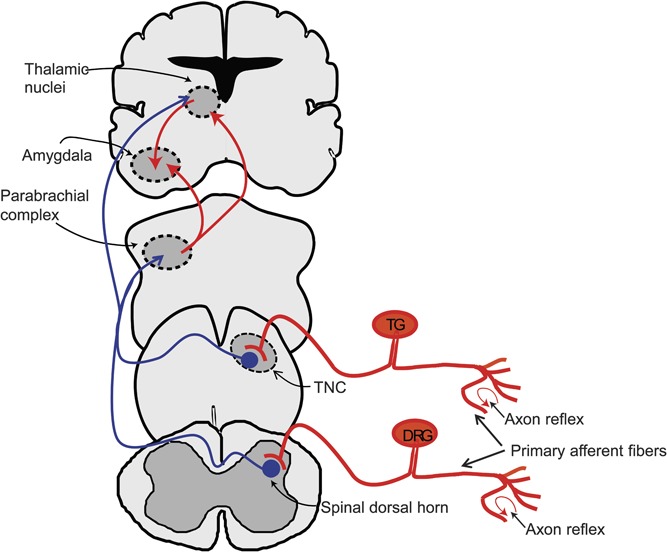
Schematic representation of pain pathways where calcitonin gene–related peptide (CGRP) is expressed. The tracts that contain nerve fibers expressing CGRP are shown in red, and the non-CGRP–expressing tracts are shown in blue. The central terminals of primary afferent fibers, some of which express CGRP, provide nociceptive inputs to second-order neurons of the spinal dorsal horns and the trigeminal nucleus caudalis (TNC). The second-order neurons project to supraspinal sites, particularly the parabrachial and thalamic nuclei, which then can send projections to cortical sites including the insula. Calcitonin gene-related peptide has been found in projections from parabrachial neurons to the posterior intralaminar thalamic complex and to the amygdala/striatal region, including the central nucleus of the amygdala (CeA). This region also receives inputs from the posterior intralaminar thalamic complex that express CGRP. In the periphery, stimulation of sensory nerve endings can elicit an axon reflex, releasing CGRP from adjacent axonal branches, thus propagating a neurogenic inflammation. DRG, dorsal root ganglion; TG, trigeminal ganglion.
High densities of binding for [125I]CGRP were found in the molecular layer and Purkinje cell layer of the human cerebellum69 and for [3H]MK-3207 in the molecular layer of rhesus cerebellum,67 suggesting the possibility of a functional role in primate cerebellar cortex. Using the radiolabeled compound [11C]MK-4232 as a tracer for identification of CGRP-binding sites, positron emission tomography (PET) found the highest concentrations of CGRP receptors in the cerebellum and brainstem of rhesus monkeys and the cerebellum of humans.92 In the same study, autoradiography and in vitro binding studies found that the highest densities of CGRP receptors were in the cerebellum and substantia nigra, whereas moderate densities were found in the meninges, brainstem, and hippocampus in both rhesus monkeys and humans.92 Although these CGRP receptors are believed to be functional, their role in the cerebellum regarding nociception or migraine remains unclear. Evidence exists showing that the cerebellum may play a role in nociception and migraine, and PET has shown activation of the cerebellum during migraine attacks.9,24,137
Most recently, autoradiography, in situ hybridization, and immunofluorescence were used to localize CGRP receptor expression in rhesus monkey brain.65 High densities of the CGRP receptor were found in the pineal gland, medial mammillary nucleus, median eminence, infundibular stem, periaqueductal gray (PAG), dorsal raphe, posterior hypothalamic area, trochlear nucleus, and the medial lemniscus.65 Binding to [3H]MK-3207, indicative of CGRP receptors, was also detected in the spinal TNC, nucleus gracilis, dorsal and ventral horns of the spinal cord, pontine raphe nucleus, pontine nuclei, area postrema, inferior olive, the centrointermediate part of the dorsal motor nucleus of vagus, and the hypoglossal nucleus.65 Many of these regions, such as the PAG, the raphe areas, and the nucleus gracilis, receive somatosensory inputs, are implicated in the transmission or modulation of nociceptive signaling, and have also been implicated in migraine headache.47,48,58,134,150
3. The presence of calcitonin gene–related peptide in dorsal root ganglion primary afferent neurons
As the distribution of CGRP and its receptor suggests, this neuropeptide is associated with activity in the gastrointestinal, cardiovascular, and nociceptive systems.152,159,160 Calcitonin gene-related peptide is expressed in nerve endings of the mesenteric plexus, coronary, and meningeal arteries, and is a very potent vasodilator.25,109 In addition, CGRP also plays a prominent role in the transmission and modulation of pain signals.14,159 Calcitonin gene-related peptide is expressed in approximately 45% of DRG neurons, predominantly in small-diameter unmyelinated C-fibers.8,158 These peptidergic nociceptors, which also coexpress substance P, represent approximately one-half of C-fiber nociceptors.8,158 The DRG neurons also express the tyrosine kinase A receptor (TrkA), and are thus responsive to nerve growth factor (NGF); approximately one-half of DRG neurons also express the transient receptor potential vanilloid type 1 (TRPV1) ion channel.133 Calcitonin gene-related peptide is also expressed in small-to-medium–diameter Aδ fibers, approximately 70% of which are nociceptors, as well as in some large-diameter Aβ sensory fibers and Aα motor neurons.8,158
Studies combining electrophysiological and histochemical approaches have shown that most CGRP-expressing neurons are either C-fiber or Aδ-fiber nociceptors and include thermal-sensitive, high-threshold mechanonociceptors and polymodal nociceptors.116,158 A small population of high-threshold mechanosensitive Aβ fibers has also been identified. Low-threshold mechanosensitive DRG neurons and those responsive to cool stimuli were not found to express CGRP.116,158 The central terminations of primary afferent neurons that express CGRP are also consistent with nociceptive processing, as these are found primarily in the outer laminae of the spinal dorsal horn and in lamina V, although there is some CGRP expression around the central canal. A recent study using immunofluorescent histochemistry and anterograde axonal tracing techniques found that the large-diameter CGRP neurons most likely are high-threshold mechanosensitive Aβ and Aδ fibers that synapse with wide-dynamic-range (WDR) neurons of laminae IV and V.106 Although it is unclear whether these fibers are nociceptive, they are believed to have polymodal mechanoreceptor functions, and their interaction with the WDR neurons would still allow these fibers to influence nociceptive inputs and central sensitization.106
4. Peripheral effects of calcitonin gene–related peptide
The DRG and TG neurons are pseudounipolar, meaning that the axon bifurcates and sends projections both to the dorsal horn of the spinal cord or brainstem and to the periphery.10 This unique morphology allows the release of transmitters at central and peripheral sites, and allows bidirectional communication between the 2 terminals.10 In the periphery, the primary afferent nociceptors terminate as free nerve endings innervating peripheral structures.10 Tissue injury results in the release of numerous proinflammatory mediators from the injured cells, mast cells, leukocytes, and the nerve terminals. These substances include the tachykinins, bradykinin, prostaglandins, serotonin, histamine, as well as the release of CGRP and substance P from the nerve endings.10 These proinflammatory mediators can directly activate the nociceptors, generate action potentials, or facilitate the firing of the nociceptors, resulting in peripheral sensitization and hyperalgesia.10 Activation of a free nerve ending can produce an axon reflex action, which causes the release of CGRP and substance P from adjacent-free endings of the same axon, resulting in the evoked release of additional proinflammatory mediators. This in turn can further excite adjacent nerve endings and thus propagate an expanding area of neurogenic inflammation, indicated by the erythematous flare response.10,93,163,165
5. Peripheral sensitization and the role of calcitonin gene–related peptide
An increase in the activity of peripheral nerves in response to local tissue injury or inflammatory processes results in sensitization of peripheral nerves. Injured tissue releases proinflammatory substances that can decrease the firing thresholds of peripheral nociceptors (ie, sensitizers) as well as mediators that activate (ie, activators) these neurons. Consequently, the peripheral afferent neurons become sensitive to further inputs, thus amplifying pain signals.10,189,192 Over time, the peripheral nerve increases the production of peptidic neurotransmitters and of ion channels, which also lend to an increase in the somatosensory signals.10,189,192 Peripheral sensitization included decreased threshold of a peripheral sensory neuron to firing, increased neuronal responses to a stimulus, and enlargement of the receptive field. Peripheral sensitization manifests clinically as hyperalgesia and allodynia.10
5.1. Peripheral sensitization
Peripheral sensitization and the associated hyperalgesia are initiated and maintained in part by the actions of CGRP (Fig. 3A). Studies performed with human volunteers showed that the intradermal injection of up to 0.25 nmol of CGRP into the skin of the forearm or back produced localized erythema and distant flare that lasted over 12 hours, consistent with its potent vasodilatory action.75 Others have shown that 15 pmol injected intradermally into the forearm produced vasodilation lasting 5 to 6 hours.25 The long duration of the vasodilatory effect of CGRP contrasts with its short half-life of only 7 minutes, suggesting perhaps prolonged receptor activation or the engagement of long-lasting intracellular mechanisms.87 The intradermal application of substance P or CGRP through dialysis tubing in the forearm of human volunteers did not produce any sensations of pain or axon reflex erythema.188 Substance P provoked vasodilation and plasma protein extravasation, whereas CGRP produced a potent vasodilation only, without plasma extravasation.188 Importantly, plasma extravasation and histamine release occurred at high concentrations of substance P (<10−8 M and 10−5 M, respectively) that exceeded normal physiologic concentrations.188 It was concluded that substance P or CGRP most likely do not directly mediate pain, but that they may contribute to sensitization and pain facilitation.188 In a different study, intense electrical stimulation of cutaneous nerves of human volunteers, which released substance P and CGRP, did not evoke mast cell degranulation, histamine release, or plasma protein extravasation.166 Moreover, capsaicin evoked the release of CGRP from human iris slices, whereas substance P was not detectable in these studies.80,81 In addition, the failure of substance P antagonists to block acute migraine attacks convincingly demonstrated that migraine is not due to meningeal plasma protein extravasation induced by substance P.81 The dermal application of capsaicin to the forearm of healthy adult volunteers, through the activation of TRPV1 receptors on cutaneous nociceptors, evokes the local release of CGRP, resulting in increased dermal blood flow which presents as the flare response.168 Oral administration of the CGRP antagonist telcagepant and injection of the humanized monoclonal CGRP antibody galcanezumab (LY2951742) both blocked capsaicin-induced increases in dermal blood flow, as measured by laser Doppler perfusion imaging in healthy human subjects.168,183 Taken together, these observations all support the contention that CGRP is the major mediator of neurogenic dilator responses arising from the activation of sensory nerves in humans.81 This situation contrasts with the rat, where these neuropeptides produce not only vasodilation and plasma protein extravasation but also histamine release and mast cell degranulation.166
Figure 3.
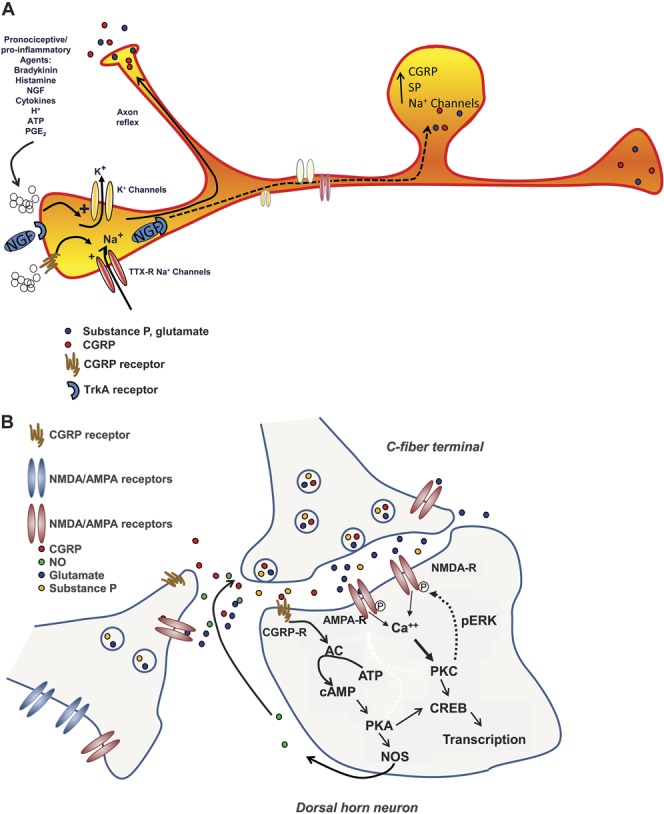
(A) Peripheral sensitization. Damage to tissue or nerves in the periphery results in the localized release of proinflammatory mediators such as bradykinin, histamine, chemokines and cytokines, nerve growth factor (NGF), adenosine triphosphate (ATP), prostaglandin E2 (PGE2) and creates acidic conditions (eg, H+). These substances can lower the activation threshold of peripheral nociceptors (ie, sensitization) or trigger action potentials, thus activating the nociceptors. Action potentials can cause release of excitatory transmitters from other branching terminals of the same axon (ie, axonal reflex), which elicits the release of inflammatory mediators from adjacent tissue and excites adjacent nerve endings, thus spreading the inflammatory response. Persistent activation of the nociceptors over time, along with the retrograde transport of NGF bound to its receptor, tyrosine kinase A (TrkA-NGF complex) causes transcriptional changes, resulting in the increased production of sodium channels, calcitonin gene–related peptide (CGRP), and substance P (SP). These changes result in enhanced excitability of the peripheral nerve and enhanced release of CGRP and SP, thus maintaining a state of peripheral sensitization. (B) Central sensitization. Increased outputs from primary afferent terminals increases the excitability of postsynaptic neurons, directly and through activation of secondary mechanisms. Activation of the N-methyl-D-aspartate (NMDA) receptor increases calcium inputs and sensitizes the neuron to further inputs, such that inputs that would normally be subthreshold are sufficient to generate an action potential. Calcitonin gene-related peptide receptor activation activates the Gs protein and elicits a number of signaling cascades that serve to enhance neuronal excitability. The downstream activation of protein kinase A (PKA) and protein kinase C (PKC) promotes phosphorylation of NMDA receptors and of calcium ion channels, increasing their activity. Protein kinase A can also increase nitric oxide synthase (NOS) activity, increasing the release of nitric oxide (NO). Nitric oxide along with glutamate and PGE2 can act as retrograde transmitters increasing the output from primary afferent terminals. Moreover, the enhanced release of CGRP can also activate presynaptic CGRP receptors (CGRP-R) of adjacent terminals, further enhancing transmitter release. Over time, the increased phosphorylation of PKA and PKC can lead to transcriptional changes, such as the upregulation of receptors and ion channels, which increase the excitability of the second-order neuron, thus maintaining a state of central sensitization. AC, adenylyl cyclase; AMPA, amino-3-hydroxy-5-methylisoxazole-4-propionic acid; AMPA-R, amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding; NMDA-R, N-methyl-D-aspartate receptor; pERK, phosphorylated extracellular signal–regulated kinase.
Studies performed in several animal models also support the idea that peripheral CGRP release supports nociceptor sensitization associated with hyperalgesia, while not eliciting nociceptive signals alone. For example, repeated subplantar injections of low doses (0.1 fmol) of CGRP into the paws of rats resulted in peripheral sensitization, indicated by a significant reduction in response threshold to a noxious mechanical stimulus.139 In that study, a challenge injection with the same low dose of CGRP after response latencies returned to baseline levels resulted in a pronounced and long-lasting reduction in response latency, signaling a restoration of peripheral sensitization.139 Intraplantar injection of a CGRP receptor antagonist blocked behavioral signs of hyperalgesia in rats with peripheral nerve injury99 and attenuated capsaicin-induced mechanical allodynia and hyperalgesia.174 Calcitonin gene-related peptide enhances tetrodotoxin-resistant Na+ currents in small- and medium-diameter–cultured DRG neurons, and this effect is blocked by the CGRP antagonist CGRP8-37.141 Importantly, the tetrodotoxin-resistant Na+ currents occur mainly in nociceptive DRG neurons.141 Tissue injury provokes the release of NGF from epithelial cells and fibroblasts, which when bound to the TrkA receptor is retrogradely transported to the cell body in the DRG of the sensory fiber.15,121,149,161 Through activation of several intracellular signaling cascades, the NGF–TrkA complex elicits marked upregulation of CGRP protein and mRNA in the DRG and increased CGRP levels in the dorsal horns of the spinal cord.15,121,149,161 Thus, one mechanism by which NGF increases excitability may be through an induced CGRP-mediated enhancement of Na+ currents. Accordingly, the up-regulation of CGRP is believed to contribute to the development of central sensitization and enhanced pain.15,149,161
5.2. Dorsal root reflex, calcitonin gene–related peptide, and peripheral sensitization
Some investigators have suggested that neurogenic inflammation also may be maintained in part through the dorsal root reflex (DRR), which is the generation of action potentials arising from the central terminals and traveling to the periphery.124,126,170,189 Primary afferent neurons express the Na+K+2Cl− cotransporter (NKCC1), which maintains high intracellular concentrations of chloride in these fibers.154,182,189 It is proposed that GABA released from GABAergic interneurons activates the GABAA receptors on primary afferents, opening the Cl− channels.154,170,182,189 When the intercellular Cl− concentrations are sufficiently high, opening the channels results in an outflow of Cl−, producing primary afferent depolarizations, which can summate to trigger action potentials that are conducted centrifugally to the peripheral terminals.154,170,182,189 Electrophysiologic studies performed in normal, uninjured rats have shown GABA-mediated depolarization of Aδ and C-fiber DRG neurons.52 Other studies have shown that electrical stimulation of a peripheral nerve generates DRRs in adjacent fibers, which would help spread the neurogenic inflammatory response from the initial site of injury.170,189 Several electrophysiologic and pharmacologic studies performed in rats with capsaicin-induced inflammation showed that Aδ and C-fibers mediate neurogenic inflammation through activation of DRRs and peripheral release of CGRP and substance P.122–126 Neurogenic inflammation was blocked by dorsal rhizotomy or intrathecal administration of the GABAA antagonist bicuculline.124 Collectively, these studies suggest the possibility that DRRs can preferentially cause release of CGRP from peripheral terminals of Aδ afferent fibers, thus contributing to the sensitization of peripheral nociceptors.122,124–126 Hence, the DRR represents another means through which CGRP may contribute to peripheral sensitization.
6. Central sensitization and the role of calcitonin gene–related peptide
Central sensitization, when considered regarding pain, refers to the enhanced responses of neurons of the central pain transmission pathways in response to stimuli. Central sensitization encompasses increased sensitivity of second-order neurons to afferent inputs, enhancement of receptive fields, and increased excitability, but it also includes long-term effects due to transcriptional changes and an increased net pain facilitatory tone from a reduction of descending inhibition and/or enhancement of descending facilitation. These changes result in enhanced pain sensitivity (hyperalgesia) and pain arising from stimuli that are normally nonnoxious (allodynia).
6.1. Central sensitization
Central sensitization, originally described in the spinal dorsal horn, arises from the concept that excessive nociceptive primary afferent discharges, which increases the release of excitatory transmitters including CGRP, substance P and glutamate, into the spinal dorsal horn, lead to neuroplastic changes that support synaptic efficacy and enhanced nociceptive transmission (Fig. 3B).115,192 Behaviorally, central sensitization is manifest as enhanced nociception, secondary hyperalgesia, and allodynia.115,176,192 Electrophysiologically, it is characterized by increased spontaneous and evoked activity of WDR neurons, lowered response thresholds, responses to novel inputs, enlargement of the receptive field, and neuronal after-discharge.115,176,192 These characteristics are considered to be electrophysiologic correlates that parallel the allodynia, hyperalgesia, and enhanced sensitivity in noninjured regions observed in human studies.115,176,192 Conditions involving a dysfunction of descending pain modulatory systems such that descending inhibition is deficient or descending pain facilitation is enhanced, so that the net drive is a pronociceptive pain facilitation, can help maintain a sensitized state.51,114,150,176,192 Emerging evidence suggests that dysfunctional pain states, such as rheumatoid arthritis, osteoarthritis, visceral pain hypersensitivity syndromes, fibromyalgia, inflammatory bowel syndrome, neuropathic pain, and headaches, including migraine headache, may be caused in part by a deficient endogenous pain inhibitory system that allows the development of central sensitization.1,33,96,108,169,192
Development and maintenance of central sensitization is largely dependent on the activation of the glutamate N-methyl-D-aspartate (NMDA) receptors.114,171,191,192 Spinal administration of NMDA antagonists or selective knock-down of the NR1 subunit of the NMDA receptor blocks development of experimentally induced central sensitization in rodents.114,171 The activation of the NMDA receptors of postsynaptic neurons elicits the release of glutamate, prostaglandin E2, and nitric oxide (NO), which act as retrograde transmitters to promote further release of excitatory transmitters from primary afferent terminals and thus maintaining a sensitized state.114,127,171,191–194 Activation of the CGRP receptor by release of the neuropeptide from primary afferent terminals activates signaling cascades that also sensitize the NMDA receptor.13 Sustained enhancement of dorsal horn neuronal activity triggers the activation of the mitogen-activated protein kinase signaling cascade, promoting the phosphorylation of the NR1 subunit of the NMDA receptor and the activation of transcription factors that increase expression of pronociceptive transmitters, thus facilitating pain transmission.100,114,171,192 The disruption of this cascade with inhibitory agents or receptor antagonists has abolished behavioral signs of hyperalgesia in several animal models of inflammation or nerve injury.100,114,150,171,192
Although most studies on central sensitization regarding pain enhancement have focused on the spinal or trigeminal medullary dorsal horns, several studies have shown that central sensitization at supraspinal sites also can support enhanced abnormal pain. Sensitization of neurons of the thalamus or of the amygdala has been associated with enhanced nociceptive responses which were blocked by NMDA receptor antagonists.88,104 Development of central sensitization has also been described in the anterior cingulate cortex, insula, and the ventrolateral orbitofrontal area, and has implications for enhanced abnormal pain.97 The spread of central sensitization is believed to contribute to mirror-image pain, where hypersensitivity or pain may be expressed on the uninjured side, and to growing allodynia and enhanced pain that occur as chronic pain conditions progress.30,34,71–73,97,192 Animal studies suggest that the development of widespread allodynia with migraine is due to development of central sensitization.30,32–34 Imaging studies with migraine patients using functional magnetic resonance imaging scans with recent blood-oxygenation-level-dependent (BOLD) signals indicated a sensitization of the thalamus associated with extracephalic allodynia.33
6.2. Calcitonin gene–related peptide physiology and central sensitization
The distribution of CGRP within the spinal cord is almost exclusively within the outer laminae of the dorsal horn, with some distribution in lamina V and very little expression in the intermediate laminae of the spinal cord. Importantly, CGRP, like substance P, is found exclusively within the terminals of primary afferent neurons innervating these laminae.98,159,200 Disruption of primary afferent inputs into the spinal cord produced near-complete loss of immunoreactive staining for CGRP in the dorsal horn.98,159,200 This distribution is consistent with a role for CGRP as a primary afferent neurotransmitter acting on second-order transmission neurons in the spinal dorsal horns.98,159,200 These neurons include the nociceptive-specific spinothalamic projections of the outer laminae and the WDR neurons of lamina V. However, the exact nature of the interactions between CGRP and second-order spinal dorsal horn neurons is still not well understood. Early studies of nerve fibers immunoreactive for CGRP in the spinal dorsal horn, including human, described them as a mesh, network, or plexus.98 A fine-scale light microscopic analyses of human spinal cord tissue revealed that CGRP fibers in lamina I and lamina V can form multiple intimate axodendritic and axosomatic synaptic connections with targeted neurons, whereas those in lamina II form a homogenous plexus.98 Thus, it seems that CGRP can act as a released neurotransmitter and also as a secreted neuromodulator.
Evidence also exists to suggest a presynaptic role for CGRP as well as a postsynaptic one on second-order neurons. Lennerz et al.119 found immunoreactivity for CGRP and RAMP1 on “glomerular structures,” but not on cell bodies, in the TNC, and suggested that the CGRP receptor probably resides on neuronal processes, including primary afferent endings. Immunohistochemical studies performed in the human TG with novel antibodies found CGRP expressed in small- and medium-sized neurons and CLR/RAMP1 in larger neurons including on nerve fibers.66 No cell bodies expressing CGRP or components of the CGRP receptor were found in the TNC, but are likely present on nerve terminals, suggesting that CGRP may act presynaptically.63 In the spinal dorsal horn, CGRP receptors were identified presynaptically, on nerve terminals of glutaminergic neurons, some of which also express enkephalins that also express presynaptic α2-adrenergic receptors.131 It is possible that these CGRP receptors promote the release of glutamate, acting in opposition to the inhibitory effect of the α2-adrenergic receptors, but the fact that some of these neurons also express the enkephalins is a confounding factor.131
Considerable evidence supports a pronociceptive role for CGRP in the spinal cord. However, it is not clear if CGRP itself is nociceptive or whether it facilitates nociceptive signals in the spinal cord. Early studies suggested that spinal CGRP might have a nociceptive role.76,105,112,128 Stimulation of peripheral nociceptors evokes release of CGRP from their central terminals in the spinal cord.128,129,159,190 The intrathecal administration of CGRP did not alter response thresholds of normal rats to noxious mechanical or thermal stimuli, but intrathecal injection of the CGRP antagonist CGRP8-37 or antiserum to CGRP significantly elevated these thresholds, suggesting an antinociceptive effect.112,199 However, many studies suggest that spinal release of CGRP may support central sensitization, rather than produce nociception. For example, spinal administration of CGRP antisera blocked behavioral signs of hyperalgesia in rats with carrageenan-induced inflammation or experimental arthritis, but did not alter nociceptive behavioral responses in the noninflamed hind paws of these animals or in normal rats.105 These results suggest that CGRP mediates the enhanced abnormal pain sensitivity but does not mediate normal acute nociceptive signals.105 Application of CGRP produces a slow, long-lasting depolarization of dorsal horn WDRs and enhances WDR responses to nerve stimulation.197,202 For example, noxious electrical stimulation of the rat hind paw evoked firing of WDRs that was enhanced by iontophoretic application of CGRP and blocked by the CGRP antagonist CGRP8-37, also applied iontophoretically.202 The convergence of sensory afferents from the bladder and the gastrointestinal organs with somatic afferents in the spinal dorsal horn can account for referral of visceral pain.26,27 Cross-organ sensitization, where inflammation of the gut can manifest also as referred hypersensitivity to cutaneous sites, is mediated in part by increased levels of CGRP in the spinal dorsal horns.26,27
The microiontophoresis of glutamate or of CGRP onto WDR or nociceptive-specific neurons in the TNC of the cat enhanced neuronal responses to stimulation of the superior sagittal sinus.172 The CGRP antagonists, BIBN4096BS and CGRP(8-37), blocked the neuronal activity evoked by glutamate microiontophoresis or stimulation of the superior sagittal sinus.172 It was concluded that release of CGRP in the TNC acts at nonpresynaptic, likely postsynaptic sites to promote transmission of trigeminovascular nociceptive inputs.172
Other studies showed that CGRP can promote central sensitization, thus enhancing synaptic function, at supraspinal sites. For example, the laterocapsular division of the central nucleus of the amygdala (CeLC) receives CGRP immunoreactive fibers from the parabrachial region and expresses the CGRP receptor.88 Patch-clamp recordings performed on coronal slices of the rat brain showed that CGRP increases the amplitude of excitatory postsynaptic currents and neuronal excitability in postsynaptic neurons of the CeLC.88
7. Calcitonin gene–related peptide and pain models
Numerous behavioral and electrophysiologic studies provide strong evidence that CGRP contributes to central sensitization in animal models of inflammation or nerve injury.13,14,159,175,200 Studies performed with α-CGRP–null mutant mice showed that these mice had markedly attenuated behavioral responses in the capsaicin, formalin, and carrageenan models of peripheral inflammation but did not change nociceptive responses to noxious radiant heat.162 Moreover, visceral responses to intraperitoneal acetic acid, which produces a delayed inflammatory effect, but not to MgSO4, which produces a noninflammatory visceral nociceptive response were significantly decreased in α-CGRP–null mutant mice.162 These results are consistent with a pronociceptive sensitizing role of CGRP. The selective ablation of DRG neurons that express α-CGRP through the use of a LoxP-stopped cell ablation construct (human diphtheria toxin receptor) resulted in a >90% loss of all α-CGRP DRG neurons and spinal afferents as well as a loss of approximately one-half of TRPV1+ DRG neurons.132 These mice had reduced behavioral and electrophysiologic nociceptive responses in models of inflammation and neuropathic pain.132
7.1. Calcitonin gene–related peptide in inflammatory pain models
Animal models of adjuvant arthritis and of chronic inflammatory pain show a marked upregulation of CGRP and mRNA in DRG neurons, as well as elevated CGRP levels in primary afferent terminals of the spinal dorsal horn.13,14,200 For example, carrageenan-induced inflammation of the hind paw of the rat increased CGRP levels in exudate and cerebrospinal fluid, but not plasma, 24 hours after the inflammation.200 In another study, inflammation induced by injection of complete Freund adjuvant into the L5-L6 facet joint increased CGRP expression in DRG from L1 through L5.147 Moreover, blocking the effects of CGRP, either with the CGRP antagonists, BIBN4096BS or CGRP8-37, or with galcanezumab, blocked behavioral and electrophysiologic signs of enhanced pain in animals with inflammation.13,14,16,89,200 Rats with inflammation induced by complete Freund adjuvant or with monoiodoacetate injected into the knee joint, which serves as a model for osteoarthritis, reduced mechanical thresholds to elicit a nocifensive response and lowered the thresholds required to evoke electrophysiologic responses of WDR neurons.89 The intravenous administration of the small-molecule CGRP antagonist BIBN4096BS abolished both the behavioral and electrophysiologic manifestations of enhanced pain.89 It was also found that peripheral inflammation can promote upregulation of CGRP in the DRG through exposure of TRPV1 receptors of primary afferent fibers to an acidic environment.140 Collagen-induced arthritis was associated with increased CGRP levels in DRG, enhanced abnormal pain, and significant microgliosis.142 These were abolished by spinal administration of CGRP8-37.142 Electrophysiologic studies performed in rats with experimentally (ie, kaolin/carrageenan) induced arthritis of the knee showed that the inflammation was associated with synaptic facilitation and enhancement of the monosynaptic excitatory postsynaptic currents of dorsal horn units.22 These signs of enhanced synaptic function were blocked by the addition of CGRP8-37 to the artificial cerebrospinal fluid superfusate.22 Taken together, these studies show that central sensitization associated with the enhanced abnormal pain responses in the inflammatory pain state is mediated in large part by elevated CGRP expression in DRG neurons. Thus, animal models of osteoarthritis or of rheumatoid arthritis showed enhanced CGRP in peripheral nerves, and the small-molecule CGRP antagonist BIBN4096BS abolished behavioral and electrophysiologic signs of enhanced pain.28,29,186 Benschop et al.16 demonstrated that systemic administration of galcanezumab blocked behavioral signs of hyperalgesia in 2 rat models of osteoarthritis (ie, monoiodoacetate and meniscal tear) and suggested that clinical studies with galcanezumab were warranted in patients with osteoarthritis. However, a preliminary abstract on a phase II randomized controlled trial (RCT) study of galcanezumab in patients with osteoarthritis found that the antibody did not show efficacy against moderate-to-severe knee pain.101 No other clinical studies have been reported to have examined the utility of CGRP receptor antagonists or CGRP antibodies in inflammatory pain.
7.2. Calcitonin gene–related peptide in neuropathic pain models
Ligation of the L5 and L6 spinal nerves produces behavioral signs of hyperalgesia and allodynia, suggestive of central sensitization associated with enhanced abnormal neuropathic pain, along with an increase in capsaicin-evoked release of CGRP from dorsal spinal cord sections.77,79,151 Ablation of descending modulation from the nucleus raphe magnus (NRM) by neurochemical lesioning of neurons expressing the μ-opiate receptor, which is believed to correlate with descending pain facilitatory neurons, or by surgical lesioning of the dorsolateral funiculus, which contains the spinopetal projections from the NRM, abolished behavioral signs of enhanced abnormal pain (ie, hind paw hyperalgesia and allodynia) and reduced the enhanced capsaicin–evoked release of CGRP from sections of the dorsal lumbar spinal cord.77,79,151 Systemic administration of the neurotrophic artemin, which restored functionality of the injured spinal nerves, produced similar results.79 Intrathecal administration of CGRP8-37 attenuated behavioral signs of mechanical hyperalgesia in rats with a peripheral nerve injury.118 Importantly, CGRP expression is reduced in the injured DRG, but there seems to be a compensatory upregulation in adjacent DRG. Spinal nerve ligation of L5 spinal nerve of rats produced a marked reduction of CGRP in the L5 DRG, but marked increases in L4 and L6.187 Repeated injections of ketanserin attenuated thermal hyperalgesia and tactile allodynia in a manner that corresponded to the reduction in upregulation of CGRP in the DRG.187 A recent study in an animal model of nerve compression and disk herniation showed mechanical hyperalgesia and upregulation of CGRP in primary afferent nerve terminals in laminae I and II, as well as in the deeper laminae.107 In other studies, the persistent exposure of rats to morphine resulted in the gradual development of enhanced abnormal pain along with upregulation of CGRP in the spinal dorsal horn and enhanced capsaicin–evoked release of CGRP from dorsal spinal cord sections.78 Manipulations that abolished endogenous pain facilitatory systems attenuated the upregulation of the neuropeptides and reduced the enhanced release of CGRP along with behavioral signs of enhanced abnormal pain.78 Studies performed with genetically similar rats that expressed either a high (HA) or low (LA) propensity to develop allodynia after peripheral nerve injury showed that the HA rats showed a 10-fold increase in CGRP in large-diameter DRG neurons.143,144 The increase in CGRP in these neurons corresponded to the development of behavioral signs of tactile allodynia and central sensitization in the HA rats.143,144The intrathecal administration of the CGRP receptor antagonist BIBN4096BS (olcegepant) blocked behavioral and neurochemical signs of central sensitization without altering baseline nociceptive thresholds.143,144 No clinical studies on neuropathic pain have been reported to date on the effects of CGRP antagonists or antibodies. Thus, to date there is limited information on clarifying the role of CGRP in this condition.
Taken together, studies overall suggest that CGRP may contribute to the development and maintenance of central sensitization, which underlies enhanced chronic pain conditions including idiopathic pain states, as well as inflammatory and neuropathic pain. However, there is a paucity of information on CGRP's direct effects on pain and the utility of CGRP receptor antagonists or CGRP antibodies in nonmigraine chronic pain conditions.
8. Supraspinal effects of calcitonin gene–related peptide
The CGRP peptide and the CGRP receptors are present in different regions of the brain that modulate nociception and that have been implicated in the pathophysiology of migraine.3,65,67,177 In a 2001 study, it was estimated that up to 80% of cell bodies in the locus coeruleus, which is implicated in cardiovascular, autonomic, and nociceptive functions, expressed CGRP.177 In that study, no CGRP was detected in cell bodies of the PAG or NRM.177 An earlier study had found CGRP expression in nerve fibers that terminated in the PAG of the cat as well as CGRP in cell bodies in the caudal aspect of the ventrolateral PAG.43 Microinjection of CGRP into the PAG blocked nociception in normal and nerve-injured rats, and this effect was reversed by CGRP(8-37).195,201 The CeA, which also contributes to the regulation of pain, was found to contain CGRP receptors as well as nerve fibers containing CGRP.196 Microinjection of CGRP into the CeA produced dose-dependent antinociception in rats blocked by intra-CeA CGRP (8-37) and systemic naloxone.196 Retrograde fluorescent tracing studies along with immunohistochemistry indicated that CGRP released within the CeA activated enkephalinergic projections to the PAG, thus producing an antinociceptive effect.195,196 In a study where the subcutaneous injection of 10 mg/kg of nitroglycerin to rats was proposed to act as an experimental migraine model, this treatment resulted in an increase in mRNA and protein levels of CGRP in the PAG.198 This increase was blocked by pretreatment with rizatriptan.198 However, no behavioral signs of pain or headache were measured in that study. In a more recent study, the responses of neurons in the trigeminocervical complex with cutaneous or meningeal receptive fields were recorded.153 Neuronal responses were enhanced by CGRP microinjected into the ventrolateral PAG and attenuated by microinjection of either olcegepant or CGRP(8-37).153 These studies suggest that CGRP may promote enhanced excitability of the trigeminocervical complex in part through activity of the PAG.153 Most recently, it was found that functional CGRP receptors are present in many sites in the brainstem of the rhesus monkey, including sites important to nociceptive processing such as the PAG, area postrema, pontine raphe nucleus, gracile nucleus, and the TNC.65 Moreover, CGRP receptors were detected in areas that lack protection of the blood–brain barrier (BBB), such as the area postrema, median eminence, and the pineal gland.65 These studies point to the involvement of CGRP in areas of the brain involved in central sensitization and descending modulation, implicated in both migraine and nociceptive processing. However, the role of CGRP in these different brain regions remains to be elucidated, as both pro- and antinociceptive roles have been suggested, depending on the region involved. In addition, the roles of the CGRP receptor as well as potential roles for the AMY1 receptor in migraine headache, and nociceptive processing in general, also require further clarification. A better understanding of the regional effects of CGRP, and of receptors stimulated by CGRP, in the brain will lead to a better understanding of the role of this peptide in pain and its modulation.
9. Calcitonin gene–related peptide, migraine, and the trigeminal system
There is considerable, growing evidence that implicates CGRP in the genesis of migraine (Fig. 4). This role is ultimately emphasized by the recent success of early phase clinical trials with CGRP receptor antagonists and humanized monoclonal antibodies.20,21,54,55,82
Figure 4.
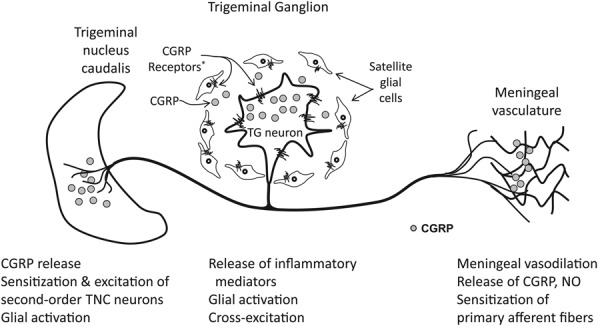
Activation of trigeminal ganglion (TG) neurons, perhaps through the release of inflammatory mediators or in response to ischemia or a localized insult, results in the release of calcitonin gene–related peptide (CGRP) into the meningeal vasculature, which results in vasodilation and release of nitric oxide (NO), producing a neurogenic inflammation. Calcitonin gene-related peptide can also be secreted from the cell body within the TG, where it excites satellite glial cells, which in turn can release inflammatory mediators, thus resulting in cross-excitation and facilitation of an inflammatory loop. The CGRP receptors are also present on TG neurons that do not express CGRP. Thus, CGRP released in the meninges and TG can initiate and maintain peripheral sensitization. The release of CGRP and other excitatory neurotransmitters from the central terminals of the TG neurons in the trigeminal nucleus caudalis (TNC) can excite second-order neurons in the TNC, leading to central sensitization and the manifestation of hyperalgesia and allodynia. Calcitonin gene-related peptide can also sensitize primary afferent nerves by acting on the presynaptic CGRP receptors that are present on presynaptic terminals of primary afferents that do not express CGRP. *CGRP receptors are found on large-diameter TG neurons that do not express CGRP.
9.1. Calcitonin gene–related peptide and migraine
The possibility that CGRP might be germane to migraine was first raised by Goadsby and Edvinsson who in 1988 found that patients who were undergoing thermocoagulation of the TG for trigeminal neuralgia and who showed facial flushing had significantly elevated CGRP levels in blood taken from the external jugular vein.85 These findings were coupled with findings that electrical stimulation of the cat TG also elevated blood CGRP levels.85 It was then discovered that blood collected from the external jugular vein, but not from the cubital fossa, from patients during a migraine attack showed elevated levels of CGRP.86 Moreover, patients with migraine also showed a normalization of CGRP levels after efficacious sumatriptan treatment.84 Studies performed in parallel showed that elevated plasma CGRP levels following electrical stimulation of the TG of cats were reduced by subsequent administration of sumatriptan.84 Although another study failed to find any change in CGRP levels in either cubital or jugular blood samples during migraine,181 this failure may have been due to the low pain levels reported in these patients.111
The administration of NO, by means of an NO donor such as glyceryl trinitrate (GTN), to some migraineurs precipitates a typical migraine attack, but after a delayed onset of several hours.2,111,179 Importantly, individuals who do not experience migraine do not develop the delayed migraine-like symptoms.2,111,179 However, both migraineurs and normal individuals report developing a mild headache immediately after NO donor administration, lasting 15 to 20 minutes.2,111,179 The intensity of GTN-induced migraine was associated with significant elevations in levels of CGRP in blood obtained from the external jugular vein.102,103 The infusion of GTN to normal, healthy volunteers produced an immediate, mild headache but did not elevate plasma CGRP levels.111 Sumatriptan attenuated the GTN-induced migraine headache symptoms and reduced the elevated blood levels of CGRP.102 Sumatriptan elicited a moderate increase in CGRP levels in blood samples obtained from cubital fossa, but not external jugular, venipuncture. However, it did not alter CGRP levels after GTN infusion.111 It was also found that patients with chronic migraine had significantly elevated blood CGRP levels during the interictal (ie, headache-free) periods when compared with healthy normal volunteers and patients with episodic migraine.38 Plasma CGRP levels were predictive of the efficacy of onabotulinumtoxinA in preventing migraine episodes in patients with chronic migraine. The probability of a patient responding to onabotulinumtoxinA was 28-fold greater for patients with interictal plasma CGRP levels of >72 pg/mL than for those with lower CGRP levels.39 Moreover, the interictal CGRP levels were significantly reduced by onabotulinumtoxinA in responsive patients, whereas levels did not change in nonresponders.40 Similar to GTN, the intravenous infusion of human α-CGRP to patients with migraine provokes a mild headache during the infusion that was followed by an attack with many of the characteristics of migraine without aura.113 Importantly, as with NO donors, CGRP infusion to nonmigraineurs does not provoke migraine-like attack.113 Taken together, these studies clearly show a prominent role of CGRP, as well as NO, in the pathogenesis of migraine. However, the fact that neither CGRP nor NO produce an immediate migraine episode, but require a substantial delay to exert its effect, strongly suggests that CGRP and NO are likely to act through the development of sensitization of the TG afferents and/or central loci that receive inputs from the TG. Several studies show that CGRP promotes the synthesis and release of NO and NO promotes that of CGRP, such that both of these substances can enhance the activity of the other.12,95,123,135
The important role of CGRP in the pathogenesis of migraine is further supported by recent phase II RCTs that show that the small-molecule CGRP antagonists, olcegepant, telcagepant, MK-3207, BI 44370 TA, rimegepant, and ubrogepant, all were efficacious for the acute treatment of migraine.53,90,91,130,148,167,184 More recently, RCTs using humanized monoclonal antibodies to CGRP or to the CGRP receptor have shown efficacy in patients with migraine.20,21,54,55,173 For instance, a 12-week study in patients with episodic migraine demonstrated that the humanized monoclonal antibody galcanezumab reduced the frequency of migraine headache days with low incidence of adverse events.55 Another recent study demonstrated that the monoclonal CGRP antibody ALD403 reduced the number of migraine headache days in patients with episodic migraine.54 Phase II studies with the CGRP antibody TEV-48125 show efficacy in episodic and chronic migraine, with a favorable safety profile.18,19 AMG 334 is a CGRP receptor antibody that was demonstrated to be effective in a phase II study for migraine prevention in patients with episodic migraine.173 Taken together, these trials present strong evidence that disruption of the effects of endogenous CGRP is an efficacious approach to attenuate migraine headaches.59 However, the means through which CGRP may initiate and/or sustain migraine, as well as the site(s) of action of the anti-CGRP therapeutics are still not well understood.
9.2. Calcitonin gene–related peptide, the trigeminal system, and sensitization
The TG and its central terminal field, the TNC, are often likened to the DRG and spinal dorsal horn. Although there are many similarities, there are also some differences that become important in the context of migraine. Like in the DRG, the TG sensory neurons are pseudounipolar, with one end of the axon terminating in the TNC and the antipodal end projecting to peripheral sites. In addition to providing cutaneous innervation of the face and scalp, the TG also supplies a dense innervation of the meningeal vasculature with nociceptive primary afferents. Moreover, CGRP is the most abundant peptidic neurotransmitter in the TG, expressed in 70% of TG neurons in rats, compared with 49% of DRG neurons.155 Also, there is a greater proportion of CGRP neurons that coexpress TRPV1 in the TG in comparison with DRG neurons.155 These differences highlight the importance of CGRP in the trigeminovascular innervation.57,155 This distribution is consistent with a prominent nociceptive, CGRP-dominant innervation of the trigeminovascular system. It was recently reported that elevated levels of CGRP in the rostral spinal cord are associated with sensitization of primary nociceptive trigeminal neurons.44 The intracisternal injection of CGRP enhanced nocifensive withdrawal of the head to sensory stimuli, and the enhancement of this response was inhibited by the CGRP antagonist CGRP(8-37).44
As reviewed above, there is considerable evidence to indicate that CGRP is instrumental in the development and maintenance of peripheral and central sensitization. Based on the results of a series of animal and clinical studies, Burstein et al. have advanced the idea that migraine is a product of peripheral and central sensitization.17,30,34,35,146 In support of this hypothesis, some patients develop cutaneous allodynia, and the allodynia spreads eventually to include extracephalic areas.17,30,34,35,146 In these patients, triptans would effectively block migraine headache only if given before the onset of cutaneous allodynia.17,30,35 Functional magnetic resonance imaging performed in patients during a migraine attack showed enhanced activation of the TG and TNC in response to innocuous mechanical stimuli applied to the periorbital skin during migraine and enhanced activity of the thalamus to innocuous mechanical stimulation applied to the dorsum of the hand.17,30,35 Thus, it was hypothesized that peripheral sensitization mediates the painful throbbing felt at the beginning phases of a migraine episode. Left unchecked, the sensitized TG neurons provoke central sensitization of the second-order TNC neurons, which mediates the development of cutaneous allodynia.17,30,35 In a recent BOLD functional magnetic resonance imaging study with normal, healthy volunteers, the intravenous infusion of CGRP increased BOLD-signal in the brainstem and insula and decreased the BOLD-signal in the caudate nuclei, thalamus, and cingulate cortex in response to the application of noxious heat stimuli to the V1 trigeminal area.7 These changes in BOLD-signal were reversed by sumatriptan. These data support the notion that CGRP modulates nociceptive transmission in the trigeminal pain pathways.7
The interpretations of these clinical results were supported by correlative studies in rats. The application of a solution of inflammatory mediators to the dura of rats increased the spontaneous activity of second-order TNC neurons. Moreover, the TNC neurons also show reduced firing thresholds, increased firing frequencies, and enlarged receptive fields in response to light mechanical stimulation of the periorbital cutaneous region or to the dura.17,31,35 These electrophysiological properties are hallmarks indicative of central sensitization. The intravenous administration of sumatriptan 2 hours after application of inflammatory mediators (ie, late application) reduced the expanded receptive fields and the neuronal responses to light tactile stimuli applied to the dura. However, the increased spontaneous firing rates and the enhanced responses to light cutaneous tactile stimuli and reduced response thresholds to heat stimuli remained.17,31,35 In contrast, administration of sumatriptan at the same time as the inflammatory mediators blocked the development of all electrophysiologic signs of central sensitization. Because the 5-HT1B/D/1F receptors are coexpressed with nitric oxide synthase (NOS) and CGRP in trigeminal neurons, it is very possible that sumatriptan acts peripherally, by inhibiting the release of CGRP from the primary afferent TG nociceptors.4,12,17,31,35,94
Although there is strong evidence that CGRP can mediate neuronal sensitization, the mechanism of action remains unclear. One study showed that CGRP neither excites nor sensitizes meningeal nociceptors directly.120 In that study, the spontaneous and noxious-evoked discharge rates of TG nociceptors with receptive fields in the dura were measured along with dural blood flow. Neither intravenous infusion nor direct application to the dura of CGRP increased ongoing neuronal activity or responses to mechanical stimulation of the dura, but both increased dural blood flow.120 These investigators suggested that the results indicate a central effect of CGRP, perhaps facilitation of the second-order neurons of the TNC. The absence of an activation of nociceptors by CGRP is consistent with studies showing that intradermal CGRP does not evoke nociceptive responses in normal individuals.188 This observation is not necessarily surprising, as intravenous infusions of CGRP, like NO donors, produce migraine-like headaches after a considerably long latency period of several hours in migraineurs but not in healthy controls.2,111,179 It should be noted that in the electrophysiologic study, neuronal recordings were performed for no more than 30 minutes after CGRP administration.120 Moreover, these studies were performed in rats that have not been sensitized, and may not mimic the sensitized state believed to exist in migraineurs.17,31,35,47 This suggests the activation of processes secondary to CGRP rather than a direct excitatory effect of CGRP. One such possibility is that CGRP acts on nonneural cells to promote sensitization. The TG neurons can secrete CGRP from the soma within the TG, and satellite glial cells in the TG express CGRP receptors. The satellite glial cells of the TG are stimulated by CGRP to upregulate proinflammatory substances, such as IL-1β and the inducible form of NOS.46 Increased IL-1β levels induced by CGRP resulted in a 3-fold increase in COX2 levels, which would increase prostaglandin E2 synthesis, ultimately further sensitizing the TG neurons.37,46 This could result in a positive feedback loop, with CGRP promoting neuronal-glial crosstalk within the TG and maintaining a state of peripheral, and ultimately central, sensitization.36,37,46,56,119,123
The interaction among the trigeminal afferents, meningeal vasculature, and the meningeal environment may also provide a means through which CGRP may sustain central sensitization. The release of CGRP provokes an intense vasodilation of the meningeal vasculature. Vasodilation itself is no longer held to be a mechanism driving migraine, and the vascular theory of migraine has been largely abandoned.41,83 However, the effects of CGRP could still be linked to a neurogenic mechanism promoting sensitization. Calcitonin gene-related peptide released from peripheral trigeminal nerve terminals can activate CGRP receptors on the endothelial cells of the meningeal vasculature, which results in cyclic adenosine monophosphate (cAMP) formation, which in turn stimulates endothelial NOS to enhance production of NO. Nitric oxide can sensitize the peripheral trigeminal afferent fibers, causing enhanced release of CGRP at the peripheral and central terminals.14,35,135,159 Because both NO and CGRP facilitate nociceptive transmission, this mechanism provides a means through which a state of enhanced excitability might be maintained.14,35,135,159 Electrophysiologic studies in rats showed that blockade of CGRP receptors by systemically administered CGRP antagonist (MK-8825) blocked the increased neuronal activity of trigeminal neurons with meningeal afferent inputs during sustained infusion of nitroglycerin.70 Tissue culture studies showed that NO donors increase CGRP release and stimulate CGRP promoter activity in TG neurons.12 Taken together, these studies provide a potential mechanism where CGRP can act in concert with NO to facilitate trigeminal nociceptive transmission and potentially promote migraine headache. The complex interaction among CGRP, meningeal vasculature, afferent fibers, and NO suggest that perhaps CGRP-induced vasodilation may not be directly responsible for migraine headache. The consequences of the action of CGRP on meningeal vasculature, involving nociceptive afferent neurons and the release of NO, could mean that meningeal vasodilation is in some way germane to migraine. Clearly, further investigations into the complex interactions among these components are warranted.
9.3. Calcitonin gene–related peptide, latent sensitization, and relevance to migraine
The concept of latent sensitization is that an initiating event can produce a sensitized condition that lies dormant, to be “awakened” after exposure to a triggering stimulus.48–50 In an animal model of latent sensitization, sustained sumatriptan infusion to rats produced the development of periorbital cutaneous allodynia along with a marked upregulation of neuronal NOS (nNOS) and CGRP, but not substance P, in trigeminal neurons with meningeal afferent inputs.48–50 After 2 weeks, behavioral signs of allodynia resolved, but the trigeminal levels of CGRP and nNOS remained elevated.48–50 The administration of an NO donor or exposure to environmental stress, which are known migraine triggers in migraineurs, precipitated periorbital allodynia that was blocked by nNOS inhibitors.48–50 Moreover, the administration of the NO donor to rats with sumatriptan-induced latent sensitization also elevated blood CGRP levels, but did not change them in the nonsensitized (ie, saline-infused) rats.50 These studies show that a state of latent sensitization can be produced in rats that, when challenged with putative migraine triggers, can provoke periorbital cutaneous allodynia and elevated blood levels of CGRP.
10. Central vs peripheral site of action of calcitonin gene–related peptide in migraine
Despite the advances in the study of migraine neuropathology and of the role of CGRP in migraine, the site of action of CGRP in alleviating migraine remains unsettled. However, there is a growing body of evidence that points to the peripheral site as being more likely in mediating the observed antimigraine activity. The triptans, which likely act by inhibition of release of CGRP and other neurotransmitters from primary afferents to abort migraines, in general have poor penetration of the BBB.4,74 Moreover, their efficacious clinical doses do not correlate well with BBB penetration.4,74 The small-molecule CGRP receptor blockers, which also show clinical efficacy in aborting migraine, show little (telcagepant) to no (olcegepant) ability to cross the BBB.59–61 Likewise, the recently developed humanized antibodies to CGRP or the receptor are unlikely to act via a central site in preventing migraine, as these substances have very little to no ability to penetrate the BBB.55,62,178,203 It has been suggested that migraine episodes are accompanied by changes in BBB permeability, which would allow penetration of these substances.4 However, increased BBB permeability during migraine has not been consistently demonstrated and would not explain how these therapeutics may access the brain to prevent migraine attacks.4 A recent study using PET with the novel radioligand 11C-dihydroergotamine showed that the radioligand was unable to penetrate into the brain of patients with migraine when migraine was provoked by exposure to GTN suggesting that the BBB is not compromised during a migraine attack.164 A recent PET study using CGRP PET tracer [11C]MK-4232 as a validated ligand for the CGRP receptor showed very low (<10%) receptor occupancy of the CGRP receptor by telcagepant at therapeutic doses.92 It was concluded that occupancy of central CGRP receptors is not required for the therapeutic effect of telcagepant, but the central occupancy that could occur with higher doses may confer some additional therapeutic benefit.92
11. Summary
Collectively, these studies provide strong evidence that an important physiologic function of CGRP seems to be its association with pain transmission and modulation. It is found in high concentrations primarily in peptidergic nociceptive Aδ and C-fiber DRG and TG neurons and in their terminals in the outer laminae of the spinal cord dorsal horn and in the TNC. The terminations of the CGRP-expressing primary afferents correlate well with the distribution of second-order neurons with ascending projections to supraspinal sites involved in pain perception and modulation. Considerable evidence indicates that peripheral sensitization and the associated hyperalgesia are initiated and maintained in part by the actions of CGRP, and enhanced release of CGRP at peripheral and central terminals of primary afferent fibers is associated with nociceptor sensitization and hyperalgesia. Upregulation of CGRP is believed to contribute to the development of central sensitization and enhanced pain in neuropathic and inflammatory pain states. Calcitonin gene-related peptide is a pronociceptive neuromediator, both at somatic and cephalic sites. Moreover, CGRP plays a critical role in the trigeminovascular innervation, where it can activate positive feed-forward circuitries that can initiate and/or sustain a painful event. Interactions between the TG neurons and satellite cells and with the meningeal vasculature can help maintain the state of enhanced excitability by promoting the synthesis and release of both NO and CGRP, which act to promote each other's activities. Disruption of this feed-forward loop, by blocking the activity of CGRP, appears to be therapeutically relevant in migraine.
Conflict of interest statement
S. Iyengar and K. W. Johnson are employed by and are stockholders of Eli Lilly and Company. M. H. Ossipov is employed by inVentiv Health Clinical, LLC.
Eli Lilly and Company contracted inVentiv Health Clinical, LLC, for writing and editorial services.
Teri Tucker of inVentiv Health Clinical, LLC provided editorial assistance. This work was sponsored and funded by Eli Lilly and Company, Indianapolis, IN, USA.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ablin JN, Buskila D, Clauw DJ. Biomarkers in fibromyalgia. Curr Pain Headache Rep 2009;13:343–9. [DOI] [PubMed] [Google Scholar]
- [2].Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. PAIN 2004;110:675–80. [DOI] [PubMed] [Google Scholar]
- [3].Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128:932–9. [DOI] [PubMed] [Google Scholar]
- [4].Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? PAIN 2005;115:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 1985;229:1094–7. [DOI] [PubMed] [Google Scholar]
- [6].Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982;298:240–4. [DOI] [PubMed] [Google Scholar]
- [7].Asghar MS, Becerra L, Larsson HB, Borsook D, Ashina M. Calcitonin gene-related peptide modulates heat nociception in the human brain—an fMRI study in healthy volunteers. PLoS One 2016;11:e0150334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci 1995;7:1484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7. [DOI] [PubMed] [Google Scholar]
- [10].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Becker KL, Nylén ES, White JC, Müller B, Snider RH., Jr Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004;89:1512–25. [DOI] [PubMed] [Google Scholar]
- [12].Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci 2006;23:2057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology 2011;77:281–7. [DOI] [PubMed] [Google Scholar]
- [14].Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 2009;9:9–14. [DOI] [PubMed] [Google Scholar]
- [15].Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist 2001;7:13–7. [DOI] [PubMed] [Google Scholar]
- [16].Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, Lynch RA, Li B, McCarty D, Nisenbaum ES, Oskins JL, Lin C, Johnson KW, Chambers MG. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage 2014;22:578–85. [DOI] [PubMed] [Google Scholar]
- [17].Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 2012;8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, Loupe PS, Burstein R, Newman LC, Lipton RB. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015;14:1081–90. [DOI] [PubMed] [Google Scholar]
- [19].Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC, Burstein R, Loupe PS, Ma Y, Yang R, Silberstein SD. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015;14:1091–100. [DOI] [PubMed] [Google Scholar]
- [20].Bigal ME, Escandon R, Bronson M, Walter S, Sudworth M, Huggins JP, Garzone P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the phase 1 program. Cephalalgia 2014;34:483–92. [DOI] [PubMed] [Google Scholar]
- [21].Bigal ME, Walter S, Bronson M, Alibhoy A, Escandon R. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia 2014;34:968–76. [DOI] [PubMed] [Google Scholar]
- [22].Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V. Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP. Mol Pain 2006;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Born W, Fischer JA. The calcitonin peptide family: what can we learn from receptor knock out and transgenic mice. In: Hay LD, Dickerson MI, editors. The calcitonin gene-related peptide family: form, function and future perspectives. Dordrecht: Springer, 2010. p. 75–86. [Google Scholar]
- [24].Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum 2008;7:252–72. [DOI] [PubMed] [Google Scholar]
- [25].Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985;313:54–6. [DOI] [PubMed] [Google Scholar]
- [26].Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization—an integrated perspective. Auton Neurosci 2010;153:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brumovsky PR, La JH, McCarthy CJ, Hökfelt T, Gebhart GF. Dorsal root ganglion neurons innervating pelvic organs in the mouse express tyrosine hydroxylase. Neuroscience 2012;223:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bullock CM, Kelly S. Calcitonin gene-related peptide receptor antagonists: beyond migraine pain–a possible analgesic strategy for osteoarthritis? Curr Pain Headache Rep 2013;17:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol 2014;66:2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004;55:19–26. [DOI] [PubMed] [Google Scholar]
- [31].Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol 2004;55:27–36. [DOI] [PubMed] [Google Scholar]
- [32].Burstein R, Jakubowski M. Managing migraine associated with sensitization. Handb Clin Neurol 2010;97:207–15. [DOI] [PubMed] [Google Scholar]
- [33].Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005;161:658–60. [DOI] [PubMed] [Google Scholar]
- [35].Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 2015;35:6619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain 2011;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain 2009;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013;81:1191–6. [DOI] [PubMed] [Google Scholar]
- [39].Cernuda-Morollón E, Martínez-Camblor P, Ramón C, Larrosa D, Serrano-Pertierra E, Pascual J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 2014;54:987–95. [DOI] [PubMed] [Google Scholar]
- [40].Cernuda-Morollón E, Ramón C, Martínez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. PAIN 2015;156:820–4. [DOI] [PubMed] [Google Scholar]
- [41].Charles A. Vasodilation out of the picture as a cause of migraine headache. Lancet Neurol 2013;12:419–20. [DOI] [PubMed] [Google Scholar]
- [42].Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem 2003;278:3293–7. [DOI] [PubMed] [Google Scholar]
- [43].Conti F, Sternini C. Calcitonin gene-related peptide (CGRP)-positive neurons and fibers in the cat periaqueductal grey matter. Somatosens Mot Res 1989;6:497–511. [DOI] [PubMed] [Google Scholar]
- [44].Cornelison LE, Hawkins JL, Durham PL. Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons. Neuroscience 2016;339:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].D'Hanis W, Linke R, Yilmazer-Hanke DM. Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala. J Comp Neurol 2007;505:268–91. [DOI] [PubMed] [Google Scholar]
- [46].De Corato A, Lisi L, Capuano A, Tringali G, Tramutola A, Navarra P, Dello Russo C. Trigeminal satellite cells express functional calcitonin gene-related peptide receptors, whose activation enhances interleukin-1β pro-inflammatory effects. J Neuroimmunol 2011;237:39–46. [DOI] [PubMed] [Google Scholar]
- [47].De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol 2013;74:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].De Felice M, Ossipov MH, Porreca F. Persistent medication-induced neural adaptations, descending facilitation, and medication overuse headache. Curr Opin Neurol 2011;24:193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].De Felice M, Ossipov MH, Wang R, Dussor G, Lai J, Meng ID, Chichorro J, Andrews JS, Rakhit S, Maddaford S, Dodick D, Porreca F. Triptan-induced enhancement of neuronal nitric oxide synthase in trigeminal ganglion dural afferents underlies increased responsiveness to potential migraine triggers. Brain 2010;133:2475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, Dodick DW, Vanderah TW, Dussor G, Porreca F. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol 2010;67:325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. PAIN 2011;152:2701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Desarmenien M, Santangelo F, Loeffler JP, Feltz P. Comparative study of GABA-mediated depolarizations of lumbar A delta and C primary afferent neurones of the rat. Exp Brain Res 1984;54:521–8. [DOI] [PubMed] [Google Scholar]
- [53].Diener HC, Barbanti P, Dahlöf C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 2011;31:573–84. [DOI] [PubMed] [Google Scholar]
- [54].Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J; ALD403 study investigators. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014;13:1100–7. [DOI] [PubMed] [Google Scholar]
- [55].Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014;13:885–92. [DOI] [PubMed] [Google Scholar]
- [56].Durham PL. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep 2016;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 2014;5:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 2009;65:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 2015;80:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics 2010;7:164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol 2007;150:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Edvinsson L, Tfelt-Hansen P. The blood-brain barrier in migraine treatment. Cephalalgia 2008;28:1245–58. [DOI] [PubMed] [Google Scholar]
- [63].Eftekhari S, Edvinsson L. Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Ther Adv Neurol Disord 2010;3:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci 2011;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Eftekhari S, Gaspar RC, Roberts R, Chen TB, Zeng Z, Villarreal S, Edvinsson L, Salvatore CA. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: a detailed study using in situ hybridization, immunofluorescence, and autoradiography. J Comp Neurol 2016;524:90–118. [DOI] [PubMed] [Google Scholar]
- [66].Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010;169:683–96. [DOI] [PubMed] [Google Scholar]
- [67].Eftekhari S, Salvatore CA, Gaspar RC, Roberts R, O'Malley S, Zeng Z, Edvinsson L. Localization of CGRP receptor components, CGRP, and receptor binding sites in human and rhesus cerebellar cortex. Cerebellum 2013;12:937–49. [DOI] [PubMed] [Google Scholar]
- [68].Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 2015;1600:93–109. [DOI] [PubMed] [Google Scholar]
- [69].Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 2013;14:1289–303. [DOI] [PubMed] [Google Scholar]
- [70].Feistel S, Albrecht S, Messlinger K. The calcitonin gene-related peptide receptor antagonist MK-8825 decreases spinal trigeminal activity during nitroglycerin infusion. J Headache Pain 2013;14:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fernández-Carnero J, Fernández-de-Las-Peñas C, de la Llave-Rincón AI, Ge HY, Arendt-Nielsen L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain 2009;25:555–61. [DOI] [PubMed] [Google Scholar]
- [72].Fernández-Carnero J, Fernández-de-las-Peñas C, Sterling M, Souvlis T, Arendt-Nielsen L, Vicenzino B. Exploration of the extent of somato-sensory impairment in patients with unilateral lateral epicondylalgia. J Pain 2009;10:1179–85. [DOI] [PubMed] [Google Scholar]
- [73].Fernández-de-las-Peñas C, de la Llave-Rincón AI, Fernández-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain 2009;132:1472–9. [DOI] [PubMed] [Google Scholar]
- [74].Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 2002;22:633–58. [DOI] [PubMed] [Google Scholar]
- [75].Fuller RW, Conradson TB, Dixon CM, Crossman DC, Barnes PJ. Sensory neuropeptide effects in human skin. Br J Pharmacol 1987;92:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gamse R, Saria A. Nociceptive behavior after intrathecal injections of substance P, neurokinin A and calcitonin gene-related peptide in mice. Neurosci Lett 1986;70:143–7. [DOI] [PubMed] [Google Scholar]
- [77].Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, Porreca F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci 2003;23:8370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci 2002;22:6747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med 2003;9:1383–9. [DOI] [PubMed] [Google Scholar]
- [80].Geppetti P, Del Bianco E, Cecconi R, Tramontana M, Romani A, Theodorsson E. Capsaicin releases calcitonin gene-related peptide from the human iris and ciliary body in vitro. Regul Pept 1992;41:83–92. [DOI] [PubMed] [Google Scholar]
- [81].Geppetti P, Rossi E, Chiarugi A, Benemei S. Antidromic vasodilatation and the migraine mechanism. J Headache Pain 2012;13:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Giamberardino MA, Affaitati G, Curto M, Negro A, Costantini R, Martelletti P. Anti-CGRP monoclonal antibodies in migraine: current perspectives. Intern Emerg Med 2016;11:1045–57. [DOI] [PubMed] [Google Scholar]
- [83].Goadsby PJ. The vascular theory of migraine—a great story wrecked by the facts. Brain 2009;132:6–7. [DOI] [PubMed] [Google Scholar]
- [84].Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48–56. [DOI] [PubMed] [Google Scholar]
- [85].Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 1988;23:193–6. [DOI] [PubMed] [Google Scholar]
- [86].Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–7. [DOI] [PubMed] [Google Scholar]
- [87].Grant AD, Tam CW, Lazar Z, Shih MK, Brain SD. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS blocks CGRP and adrenomedullin vasoactive responses in the microvasculature. Br J Pharmacol 2004;142:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 2010;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. PAIN 2013;154:700–7. [DOI] [PubMed] [Google Scholar]
- [90].Ho TW, Olesen J, Dodick DW, Kost J, Lines C, Ferrari MD. Antimigraine efficacy of telcagepant based on patient's historical triptan response. Headache 2011;51:64–72. [DOI] [PubMed] [Google Scholar]
- [91].Hoffmann J, Goadsby PJ. New agents for acute treatment of migraine: CGRP receptor antagonists, iNOS inhibitors. Curr Treat Options Neurol 2012;14:50–9. [DOI] [PubMed] [Google Scholar]
- [92].Hostetler ED, Joshi AD, Sanabria-Bohórquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther 2013;347:478–86. [DOI] [PubMed] [Google Scholar]
- [93].Hotta H, Sato A, Sato Y, Uchida S. Stimulation of saphenous afferent nerve produces vasodilatation of the vasa nervorum via an axon reflex-like mechanism in the sciatic nerve of anesthetized rats. Neurosci Res 1996;24:305–8. [DOI] [PubMed] [Google Scholar]
- [94].Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 2001;909:112–20. [DOI] [PubMed] [Google Scholar]
- [95].Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol 1994;111:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A 2005;102:18195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res 2011;1381:187–201. [DOI] [PubMed] [Google Scholar]
- [98].Jakab G, Salamon I, Petrusz P, Réthelyi M. Termination patterns of calcitonin gene-related peptide-immunoreactive nerve fibers in the dorsal horn of the human spinal cord. Exp Brain Res 1990;80:609–17. [DOI] [PubMed] [Google Scholar]
- [99].Jang JH, Nam TS, Paik KS, Leem JW. Involvement of peripherally released substance P and calcitonin gene-related peptide in mediating mechanical hyperalgesia in a traumatic neuropathy model of the rat. Neurosci Lett 2004;360:129–32. [DOI] [PubMed] [Google Scholar]
- [100].Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003;26:696–705. [DOI] [PubMed] [Google Scholar]
- [101].Jin Y, Smith C, Monteith D, Brown R, Camporeale A, McNearney T, Deeg M, Raddad E, de la Pena A, Kivitz A, Schnitzer T. LY2951742, a monoclonal antibody against CGRP, failed to reduce signs and symptoms of knee osteoarthritis. Osteoarthritis Cartilage 2016;24(suppl 1):S50. [DOI] [PubMed] [Google Scholar]
- [102].Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005;25:179–83. [DOI] [PubMed] [Google Scholar]
- [103].Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, Szolcsanyi J, Vitrai J, Bagdy G. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. PAIN 2003;106:461–70. [DOI] [PubMed] [Google Scholar]
- [104].Kaneko M, Kaneko T, Kaneko R, Chokechanachaisakul U, Kawamura J, Sunakawa M, Okiji T, Suda H. The role of N-methyl-D-aspartate receptor subunits in the rat thalamic mediodorsal nucleus during central sensitization. Brain Res 2011;1371:16–22. [DOI] [PubMed] [Google Scholar]
- [105].Kawamura M, Kuraishi Y, Minami M, Satoh M. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res 1989;497:199–203. [DOI] [PubMed] [Google Scholar]
- [106].Kestell GR, Anderson RL, Clarke JN, Haberberger RV, Gibbins IL. Primary afferent neurons containing calcitonin gene-related peptide but not substance P in forepaw skin, dorsal root ganglia, and spinal cord of mice. J Comp Neurol 2015;523:2555–69. [DOI] [PubMed] [Google Scholar]
- [107].Kimura S, Sakuma Y, Suzuki M, Orita S, Yamauchi K, Inoue G, Aoki Y, Ishikawa T, Miyagi M, Kamoda H, Kubota G, Oikawa Y, Inage K, Sainoh T, Sato J, Nakamura J, Toyone T, Takahashi K, Ohtori S. Evaluation of pain behavior and calcitonin gene-related peptide immunoreactive sensory nerve fibers in the spinal dorsal horn after sciatic nerve compression and application of nucleus pulposus in rats. Spine (Phila Pa 1976) 2014;39:455–62. [DOI] [PubMed] [Google Scholar]
- [108].Kranzler JD, Gendreau RM. Role and rationale for the use of milnacipran in the management of fibromyalgia. Neuropsychiatr Dis Treat 2010;6:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kresse A, Jacobowitz DM, Skofitsch G. Distribution of calcitonin gene-related peptide in the central nervous system of the rat by immunocytochemistry and in situ hybridization histochemistry. Ann N Y Acad Sci 1992;657:455–7. [DOI] [PubMed] [Google Scholar]
- [110].Kruger L, Sternini C, Brecha NC, Mantyh PW. Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J Comp Neurol 1988;273:149–62. [DOI] [PubMed] [Google Scholar]
- [111].Kruuse C, Iversen HK, Jansen-Olesen I, Edvinsson L, Olesen J. Calcitonin gene-related peptide (CGRP) levels during glyceryl trinitrate (GTN)-induced headache in healthy volunteers. Cephalalgia 2010;30:467–74. [DOI] [PubMed] [Google Scholar]
- [112].Kuraishi Y, Nanayama T, Ohno H, Minami M, Satoh M. Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett 1988;92:325–9. [DOI] [PubMed] [Google Scholar]
- [113].Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- [114].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Latremoliere A, Woolf CJ. Synaptic plasticity and central sensitization: author reply. J Pain 2010;11:801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol 2002;540:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Le Grevès P, Andersson K, Silberring J. Isolation and identification of CGRP C-terminal fragments in the rat spinal cord. Neuropeptides 1997;31:19–23. [DOI] [PubMed] [Google Scholar]
- [118].Lee SE, Kim JH. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res 2007;58:245–9. [DOI] [PubMed] [Google Scholar]
- [119].Lennerz JK, Rühle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 2008;507:1277–99. [DOI] [PubMed] [Google Scholar]
- [120].Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol 2005;58:698–705. [DOI] [PubMed] [Google Scholar]
- [121].Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 1993;16:353–9. [DOI] [PubMed] [Google Scholar]
- [122].Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J Pain 2008;9:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res 2008;1196:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain 2007;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lin Q, Zou X, Willis WD. Adelta and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol 2000;84:2695–8. [DOI] [PubMed] [Google Scholar]
- [126].Lobanov OV, Peng YB. Differential contribution of electrically evoked dorsal root reflexes to peripheral vasodilatation and plasma extravasation. J Neuroinflammation 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. PAIN 1995;61:383–90. [DOI] [PubMed] [Google Scholar]
- [128].Ma W, Chabot JG, Schorscher-Petcu A, Hong Y, Wang Z, Quirion R. CGRP and adrenomedullin as pain-related peptides. In: Hay LD, Dickerson MI, editors. The calcitonin gene-related peptide family: form, function and future perspectives. Dordrecht: Springer, 2010. p. 151–71. [Google Scholar]
- [129].Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol 1988;19:1–43. [DOI] [PubMed] [Google Scholar]
- [130].Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 2014;34:114–25. [DOI] [PubMed] [Google Scholar]
- [131].Marvizón JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience 2007;148:250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013;78:138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].McCoy ES, Taylor-Blake B, Zylka MJ. CGRPα-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PLoS One 2012;7:e36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Meng ID, Dodick D, Ossipov MH, Porreca F. Pathophysiology of medication overuse headache: insights and hypotheses from preclinical studies. Cephalalgia 2011;31:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache 2012;52:1411–27. [DOI] [PubMed] [Google Scholar]
- [136].Miller S, Liu H, Warfvinge K, Shi L, Dovlatyan M, Xu C, Edvinsson L. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016;328:165–83. [DOI] [PubMed] [Google Scholar]
- [137].Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev 2010;65:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mulderry PK, Ghatei MA, Spokes RA, Jonhs PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S, Polak JM, Bloom SR. Differential expression of α-CGRP and β-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 1988;25:195–205. [DOI] [PubMed] [Google Scholar]
- [139].Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett 1991;124:49–51. [DOI] [PubMed] [Google Scholar]
- [140].Nakanishi M, Hata K, Nagayama T, Sakurai T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S, Yoneda T. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: a potential mechanism of inflammatory pain. Mol Biol Cell 2010;21:2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. PAIN 2005;116:194–204. [DOI] [PubMed] [Google Scholar]
- [142].Nieto FR, Clark AK, Grist J, Chapman V, Malcangio M. Calcitonin gene-related peptide-expressing sensory neurons and spinal microglial reactivity contribute to pain states in collagen-induced arthritis. Arthritis Rheumatol 2015;67:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nitzan-Luques A, Devor M, Tal M. Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. PAIN 2011;152:2413–26. [DOI] [PubMed] [Google Scholar]
- [144].Nitzan-Luques A, Minert A, Devor M, Tal M. Dynamic genotype-selective phenotypic switching of CGRP expression contributes to differential neuropathic pain phenotype. Exp Neurol 2013;250:194–204. [DOI] [PubMed] [Google Scholar]
- [145].Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Co-expression of alpha-CGRP and beta-CGRP mRNAs in the rat dorsal root ganglion cells. Neurosci Lett 1990;108:1–5. [DOI] [PubMed] [Google Scholar]
- [146].Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. PAIN 2013;154(suppl 1):S44–53. [DOI] [PubMed] [Google Scholar]
- [147].Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Phenotypic inflammation switch in rats shown by calcitonin gene-related peptide immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints. Spine (Phila Pa 1976) 2001;26:1009–13. [DOI] [PubMed] [Google Scholar]
- [148].Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM; BIBN4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004;350:1104–10. [DOI] [PubMed] [Google Scholar]
- [149].Ossipov MH. Growth factors and neuropathic pain. Curr Pain Headache Rep 2011;15:185–92. [DOI] [PubMed] [Google Scholar]
- [150].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci 2001;21:5281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 2002;54:233–46. [DOI] [PubMed] [Google Scholar]
- [153].Pozo-Rosich P, Storer RJ, Charbit AR, Goadsby PJ. Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 2015;35:1298–307. [DOI] [PubMed] [Google Scholar]
- [154].Price TJ, Cervero F, de Konick Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem 2005;5:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 2007;8:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Roeder Z, Chen Q, Davis S, Carlson JD, Tupone D, Heinricher MM. Parabrachial complex links pain transmission to descending pain modulation. PAIN 2016;157:2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983;304:129–35. [DOI] [PubMed] [Google Scholar]
- [158].Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol 2007;502:325–36. [DOI] [PubMed] [Google Scholar]
- [159].Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Russo AF, Dickerson IM. CGRP: a multifunctional neuropeptide. In: Lajtha A, Lim R, editors. Handbook of neurochemistry and molecular neurobiology: neuroactive proteins and peptides. Boston: Springer, 2006. p. 391–426. [Google Scholar]
- [161].Sah DW, Ossipo MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov 2003;2:460–72. [DOI] [PubMed] [Google Scholar]
- [162].Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in [alpha]CGRP-deficient mice. Nat Neurosci 2001;4:357–8. [DOI] [PubMed] [Google Scholar]
- [163].Sann H, Pierau FK. Efferent functions of C-fiber nociceptors. Z Rheumatol 1998;57(suppl 2):8–13. [DOI] [PubMed] [Google Scholar]
- [164].Schankin CJ, Maniyar FH, Seo Y, Kori S, Eller M, Chou DE, Blecha J, Murphy ST, Hawkins RA, Sprenger T, VanBrocklin HF, Goadsby PJ. Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain 2016;139:1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neurosci Lett 1997;230:117–20. [DOI] [PubMed] [Google Scholar]
- [166].Schmelz M, Petersen LJ. Neurogenic inflammation in human and rodent skin. News Physiol Sci 2001;16:33–7. [DOI] [PubMed] [Google Scholar]
- [167].Silberstein SD. Emerging target-based paradigms to prevent and treat migraine. Clin Pharmacol Ther 2013;93:78–85. [DOI] [PubMed] [Google Scholar]
- [168].Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depré M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974). Br J Clin Pharmacol 2010;69:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sluka KA, Willis WD, Westlund KN. The role of dorsal root reflexes in neurogenic inflammation. Pain Forum 1995;4:141–9. [Google Scholar]
- [171].South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci 2003;23:5031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 2004;142:1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Lenz R. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382–90. [DOI] [PubMed] [Google Scholar]
- [174].Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. PAIN 2003;104:201–8. [DOI] [PubMed] [Google Scholar]
- [175].Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 2004;92:2859–66. [DOI] [PubMed] [Google Scholar]
- [176].Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals 2005;14:175–81. [DOI] [PubMed] [Google Scholar]
- [177].Tajti J, Uddman R, Edvinsson L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 2001;21:96–101. [DOI] [PubMed] [Google Scholar]
- [178].Tfelt-Hansen P. Site of effect of LY2951742 for migraine prophylaxis. Lancet Neurol 2015;14:31–2. [DOI] [PubMed] [Google Scholar]
- [179].Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerine) triggers genuine migraine attacks. Eur J Neurol 1994;1:73–80. [DOI] [PubMed] [Google Scholar]
- [180].Tschopp FA, Henke H, Petermann JB, Tobler PH, Janzer R, Hökfelt T, Lundberg JM, Cuello C, Fischer JA. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci U S A 1985;82:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 2005;58:561–8. [DOI] [PubMed] [Google Scholar]
- [182].Valencia-de Ita S, Lawand NB, Lin Q, Castañeda-Hernandez G, Willis WD. Role of the Na+-K+-2Cl- cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol 2006;95:3553–61. [DOI] [PubMed] [Google Scholar]
- [183].Vermeersch S, Benschop RJ, Van Hecken A, Monteith D, Wroblewski VJ, Grayzel D, de HJ, Collins EC. Translational pharmacodynamics of calcitonin gene-related peptide monoclonal antibody LY2951742 in a capsaicin-induced dermal blood flow model. J Pharmacol Exp Ther 2015;354:350–7. [DOI] [PubMed] [Google Scholar]
- [184].Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, Assaid C, Aurora SK, Michelson D. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 2016;36:887–98. [DOI] [PubMed] [Google Scholar]
- [185].Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, Waldvogel HJ, Jamaluddin MA, Russo AF, Hay DL. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2015;2:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol 2015;80:965–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wang D, Gao Y, Ji H, Hong Y. Topical and systemic administrations of ketanserin attenuate hypersensitivity and expression of CGRP in rats with spinal nerve ligation. Eur J Pharmacol 2010;627:124–30. [DOI] [PubMed] [Google Scholar]
- [188].Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin—a microdialysis study. J Invest Dermatol 2000;115:1015–20. [DOI] [PubMed] [Google Scholar]
- [189].Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 1999;124:395–421. [DOI] [PubMed] [Google Scholar]
- [190].Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 1996;17:533–85. [DOI] [PubMed] [Google Scholar]
- [191].Woolf CJ. Windup and central sensitization are not equivalent. PAIN 1996;66:105–8. [PubMed] [Google Scholar]
- [192].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152(3 suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993;77:362–79. [DOI] [PubMed] [Google Scholar]
- [194].Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. PAIN 1991;44:293–9. [DOI] [PubMed] [Google Scholar]
- [195].Xu S, Lundeberg T, Yu L. Antinociceptive effects of calcitonin gene-related peptide injected into periaqueductal grey of rats with mononeuropathy. Brain Res 2000;859:358–60. [DOI] [PubMed] [Google Scholar]
- [196].Xu W, Lundeberg T, Wang YT, Li Y, Yu LC. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience 2003;118:1015–22. [DOI] [PubMed] [Google Scholar]
- [197].Xu XJ, Wiesenfeld-Hallin Z. Neuropeptides: pain A2. In: Squire LR, editor. Encyclopedia of neuroscience. Oxford: Academic Press, 2009. p. 931–4. [Google Scholar]
- [198].Yao G, Han X, Hao T, Huang Q, Yu T. Effects of rizatriptan on the expression of calcitonin gene-related peptide and cholecystokinin in the periaqueductal gray of a rat migraine model. Neurosci Lett 2015;587:29–34. [DOI] [PubMed] [Google Scholar]
- [199].Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res 1994;653:223–30. [DOI] [PubMed] [Google Scholar]
- [200].Yu LC, Hou JF, Fu FH, Zhang YX. Roles of calcitonin gene-related peptide and its receptors in pain-related behavioral responses in the central nervous system. Neurosci Biobehav Rev 2009;33:1185–91. [DOI] [PubMed] [Google Scholar]
- [201].Yu LC, Weng XH, Wang JW, Lundeberg T. Involvement of calcitonin gene-related peptide and its receptor in anti-nociception in the periaqueductal grey of rats. Neurosci Lett 2003;349:1–4. [DOI] [PubMed] [Google Scholar]
- [202].Yu Y, Lundeberg T, Yu LC. Role of calcitonin gene-related peptide and its antagonist on the evoked discharge frequency of wide dynamic range neurons in the dorsal horn of the spinal cord in rats. Regul Pept 2002;103:23–7. [DOI] [PubMed] [Google Scholar]
- [203].Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics 2013;10:459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


