Abstract
Case summary
A case of cerebral phaeohyphomycosis caused by Cladophialophora bantiana is described in a 15-week-old domestic shorthair kitten.
Relevance and novel information
Cerebral phaeohyphomycosis is a rare condition in cats caused by dematiaceous fungi. This report describes the clinical and histopathological findings in the youngest case documented in a feline, provides a brief review of aetiology, diagnosis, treatment and prognosis of cerebral phaeohyphomycosis and demonstrates the importance of molecular diagnostics in accurate mycotic species identification.
Introduction
Phaeohyphomycosis is an infection caused by dematiaceous fungi, a group of fungi that have melanin pigment in their cell walls. Dematiaceous fungi are ubiquitous and found in soil worldwide.1 Taxonomy is confusing, with frequent name changes reflecting new information in this emerging area of mycoses. The agent in this case, Cladophialophora bantiana, has gone through several taxonomical changes in the past, previously named Cladosporium bantianum, Cladosporium trichoides and Xylohypha bantiana.
Several clinical manifestations of phaeohyphomycosis have been reported in cats, most frequently subcutaneous infections.2–14 Central nervous system (CNS),15–23 pulmonary,24 renal,25,26 nasal27 and disseminated28–30 infections have also been reported.
CNS phaeohyphomycosis is a rare condition with 11 reported cases in cats.15–23,28 Clinical signs of CNS phaeohyphomycosis depend on the location of the infection; they include seizures, vertical nystagmus, cranial nerve deficits, altered mentation, ataxia, head tilt and paresis. Thus far, the youngest reported cases of CNS phaeohyphomycosis in cats have involved 6-month-old cats.15,18 The following report is the youngest feline case of phaeohyphomycosis documented to date.
Case description
An approximately 13-week-old, male, entire, domestic shorthair kitten weighing 1.59 kg was surrendered to a Brisbane clinic. Physical examination was unremark-able. After a 1 week isolation period, the kitten was castrated, microchipped, treated for parasites, vaccinated and adopted by a member of the public.
Six days later the kitten presented to the clinic with a 1 day history of vomiting, anorexia and lethargy.
The kitten had lost 170 g since adoption and was subdued. Anisocoria was noted with a mydriatic right pupil. No other significant abnormalities were identified. The kitten was started on once-daily subcutaneous amoxicillin/clavulanate (Clavulox; Pfizer) injections at 8.75 mg/kg.
Results of haematological and biochemical testing, pre- and postprandial serum bile acid concentrations and in-house urinalysis are presented in Tables 1 and 2. Absolute lymphopenia and moderate monocytosis were identified. Testing for feline leukaemia virus antigen and feline immunodeficiency virus antibodies was negative (SNAP FIV/FeLV Combo Test; IDEXX).
Table 1.
Haematological, biochemistry, bile acid and endocrine test results
| Parameter | Result | Reference interval |
|---|---|---|
| Haematology | ||
| RBC (× 1012/l) | 8.2 | 4.9–10.0 |
| Haemoglobin (g/l) | 111 | 77–156 |
| Haematocrit (l/l) | 0.33 | 0.25–0.48 |
| Reticulocyte (%) | 0.1 | 0.0–0.4 |
| Reticulocyte ABS (× 109/l) | 8 | 3–50 |
| MCV (fl) | 40 | 43–55 |
| MCH (pg) | 14 | 13–17 |
| MCHC (g/l) | 336 | 282–333 |
| Platelet count (× 109/l) | 89 (clumped and adequate) | 300–800 |
| WBC (× 109/l) | 11.8 | 5.5–19.0 |
| Neutrophils (%) | 89 | – |
| Neutrophils (× 109/l) | 10.5 | 2.0–13.0 |
| Lymphocytes (%) | 0 | |
| Lymphocytes (× 109/l) | 0.0 | 0.9–7.0 |
| Monocytes (%) | 10 | |
| Monocytes (× 109/l) | 1.2 | 0.0–0.6 |
| Eosinophils (%) | 1 | |
| Basophils (%) | 0 | |
| Blood smear examination | Red cell and white cell morphology normal | |
| Biochemistry | ||
| Sodium (mmol/l) | 147 | 144–158 |
| Potassium (mmol/l) | 4.6 | 3.7–5.4 |
| Chloride (mmol/l) | 113 | 106–123 |
| Bicarbonate (mmol/l) | 17 | 12–24 |
| Na:K ratio | 32 | >29.0 |
| Anion gap (mmol/l) | 21.6 | 15.0–31.0 |
| Glucose, serum (mmol/l) | 5.1 | 3.2–7.5 |
| Urea (mmol/l) | 8.7 | 5.0–15.0 |
| Creatinine (mmol/l) | 0.07 | 0.08–0.20 |
| Calcium (mmol/l) | 2.3 | 2.1–2.8 |
| Phosphate (mmol/l) | 2.8 | 1.0–2.3 |
| Ca:P ratio | 0.8 | 1.1–2.3 |
| Protein, total (g/l) | 64 | 60–84 |
| Albumin (g/l) | 30 | 25–38 |
| Globulin (g/l) | 34 | 31–52 |
| A:G ratio | 0.9 | 0.5–1.1 |
| Bilirubin, total (µmol/l) | 4 | 0–7 |
| Alkaline phosphatase (IU/l) | 69 | 5–50 |
| Aspartate aminotransferase (IU/l) | 42 | 2–62 |
| Alanine transaminase (IU/l) | 111 | 19–100 |
| Creatinine kinase (IU/l) | 192 | 64–400 |
| Cholesterol (mmol/l) | 3.5 | 2.2–5.5 |
| Bile acids | ||
| Fasting (µmol/l) | 1 | <11 |
| Postprandial (µmol/l) | 1 | <21 |
| Endocrinology | ||
| T4 total (nmol/l) | 34 | 10–60 |
Entries in bold are outside the reference interval.
RBC = red blood cells; ABS = absolute; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; WBC = white blood cells; T4 = thyroxine
Table 2.
Results of in-house urinalysis
| Parameter | Result |
|---|---|
| Urine-specific gravity | 1.042 |
| pH | 6.0 |
| Protein | 1+ |
| Glucose | Negative |
| Ketones | Negative |
| Urobilinogen | 1+ |
| Bilirubin | Negative |
| Blood | Negative |
On day 3 of hospitalisation the kitten developed vertical nystagmus and ataxia, and was euthanased.
Necropsy demonstrated a 1.5 × 1.5 cm dark brown-to-black soft area that predominantly involved the left parietal lobe and extended across the longitudinal fissure to include a 0.5 × 0.5 cm region of the right parietal lobe of the cerebrum (Figure 1). On cut surface the lesion extended 1 cm into subcortical white matter. The left cerebral hemisphere was swollen with flattening and widening of gyri in comparison. The meninges were diffusely slightly reddened. No other gross lesions were identified on examination of the nasal cavity, sinuses, thoracic or abdominal cavities. Histopathology was performed on the brain.
Figure 1.
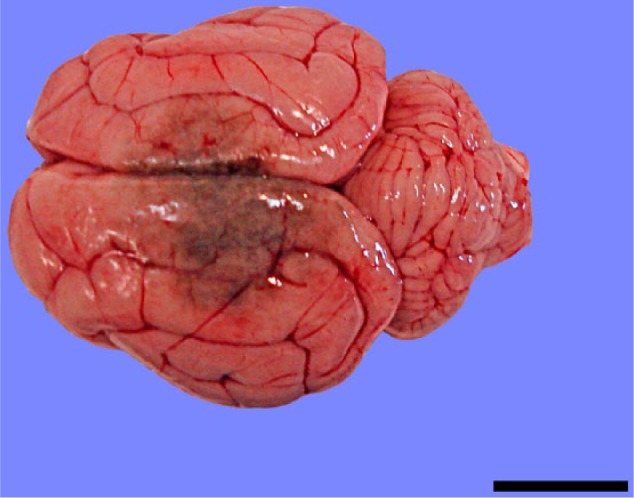
The meninges are reddened and the gyri are swollen and flattened, especially on the left side. There is a dark brown region, which spans the parietal lobes of both hemispheres. Bar = 1.5 cm
Histological examination (Figures 2 and 3) showed fulminating, focal, pyogranulomatous meningoencephalitis with intralesional pigmented fungus, consistent with phaeohyphomycosis. Leptomeninges and Virchow–Robin spaces were expanded by severe infiltrate of macrophages, lesser neutrophils, lymphocytes, multinucleate giant cells and occasional plasma cells. Leukocytes were often centred on golden-brown pigmented fungi, present extracellularly and within the cytoplasm of macrophages and multinucleate giant cells. Fungal hyphae were 2–5 µm in diameter, poorly septate, sparsely and non-dichotomously branched, and had thin, slightly bulbous walls. Conidiophores were 2–10 µm, oval, present in chains (pseudohyphae), and sometimes showed a thin, translucent capsule. In severely affected areas, leukocytes extended into the adjacent white or grey matter parenchyma. Such regions occasionally showed gliosis, gemistocytic astrocytes, rarefaction and malacia. Rare meningeal veins had fibrin thrombi, rarely containing fungal elements.
Figure 2.

The meninges (M) are markedly expanded by pyogranulomatous leukocyte infiltrate, which extends along Virchow–Robin spaces (arrows) and into the cortical grey matter (C). Haematoxylin and eosin. Bar = 250 μm
Figure 3.
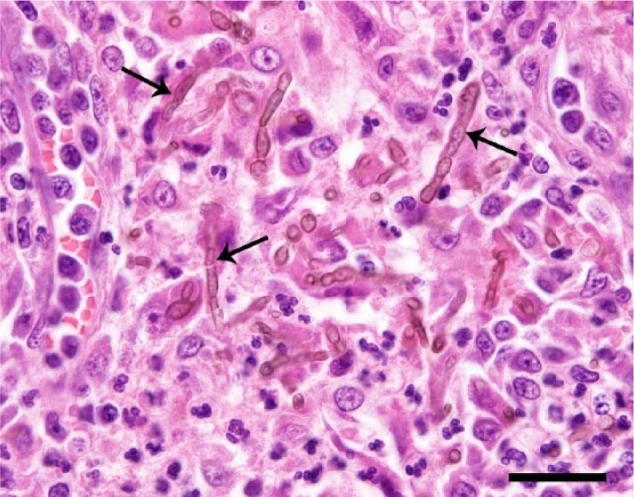
Leukocyte infiltrate predominantly consists of macrophages, and lesser multinucleate giant cells, neutrophils, lymphocytes and plasma cells. The leukocytes are centred on intra- and extracellular fungal hyphae and pseudohyphae (arrows). Haematoxylin and eosin. Bar = 25 μm
Brain tissue was plated on Sabouraud agar and incubated at 28°C for 28 days, until growth of an olivaceous-to-greyish-green woolly fungus was detected. The microscopic morphology showed dark septate hyphae with conidiophores producing long chains of oval conidia. The fungus was given a presumptive identification of Cladosporium species and referred for molecular identification.
DNA sequence analysis of the internal transcribed spacer (ITS) 1, 5.8S and ITS2 regions, and the D1/D2 region of the 28S (large subunit) ribosomal DNA gene cluster, using published primers and standard sequencing methodologies31 identified the fungus as C bantiana. The isolate’s sequence was compared with those in the ISHAM ITS database and the CBS database using the BLASTn search tool and showed >99.8% identity to MITS1144 and CBS118738, respectively.
Discussion
The most frequently isolated fungus in CNS phaeohyphomycosis in cats has been C bantiana;15,17,23,26 however, there have been single case reports of Verruconis gallopava,22 Phoma eupyrena16 and Exophiala jeanselmei.28 Similarly, in human beings Cladophialophora species are responsible for 48% of cases of CNS phaeohyphomycosis.32 C bantiana is regarded as being neurotropic;1,33,34 however, there have been two feline cases caused by C bantiana with renal26 and systemic29 involvement without CNS lesions.
This is the youngest feline case of phaeohyphomycosis documented to date. Previously, the youngest have both involved 6-month-old cats.15,18 While C bantiana is typically associated with chronic and slowly progressive infections in human35 and veterinary literature,36 it may also cause acute infections.33 Two reported cases of phaeohyphomycosis in human neonates, who developed clinical signs at 637 and 738 days old, respectively, after presumed postnatal infection, illustrate how rapidly clinical infection can develop.
The method of infection is incompletely understood and in most cases the route of entry is not identified. CNS infections are thought to occur via the respiratory route.1 Ocular23 and aural39 routes of entry have also been suggested. In this case no gross pulmonary, sinus, aural or skin wounds were identified, and the route of infection was undetermined. Given the strong localisation of the infection to the meninges and the presence of meningeal venous thrombi, meningeal haematogenous entry was considered most likely. Unfortunately, owing to cost constraints, in this case histopathology was only performed on the brain. Histopathology of other tissues such as the nasal cavity, sinuses, hilar lymph nodes, lung and gastrointestinal tract may have better helped define the route of entry and it is recommended that, where possible, gross and histopathological examination of all tissues is performed at necropsy in future cases.
Most feline cases have not identified an underlying cause; however, there have been cases associated with corticosteroid administration19 and lymphocytic leukaemia.26 Cerebral phaeohyphomycosis has also been described in a dog with persistent lymphopenia.40 Risk factors in humans include HIV infection, solid organ and bone marrow transplantation, corticosteroid therapy and trauma; however, over half of human patients do not have an underlying disease or risk factor identified.32 This reflects the ability of dematiaceous fungi to act as primary pathogens. In the present case absolute lymphopenia was identified. Possible explanations for this include stress leukogram, an infection associated, spurious laboratory result or a primary immunodeficiency such as severe combined immunodeficiency, which has been described in human neonates, foals, dogs and mice.41 The significance of the lymphopenia is unknown.
Standard haematological and biochemical testing in patients with CNS phaeohyphomycosis are generally within reference intervals. In this case monocytosis was considered to be a component of a stress leukogram. Other haematological and biochemical aberrations were considered unlikely to be of clinical significance.
There are no serological tests to diagnose C bantiana infection. CT and MRI may reveal changes consistent with intracranial abscessation, ependymitis or meningitis; however, diagnosis relies on biopsies and fungal culture.16,25 In the present case the culture isolate was initially incorrectly identified as Cladosporium species, despite Cladophialophora species’ distinctive microscopic morphology. This emphasises the important role of reference laboratories for accurately identifying these rare mycoses and the benefits of sequencing as the gold standard.
Only one feline case42 has been diagnosed ante-mortem; this was achieved by craniectomy subsequent to MRI. In dogs, diagnosis has been achieved ante-mortem by CT-guided biopsy43 and craniotomy with excisional biopsy following MRI.44 Cerebrospinal fluid (CSF) analysis is generally regarded as being non-specific,45 but may demonstrate CNS inflammation by increased protein or pleocytosis. In one human case fungal hyphae and conidia were observable cytologically in CSF, and culture grew C bantiana.37
There is no standard treatment for CNS phaeohyphomycosis and many different combinations of surgical and antifungal regimens have been reported. Response to therapy is unpredictable, and in vitro sensitivities may not correlate with in vivo results.38,46 Published experience even in the human field is too small to reach definitive conclusions about which antifungal agents are most effective, but a recent review showed treatment with combined itraconazole, flucytosine and amphotericin B to be most effective.32 In this study surgery was not associated with an improved outcome; however, cases where complete excision of brain abscesses was achieved had lower mortality rates than those who underwent therapeutic aspiration or partial excision. Prognosis was reported as poor, with overall mortality rates of 73% in humans, and 100% in untreated cases mortality rates were 100% (as referring to previous study in humans). Two veterinary cases with successful treatment have been reported; one in a dog44 and one a cat.42 Both were treated with surgical debulking and fluconazole initially, and the dog was changed to voriconazole long-term.
Conclusions
This case report describes a CNS infection with C bantiana in a kitten. Cerebral phaeohyphomycosis should not be excluded from the differential list of cats presenting with neurological signs, regardless of age, rapidity of clinical course or immune status. Ante-mortem diagnosis requires advanced imaging and biopsy in most cases, and correct identification to the species level may be aided by the use of molecular techniques. Despite high mortality rates in humans and cats, long-term survival is possible with aggressive treatment.
Acknowledgments
The authors thank colleagues at The Cat Clinic, Brisbane and QML Laboratories.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Reiss E, Shadomy HJ, Lyon GM. Phaeohyphomycosis. In: Mindy O-M. (ed). Fundamental medical mycology. Hoboken NJ: Wiley, 2011, pp 493–512. [Google Scholar]
- 2. McKenzie RA, Connole MD, McGinnis MR, et al. Subcutaneous phaeohyphomycosis caused by Moniliella suaveolens in two cats. Vet Pathol 1984; 21: 582–586. [DOI] [PubMed] [Google Scholar]
- 3. Dye C JE, Gruffydd-Jones TJ. Alternaria species infection in nine domestic cats. J Feline Med Surg 2009; 11: 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fondati A, Gallo GM, Romano E, et al. A case of feline phaeohyphomycosis due to Fonsecaea pedrosoi. Vet Dermatol 2001; 12: 297–301. [DOI] [PubMed] [Google Scholar]
- 5. McKay JS, Cox CL, Foster AP. Cutaneous alternariosis in a cat. J Small Anim Pract 2001; 42: 75–78. [DOI] [PubMed] [Google Scholar]
- 6. Overy DP, Martin C, Muckle A, et al. Cutaneous phaeohyphomycosis caused by Exophiala attenuata in a domestic cat. Mycopathologia 2015; 180: 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abramo F, Bastelli F, Nardoni S, et al. Feline cutaneous phaeohyphomycosis due to Cladophyalophora bantiana. J Feline Med Surg 2002; 4: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maeda H, Shibuya H, Yamaguchi Y, et al. Feline digital phaeohyphomycosis due to Exophiala jeanselmei. J Vet Med Sci 2008; 70: 1395–1397. [DOI] [PubMed] [Google Scholar]
- 9. Sisk DB, Chandler FW. Phaeohyphomycosis and cryptococcosis in a cat. Vet Pathol 1982; 19: 554–556. [DOI] [PubMed] [Google Scholar]
- 10. Beccati M, Vercelli A, Peano A, et al. Phaeohyphomycosis by Phialophora verrucosa: first European case in a cat. Vet Rec 2005; 157: 93–94. [DOI] [PubMed] [Google Scholar]
- 11. Knights CB, Lee K, Rycroft AN, et al. Phaeohyphomycosis caused by Ulocladium species in a cat. Vet Rec 2008; 162: 415–416. [DOI] [PubMed] [Google Scholar]
- 12. Roosje PJ, de Hoog GS, Koeman JP, et al. Phaeohyphomycosis in a cat caused by Alternaria infectoria E. G. Simmons. Mycoses 1993; 36: 451–454. [DOI] [PubMed] [Google Scholar]
- 13. Outerbridge CA, Myers SL, Summerbell RC. Phaeohyphomycosis in a cat. Can Vet J 1995; 36: 629–630. [PMC free article] [PubMed] [Google Scholar]
- 14. Hill JR, Migaki G, Phemister RD. Phaeomycotic granuloma in a cat. Vet Pathol 1978; 15: 559–561. [DOI] [PubMed] [Google Scholar]
- 15. Bouljihad M, Lindeman CJ, Hayden DW. Pyogranulomatous meningoencephalitis associated with dematiacous fungal (Cladophialophoroa bantiana) infection in a domestic cat. J Vet Diagn Invest 2002; 14: 70–72. [DOI] [PubMed] [Google Scholar]
- 16. Lapointe JM, Higgins RJ, Sturges B. Phaeohyphomycotic ependymitis in a cat. J Vet Diagn Invest 1998; 10: 202–204. [DOI] [PubMed] [Google Scholar]
- 17. Shinwari MW, Thomas AD, Orr JS. Feline cerebral phaeohyphomycosis associated with Cladosporium bantianum. Aust Vet J 1985; 62: 383–384. [DOI] [PubMed] [Google Scholar]
- 18. Julian AF. Cerebral phaeohyphomycosis in a Birman kitten. New Zealand Vet J 2004; 52: 50. [Google Scholar]
- 19. Foster SF, France MP, Malik R. Cerebral phaeohyphomycosis in a cat. Aust Vet Pract 1999; 29: 28–30. [Google Scholar]
- 20. Dillehay DL, Ribas JL, Newton JCJ, et al. Cerebral phaeohyphomycosis in two dogs and a cat. Vet Pathol 1987; 24: 192–194. [DOI] [PubMed] [Google Scholar]
- 21. Mariani CL, Platt SR, Scase TJ, et al. Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthair cats. J Am Anim Hosp Assoc 2002; 38: 225–230. [DOI] [PubMed] [Google Scholar]
- 22. Padhye AA, Amster RL, Browning M, et al. Fatal encephalitis caused by Ochroconis gallopavum in a domestic cat (Felis domesticus). J Med Vet Mycol 1994; 32: 141–145. [PubMed] [Google Scholar]
- 23. Jang SS, Biberstein EL, Rinaldi MG, et al. Feline brain abscesses due to Cladosporium trichoides. Med Mycol 1977; 15: 115. [PubMed] [Google Scholar]
- 24. Evans N, Gunew M, Marshall R, et al. Focal pulmonary granuloma caused by Cladophialophora bantiana in a domestic short haired cat. Med Mycol 2011; 49: 194–197. [DOI] [PubMed] [Google Scholar]
- 25. Coldrick O, Brannon CL, Kydd DM, et al. Fungal pyelonephritis due to Cladophialophora bantiana in a cat. Vet Rec 2007; 161: 724–727. [DOI] [PubMed] [Google Scholar]
- 26. Reed C, Fox JG, Campbell LH. Leukaemia in a cat with concurrent Cladosporium infection. J Small Anim Pract 1974; 15: 55. [DOI] [PubMed] [Google Scholar]
- 27. Kettlewell P, McGinnis MR, Wilkinson GT. Phaeohyphomycosis caused by Exophiala spinifera in two cats. J Med Vet Mycol 1989; 27: 257–264. [PubMed] [Google Scholar]
- 28. Helms SR, McLeod CG. Systemic Exophiala jeanselmei infection in a cat. J Am Vet Med Assoc 2000; 217: 1858–1861. [DOI] [PubMed] [Google Scholar]
- 29. Elies L, Balandraud V, Boulouha L, et al. Fatal systemic phaeohyphomycosis in a cat due to Cladophialophora bantiana. J Am Vet Med Assoc 2003; 50: 50–53. [DOI] [PubMed] [Google Scholar]
- 30. Zambelli AB, Griffiths CA. South African report of first case of chromoblastomycosis caused by Cladosporium (syn Cladophialophora) carrionii infection in a cat with feline immunodeficiency virus and lymphosarcoma. J Feline Med Surg 2015; 17: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White T, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, et al. (eds). PCR protocols: a guide to methods and applications. New York: Academic Press, 1990, pp 315–322. [Google Scholar]
- 32. Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 2004; 38: 206–216. [DOI] [PubMed] [Google Scholar]
- 33. Horre R, de Hoog GS. Primary cerebral infections by melanized fungi: a review. Stud Mycol 1999; 43: 176–193. [Google Scholar]
- 34. Perfect JR, Alexander BD, Schell WA. Phaeohyphomycoses (brown-black moulds). In: Kauffman CA, Pappas PG, Sobel JD, et al. (eds). Essentials of clinical mycology. 2nd ed. New York: Springer-Verlag, 2011, pp 305–317. [Google Scholar]
- 35. Sutton DA, Rinaldi MG, Sanche SE. Dematiaceous fungi. In: Anaissie EJ, McGinnis MR, Pfaller MA. (eds). Clinical mycology. 2nd ed. Edinburgh: Churchill Livingstone/Elsevier, 2009, pp 329–354. [Google Scholar]
- 36. Carter GR, Wise DJ. Subcutaneous and inoculation mycoses. Essentials of veterinary bacteriology and mycology. 6th ed. Ames, IA: Iowa State Press, 2004, p 264. [Google Scholar]
- 37. Banerjee TK, Patwari AK, Dutta R, et al. Cladosporium bantianum meningitis in a neonate. Indian J Pediatr 2002; 69: 721–723. [DOI] [PubMed] [Google Scholar]
- 38. Moore ML, Collins GR, Hawk BJ, et al. Disseminated Bipolaris spicifera in a neonate. J Perinatol 2001; 21: 399. [DOI] [PubMed] [Google Scholar]
- 39. Kwon-chung KJ, Bennett JE. Phaeohyphomycosis. In: Cann C. (ed). Medical mycology. Malvern, PA: Lea & Febiger, 1992, pp 620–677. [Google Scholar]
- 40. Migaki G, Casey HW, Bayles WB. Cerebral phaeohyphomycosis in a dog. J Am Vet Med Assoc 1987; 191: 997–998. [PubMed] [Google Scholar]
- 41. Bell TG, Butler KL, Sill HB, et al. Autosomal recessive severe combined immunodeficiency of Jack Russell terriers. J Vet Diagn Invest 2002; 14: 194–204. [DOI] [PubMed] [Google Scholar]
- 42. Lavely J, Lipsitz D. Fungal infections of the central nervous system in the dog and cat. Clin Tech Small Anim Pract 2005; 20: 212–219. [DOI] [PubMed] [Google Scholar]
- 43. Añor S, Sturges BK, Lafranco L, et al. Systemic phaeohyphomycosis (Cladophialophora bantiana) in a dog: clinical diagnosis with stereotactic computed tomographic-guided brain biopsy. J Vet Intern Med 2001; 15: 257–261. [DOI] [PubMed] [Google Scholar]
- 44. Bentley RT, Faissler D, Sutherland-Smith J. Successful management of an intracranial phaeohyphomycotic fungal granuloma in a dog. J Am Vet Med Assoc 2011; 239: 480–485. [DOI] [PubMed] [Google Scholar]
- 45. Richardson MD, Warnock DW. Phaeohyphomycosis. Fungal infection: diagnosis and management. 4th ed. Chichester: Wiley-Blackwell, 2012, pp 383–395. [Google Scholar]
- 46. Grooters AM, Foil CS. Miscellaneous fungal infections. In: Greene CE. (ed). Infectious diseases of the dog and the cat. 4th ed. St Louis, MO: Elsevier/Saunders, 2012, pp 685–688. [Google Scholar]


