Abstract
Background
HCMV phosphoprotein 65 (HCMVpp65) is a putative immunogen that acts as an accelerator, inducing autoantibody and exacerbating autoimmune response in susceptible animals. The immunity to pp65336-439 instigates autoimmunity, suggesting that pp65336-439 contains crucial B cell epitope(s) for the development of nephritis. This study narrowed down the target epitope to pp65422-439 for immunization of BALB/c mice and mapping of B cell epitope.
Methods
The target epitope pp65422-439 reactivity and B cell epitope mapping was examined in serum from pp65422-439-immunized mice and patients with systemic lupus erythematosus (SLE). Kidney tissue from immunized mice was examined for signs of immune complex nephritis.
Results
Anti-pp65422-439 antibody in serum either from patients with SLE or from pp65422-439-immunized mice exhibited cross-reactivity to several nuclear components such as double-stranded DNA (dsDNA). Moreover, the pp65422-439-immunized mice developed initial signs of glomerulonephritis such as deposition of immunoglobulin G/M (IgG/IgM) and third complement component (C3). With B cell epitope mapping by pp65422-439-derived decapeptides, one dominant epitope, pp65428-437, was identified in serum from pp65422-439-immunized mice and patients with SLE with anti-pp65422-439 antibody. Epitope spreading from pp65428-437 to pp65430-439 was found in pp65422-439-immunized mice in which we generated monoclonal antibodies to pp65425-434 and pp65430-439. However, dsDNA positive reactivity was exclusively observed in Crithidia luciliae stains with pp65430-439-reactive monoclonal antibody. Additionally, we observed the amelioration of autoimmunity following the elevation of IgM targeting pp65428-437.
Conclusions
Our data suggest that pp65428-437 may be an autoimmune or lupus-prone B cell epitope and may catalyze further epitope spreading for inducing autoantibodies in lupus-susceptible individuals.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-017-1268-2) contains supplementary material, which is available to authorized users.
Keywords: Systemic lupus erythematosus, Human cytomegalovirus phosphoprotein 65, Glomerulonephritis, Anti-dsDNA antibody
Background
Systemic lupus erythematous (SLE) is a chronic autoimmune disease characterized by widespread loss of immune tolerance to self-antigens. Pathogen recognition and subsequent immune responses are potentially the important initiators of autoimmunity in genetically predisposed persons. Emerging evidence indicates that in patients with lupus, exposure to human cytomegalovirus (HCMV) or Epstein-Barr virus (EBV), often precedes the onset of tolerance break [1–3]. EBV is the most studied example for cross-reactive autoantibody-mediated autoimmunity. Cross-reactivity of anti-Epstein Barr virus antigen-1 (EBNA-1) antibody to Ro or spliceosomal proteins has been reported [4–6]. Anti-Sm antibody has been found to cross-react in EBNA-1-immunized animals, underlying the molecular mimicry between these antigens [7–10].
HCMV, a ubiquitous opportunistic pathogen, induces 60 kD/Ro expression on the surface of human keratinocytes [11]. Immunization of lupus-prone mice by HCMV recombinant glycoprotein B (gB) results in the production of significant autoantibody to the U1-70 kDa spliceosome protein [12]. Also, the significant correlation between antibody to HCMV and U1 small nuclear ribonucleoprotein (snRNP) in HCMV-infected patients with SLE implies that HCMV infection is associated with the development of SLE [13]. In addition, immunization of BALB/c mice with a surrogate octapeptide, DWEYSVWLSN, which induces anti-dsDNA antibody, suggests that the shared structural similarity of antigenic determinants among pathogens and self-proteins leads to autoantibody production [14]. The DNA-interacting amino acids of necrotic cells from post-infected hosts may contribute to induction of anti-dsDNA antibodies [15].
HCMV phosphoprotein 65 (pp65) is a viral scaffold protein and the most abundant constituent of the extracellular viral particle [16]. The pp65 is involved in modulating viral kinase activity and attenuating host antiviral responses [17, 18]. The pp65 protein is a target of both cellular and humoral immunity in healthy individuals, but dominant T cell epitope(s) leads to the robust cellular responses such as cytotoxic T lymphocyte response [19, 20]. Highly elevated anti-pp65 titers in patients with SLE and immunization of NZB/W F1 mice by pp65 induces early onset of lupus-like symptoms, implying a potential role of pp65 in SLE [21].
The immunization of truncated pp65336-439-conjugated C3d has been shown to induce lupus-like autoantibodies and subsequent development of autoimmunity [22]. The current study aims to further identify the autoantibody-inducing B cell epitope(s) within pp65386-439 and the potential pathogenic immune response.
Methods
Characteristics of the study populations
All patients were recruited from the clinics of Chang Gung Memorial Hospital, and rheumatology specialists confirmed that all patients fulfilled the 1982 and 1997 American College of Rheumatology (ACR) diagnostic criteria for SLE [23, 24] This study was approved by the Institutional Review Board of Chang Gung Medical Foundation. The study of methods was carried out in accordance with the relevant guidelines and informed consent was obtained from all subjects.
Mice
Normal female BALB/c mice, 3–5 weeks old, were purchased from the National Laboratory Animal Center (NLAC), Taiwan. Animals were housed in a pathogen-free facility with an independent ventilation cage system at the laboratory animal center of Chang Gung Memorial Hospital. All BALB/c mice were 8 weeks old at inoculation.
Synthetic peptides
For all synthetic peptides, the purity of the peptide was >95%, per the peptide manufacturer (GenScript, NJ, USA). The preparation of peptides followed the manufacturer’s instructions (20 μg/μl), with storage at -80 °C prior to use. Six histidines and one cysteine were added at the C terminus of the peptide as a target or for crosslinking to a carrier protein via a disulfide bond.
Plasmid construction
The full-length pp65 sequence was amplified from pCMV6-pp65 (SKU VC101263, Origene, FJ527563) using the following paired primers (forward 5′GCGGATATCATGGAGAGCCGGGGCCGG, reverse 5′ GCGGGATCCGCCTCTATGCTTCTTGGG). The pp65 sequence was prepared from PCR and digested by EcoRV/BamHI, then ligated into pET30. The murine C3d encoding sequence (GenBank: DQ408205) was PCR-amplified with C3d primers (forward 5′CGCGGATCCATGACCCCCGCAGGCTGTGGG, reverse 5′CGCGCTCGAGGCTACGGCTGGGGAGGTG) and ligated into pET30.
Antigen preparation
The C3d biotinylation (Pierce, Thermo Scientific, IL, USA) and streptavidin (SA) (Pierce) conjugation were performed as per the manufacturers’ protocol. In brief, maleimide-activated streptavidin (Pierce) was conjugated with peptide containing reduced disulfide bonds from a disulfide reducing gel (Pierce) and mixed with biotinylated C3d to form the peptide-SA-biotin-C3d tetramer, including pp65386-403, pp65422-439 and SA-C3d only. Tetramers were generated and prepared for immunization within 4 hours.
Immunization and serum collection
Female BALB/c mice (n = 28) were randomly separated into groups receiving pp65386-403- (n = 9), pp65422-439-C3d (n = 9), SA-C3d (n = 5) or PBS (n = 5). Mice were inoculated subcutaneously with 100 μg (2 μg/μl) pp65386-403-C3d, pp65422-439-C3d, SA-C3d or 50 μl PBS in complete Freund’s adjuvant (CFA, Sigma Aldrich, MO, USA) at day 0, respectively. Boosting was performed with antigens in incomplete Freund’s adjuvant (IFA, Sigma Aldrich) at day 14, day 28 and day 42. Mice were bled via the retro orbital vein one day prior to each assay and at 2-week intervals. Unused serum was stored at -80 °C and the PBS-diluted seruma was kept at 4 °C.
Antibody preparation, biotinylation and streptavidin conjugation
Recombinant proteins were over-expressed in Escherichia coli with 1 mM isopropyl β-D-thiogalactoside induction (IPTG, Sigma Aldrich) and purified by a nickel affinity column (Sigma Aldrich). Antibody preparation was performed as previously described [22]. In brief, moderated cyanogen bromide (CnBr) powder (Sigma Aldrich) was activated following the manufacturer’s protocol. A total of 2 mg of four tandem repeats of the pp65422-439 peptides (GGGSGGGAMAGASTSAGRKRKS) was dissolved by gentle rotation in a coupling buffer (0.1 M NaHCO3, 0.5 M NaCl, pH 8.3) with activated CnBr gel at 4 °C overnight. The free active groups on CnBr were deactivated by 0.1 M Tris-HCl (pH 8.0) at room temperature (RT) for 2 hours. After deactivation, CnBr gel was washed with alternating buffer (0.1 M NaAc, 0.5 M NaCl, pH 4.0 and 0.1 M Tris-HCl, 0.5 M NaCl, pH 8.0) twice and washed with 10 ml PBS once. For purification, 10 ml of serum from twenty dsDNA-positive or dsDNA-negative patients with SLE with pp65422-439 antibody in 20 ml PBS, respectively, were added to pp65422-439-conjugated CnBr gel and rolled at 4 °C overnight. The flow-through was collected and concentrated as a negative control, while bound antibodies were eluted by 1 ml of 0.1 M glycine (pH 2.0). The eluted samples were neutralized immediately with 30 μl of neutralizing buffer (1 M Tris-HCl, 2 M NaCl, pH 8.8).
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as previous described [22]. Briefly, for the anti-pp65 peptide (pp65386-439, pp65386-403, pp65396-413, pp65404-421, pp65414-431, pp65422-439 and nine pp65422-439-derived decapeptides) or anti-dsDNA antibody assay, 1 μg/well of synthetic peptide or purified calf thymus dsDNA (Sigma Aldrich) in coating buffer (150 mM Na2CO3, 150 mM NaHCO3, pH 9.6) was coated to a microtiter 96-well plate (Greiner Bio-One, CA, USA) at 4 °C overnight. After blocking with 5% skimmed milk, 250× diluted human or mice serum, 3 μg purified pp65422-439 antibody or 1 μg monoclonal antibodies in PBS were added and incubated at 37 °C for 2 hours.
For the competitive inhibition assay, anti-pp65422-439 purified antibody was co-incubated with 1 μg pp65422-439 or dsDNA in 200 μl PBS at RT for one hour. The mixture was transferred to one well of a 96-well plate coated with dsDNA or pp65422-439 for incubation at 37 °C for 2 hours. At the end of the incubation, the microtiter plate was washed four times with PBST (PBS with 0.05% Tween 20) and bound antibody was detected by horseradish peroxidase (HRP)-conjugated anti-human/mouse G/M or anti-mouse IgG subclasses (IgG1, IgG2a, IgG2b and IgG3) at a dilution of 1:5000 (Jackson ImmunoResearch Laboratories, PA, USA) at 37 °C for 2 hours. For detection of cross-reactivity to host proteins, 1 μg/well of homogenized HEK293T cell lysate was coated on a microtiter plate at 4 °C overnight. After blocking, mice serum was diluted and bound antibodies were detected as described above. O-phenylenediamine dihydrochloride (OPD, Sigma Aldrich) was used as the substrate in ELISA buffer (250 mM Na2HPO4, 175 mM C6H8O7, pH 5.0) and HRP activity was read at 450 nm with a micro ELISA reader (Molecular Devices).
Western blot/slot blot
Full-length pp65 protein (40 μg/per gel) was separated by 12% SDS-PAGE (slab gel format). Separated protein was transferred to nitrocellulose paper, blocked by 5% skimmed milk and then analyzed with 1 μg/ml anti-His-tag antibody (eBioscience, CA. USA), 100× diluted human sera or 3 μg purified pp65422-439 antibody in PBS at RT for 2 hours. Antibody reactivity was detected by HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) and chemiluminescent detection reagent (Millipore, MA. USA).
Anti-nuclear antibodies, C. luciliae and kidney immunofluorescence stain
Mouse serum was tested for anti-nuclear antibodies (ANAs) at 1:100 dilutions in PBS using a standard anti-nuclear antibody (ANA) test (Diasorin, Saluggia, Italy). The reactivity of anti-dsDNA antibody was examined by immunofluorescence stain using the C. luciliae test (Diasorin) at dilutions of 1:20, 1:40 and 1:80 in PBS, as per manufacturer’s instruction. In brief, 30 μl of diluted mice serum, 3 μg purified pp65422-439 antibody or 1 μg monoclonal antibodies were incubated on a slide coated with HEp-2 or C. luciliae at RT for 30 minutes in a humidified chamber. Slides were washed three times in 50 ml PBS at RT for 10 minutes each.
Bound antibodies were detected by 100× diluted FITC-conjugated anti-mouse IgG/M (Jackson ImmunoResearch Laboratories) at RT for 30 minutes in a darkened and humidified chamber. For nuclear visualization, the HEp-2 slide was incubated in 30 μl of 4',6-diamidino-2-phenylindole (DAPI) (1 mg/ml, Sigma Aldrich) at RT for 5 minutes in the dark. At the end of staining, slides were washed with PBS for 30 seconds and mounted via mounting medium (Diasorin) for investigation by fluorescence microscopy (Olympus DP72). For immunofluorescence staining of the glomerulus, kidneys were removed from the mice, immediately placed in optimal cutting temperature (OCT) gel and frozen at -80 °C for 24 hours. The 5-μm-thick frozen sections were stained with FITC-conjugated anti-mouse IgM/G (Jackson ImmunoResearch Laboratories) at a 1:100 dilution in PBS at RT for 30 minutes in a humidified chamber in the dark. After PBS washing, coverslips with mounting medium (Diasorin) on tissue slides were prepared for investigation by fluorescence microscopy.
Hybridoma preparation
The hybridoma was prepared following the manufacturer’s instructions (Roche, Basel, Switzerland) with minor modifications. Briefly, the mouse spleen cells were mixed at a ratio to Sp2/0-Ag14 of 5:1 (ATCC, VA, USA) in a sterile 50-ml conical tube, which was centrifuged to pellet the cells at 800 rpm for 10 minutes. After discarding the supernatant, 1 ml of 50% PEG 1500 (Roche) was slowly added to the cell pellet dropwise over a 1-minute period and the cells were swirled for 90 seconds in a 37 °C water bath. Cell fusion was stopped by adding Roswell Park Memorial Institute medium (RPMI) 1640 (Gibco, CA, USA) containing 10% fetal bovine serum (FBS, Invitrogen, CA, USA) with gentle swirling at RT for 10 minutes. After washing with RPMI 1640 twice, cells were suspended in 30 ml of RPMI1640 supplemented with 10% FBS, 10% BM Condimed H1 (Roche) and 1x HAT (Gibco), plated 2.5 ml per well in a 6-well culture dish and incubated at 37 °C in a 5% CO2 incubator. Limiting dilution was carried out for selection of a single colony, which was amplified in RPMI 1640 supplemented with 10% FBS, 10% BM Condimed H1 (Roche), 1× HT (Gibco) and 1x hybridoma fusion & cloning supplement (Roche). The supernatant was harvested for ELISA of antibody activity to pp65422-439.
Statistical analysis
Statistical differences in titer and prevalence were analyzed using GraphPad Prism (GraphPad Software Inc.). The Student t test, two-tailed Fisher’s test, and Mann-Whitney test were used for these comparisons with graphs depicting mean ± SEM. A 5% level of significance for p values was used for all analyses.
Results
Elevated anti-pp65422-439 reactivity related to dsDNA positivity in SLE
The fragment of pp66336-439 induced autoantibodies and immunoglobulin (Ig) deposition on glomeruli in BALB/c mice has been reported [22]. Due to a poor humoral response to pp65336-385 in patients with SLE, we examined anti-pp65386-439 reactivity to reveal the critical B cell epitopes using serum from 238 patients with SLE (119 with and 119 without anti-dsDNA reactivity), 86 patients with ankylosing spondylitis (AS), 78 patients with rheumatoid arthritis (RA) and 84 healthy controls. As shown in Table 1, 83 of 238 patients with SLE (34.87%) had higher incidence of antibody reactivity to pp65386-439 compared to the 1/86 patients with AS (1.16%), 4/78 patients with RA (5.28%) or 1/84 normal controls (1.20%). Of the 119 Patients with SLE with anti-dsDNA positive serum (termed SLE-dsDNA(+)), 52/119 patients were also positive for anti-pp65386-439 activity (43.70%). On the other hand, 31/119 patients with SLE with anti-dsDNA negative sera (termed as SLE-dsDNA(-)) (26.05%) were anti-pp65386-439 positive. Human serum positive for pp65386-439 was reconfirmed by western blotting (Additional file 1: Figure S1).
Table 1.
The prevalence of antibody to HCMVpp65386-439 in patients with autoimmunity and healthy controls
| SLE-dsDNA(+) | SLE-dsDNA(-) | AS | RA | Normal | ||
|---|---|---|---|---|---|---|
| Age (years) | 16-77 | 22-75 | 15-66 | 20-89 | 32-64 | |
| Mean (years) | 39.5 | 35.2 | 37.1 | 56.2 | 43.2 | |
| Total specimen | 119 | 119 | 86 | 78 | 84 | |
| Female (%) | 100 | 100 | 18 | 82 | 100 | |
| Responsiveness | pp65386 to 439 (%) | 52/119 | 31/119 | 1/86 (1.16) | 4/78 (5.13) | 1/84 (1.19) |
| (43.70) | (26.05) |
RA: rheumatoid arthritis; AS: ankylosing spondylitis
Next, we synthesized pp65386-403, pp65404-421 and pp65422-439, covering the entire pp65386-439, and re-screened serum from patients with SLE and healthy controls using ELISA to identify the dominant epitope(s). As shown in Fig. 1, in the SLE subgroups there was significant elevation of IgG antibody to pp65422-439 (SLE-dsDNA(+) 0.254 ± 0.014, p < 0.001; SLE-dsDNA(-), 0.186 ± 0.009, p = 0.026) and pp65404-421 (SLE-dsDNA(+), 0.180 ± 0.011, p = 0.004; SLE-dsDNA(-), 0.151 ± 0.008) compared to normal controls (0.156 ± 0.009; 0.144 ± 0.005). The IgG antibody titer for pp65422-439 was greater than that for pp65404-421 in the SLE subgroups (SLE-dsDNA(+), p < 0.001; SLE-dsDNA(-), p = 0.003). Moreover, SLE-dsDNA(+) had greater anti-pp65422-439 reactivity than SLE-dsDNA(-) (p < 0.001).
Fig. 1.
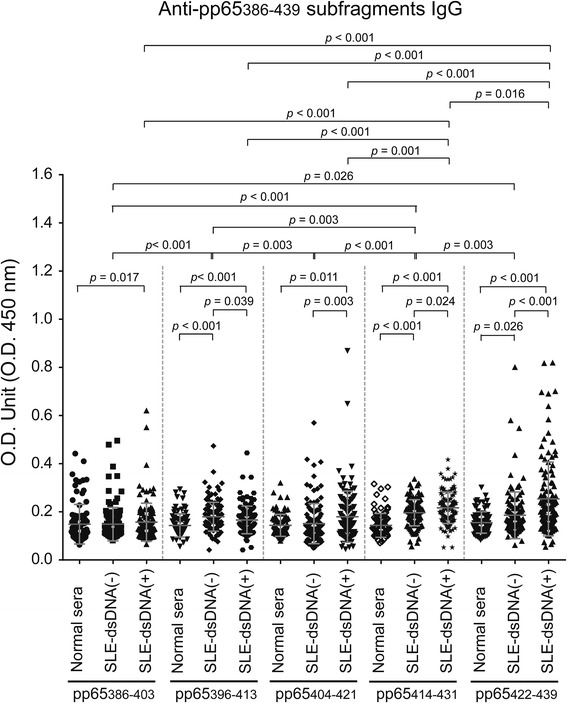
Detection of IgG antibody against pp65422-439 subfragments by serum from patients with systemic lupus erythematosus (SLE) and healthy controls. ELISA was performed for IgG against five pp65 subfragments, pp65386-403, pp65396-413, pp65404-421, pp65414-431 and pp65422-439, using serum from patients with SLE with or without anti-double-stranded DNA antibody (SLE-dsDNA(+), n = 119; SLE-dsDNA(-), n = 119) and normal controls (n = 84). 250× diluted sera were used. Data are presented as the mean ± SEM of three independent experiments. O.D. optical density
In further epitopes analysis, antibody against pp65422-439 was significantly elevated in patients with SLE-dsDNA(+) compared to pp65396-413 (0.163 ± 0.005, p < 0.001) or pp65414-431 (0.180 ± 0.01, p = 0.016). On the other hand, we were unable to purify anti-pp65422-439 antibody using CnBr conjugated with four tandem repeated pp65422-439 peptide from disease or healthy controls, suggesting that the lower titer of anti-pp65422-439 antibody is unavailable for purification. Together, these findings suggested anti-pp65422-439 reactivity is specific to patients with SLE and related to dsDNA positivity.
Anti-pp65422-439 antibody showed cross-reactivity to nuclear proteins and dsDNA
To elucidate the relationship between pp65422-439 and autoantibodies developed in patients with SLE, antibodies to pp65422-439 were affinity-purified in pooled serum from SLE-dsDNA(+) or SLE-dsDNA(-). The anti-pp65422-439 antibodies from both SLE subgroups exhibited anti-pp65 activities (Fig. 2a). The purified anti-pp65422-439 antibodies could be inhibited by pp65422-439 or partially inhibited by dsDNA in both SLE groups (Fig. 2b). Also, there was cross-reactivity of anti-pp65422-439 antibody to dsNDA suppressed by pp65422-439 or dsDNA in the two SLE groups (Fig. 2c). Notably, the titer of anti-dsDNA antibody was significantly higher in SLE-dsDNA(+) (0.833 ± 0.056) than in SLE-dsDNA(-) (0.418 ± 0.037, p = 0.004; Fig. 2c). However, the anti-dsDNA activity was solely exhibited by C. luciliae stain with anti-pp65422-439 antibody from SLE-dsDNA(+) (Fig. 2d). Indirect immunofluorescence stain on purified serum from patients with SLE revealed clear speckle stains (Figs. 2e1, e4). Notably, stain from nucleosome/chromatin is unique to pp65422-439 specific antibody from SLE-dsDNA(+) (Fig. 2e2, e5). In contrast, no nuclear responses were observed in normal serum stain (Fig. 2e3, e6).
Fig. 2.
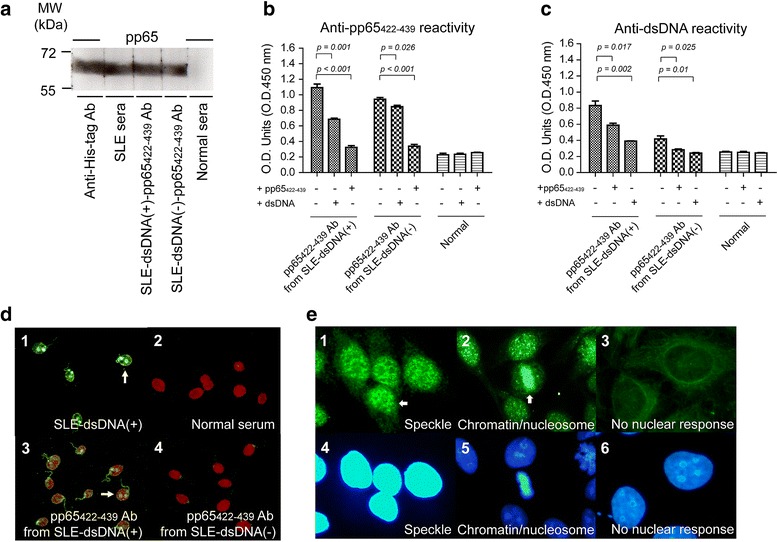
Detection of cross-reactivity in affinity-purified pp65422-439-specific IgG in serum from patients with systemic lupus erythematosus (SLE). a Immunoblot analysis with serum from patients with SLE, healthy controls and anti-pp65422-439 antibody from ten pooled SLE-double-stranded DNA (SLE-dsDNA(+) or SLE-dsDNA(-)) serum against human cytomegalovirus (HCMV)pp65. We used 100× diluted serum from patients with SLE or healthy controls and anti-His-tag antibody as positive and negative controls. Molecular mass markers (kD) are shown on the left. MW molecular weight, kDa kilodalton. b-c ELISA for anti-pp65422-439 and anti-dsDNA activity: 100× diluted normal serum and 3 μg anti-pp65422-439 antibody from SLE-dsDNA(+) or SLE-dsDNA(-) sera were used. For the competitive inhibitory assay, 1 μg/well of pp65422-439 or dsDNA was used. d Representatives of C. luciliae stain by 100× diluted serum from SLE-dsDNA(+) (d1) or healthy control and anti-pp65422-439 antibody (d2) from SLE-dsDNA(+) (d3) or SLE-dsDNA(-) (d4) serum. White arrowheads indicate the positive stains. e HEp-2 substrate slides were used for detection of anti-nuclear antibodies. Patterns of speckle (e4) and nucleosome/chromatin (e2, e5) stains were revealed by anti-pp65422-439 antibody from SLE-dsDNA(+) or SLE-dsDNA(-) serum. Nuclear reactivity was not observed from 100× diluted normal serum (e3, e6). White arrowheads indicate the patterns of nuclear response. These results are representative of triplicated experiments. O.D. optical density
The pp65422-439 immunization induced cross-reactive antibodies to nuclear components
To evaluate the induction of autoantibodies following exposure of pp65422-439, BALB/c mice were immunized with pp65422-439, pp65386-403, streptavidin (SA) or PBS, using mouse C3d as a molecular adjuvant to improve the immunogenicity of these peptides through CR2-C3d interaction [22, 25]. Both pp65422-439 and pp65386-403 immunization induced anti-pp65386-439-reactive IgG at 4 weeks post immunization and continued until the completion of the observation period (14 weeks post immunization). Quasi-quantitative analysis showed that the pp65422-439 induced twice as much of pp65386-439-specific antibody titers than pp65386-403. The anti-pp65386-439 reactive IgG was not detected from either SA-C3d or PBS immunized mice (Fig. 3a and Additional file 2: Figure S2).
Fig. 3.
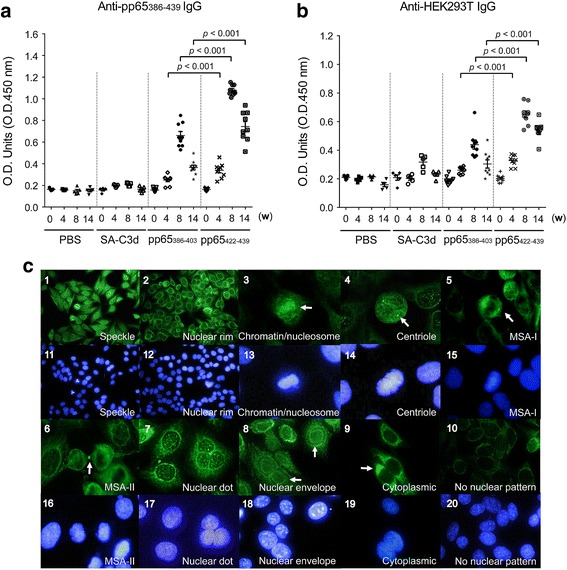
Detection of anti-pp65 and anti-nuclear reactivity from pp65386-403, pp65422-439, streptavidin-complement C3 (SA-C3d) or PBS-immunized serum. The IgG against pp65386-439 and HEK293T extract from pp65386-403 (n = 9), pp65422-439 (n = 9), SA-C3d (n = 5) or PBS (n = 5)-immunized serum at 0, 4, 8 and 14 weeks post immunization were performed at 1:250 dilution. a ELISA for anti-pp65386-403 and anti-pp65422-439 reactivity against pp65386-439 peptide. b ELISA for anti-HEK293T reactivity against total HEK293T lysate. c HEp-2 substrate slides were used for detection of anti-nuclear antibodies. Serum 8 weeks post immunization was 100× diluted for anti-nuclear antibodies (ANA) stain. Nuclear patterns of speckle (b1, b11), nuclear rim (b2, b12), chromatin/nucleosome (b3, b13), centriole (b4, b14), mitotic spindle type I (MSA-I) (b5, b15), MSA-II (b6, b16), nuclear dot (b7, b17) and nuclear envelope (b8, b18) were revealed in serum from pp65422-439-immunized mice. Cytoplasmic response (b9, b19) was detected in serum from pp65386-403-immunized and pp65422-439-immunized mice. Nuclear reactivity was not found in PBS, SA-C3d or pp65386-403-immunized mice (b10, b20). White arrowheads indicate the patterns of nuclear or cytoplasmic response. Data are presented as the mean ± SEM of three independent experiments. w weeks post immunization, O.D. optical density
The serum from pp65 epitope immunization was tested against HEK293T cell lysate. The immunization of pp65422-439 or pp65386-403 elicited anti-HEK293T IgG, which was first detected at 6 weeks (data not shown), peaked at 8 weeks and was sustained until 14 weeks post immunization. The pp65422-439 immunization (0.667 ± 0.027) induced higher titers than pp65386-403 immunization (0.447 ± 0.034, p < 0.001) at 8 and 14 weeks post immunization (Fig. 3b). Multiple ANA patterns can be identified following immunization of pp65422-439 (Fig. 3c) including speckle (Fig. 3c1, c11), nuclear rim (Fig. 3c2, c12), chromatin/nucleosome (Fig. 3c3, c13), centrioles (Fig. 3c4, c14), MSA I (Fig. 3c5, c15), MSA II (Fig. 3c6, c16), nuclear dots (Fig. 3c7, c17), nuclear envelope (Fig. 3c8, c18) and cytoplasmic proteins stains (Fig. 3c9, c19) at 1:100 dilution 8 weeks post immunization (Additional file 3: Table S1). Immunization with pp65386-403 induced a pattern of nuclear dots but it was only detected at 1:40 dilution (data not shown). Nuclear staining was not observed in control mice at 1:40 or higher dilution (Fig. 3c10, c20). Antibodies against cytoplasmic components were found in seven out of nine pp65422-439-immunized mice (78%) and three out of nine pp65386-403-immunized mice (33%) at 1:100 dilution.
Immunization of pp65422-439 induced high titers of anti-dsDNA antibodies.
Anti-dsDNA antibody is pathognomonic for SLE. To verify the cross-reactivity of anti-pp65422-439 antibodies to dsDNA, anti-dsDNA reactivity from immunized animals was tested by both anti-dsDNA ELISA and C. luciliae assays. In the ELISA, the pp65422-439 immunized mice exhibited significantly higher titers of IgG to dsDNA compared to pp65386-403 immunization (Fig. 4a). The anti-dsDNA titers were not elevated in the remaining groups. Isotype analysis of pp65422-439 immunization showed that the enhancement of anti-dsDNA is likely contributed by IgG1 (dsDNA(+) vs. dsDNA (-), 0.44 ± 0.05 vs. 0.32 ± 0.02, p = 0.049) and IgG3 (dsDNA(+) vs. dsDNA (-), 0.295 ± 0.01 vs. 0.217 ± 0.03, p = 0.043) (Fig. 4b). In the C. luciliae stain, three serial dilutions, 1:20, 1:40 and 1:80 were used (Fig. 4c and Table 2). At the lowest dilution (1:20), anti-dsDNA activities were detected from all groups.
Fig. 4.
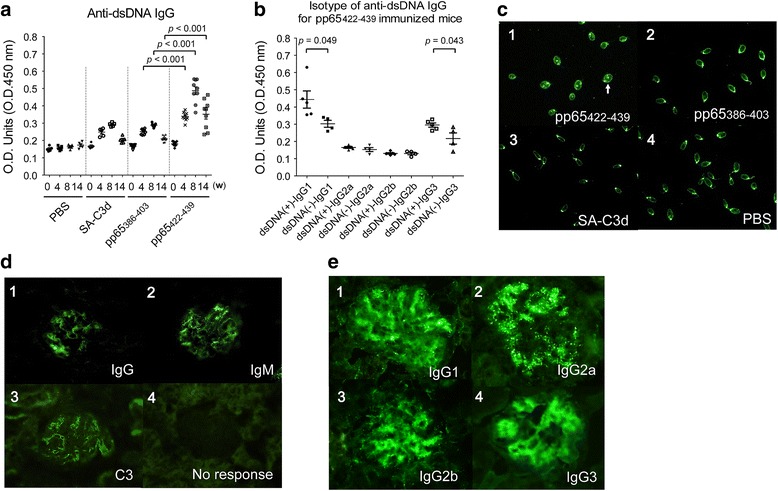
Detection of serum anti-double-stranded DNA (anti-dsDNA) antibody and immunoglobulin deposition in glomeruli from immunized mice. a ELISA for anti-dsDNA activity with serum at 1:250 dilution. b Isotyping of anti-dsDNA antibody for pp65422-439 immunized mice (n = 9) at 8 weeks post immunization. Serum was used at 1:250 dilution. dsDNA(+) seropositive for dsDNA, dsDNA(-) seronegative for dsDNA. c Representatives of C. luciliae stain by serum from pp65422-439 (c1), pp65386-403 (c2), streptavidin-complement 3d (SA-C3d) (c3) or PBS (c4) immunized animals at 8 weeks post immunization at 1:80 dilution. White arrowheads indicate dsDNA-positive stains. d Kidney sections from pp65422-439- immunized mice were stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (d1), IgM (d2) and C3 (d3). Immunoglobulin deposition was not observed in glomeruli from SA-C3d-immunized (d4) or PBS-immunized mice. e Kidney sections from pp65422-439-immunized mice were stained with FITC-conjugated anti-mouse IgG1 (e1), IgG2a (e2) IgG2b (e3) and IgG3 (e4). Kidneys were collected at 22 weeks of mice age. Data are presented as the mean ± SEM of three independent experiments. w weeks post immunization, O.D. optical density
Table 2.
Summary of anti-dsDNA activity in immunized mice
| Weeks post immunization | PBS n=5 | SA-C3d n=5 | pp65386-403 n=9 | pp65422-439 n=9 |
|---|---|---|---|---|
| 4 | 2w/5, 2w/5, 0/5 | 3w/5, 3w/5, 0/5 | 3(2,1w)/9, 3(1,2w)/9, 0/9 | 8/9, 8(5,3w)/9, 5(4,1w)/9 |
| 8 | 0/5, 0/5, 0/5 | 0/5, 0/5, 0/5 | 2(1,1w)/9, 1w/9, 0/9 | 9(8,1w)/9, 6(5,1w)/9, 5/9 |
| 14 | 0/5, 0/5, 0/5 | 0/5, 0/5, 0/5 | 0/9, 0/9, 0/9 | 4(2,2w)/9, 3(2,1w)/9, 2/9 |
Mice sera were used at dilution of 1:20, 1:40 or 1:80. W: weak response
The anti-dsDNA activities from controls and pp65386-403 gradually disappeared during serial dilutions to 1:40 or 1:80. In contrast, after pp65422-439 immunization, 8/9 and 5/9 positive rates were detected at 4 weeks at 1:40 and 1:80 dilutions, respectively. None of the animals with PBS or SA-C3d demonstrated any anti-dsDNA activity at 8 weeks post immunization. One animal with pp65386-403 immunization had activity to dsDNA at 1:20 and 1:40 dilutions but this disappeared at 1:80 dilution. In pp65422-439 immunization, 9/9, 6/9 and 5/9 anti-dsDNA serum was identified at 1:20, 1:40 and 1:80 dilutions, respectively. At 14 weeks post immunization, all animals except those with pp65422-439 immunization had negative dsDNA reactivity. The dsDNA reactivity of pp65422-439 immunization at 14 weeks post immunization was reduced to 4/9, 3/9 and 2/9 at 1:20, 1:40 and 1:80 dilutions, respectively.
To study the pathogenicity of pp65422-439 reactive antibodies, kidney tissue from immunized mice was examined for signs of immune complex nephritis. Indirect immunofluorescent stains with anti-mouse IgG or IgM revealed that pp65422-439-immunized mice developed intense IgG (6/9), IgM (5/9) and C3 (2/9) deposition in the glomeruli (Fig. 4d and Additional file 3: Table S2). In contrast to pp65422-439, pp65386-403 immunization induced only mild IgM deposition (2/9). No pathological staining was found in PBS (0/5) or SA-C3d immunized mice (0/5). Immunoglobulin isotype deposition in pp65422-439-immunized mice revealed that IgG1 (6/9) and IgG3 (4/9) were dominant isotypes compared to IgG2a (2/9) and IgG2b (1/9) in pp65386-403-immunized mice (Fig. 4e).
The dominant target of HCMV pp65428-437 epitope exhibited dsDNA reactivity
In order to map the B cell epitopes within pp65422-439, nine pp65422-439-derived decapeptides were synthesized and tested with SLE-dsDNA(+) serum and serum from immunized animals (Fig. 5a). Monoclonal antibodies were also generated from animals immunized with pp65422-439. We observed pp65422-439-specific reactive monoclonal antibodies (mAb) against pp65430-439 (P1, P2) and pp65425-434 (P3, P4) (Fig. 5b). The P1 and P2 mAbs also reacted positively in the ELISA and C. lucilliae assay (Fig. 5c, d). In human serum assays, pp65426-437, which expands three decapeptides, is targeted by anti-pp65422-439-specific antibody from SLE-dsDNA(+) (Fig. 5e).
Fig. 5.

Antibody activity against pp65422-439-derived decapeptides and double-stranded DNA (dsDNA) from anti-pp65422-439 antibodies in human systemic lupus erythematosus (SLE), immunized mice serum and monoclonal antibodies from pp65422-439-immunized mice. a Representatives of nine overlapping pp65422-439-derived decapeptides. Each peptide is shifted by one amino acid. ELISA for anti-decapeptide reactivity with four monoclonal antibodies (b) and anti-pp65422-439 antibody (c) from SLE-dsDNA(+) serum: 100× diluted normal serum and flow-through were used as negative controls. d ELISA for IgG against pp65422-439 and dsDNA with monoclonal antibodies and 100× diluted normal serum. e Representatives of C. luciliae stain by P1 (e1), P2 (e2), P3 (e3) or P4 (e4) monoclonal antibodies, respectively. White arrowheads indicate the positive stains. Data are presented as the mean ± SEM of three independent experiments. O.D. optical density
In immunized animals, pp65422-439-induced IgM reacted to all decapeptides with elevated titers to pp65425-434 at 4 weeks post immunization (Fig. 6a). In addition to pp65425-434, few mice also have elevated IgM to pp65428-437 and pp65429-438. At 8 weeks post immunization, IgM activity in response to decapeptides was enhanced roughly twofold optical density (OD) with the exception of pp65428-437, which almost tripled the OD at week 4 (Fig. 6a and b). The IgM activities in response to decapeptides at 14 weeks post immunization were reduced to an OD level similar to week 4. The IgG at 4 weeks post immunization was greatly enhanced in response to pp65428-437 in all mice but there was poor response to pp65425-434 (Fig. 6c). At 8 weeks post immunization, anti-pp65428-437 IgG represented the dominant immune activity, followed by anti-pp65430-439 and anti-pp65429-438 IgG (Fig. 6d). At 14 weeks post immunization, the IgG activities in response to pp65430-439 and pp65425-434 were further enhanced and associated with drastic reduction of anti-pp65428-437 to its basal level. This reduction of anti-pp65428-437 IgG to basal level occurred universally in all of the animals of this group.
Fig. 6.
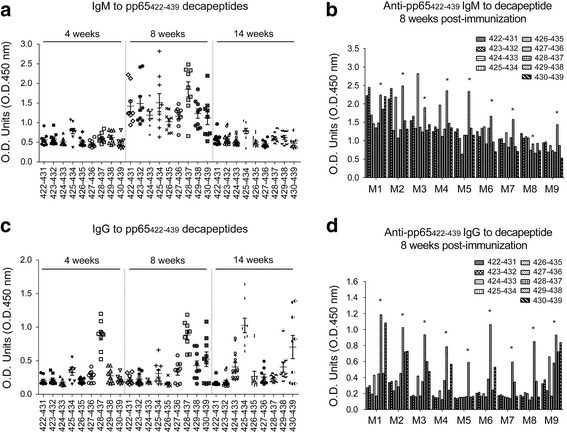
ELISA for IgG/M reactivity against decapeptides from pp65422-439-immunized serum. Nine pp65422-439 derived decapeptides and 250× diluted mice serum was used. a ELISA for IgM against decapeptides from pp65422-439-immunized mice (n = 9) at 4, 8 and 14 weeks post immunization. b The IgM against nine decapeptides from each pp65422-439-immunized mice (n = 9) at 8 weeks post immunization; IgM reactivity to each pp65422-439-derived decapeptide. c ELISA for IgG against decapeptides from pp65422-439-immunized mice (n = 9) at 4, 8 and 14 weeks post immunization. d IgG against decapeptides from pp65422-439-immunized mice (n = 9) at 8 weeks post immunization: IgG reactivity to each pp65422-439-derived decapeptide. *IgM/G against pp65428-437. Data are presented as the mean ± SEM of three independent experiments. O.D. optical density, M mouse
Discussion
Viral peptide-induced autoimmunity in animal models is an emerging field but the underlying mechanisms are not well-understood. Immunization of EBNA-1 or its fragment has been demonstrated to elicit not only immune response to viral antigen, but also IgG activity to 60 kD Ro, SmB/B’ and dsDNA [4, 26, 27]. Herein, we report the high prevalence of serum anti-pp65422-439 antibody in patients with SLE. Also, immunization of BALB/c mice with pp65422-439-induced cross-reactive autoantibodies against nuclear antigens of host cells, particularly dsDNA, and developed initial signs of nephritis with Ig deposition at 14 weeks post immunization. However, our mapping is unable to completely exclude the possibility that there were discontinuous epitopes, because the B cell epitopes were examined from pp65386-439 to pp65422-439. The higher incidence of anti-pp65422-439 activity in patients with SLE and the instigation of autoimmune-like antibodies through immunization of pp65422-439 in BALB/c mice suggested that immunity to pp65422-439 might drive pathogenic potential for SLE through epitope spreading and triggering autoantibody production in genetically susceptible individuals.
In our competitive inhibitor assay (Fig. 2b, c), pp65422-439 antibody from SLE-dsDNA(+) cross-reactive with dsDNA was not inhibited completely by pp65422-439, suggesting that more complex antibody repertoires, for example antibodies that recognize discontinuous epitopes, were obtained from SLE-dsDNA(+) through affinity purification by four tandem-repeats of pp65422-439. In addition, pp65422-439 antibody from SLE-dsDNA(-) exhibited anti-dsDNA reactivity, but was negative on C. luciliae stain. These results were suggested that the anti-dsDNA activity in the ELISA might be due to relatively weak anti-dsDNA reactivity of concentrated anti-pp65422-439 antibody from SLE-dsDNA(-). On the other hand, the increase in antibodies to HEK293 and dsDNA observed in SA-C3d-immunized mice at 8 week post-immunization might result from polyclonal B cell activation. However, we did not observe this phenomenon in our analysis of anti-pp65422-439 antibody against pp65386-439. The absorption and analysis of the B cell repertoire in response to pp65422-439 may play a critical part in autoimmunity require further validation.
HCMVpp65 is a well-known T cell antigen in healthy individuals [19, 20]. HCMV pp65 and pp65336-439-induced weak humoral responses were verified in healthy humans and BALB/c mice [21, 22]. Unlike normal or other disease controls, anti-pp65422-439 antibody occurs more frequently and has higher specificity in patients with SLE, particularly in anti-dsDNA-positive patients. Elevated anti-pp65336-439 antibody titers were measured in patients with SLE, but there was no statistically significant relationship between anti-pp65336-439 reactivity and serum dsDNA antibody [22].
These observations imply that pp65422-439 may contain one more representative epitope, which is associated with the production of anti-dsDNA antibody. Regarding the improvement of immunogenicity of pp65 peptides in the BALB/c model, mouse C3d acts as molecular adjuvant for interplay between innate and adaptive immunity [25]. Immunization of truncated pp65336-439 attached to C3d has been demonstrated to induce the development of autoimmunity [22]. In contrast, complete Freund’s adjuvant alone was unable to elicit chronic autoimmunity (data not shown). Immunization of pp65422-439 with C3d to BALB/c mice was sufficient to induce anti-pp65422-439 antibody. The transient humoral response to pp65422-439, also observed in pp65336-439-immunized BALB/c mice, indicates that genetic background plays a vital role in exacerbation of SLE.
Anti-dsDNA antibody has served as a critical immunological biomarker and diagnostic criterion for SLE [23, 24]. BALB/c mice challenged by a surrogate peptide have been reported to induce anti-dsDNA antibodies [14]. The nephritogenicity of anti-dsDNA antibody has been shown to mediate cross-reactivity to alpha actinin and annexin II [28, 29]. Also, lupus autoantibodies binding to DNA/nucleosome fragments released from apoptotic cells were observed in the glomerular matrix [30]. Immunization using pp65 or its truncated form has been previously shown to induce multiple anti-nuclear antibodies and anti-dsDNA antibody in BALB/c mice [22]. As expected, anti-dsDNA serum from patients with SLE had anti-pp65 reactivity, particularly to the pp65422-439 region. Notably, patients with SLE were double positive to pp65, and simultaneously dsDNA chromatin/nucleosome stain was positive. The anti-pp65 antibody that reacted to dsDNA and chromatin/nucleosome was previously verified in animals immunized for pp65336-439 [22].
It has been suggested that anti-nucleosome antibodies are sensitive and specific for lupus nephropathy and the correlation of the antibody titers represent a better biomarker of SLE global disease activity [31, 32]. These consistent results of human and animal studies imply that pp65422-439 peptide may possess one critical epitope contributing to the development of SLE. However, the limitations of the present study using stored serum from a cross-sectional study require future study to document their clinical associations with lupus nephropathy and the SLE disease activity damage index.
Following immunization of pp65422-439, antigen-specific IgG and IgM were analyzed at 4, 8 and 14 weeks post immunization. This pp65422-439 immunization scheme elicited antibodies reactive against antigens from HEK293T cells and produced ANA stain patterns resembling those found in anti-pp65422-439-purified antibody stains from patients with SLE. The appearance of autoantibodies in patients with SLE is an indicator of subsequent lupus disease onset [33]. The anti-dsDNA antibodies play critical roles in lupus nephritis; however, elevation of autoantibodies, particularly anti-dsDNA antibodies, has been identified in double-transgenic BALB/c mice expressing both the R4A-gamma2b heavy chain and the anti-apoptotic bcl-2 gene, but the mice did not develop nephritis [34]. In the current study ELISA and the C. luciliae assay demonstrated anti-dsDNA reactivity to pp65-purified human antibodies and pp65422-439-immunized serum. The pp65422-439 immunization scheme not only elicited anti-dsDNA antibodies, but also initiated early-phase kidney damage in BALB/c mice. In the near future, we speculate that pp65422-439 reactivity in combination with anti-chromatin/nucleosome and dsDNA antibodies may better fit as a surrogate biomarker of lupus nephropathy inflammation and damage [35, 36].
On terms IgG isotype analysis, both dsDNA and pp65422-439-specific IgG were detected in serum from immunized animals, with IgG1 and IgG3 isotypes. Mouse IgG3 is involved in the pathogenic autoimmunity, especially immune complex depositions and glomerulonephritis [37]. IgG3 production has been proposed as a critical factor in nephritis among MRL/lpr mice [38]. Similar to human IgG2, T-cell-independent mouse IgG3 mainly recognizes carbohydrate epitopes [39]. Human IgG1 and IgG2 isotypes of anti-nucleohistone and anti-dsDNA antibodies are the predominant isotypes found in plasma from patients with lupus who have renal disease [40]. In pp65422-439 immunization, elevated serum titers of anti-dsDNA IgG1 and IgG3 antibodies positively correlated with the severity of immunoglobulin deposition in glomeruli. Nevertheless, the current study did not provide sufficient evidence to fully explain the causal relationship between pp65-induced anti-dsDNA antibodies and nephritis development in BALB/c mice. The role of pp65422-439-induced autoantibodies in glomerular injury required verification by further study.
Three dominant immunological epitopes, pp65425-434, pp65428-437 and pp65430-439, elicited IgG and/or IgM activities at different immunological stages. In the first 4 weeks of immunization, IgG was targeting pp65428-437. By 8 weeks post immunization, IgG reacted to pp65425-434, pp65428-437 and pp65430-439, likely as a consequence of epitope spreading. After 14 weeks post immunization, IgG remained active in response to pp65425-434 and pp65430-439 but lost its activity in response to pp65428-437. These findings correlated with our mAb, which had reactivity to pp65425-434 and pp65430-439. The positive response of mAb P1 and P2 to both dsDNA and pp65430-439 suggests that pp65430-439 may contain elements that induce the anti-dsDNA response. The anti-dsDNA IgG activities were detected at 4 weeks post immunization with pp65425-434 and pp65428-437. As mAb P3 and P4 did not possess anti-dsDNA activity, this implies a strong association between anti-pp65428-437 and anti-dsDNA activity. Moreover, the anti-pp65428-437 activity was well-aligned with anti-dsDNA responses, as seen at weeks 4, 8 and 14 post immunization (Table 2). In humans, pp65428-437 is a target for pp65 and dsDNA-specific serum from patients with SLE. These findings suggest that pp65428-437 is a potential candidate epitope for promoting anti-dsDNA responses.
The issues of possible factors involved in molecular mimicry and epitope spreading have been widely discussed. The specific amino acid residues interacting with DNA, arginine (R), asparagine (N) and lysine (K), from either virus or necrotic cells, for somatic mutation, occurred during clonal expansion supports the hypothesis that peptide antigen has the potential to elicit the generation of anti-dsDNA antibody [15]. The amino acid 428-439, ASTSAGRKRKSA, of pp65 may contain one hot spot to provoke anti-dsDNA antibody production. However, this hypothesis cannot fully explain the discrepancy in the function of anti-pp65422-439 antibodies in dsDNA-positive and dsDNA-negative patients with SLE. We speculate that genetic background bias and preference of major histocompatibility complex (MHC) presentation may be together implicated in autoantibody production and subsequent SLE development.
Over the past few decades, study of HCMV has focused on the high-passage HCMV strain Towne, and AD169, and research into their potential capacity through efficient replication in human fibroblasts. In HCMV infection, pp65 is transported into the nucleus immediately through two nuclear localization sequences, pp65418-438 and pp65537-561 [41]. The binding of pp65 to metaphase-arrested chromosomes in pp65-expressing fibroblasts during virus infection implies that pp65 may not bind to host proteins, but also forms immune-complex to genetic materials and nuclear components [42]. The SV40 large T-antigen of human polyomaviruses has been demonstrated to form a T-antigen/nucleosome complex, subsequently targeted by host immune responses and accelerates the generation of cross-reactive antibodies against both virus and host during viral replication [43]. Therefore, full-length or fragmented pp65 binding to immune-complexes formed from nuclear binding proteins may not only be targeted by antiviral antibodies but also increase the opportunity for B cell epitope spreading and lead to autoimmunity in genetically susceptible individuals. It is worth mentioning that pp65 shares high homology among different HCMV strains and the fragment of pp65428-437, GASTSAGRKR, is highly conserved in HCMV strains such as Towne (pp65418-427), AD169 (pp65428-437) and Toledo (pp65428-437).
In patients with SLE, dsDNA-reactive IgM has been proposed as a protective mechanism that ameliorates autoimmunity and exhibits a negative association with lupus nephritis [44]. Up to now, three possible hypotheses have been proposed to explain how IgM antibody modulates autoimmunity. First, the elevated titer of IgM antibody acts as a competitive role binding to circulating antigens to decrease the formation of the IgG immune complex [45]. Second, IgM antibody downregulates autoreactive B cells to reduce the secretion of pathogenic IgG antibody [46]. Third, the uptake of IgM immune complex by phagocytic cells is more effective in preventing glomerular deposition of immune complex [47]. In pp65422-439 immunization, after immunization IgM initially targets the entire pp65422-439 with elevated titers to pp65425-434. Elevation of IgM to pp65428-437 at 8 weeks post immunization was detected after major elevation of IgG response to the same epitope. The IgM response to pp65428-437 is linked to anti-dsDNA activities (Additional file 4: Figure S3). However, after autoreactive anti-pp65428-437 IgG production, the upregulated IgM subsequently reduced anti-pp65428-437 IgG levels, suggesting that pp65428-437-specific IgM may be involved in alleviating the autoimmune response through the immune system in the non-autoimmune strain of BALB/c mice. More studies are needed to test the correlation between different classes of Ig and immune responses to specific autoantigens.
Conclusions
In conclusion, we report here that following immunization of HCMV pp65422-439, which is an 18-amino-acid peptide, non-autoimmune-prone animals developed autoimmunity, and exhibited autoantibodies to nuclear components and early signs of nephritis that resemble human SLE. The epitope pp65428-437 is the most likely candidate to trigger autoimmunity. The occurrence of epitope spreading in HCMV infection may be a driving force to induce cross-reactive autoantibodies in individuals with genetic predisposition. The amelioration of autoimmunity after elevated production of IgM targeting pp65428-437 may be ascribed to the modulation of pathogenic autoreactive IgG response.
Acknowledgements
We greatly appreciate Miss Chia Chen Hsu for providing technical support.
Funding
This work was supported by the Ministry of Science and Technology Taiwan (grant number 103-2314-B-182A-067-MY3 and Chang Gung Memorial Hospital (grant number CMRPG3E0532).
Availability of data and materials
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Authors’ contributions
AHH and JYC designed the project. JYC, CMW and YJJW performed clinical diagnosis and acquisition of data for analyses in the clinical studies and intellectual content. AHH and JYC performed experiments, acquisition of data and interpretation of data analyses. AHH, AC, MIC and JYC participated in the interpretation of data, and drafted and revised the manuscript. All authors reviewed the manuscript, approved the final version to be published and agreed to be accountable for all aspects of the work.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The participants gave their written consent to the use of their clinical samples for data publication.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Chang Gung Memorial Hospital with the following reference number 102-5607B. Written informed consent was obtained from each participant prior to sample collection. Animal experiments were approved by the Institutional Review Board of Chang Gung Medical Foundation. All experiments were performed in accordance with relevant guidelines and regulations.
Abbreviations
- ANAs
Anti-nuclear antibodies
- C3d
Complement 3d
- CFA
Complete Freund’s adjuvant
- CnBr
Cyanogen bromide
- DAPI
4',6-Diamidino-2-phenylindole
- dsDNA
Double-stranded DNA
- EBNA-1
Epstein-Barr virus nuclear antigen 1
- EBV
Epstein-Barr virus
- ELISA
Enzyme-linked immunosorbent assay
- FITC
Fluorescein isothiocyanate
- HCMV
Human cytomegalovirus
- HRP
Horseradish peroxidase
- IFA
Incomplete Freund’s adjuvant
- kDa
KiloDalton
- mAb
Monoclonal antibodies
- MSA-I/II
Mitotic spindle type I/II
- OPD
O-phenylenediamine dihydrochloride
- PBS
Phosphate-buffered saline
- pp65
Phosphoprotein 65
- RA
Rheumatoid arthritis
- RPMI
Roswell Park Memorial Institute medium
- RT
Room temperature
- SA
Streptavidin
- SLE
Systemic lupus erythematosus
- SLE-dsDNA(-)
systemic lupus erythematosus without dsDNA antibody
- SLE-dsDNA(+)
systemic lupus erythematosus with dsDNA antibody
- SmB/B’
Smith antigen B/B’
- snRNP
Small nuclear ribonucleoprotein
Additional files
Detection of IgG against pp65386-439 in serum from patients with SLE, AS, RA and normal controls. (PDF 2112 kb)
Detection of anti-pp65 reactivity from pp65386-403, pp65422-439, SA-C3d and PBS immunized serum. (PDF 1144 kb)
Summary of ANA patterns in mice against cellular components and isotypes of antibody deposition on glomeruli. (PDF 53 kb)
Detection of IgM anti-dsDNA antibodies in PBS, SA-C3d, pp65386-403 and pp65422-439 immunized sera. (PDF 773 kb)
Contributor Information
Ao HoHsieh, Email: mayitsu2006@gmail.com.
Chin Man Wang, Email: cmw1314@adm.cgmh.org.tw.
Yeong-Jian Jan Wu, Email: yjwu1962@gmail.com.
Albert Chen, Email: acchen@gmail.com.
Ming-I Chang, Email: mingi.chang@gmail.com.
Ji-Yih Chen, Phone: 886-3-3281200, Email: jychen31@adm.cgmh.org.tw.
Reference
- 1.Incaprera M, Rindi L, Bazzichi A, Garzelli C. Potential role of the Epstein-Barr virus in systemic lupus erythematosus autoimmunity. Clin Exp Rheumatol. 1998;16:289–94. [PubMed] [Google Scholar]
- 2.Lu JJ, Chen DY, Hsieh CW, Lan JL, Lin FJ, Lin SH. Association of Epstein-Barr virus infection with systemic lupus erythematosus in Taiwan. Lupus. 2007;16:168–75. doi: 10.1177/0961203306075800. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Mercado AE, Vila-Perez S. Cytomegalovirus as a trigger for systemic lupus erythematosus. J Clin Rheumatol. 2010;16:335–7. doi: 10.1097/RHU.0b013e3181f4cf52. [DOI] [PubMed] [Google Scholar]
- 4.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 5.Tseng CE, Chan EK, Miranda E, Gross M, Di Donato F, Buyon JP. The 52-kd protein as a target of intermolecular spreading of the immune response to components of the SS-A/Ro-SS-B/La complex. Arthritis Rheum. 1997;40:936–44. doi: 10.1002/art.1780400523. [DOI] [PubMed] [Google Scholar]
- 6.James JA, Scofield RH, Harley JB. Lupus humoral autoimmunity after short peptide immunization. Ann NY Acad Sci. 1997;815:124–7. doi: 10.1111/j.1749-6632.1997.tb52054.x. [DOI] [PubMed] [Google Scholar]
- 7.Arbuckle MR, Reichlin M, Harley JB, James JA. Shared early autoantibody recognition events in the development of anti-Sm B/B’ in human lupus. Scand J Immunol. 1999;50:447–55. doi: 10.1046/j.1365-3083.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 8.Poole BD, Gross T, Maier S, Harley JB, James JA. Lupus-like autoantibody development in rabbits and mice after immunization with EBNA-1 fragments. J Autoimmun. 2008;31:362–71. doi: 10.1016/j.jaut.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen J, Rhodes G, Roudier J, Vaughan JH. Altered immune response to glycine-rich sequences of Epstein-Barr nuclear antigen-1 in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1990;33:993–1000. doi: 10.1002/art.1780330711. [DOI] [PubMed] [Google Scholar]
- 10.Sabbatini A, Bombardieri S, Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein-Barr virus-encoded nuclear antigen EBNA I. Eur J Immunol. 1993;23:1146–52. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J. Cytomegalovirus infection induces expression of 60 KD/Ro antigen on human keratinocytes. Lupus. 1995;4:396–406. doi: 10.1177/096120339500400511. [DOI] [PubMed] [Google Scholar]
- 12.Curtis HA, Singh T, Newkirk MM. Recombinant cytomegalovirus glycoprotein gB (UL55) induces an autoantibody response to the U1-70 kDa small nuclear ribonucleoprotein. Eur J Immunol. 1999;29:3643–53. doi: 10.1002/(SICI)1521-4141(199911)29:11<3643::AID-IMMU3643>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Newkirk MM, van Venrooij WJ, Marshall GS. Autoimmune response to U1 small nuclear ribonucleoprotein (U1 snRNP) associated with cytomegalovirus infection. Arthritis Res. 2001;3:253–8. doi: 10.1186/ar310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 16.Reyda S, Tenzer S, Navarro P, Gebauer W, Saur M, Krauter S, Buscher N, Plachter B. The tegument protein pp 65 of human cytomegalovirus acts as an optional scaffold protein that optimizes protein uploading into viral particles. J Virol. 2014;88:9633–46. doi: 10.1128/JVI.01415-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp 65 (pp UL83) prevents interferon response factor 3 activation in the interferon response. J Virol. 2004;78:10995–1006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Chen J, Cristea IM. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe. 2013;14:591–9. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, Greenberg PD, Riddell SR. Identification of the major late human cytomegalovirus matrix protein pp 65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–10. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 20.Jahn G, Scholl BC, Traupe B, Fleckenstein B. The two major structural phosphoproteins (pp 65 and pp 150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987;68(Pt 5):1327–37. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- 21.Chang M, Pan MR, Chen DY, Lan JL. Human cytomegalovirus pp 65 lower matrix protein: a humoral immunogen for systemic lupus erythematosus patients and autoantibody accelerator for NZB/W F1 mice. Clin Exp Immunol. 2006;143:167–79. doi: 10.1111/j.1365-2249.2005.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh AH, Jhou YJ, Liang CT, Chang M, Wang SL. Fragment of tegument protein pp 65 of human cytomegalovirus induces autoantibodies in BALB/c mice. Arthritis Res Ther. 2011;13:R162. doi: 10.1186/ar3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 25.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 26.McClain MT, Lutz CS, Kaufman KM, Faig OZ, Gross TF, James JA. Structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. Proc Natl Acad Sci USA. 2004;101:3551–6. doi: 10.1073/pnas.0306267101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol. 2010;21:1912–27. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deocharan B, Qing X, Lichauco J, Putterman C. Alpha-actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J Immunol. 2002;168:3072–8. doi: 10.4049/jimmunol.168.6.3072. [DOI] [PubMed] [Google Scholar]
- 30.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168:1779–92. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervera R, Vinas O, Ramos-Casals M, Font J, Garcia-Carrasco M, Siso A, Ramirez F, Machuca Y, Vives J, Ingelmo M, et al. Anti-chromatin antibodies in systemic lupus erythematosus: a useful marker for lupus nephropathy. Ann Rheum Dis. 2003;62:431–4. doi: 10.1136/ard.62.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Prokopec SD, Morrison S, Lou W, Reich H, Gladman D, Urowitz M, Scholey J, Fortin PR, Boutros PC, et al. Anti-nucleosome antibodies outperform traditional biomarkers as longitudinal indicators of disease activity in systemic lupus erythematosus. Rheumatology. 2015;54:449–57. doi: 10.1093/rheumatology/keu326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 34.Kuo P, Bynoe MS, Wang C, Diamond B. Bcl-2 leads to expression of anti-DNA B cells but no nephritis: a model for a clinical subset. Eur J Immunol. 1999;29:3168–78. doi: 10.1002/(SICI)1521-4141(199910)29:10<3168::AID-IMMU3168>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. doi: 10.1681/ASN.2008010112. [DOI] [PubMed] [Google Scholar]
- 36.Steiman AJ, Urowitz MB, Ibanez D, Li TT, Gladman DD, Wither J. Anti-dsDNA and antichromatin antibody isotypes in serologically active clinically quiescent systemic lupus erythematosus. J Rheumatol. 2015;42:810–6. doi: 10.3899/jrheum.140796. [DOI] [PubMed] [Google Scholar]
- 37.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J Immunol. 1998;160:20–3. [PubMed] [Google Scholar]
- 38.Takahashi S, Nose M, Sasaki J, Yamamoto T, Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991;147:515–9. [PubMed] [Google Scholar]
- 39.Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Lab Immunol. 1995;2:726–32. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bijl M, Dijstelbloem HM, Oost WW, Bootsma H, Derksen RH, Aten J, Limburg PC, Kallenberg CG. IgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosus. Rheumatology. 2002;41:62–7. doi: 10.1093/rheumatology/41.1.62. [DOI] [PubMed] [Google Scholar]
- 41.Schmolke S, Drescher P, Jahn G, Plachter B. Nuclear targeting of the tegument protein pp 65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol. 1995;69:1071–8. doi: 10.1128/jvi.69.2.1071-1078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dal Monte P, Bessia C, Landini MP, Michelson S. Expression of human cytomegalovirus pp UL83 (pp 65) in a stable cell line and its association with metaphase chromosomes. J Gen Virol. 1996;77(Pt 10):2591–6. doi: 10.1099/0022-1317-77-10-2591. [DOI] [PubMed] [Google Scholar]
- 43.Andreassen K, Bredholt G, Moens U, Bendiksen S, Kauric G, Rekvig OP. T cell lines specific for polyomavirus T-antigen recognize T-antigen complexed with nucleosomes: a molecular basis for anti-DNA antibody production. Eur J Immunol. 1999;29:2715–28. doi: 10.1002/(SICI)1521-4141(199909)29:09<2715::AID-IMMU2715>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Bootsma H, Spronk PE, Hummel EJ, de Boer G, ter Borg EJ, Limburg PC, Kallenberg CG. Anti-double stranded DNA antibodies in systemic lupus erythematosus: detection and clinical relevance of IgM-class antibodies. Scand J Rheumatol. 1996;25:352–9. doi: 10.3109/03009749609065646. [DOI] [PubMed] [Google Scholar]
- 45.Forger F, Matthias T, Oppermann M, Becker H, Helmke K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus. 2004;13:36–44. doi: 10.1191/0961203304lu485oa. [DOI] [PubMed] [Google Scholar]
- 46.Heltemes-Harris L, Liu X, Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J Immunol. 2004;172:823–33. doi: 10.4049/jimmunol.172.2.823. [DOI] [PubMed] [Google Scholar]
- 47.Werwitzke S, Trick D, Kamino K, Matthias T, Kniesch K, Schlegelberger B, Schmidt RE, Witte T. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB x NZW)F1 mouse. Arthritis Rheum. 2005;52:3629–38. doi: 10.1002/art.21379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.


