Abstract
Vasculogenic mimicry (VM) is a blood supply system independent of endothelial vessels in tumor cells from different origins. It reflects the plasticity of aggressive tumor cells that express vascular cell markers and line tumor vasculature. The presence of VM is associated with a high tumor grade, short survival, invasion and metastasis. Endothelial cells (ECs) express various members of the cadherin superfamily, in particular vascular endothelial (VE-) cadherin, which is the main adhesion receptor of endothelial adherent junctions. Aberrant extra-vascular expression of VE-cadherin has been observed in certain cancer types associated with VM. In this review we focus on non-endothelial VE-cadherin as a prominent factor involved in the acquisition of tubules-like structures by aggressive tumor cells and we summarize the specific signaling pathways, the association with trans-differentiation and stem-like phenotype and the therapeutic opportunities derived from the in-depth knowledge of the peculiarities of the biology of VE-cadherin and other key components of VM.
Background
Solid tumors require blood vessels for growth, and access to oxygen and nutrients and anti-angiogenic therapies are designed to target vascular ECs to form tumor blood vessels. Whereas numerous preclinical models have recognized the efficient use of angiogenesis inhibitors to limit tumor growth, collectively only a growth delay has been achieved in the clinic [1]. This is in part due to the fact that tumor vasculature is more complex than expected and alternative mechanisms for re-vascularization might be taking place. A large number of studies in pathology have described a high degree of plasticity associated with aggressive cancer. In 1999, Maniotis et al. [2] presented a new interpretation of previous findings describing cancer cells covering non-endothelial vascular channels that contained red blood cells. This was the initial report defining tumor cell VM as the de novo formation of perfusable, matrix rich, vasculogenic-like network in 3D matrix in vitro, which resembled the matrix-rich network observed in aggressive tumors in patients [3]. The initial morphological, clinical and molecular characterization of VM was performed using human melanoma as a model. In addition to melanoma, vasculogenic mimicry (VM) has also been characterized in carcinomas of lung, prostate, bladder, kidney, ovary and breast, sarcomas and gliomas. Kaplan-Meier survival analyses indicated that patients with VM in their tumors have a poor clinical outcome compared with patients with tumors that do not exhibit VM. Table 1 shows the main differences at molecular level between blood vessels and VM networks.
Table 1.
Differences in tumor-VM and ECs-dependent angiogenesis and VM inducer and suppressor molecules
| Normal endothelial cells | Vasculogenic mimicry cells |
|---|---|
| Similarities | |
| VE-cadherin positive | |
| E-selectin positive | |
| CD34 positive | |
| Differences | |
| TIE-2 positive | TIE-2 negative |
| VEGFR-1, 2 positive | VEGFR-1, 2 negative |
| P-selectin positive | P-selectin negative |
| VCAM-1/CD106 positive | VCAM-1/CD106 negative |
| CD31/PECAM-1 positive | CD31/PECAM-1 negative *13 (Subpopulations PECAM-1 positive melanoma cells) |
| TIE-1 negative | TIE-1 positive |
| VEGF-C negative | VEGF-C positive |
| Neuropilin 1 negative | Neuropilin 1 positive |
| Endoglin negative | Endoglin positive |
| Tissue factor pathway inhibitor 1 (TFPI1) negative | Tissue factor pathway inhibitor 1 (TFPI1) positive |
| Laminin 5 gamma 2 chain (LAMC2) negative | Laminin 5 gamma 2 chain (LAMC2) positive |
| EphA-2 negative | EphA-2 positive |
The distinctive pattern of VM networks appears to recapitulate embryonic vasculogenesis patterns and this resemblance suggests that aggressive tumor cells convert to an undifferentiated, embryonic-like phenotype. Gene expression analysis demonstrated that aggressive melanomas capable of VM express genes associated with multiple cellular phenotypes, including characteristics of epithelial cells, endothelial cells and fibroblasts [4, 5]. However, the different molecular mechanisms that generate VM are still unclear. One of the most conspicuous molecular determinants in the acquisition of VM capabilities is the expression of the endothelial cell marker VE-cadherin. In the current review, we focus on the connection between VE-cadherin and its consequences in the gain of the VM phenotype which is also associated with cell plasticity and trans-differentiation of cancer stem cells present in VM.
VE-cadherin in VM
VE-cadherin, Notch or hypoxia-inducible factor 1-α (HIF1-α) are among the most relevant signaling molecules involved in the three leading pathways that control VM: vascular, hypoxia and embryonic/stem cell signaling pathways. All these pathways are complex and interconnected, with a large number of different molecules performing together and modulating the outcome effect in a different way (reviewed in [6–9]) (Fig. 1).
Fig. 1.
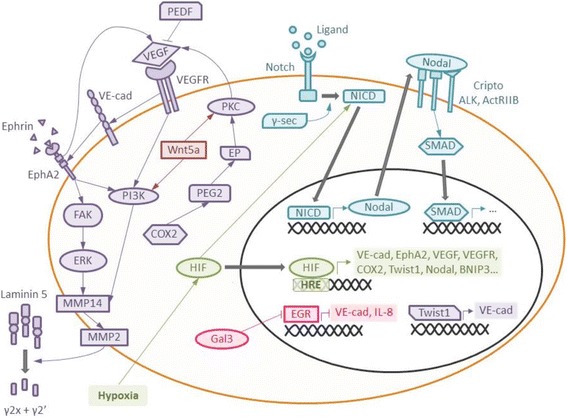
Main signaling pathways involved in vasculogenic mimicry. In vascular signaling (purple), VE-cadherin, EphA2 and VEGF lead to proteolytic cleavage of laminin 5 and release of pro-migratory γ2x and γ2’ fragments in the extracellular matrix. Galectin 3 supports the vascular pathway, since it enhances the expression of VE-cadherin. Stem cell signaling (blue), controlled by Notch and Nodal, up-regulates genes for pluripotency and de-differentiation. Hypoxia (green) contributes to all previous pathways by mediating expression of some crucial signaling molecules. Finally, Wnt proteins may promote vasculogenic mimicry through the activation of PKC and PI3K signaling, though it could play a role in tumor suppression in certain cases
VE-cadherin is a trans-membrane protein commonly expressed in endothelium, where it is responsible for cell-cell adhesion [10]. Although VE-cadherin used to be considered specific for ECs, its expression has been strongly associated with aggressiveness and VM in melanoma. Surprisingly, VE-cadherin can be found in highly aggressive tumor cells but not in non-aggressive ones. Moreover, its down-regulation in melanoma implied the loss of VM formation [11].
VE-cadherin is the best known cadherin in the context of vascular adhesion but the insights of its role in VM in aggressive tumor cells are only beginning to be well understood. The fact that VE-cadherin is key to understand VM was discovered by Hendrix and col in 2001 [12]. In this seminal study, they showed that VE-cadherin is expressed in aggressive melanoma cells while its knockdown prevented VM. On the other hand, in vivo experimental models also demonstrated that mice deficient in VE-cadherin die by severe vascular defects [13]. The reason for the expression of VE-cadherin in non-endothelial cells is mostly unclear, but in hepatocellular carcinoma (HCC) it may be due the to nuclear localization of Twist1. This transcription factor has been shown to bind VE-cadherin promoter, enhancing its activity [14]. In melanoma, VE-cadherin expression has been related with the activation of the Nodal/Notch pathway [15, 16] and hypoxia-inducible factors [17, 18]. Finally, it has been suggested that human epidermal growth factor receptor 2 (HER2) up-regulates VE-cadherin in breast cancer cells [19].
Structurally, VE-cadherin has five extracellular calcium domains (ECD:aa46-aa599) that can form cis-homodimers with other VE-cadherin or similar dimers in trans- through ECDI-ECDIV, that present on adjoining cells to support cell-to-cell recognition and adhesion. ECDV is required for binding to VE-PTP. VE-cadherin also has a trans-membrane domain (TMD: aa600-aa620) and an intracellular domain involved in post-translational modifications (PTM) (Fig. 2). In fact, 13 possible residues of VE-cadherin that can be phosphorylated in humans (Uniprot KB/Phosphosite Plus) have been described but the most relevant ones, and also the most studied, are residues Y658, S665, Y685 and Y731. Phosphorylation in S665 of VE-cadherin takes place through serine/threonine kinase P21 protein (Cdc42/Rac)-activated kinase 1 (PAK) in response to VEGF that also promotes clathrin-dependent internalization of VE-cadherin [20]. Recent studies have shown that Y658 residue is a target of focal adhesion kinase (FAK) in tumor-associated endothelial cells, and identify FAK as a key regulator of endothelial cell barrier function controlling tumor metastasis [21]. Tyrosine phosphorylation of VE-cadherin Y685 causes its internalization mediated by Src, thus playing an important role in in vivo vascular permeability [22, 23]. In contrast, mice expressing Y731F VE-cadherin mutant display deficient neutrophil-extravasation; indeed, phosphorylation of Y731 is induced through inflammatory mediators, such as histamine, that promote neutrophil-extravasation [23] (see Fig. 2).
Fig. 2.
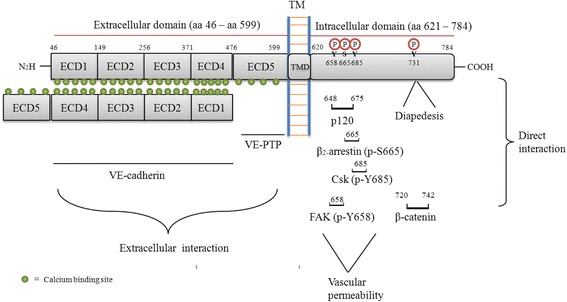
Structural features of VE-cadherin and implication in signalling. Further details are given in the text for the VE-cadherin subheading
Vascular endothelial growth factor A (VEGF-A) is known to contribute to endothelial cell proliferation, including tumor-induced angiogenesis, and has been associated to VM in ovarian carcinoma and melanoma [8]. In fact, in ovarian cancer cells VEGF-A may up-regulate most of the signaling molecules in the VE-cadherin signaling cascade: VE-cadherin itself, Epithelial cell kinase (EphA2) and MMP-2 [7]. We will analyze below different key pathways and molecules involved in VM and their connection with VE-cadherin.
VE-cadherin-dependent and independent signaling in VM
Vascular endothelial growth factor (VEGF) signaling
When we refer to VE-cadherin it is necessary to describe VEGF receptors (VEGFRs) because they represent the most important pathway to regulate VE-cadherin function and turn-over. VEGFR-2 (flk-1/KDR) and VEGFR-1 (FLT-1) are the most important receptors in this pathway although at least five different VEGF isoforms are also known in human. These receptors are stimulated by vascular endothelial growth factor (VEGF-A) and represent a crucial regulatory system of endothelial growth in a normal cell physiology context; in contrast VEGF is also an endothelial-specific mitogen and potent activator of vascular permeability secreted by tumor cells [24]. VEGFR-2 is highly expressed in vascular ECs thus forming the primitive tubular vessels called vasculogenesis. In contrast, VEGFR-1 is also highly expressed in tumor cells with capacity to form VM; this is the case of malignant melanoma [25]. As mentioned above, VEGF-A promotes vascular permeability by weakening adherent junctions and tight junctions, resulting in transient opening of the endothelial cell-cell contacts [26]. In fact, VEGF-A promotes tyrosine phosphorylation of VE-cadherin and its binding to the partner β-catenin, plakoglobin and p120, via Src-dependent mechanism [27]. VE-cadherin is inhibited following its phosphorylation in mice deficient in Src [27]. VE-cadherin may also be associated with and inhibit VEGFR-2 phosphorylation and subsequent internalization [28]. This association promotes the phosphorylation of components of the adherent junctions by Src, thus impairing the integrity of the endothelial barrier and promoting tumor cell extravasation and diffusion in pathological models [29]. In addition, VEGF-A mediates phosphorylation of VE-cadherin internalization through sequential activation of Src, the nucleotide exchange factor of Vav2, Rho GTPase Rac, leading to a downstream effect on the serine/threonine kinase P21 protein (Cdc42/Rac)-activated kinase 1 (PAK). Finally, phosphorylated PAK phosphorylates VE-cadherin, triggering their internalization [26]. Moreover, VEGF signaling reduces the association between VE-cadherin and p120-catenin promoting clathrin-dependent VE-cadherin endocytosis [30]; binding of p120 to VE-cadherin prevents its internalization, while p120 silencing leads to degradation of VE-cadherin, and loss of cell-cell contacts [30].
VEGF binding to VEGFR-1 can enhance other variety of signaling pathways. Activation of tyrosine kinase Src and extracellular regulatory kinases 1 and 2 (ERK1/2) leads to cancer cell invasion and migration. In melanoma, VEGFR-1 seems to promote VM via PI3K/PKC pathway. VEGFR-1 also mediates angiogenesis through activation of PI3K/Akt pathway [7, 8]. Apart from this, VEGFR-1 is necessary for the expression of VE-cadherin [25]. As for VEGFR-3, it has been proved in melanoma that endothelin-1 (ET-1) can enhance its expression and also the expression of its ligands VEGF-C and -D. The signaling pathway involves ET-1 binding to its receptor ETBR, which activates hypoxia-inducible factors. The binding also leads to phosphorylation of VEGFR-3 (probably mediated by Src and β-arrestin-1), which becomes able to trigger the activation of MAPK signaling cascade. All of these events lead finally to the promotion of cell migration and VM [31].
Different evidences support the fact that VEGF expression is determined by EphA2 in breast cancer and pancreatic islet carcinoma cells. Cyclooxygenase-2 (COX-2) also stimulates the expression of VEGF in other tumor cell lines [7–9].
Cyclooxygenase-2 catalyzes the reaction that converts arachidonic acid into primarily prostaglandin E2 (PGE2). This molecule binds to prostanoid receptors (EP1-4) that activate protein kinase C (PKC) and epidermal growth factor receptor (EGFR) signaling pathways. Both of them lead to decreased apoptosis and increased tumor proliferation, invasion and angiogenesis. In particular, PKC signaling up-regulates VEGF expression [7–9]. In breast cancer, elevated COX-2 expression results in the increased ability to form VM networks, while its knockdown leads to a reduced VM. Importantly, VM capability of breast cancer cells with low COX-2 can be restored if PGE2 is added to the culture [32]. It has been reported that EP3, but not EP4, modulates VM in breast cancer [33].
On the contrary, VEGF/VEGFR-1 signaling can be inhibited by the pigment epithelium-derived factor (PEDF), a glycoprotein that belongs to the family of serine protease inhibitors. Furthermore, PEDF induces tumor cell differentiation and apoptosis, yet probably prevents VM development, since it is usually down-regulated in aggressive tumor cells. Even more, PEDF silencing favors VM in melanoma [7, 8].
Tissue factor (TF) and TF pathway inhibitors (TFPI-1/2) are involved in VM as well. Specifically, knockdown of TFPI2 inhibited MMP-2 activity, suggesting a role for TFPI2 in extracellular matrix remodeling associated with VM channel formation [7, 8].
Finally, an opposite role of VEGF signaling in VM has been proposed too. All the evidence presented so far indicates that VEGF promotes VM, but it has been suggested that, on the contrary, VM might increase in the absence of this signaling pathway: VEGF would promote angiogenesis, while VEGF blockade could enhance some other strategies for tumor cell survival, including VM [34].
VE-cadherin and EphA2 in VM
EphA2 has also been shown to be related to VM. EphA2 is a protein tyrosine kinase whose phosphorylation and activity depend on the binding of ephrin-A1, although it has been reported that EphA2 can also be constitutively active in some tumor cells [35]. As VE-cadherin, EphA2 was found to be expressed only in highly aggressive tumors, where it was tyrosine-phosphorylated. When cells were cultured on a three-dimensional matrix and labeled with anti-phosphotyrosine antibodies, the staining showed that tyrosine-phosphorylation was present mainly in the areas of tubular network formation. General inhibitors of protein tyrosine kinases as well as specific silencing of EphA2 hindered the development of vascular networks, suggesting a potential role for phosphorylated EphA2 in this process [35]. In VM, VE-cadherin and EphA2 co-localize at the plasma membrane, specifically in cell-to-cell contact regions. Knockdown of VE-cadherin resulted in a reorganization of EphA2 location, which seemed to move into the cytoplasm. Moreover, there was a decrease in EphA2 phosphorylation. On account of these results, it seems that VE-cadherin may help EphA2 translocate to the plasma membrane, [36, 37].
PI3K up-regulates both the activity and expression of matrix metalloproteinase-14 (MMP-14) in highly aggressive cells. MMP-14 in turn activates MMP-2, and finally cleaves laminin 5γ2 chain to produce the γ2’ and γ2x fragments, which are secreted to the extracellular matrix to promote migration in various tumor cell types, like breast, colon carcinomas and hepatoma [38]. More precisely, they activate the secretion of γ2’ and γ2x pro-migratory fragments leading to VM in melanoma, gallbladder and ovarian carcinomas [39–41]. Furthermore, poorly aggressive melanoma cells (which cannot normally engage in VM) could form vasculogenic-like networks when seeded on collagen gels that had been pre-conditioned by highly aggressive melanoma cells. Aggressive cells were removed before apparent formation of tubular networks, but the examination of the a-cellular matrices showed the presence of laminin-positive patterned networks. If these matrices where treated with antibodies anti-laminin-5γ2 chain before seeding the poorly aggressive melanoma cells, they could no longer develop tubular networks. Altogether, these results confer great importance to this signaling cascade and laminin-5γ2 in particular.
As for FAK, over-expression of EphA2 caused an increase of MMP-2 in a way dependent on FAK [42]. FAK itself is highly phosphorylated at positions Y397 and Y576 in highly aggressive melanoma, but not in poorly aggressive melanoma cells; this post-translational changes are indicative of a fully active FAK. FAK-related non-kinase (FRNK) can interact with focal adhesion proteins but it lacks kinase activity, so it is considered a dominant-negative FAK protein. Expression of FRNK in aggressive melanoma decreased invasiveness, migration and ability to form tubular networks on collagen gels. It also reduced the levels of phosphorylated ERK1/2, whose inhibition decreased the levels of urokinase as well as the activity of MMP-14 and MMP-2. These results hint at a signaling cascade where EphA2 promotes VM via FAK and ERK, meeting the PI3K pathway at MMP-14 and leading henceforth to the cleavage of laminin-5γ2 [43].
Notch/Nodal
Nodal/Notch belongs to the superfamily of the transforming growth factor β (TGF-β). It is essential during embryonic development, since it maintains undifferentiated status of embryonic stem cells in order to ensure growth and development. Notch is a trans-membrane receptor with four different isoforms (Notch1-4), all of which undergo cleavage by γ-secretase after binding of one out of five possible ligands: Delta-like-1/3/4 and Jagged1/2. Notch intracellular domain (NICD) is released into the cytoplasm and it is imported to the nucleus, where it regulates gene expression, including expression of Nodal. Notch signaling pathway is involved in differentiation, although cell fate depends on cellular context. Also Notch is associated with the development of vascular networks in embryonic stages, so it may have a relationship with VM in cancer. Nodal binds several membrane receptors (Cripto-1, activing-like kinase receptors (ALK4/7), ActRIIB, and/or TGF-β receptor I and II), causing phosphorylation of SMAD2/3, enabling its association with SMAD4 and their translocation to the nucleus to activate the expression of Nodal target genes, including LEFTY, which is responsible of negative feedback in this signaling pathway, as it directly inhibits Nodal.
It has been reported that disruption of the Notch signaling cascade with γ-secretase inhibitors stabilizes VM networks in melanoma, that is, Notch might have an attenuating effect in VM [44]. Nevertheless, there are evidences supporting a positive relationship between Notch and VM: Notch-4 was shown to be highly expressed in several aggressive melanoma cell lines capable of VM, where it seemed to play a crucial role in the up-regulation of Nodal expression as well. Furthermore, antibody-mediated blocking of Notch signaling resulted in an impairment of VM ability, which could be restored with the addition of Nodal [45]. Nodal expression normally stops after cell differentiation during human development, but it can be rescued in tumor cells, for instance, melanoma and breast cancer cells. This pathway is related to tumor progression, aggressiveness, VM and VE-cadherin expression in aggressive melanoma cells [15, 16].
Hypoxia
Oxygen depletion is a common situation in growing tumors; therefore tumor cell survival and, consequently, malignancy are often dependent on their adaptation to hypoxia. Hypoxia-inducible factors (HIFs) and hypoxia responsive elements (HRE) play a crucial role in this context; HIFs are transcription factors stable in hypoxic conditions, while HRE are the genomic regions where HIF bind to mediate gene expression. These transcription factors are stabilized during conditions of hypoxia and they are critical to induce genes for tumor cell adaption to a hostile and changing microenvironment.
Notably, some potential hypoxia target genes containing HRE are involved in VM, such as VEGF-A, VEGFR-1, EphA2, Twist, Nodal, COX-2 and VE-cadherin [8, 46]. The later has been found to contain up to six HRE upstream of the promoter [6]. As a result, hypoxia has been reported to promote VM in a wide variety of tumor cell lines [47–49]. In murine models of melanoma, VM development in conditions of ischemia was significantly increased. Moreover, there is a positive relationship between expression of HIF-1α and VEGF in ischemic tumor cells [50]. Instead of VEGF, in human fibrosarcoma, HIF-1α promoted VM by up-regulating Neuropilin-1 (NRP-1), a VEGF receptor and co-receptor of VEGFR-2. Unexpectedly, silencing of NRP-1 completely disrupted tumor formation in fibrosarcoma [51].
In human melanoma, hypoxia induced over-expression of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2), which in turn increased VE-cadherin expression [17]. VE-cadherin was also up-regulated by HIF-1α in esophageal carcinoma [18]. To summarize, hypoxia is an essential trigger for vascular signaling pathways in the process of vasculogenic mimicry. Hypoxia may also influence VM through BNIP3, a protein that belongs to the family of Bcl-2. Expression of BNIP3 is remarkably up-regulated under hypoxia, allowing its contribution to cell migration and VM development in melanoma. BNIP3 enhanced these processes by modulating the organization of the actin cytoskeleton while BNIP3 knockdown completely inhibits VM, changed cell size and shape, driving to formation of actin stress fibers, and reduced tight and adherents junctions [52].
In glioblastoma, HIF-1α expression is mediated by mammalian target of rapamycin (mTOR). Specific inhibition or silencing of mTOR disrupted VM formation, especially under hypoxia but also in normoxic conditions, since some VM signaling molecules (HIF-1α, MMP-2 and MMP-14) were down-regulated [47]. In 2007, A. Le Bras and col [53] showed that the expression of VE-cadherin is controlled and regulated by a basal, non-endothelial specific promoter, containing six putative hypoxia responsive elements (HRE) which are binding sites for HIF-1α and HIF-2α. Consistent with this, HIF-2α (but not HIF-1α) regulated the expression of VE-cadherin in hypoxia as well as in normoxia. HIF-1α also induced the expression of VE-cadherin and modulated VM in esophageal carcinoma cells [18]. Following knockdown of HIF-1αVM in inhibited and the expression of VM-related genes reduced, for example EphA2, VE-cadherin or laminin-5γ2 but not MMP-2 in vitro and also in vivo [18]. Finally, it should be noted that mediating gene expression is not the only way by which hypoxia can influence VM: HIF-1α is known to stabilize NICD [54], whose role was explained above.
Galectin-3
Galectins are carbohydrate-binding proteins whose functions include cell adhesion and migration [9]. Galectin-3 (Gal-3) was proven to have oncogenic and angiogenic properties, being up-regulated, for example, in metastatic melanoma and breast cancer cells. Gal-3 silencing reduced invasiveness and inhibited VM formation in melanoma through down-regulation of the expression of some endothelial markers, such as VE-cadherin, together with the release of interleukin-8 (IL-8) was found to decrease. IL-8 is pro-angiogenic interleukin which modulates the expression of MMP-2, whose important role in VM was mentioned above [55]. Gal-3 mediates gene expression by inhibiting early growth response protein 1 (EGR-1), which loses its ability to bind (and thereby to repress) VE-cadherin and IL-8 promoter regions [55]. Therefore, the presence of Gal-3 allows the transcription of EGR-1 target genes. Consistent with this, gene expression microarrays analysis after silencing Gal-3 showed that Gal-3 regulates the expression of multiple genes, and has a negative influence on endothelial markers aberrantly expressed in highly aggressive melanoma cells such as VE-cadherin, IL-8, fibronectin-1, endothelial differentiation sphingolipid G-protein receptor-1 and MMP-2 [56]. It has been shown that shRNA of Gal-3 decreased VE-cadherin, and IL-8 promoter activity due to enhanced transcription of factor early growth response-1 (EGR-1) [57].
Wnt family
The Wingless (Wnt) family proteins is related to a large range of physiological processes, including embryonic patterning, cell proliferation, migration, cell differentiation. It plays an important role in endothelial cell differentiation, vascular development and angiogenesis [58], especially Wnt5a, a member of the non-canonical Wnt signaling. However, the role of Wnt5a in cancer is still being debated: in certain settings has been described as a tumor suppressor, but it is generally involved as a pro-metastatic factor [59].
Wnt5a is known to promote cell migration by modulating several proteins of the cytoskeleton, providing tumor cells with abilities in relocation. Moreover, Wnt5a releases intracellular calcium, activating calcium-dependent proteins such as protein kinase C (PKC), which is essential for invasion in cancer cells. All these properties, among others, suggest that Wnt5a is a cancer-promoting molecule [60]. PKCα has been shown to be involved with Wnt5a in EMT and VM in ovarian cancer cells, where the expression of Wnt5a and PKCα were correlated and PI3K levels were enhanced upon up-regulation of Wnt5a [61]. Over-expression of Wnt5a mediates VM formation in ovarian cancer and lung cancer [62, 63]. In 2015, Lisha Qi et al. [62] reported that Wnt3a expression in HT29 colon cancer cells promoted the capacity to form tube-like structures and increased the levels of proteins involved in VM such as VEGFR-2 and VE-cadherin. In addition, the antagonist Dickkopf-1(Dkk-1) reverted the capacity to form VM and decreased the expression of VEGFR2 and VE-cadherin in Wnt3a-overexpressing cells.
On the other hand, Wnt5a may perform as a tumor suppressor in certain cancers by inhibiting β-catenin-mediated transcription via several different molecules, preventing the expression of potential oncogenes. For this reason, the opposing effects of Wnt proteins remain controversial [60].
Epithelial Mesenchymal Transition (EMT) in VM
EMT is a dynamic biological process where polarized epithelial cells lose their epithelial properties and gain typical characteristics of mesenchymal cells [64]. The best known regulator of EMT is Transforming Growth Factor-beta (TGF-β), whose role in EMT is well-established [64, 65]. In 2008, Myriam Labelle and col [66] showed that VE-cadherin is induced in EMT in mammary tumor cells and it is also aberrantly expressed in invasive human breast carcinomas. In addition, VE-cadherin influenced the levels of SMAD2 phosphorylation and the expression of TGF-target genes. Thus, VE-cadherin might promote tumor progression by contributing to tumor angiogenesis as well as by enhancing tumor cell proliferation via TGF-β signaling. Recently, it has been reported that Zinc finger E-box binding homeobox 2 (ZEB2) fosters VM by TGF-β induction of EMT in HCC where ZEB2 over-expression significantly enhanced cell mobility and VM formation. Up-regulation of ZEB2 increased VE-cadherin, VEFGR-2 and VEGFR-1 expression as well as MMP-2 and MMP-9 [67]. ZEB1 down-regulation decreased the expression of VE-cadherin and VEGFR-2 in colorectal carcinoma, which are characteristic of ECs. In conclusion, ZEB1 promote VM formation by inducing EMT in colorectal carcinoma through the inhibition of VE-cadherin.
Other protein that has been identified to have a prominent role in EMT is Twist1. This protein binds DNA using similar E-box sequence motifs repressing E-cadherin and up-regulating mesenchymal markers expression; over-expression of Twist1 significantly enhanced cell mobility, invasiveness and promoted VM formation in HepG2 cells while chromatin immunoprecipitation showed that Twist1 binds to the VE-cadherin promoter and enhances its activity [68].
Cyclic adenosine monophosphate (cAMP)
cAMP is an essential second messenger involved in a number of cellular processes, such as cell growth and differentiation. It has also been linked to VM in cancer [69, 70]. Firstly, a rise in cAMP levels reduced VM in cutaneous and uveal melanoma. This inhibition appeared to be dependent mostly on the exchange protein directly activated by cAMP (Epac), but not on protein kinase A. Secondly, VM impairment was associated with an inhibition of ERK and PI3K/Akt signaling [70, 71], both of which were previously mentioned as important participants in the vascular pathways.
Apart from vascular signaling, cAMP may be involved in Notch signaling during endothelium development, so there might be an association with VM, too. The exact relationship between cAMP and Notch in endothelial cell differentiation remains unknown, but cAMP has been shown to modulate presenilin-1 (a component of γ-secretase) in neurons [72].
Besides the different signaling described above other key elements regulating VM are presented in Table 2.
Table 2.
VM inducer and suppressor molecules
| VM inducers | References |
| HIF1α | [50] |
| Twist1 | [14] |
| Nodal | [45] |
| Wnt5a | [61] |
| VE-cadherin | [111] |
| EphA2 | [35] |
| Laminin 5γ2 | [39] |
| CD133 | [116] |
| VEGFR-1/2/3 | [25] |
| [31] | |
| [118] | |
| PECAM1 | [133] |
| Desmoglein 2 | [134] |
| VM suppressors | |
| FAK-related nonkinase | [133] |
| PEDF | [135] |
| cAMP | [71] |
| TIMP-2 | [136] |
| AP-2α | [133] |
| miR-26b (EphA2) | [137] |
| miR-200a (EphA2) | [138] |
| miR-1236 (PI3K) | [139] |
| miR-27a-3p (VE-cadherin) | [140] |
| miR186 (Twist1) | [141] |
| hsa-mir-299–5p | [142] |
| miR-409-3p | [143] |
| miR-124 | [144] |
| [145] | |
| miR-Let-7f | [146] |
Vasculogenic mimicry and tumor microenvironment
For a long time, it has been assumed that tumor microenvironment (TME) plays an important role in development and progression of tumors, including invasion and metastasis [73, 74]. This physico-chemical niche includes stromal cells and fibroblasts, blood vessels and oxygen availability, immune cells, extracellular matrix (ECM), and cytokines [75]. Both, TME and tumor, present a bidirectional interaction that regulates several processes in tumor progression. One if these regulated processes is VM.
The first evidence of the influence of TME in VM was proposed by Hendrix et al. in 2002 [76]. They induced ischemic environment by surgery of the femoral artery in nude mice and inoculated highly and poorly aggressive human melanoma cells. They observed that highly aggressive cells, but not poorly aggressive, overlapped with endothelial cells in vasculogenesis of ischemic muscle. They also found by immunohistochemistry that only highly aggressive melanoma cells, but not control cells, strongly expressed Notch-3 and Notch-4 [76]. In a different study, they demonstrated that aggressive melanoma cells can modify the ECM and reprogram the poorly aggressive ones and inducing VM. Microarray analysis confirmed differential gene expression in poorly aggressive melanoma cells preconditioned with microenvironment of aggressive melanoma cells. As mentioned before, among up-regulated genes Eph2A, VE-Cadherin, TIE-1, VEGF-C, metalloproteases (MMP) and γ2 chain of laminin 5 (Ln5γ2) were present [77]. Cooperation between endothelial cells and tumor cells in VM formation has also been demonstrated in lung cancer [78]. Moreover, aggressive melanoma cells but not poorly aggressive melanoma cells induce VM in normal human mesenchymal stromal cells in co-culture. This effect is mediated by VEGFA [79]. Treatment against aggressive melanoma cells may ignore changes in ECM, decreasing the effectiveness of the therapy.
Relationship between cancer-associated fibroblast (CAFs) and VM has also been demonstrated. Conditioned medium of CAFs induces VM in hepatic tumor cells in vitro, and co-implantation of CAFs and hepatic tumor in mouse xenografts showed an increase in VM formation [80]. Additionally, CAFs were present among tumor cells involved in VM formation. This effect is mediated by paracrine effect of TGF-β and SDF1, which promote VE-cadherin, MMP-2 and LAMC2 expression [80]. A recent study demonstrates that TGF-β1 plays an essential role to recruit bone marrow-derived mesenchymal stem cells (MSC) to the tumor. TGF-β1 is involved in MSC differentiation to CAFs which indeed develop VM. Besides, MSC showed tropism with M2 macrophages, that have been related to tumor aggressiveness [81].
Macrophages drill channels and promote microcirculation in ischemic heart [82]. In a tumor context, macrophages have capacity of produce tubular network and they have been also identified in VM formation. These macrophages connect to blood vessels and express EMC degrading proteases [83]. High number of infiltrated M2 macrophages have also been observed in GBM tumor samples. These tumor associated macrophages induce COX-2 dependent VM and their effect is inhibited by Celecoxib [84].
Otherwise, conditioned medium from co-culture of umbilical vein endothelial cells (HUVEC) and HTR-8 trophoblast cells in a three-dimensional system induces VM in ovarian tumor cells (OVCAR-3). These results show that chorionic gonadotropin (HCG) is essential in this induction. HCG enhances HIF-1α and vascular markers. Anti-HCG antibodies reverse this effect. However, siRNA of HCG in OVCAR-3 do not inhibit VM, demonstrating that this induction is due to microenvironment [85].
CSCs in vasculogenic mimicry
VM requires an adaptive response of tumor cells and cellular “plasticity” is an essential property for this purpose. Cancer Stem Cells (CSCs) are by definition the tumor cell subpopulation with highest plasticity. In this context, “plasticity” is defined as the capability of pluripotent CSCs for trans-differentiation.
In 1994, Lapidot et al. and Caceres-Cortes et al. reported the first evidence of cancer initiating stem-like cells by injection of different leukaemia cell subpopulations in mice [86, 87]. Today CSCs have been determined in a wide range of solid tumors like breast [88], gliomas [89], prostate [90], melanoma [91, 92], lung [93, 94], colon [95–97], pancreatic [98], head and neck squamous cell [99], liver [100], non-melanoma skin cancer [101] and renal carcinoma [102].
CSCs represent an aggressive subpopulation of tumor cells with capacity of self-renewal, multi-lineage differentiation (stemness), tumor initiation and resistance of radio- and chemotherapy [89, 103, 104]. CSCs phenotype is enhanced in perivascular niche of several tumors and this correlates with aggressiveness [105, 106]. Furthermore, CSCs may trans-differentiate to ECs. This fact demonstrates the implication of CSCs in vascularization [107]. Also, several works have linked CSCs with VM capacity in different tumor types. In triple-negative breast cancer (TNBC), VM capacity statistically correlates with CD133 CSCs marker expression [108], and this co-relation was also demonstrated in other tumors like uveal and cutaneous melanoma, gliomas, hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC) [108–110].
To have a global view of the importance of VM and its interaction with CSCs we summarized above some key findings linking these two aspects in different tumor types:
Melanoma
Aggressive melanoma produces VM and it is inhibited by down-regulation of VE-cadherin [111]. Melanoma cancer stem cells (MCSCs) are identified by the expression of CD133 and ABCB5. These cells express VE-cadherin and they are located in the perivascular niche and implicated in VM [112]. MCSCs also express VEGFR-1 and are required for VE-cadherin-dependent VM and tumor growth [25]. Anti-VEGF treatments have been proposed in the treatment of melanoma although with low efficiency to target abnormal tumor angiogenesis, due to the acquisition of anti-VEGF by VM induction. This process is mediated by HIF-1α and involves MCSC in perivascular niche [34]. It has also been reported that PECAM1+/VEGFR-2– subpopulation of melanoma cells induces VM in a PECAM1-dependent process. This process is also dependent on AP-2α, a transcription factor that represses PECAM1 expression: while knockdown of AP-2α up-regulates PECAM1 and promotes tube formation, lentiviral re-introduction of AP-2α down-regulates PECAM1 and inhibits tube formation [113].
Gliomas
VM is proposed as an important target in glioblastoma because abnormal vasculature induces the loss of blood–brain barrier and contributes to brain edema formation. The expression of VE-cadherin in glioblastoma stem-like cells (GSLCs) has been reported [114]. Based on this premise, Mao et al. demonstrated that VE-cadherin is up-regulated under hypoxic conditions in a way dependent on HIF-1α and HIF-2α and contributes to hypoxia-induced VM [115]. In addition, GSLCs express vascular endothelial growth factor receptor-2 (VEGFR-2) as well as other VM markers. shRNA for VEGFR-2 inhibits tube-like structures formation, VM and vascularization, but also self-renewal and tumor xenograft initiation. For this reason, VEGFR-2 has been proposed as an essential component for the maintenance of stemness capacity and vascularization. VEGFR-2 is necessary for the trans-differentiation of GCSCs to mural cells [116–118]. Other stem-like cells implicated in abnormal vasculogenesis of gliomas are endothelial progenitor cells (EPCs). These cells are recruited from bone marrow and contribute to tumor vascularization. Strictly, EPCs are not tumoral but stem-like cells with trans-differentiation capacity that contribute to tumors aggressiveness [119].
Hepatocellular Carcinoma (HCC)
The transcription factor Twist1 is frequently expressed in the nucleus of HCC cells. Up-regulation of Twist1 enhances VM and its knockdown prevents VM formation. Twist1 increases HCC cells plasticity by up-regulation of VE-cadherin and down-regulation of E-cadherin [14]. Another report also suggests that Twist mediates hypoxia-induced VM [48].
Mesenchymal phenotype and poor differentiation in hepatocarcinoma correlates with VM acquisition. No co-relation has been shown between expression of stemness genes and intrinsic VM capacity and it has been proposed that the role of stemness genes in VM capacity of HCC cells is likely to depend on differentiation status [120].
Non–small cell lung cancer (NSCLC)
In NSCLCs, a Hoechst 33342 dye effluxing side population (SP) cells present features of CSCs. These cells display tubular formation capacity in matrigel [121, 122]. The knockdown of Gli-l (a transcription factor that positively regulates Sox2) by siRNA inhibits VM and stemness capacities of SP cells [123]. YAP1 also regulates the expression of Sox2 by physical interaction with Oct4. NSCLC presents high levels of YAP1 and depletion by knockdown reduces tubules formation capacity in matrigel SP cells [124].
Breast cancer
Breast cancer stem cells (BCSCs) are identified by CD24-, CD44+ and ALDH+ markers or mammosphere-forming capacity. ALDH+, but not ALDH- cells, have VM formation capacity in matrigel. Epidermal growth factor (EGF) mediates this capacity and can be suppressed by EGFR inhibitor gefitinib or shEGFR. Hsp27 is downstream regulated by EGFR pathway and modulates EGF-mediated VM capacity of BCSCs. Therefore, knockdown of Hsp27 suppresses VM capacity of ALDH+ cells [125]. Ubiquitin-specific protease 44 (UPS44) also contributes to VM formation whereas UPS44 knockdown inhibits VM formation of CSCs in vivo [126].
Targeting vasculogenic mimicry
As the use of anti-angiogenic therapies against tumor development has had limited results there is a critical need to attempt novel anti-tumor angiogenesis strategies centered in targeting alternative mechanism used by the tumor cells (by way of trans-differentiation) to become pseudo-vascular cells leading to VM.
Numerous studies have attempted to specifically disable VM in different tumor models (Table 3). In a recent report our group has shown that the use of PARP inhibitors was effective in preventing melanoma-derived lung metastasis in a murine model and this effect was in part explained by PARP inhibitor’s faculty to counteract VM through down-regulation of VE-cadherin concomitant to the loss of EMT attributes linked to a decreased vimentin expression and Integrin-Linked Kinase/GSK3β axis down-regulation [127].
Table 3.
Pharmacological agents targeting VM
| Therapeutic agents | Molecular target or function | Effect on VM | References |
|---|---|---|---|
| Bevacizumab (Avastin) | VEGF | no effect | [114] |
| PARP inhibition | VE-cadherin | inhibition | [127] |
| Thalidomide (Thalomid) | TNFα; ROS producer | inhibition | [147] |
| TNP-470 (AGM-1470) | TK inhibition | no effect | [148] |
| Endostatin (rhEndostatin, Endostar) | integrin signaling | no effect | [149] |
| Rapamycin (Rapamune) | mTOR, VEGF | inhibition | [150] |
| Curcumin | EPHA2, PI3K, MMPs | inhibition | [151] |
| Isoxanthohumol | TGF-β signaling | inhibition | [152] |
| Vadimezan (ASA404, AS1404, DMXAA) | MAPK, VE-cadherin | inhibition | [140] |
| Resveratrol | VEGF-R1, VEGF-R2 | inhibition | [153] |
| Ginsenoside Rg3 | VE-cadherin/MMPs/EPHA2 | inhibition | [154] |
The Rho kinase inhibitors fasudil and Incarvin C have also been shown to prevent VM of B16 mouse melanoma cells and HCC in Matrigel and xenograft tumor growth as therapeutic option for targeting cancer VM [128, 129]. An interesting study by Orechia et al. [130] has shown the syndecan-1 co-expression with VM markers in melanoma patient cell lines having vasculogenic/stem-cell like phenotype; melanoma cells lose their ability to form tubule-like structures in vitro after blocking syndecan-1 activity by the specific human recombinant antibody, OC-46 F2 while the combined therapy using OC-46 F2 and L19-IL2, led to a complete inhibition of tumor growth until day 90 from tumor implantation in 71% of treated mice [130].
Another example of VM targeting has used TNBC as proof-of-concept. TNBC cells remaining after conventional chemotherapy readily form VM, which leads to the relapse of cancer after treatment. Functional liposomes vincristine plus dasatinib modified by a targeting molecule DSPE-PEG2000-c(RGDyK), exhibited the superior performances in the enhancement of cellular uptake via targeted action and the induction of apoptosis and removal of VM channels in the TNBC-bearing mice [131].
A similar approach has been reported for VM in glioblastoma multiforme (GBM). Current prognosis of GBM remains extremely poor attributed to the formation of VM and the presence of glioma initiating cells (GICs) responsible for resistence to current therapies and disease recurrence; paclitaxel-loaded liposomes modified with a peptide R8-c(RGD) (R8-c(RGD)-Lip) were used for the treatment of glioma [132]. An in vitro cellular uptake study proved the strongest targeting ability to be that of R8-c(RGD)-Lip to glioma stem cells. Drug loaded R8-c(RGD)-Lip exhibited an efficient anti-proliferation effect on BCSCs and could induce the destruction of VM channels in vitro. The pharmacodynamics study demonstrated that R8-c(RGD)-modified drug-loaded liposomes achieved both anti-VM and anti-BCSC effects in vivo. Finally, no significant cytotoxicity of the blood system or major organs of the drug-loaded liposomes was observed under treatment dosage in the safety evaluation. In conclusion, all of the results proved that R8-c(RGD)-Lip was a safe and efficient anti-glioma drug delivery system [132].
Conclusion
This review features on one specific strategy that tumor cells utilize to escape from the hostile microenvironment through trans-differentiation leading to a “caricature” of endothelial-like cells, VM. This trans-differentiation allows the tumor mass to evolve to an irrigated, avascular network to avoid shortage of nutrients and oxygen inside the tumor. As we have reviewed, this phenotype change is pertinent to aggressive metastatic behavior and has functional and translational relevance. The molecular pathways underlying VM have lighted up VE-cadherin as a critical component of VM and, therefore it needs to be taken into account for the development of innovative treatment strategies that target tumor cell plasticity and the metastatic properties affiliated with disease recurrence and drug resistance. As a prominent future challenge, new concepts are needed to discern the specific signaling peculiarities of VE-cadherin (different from its role in the endothelial context) affecting tumor cell biology and related to VM/aggressive development. Particular attention is needed to identify the precursors of tumor cells committed to acquire VE-cadherin expression/VM phenotype and to the interactome connecting VE-cadherin with cell trans-differentiation. In this regard, the presence of elevated levels of nuclear VE-cadherin and phospho-VE-cadherin in VM-prone cells (unpublished results) could be crucial to understand the specific role non vascular VE-cadherin in the acquisition of invasive properties. Our ability to effectively abolish cancer is limited in part by the diverse vasculature development in relation with heterogeneous subpopulations contributing to tumors. Moreover, the unintended consequences of hypoxia induced by rapid tumor growth or by some conventional therapies may serve as a catalyst for the VM and CSCs phenotype. Through a profound elucidation of VM biology these limitations could be override. Therefore, it seems prudent, and definitely appropriate, to consider the application of new agents to target VM pathways associated with the stem cell phenotype and resistant to most conventional agents. Targeting VM with specific molecular compounds used in a combinatorial manner with front-line therapies may hold the greatest promise in the war on cancer.
Acknowledgements
SSS has been funded by a fellowship by RTICC RD12/0036/0026. This work was supported by Junta de Andalucía, project of Excellence from Junta de Andalucía P07-CTS-0239, P10-CTS-0662, P10-CTS-383, Spanish Ministry of Economy and Competitiveness SAF2009-13281-C02-01, SAF2012-40011-C02-01, SAF2015-70520-R,RTICC RD12/0036/0026 and CIBERONC ISCIII CB16/12/00421.
Authors’ contributions
DD-B, SS-S, MF-L wrote the manuscript and designed the figures and tables. FJO concieved, wrote and contributed to the design and coordination. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ellis LM, Fidler IJ. Finding the tumor copycat. Therapy fails, patients don’t. Nat Med. 2010;16:974–5. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- 2.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 5.Seftor EA, Meltzer PS, Kirschmann DA, Pe’er J, Maniotis AJ, Trent JM, Folberg R, Hendrix MJ. Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metastasis. 2002;19:233–46. doi: 10.1023/A:1015591624171. [DOI] [PubMed] [Google Scholar]
- 6.Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta. 1806;2010:18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726–32. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–25. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, Yu X, Tian Y. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med. 2015;19:315–26. doi: 10.1111/jcmm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breier G, Grosser M, Rezaei M. Endothelial cadherins in cancer. Cell Tissue Res. 2014;355:523–7. doi: 10.1007/s00441-014-1851-7. [DOI] [PubMed] [Google Scholar]
- 11.Williamson SC, Metcalf RL, Trapani F, Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N, Polanski R, Nonaka D, Priest L, Fusi A, Carlsson F, Carlsson A, Hendrix MJ, Seftor RE, Seftor EA, Rothwell DG, Hughes A, Hicks J, Miller C, Kuhn P, Brady G, Simpson KL, Blackhall FH, Dive C. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–57. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 14.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51:545–56. doi: 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- 15.Topczewska JM, Postovit L-M, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 16.McAllister JC, Zhan Q, Weishaupt C, Hsu MY, Murphy GF. The embryonic morphogen, Nodal, is associated with channel‐like structures in human malignant melanoma xenografts. J Cutan Pathol. 2010;37:19–25. doi: 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu ZY, Dong XY, Che N, Mo J, Gu Q. Hypoxia-induced vasculogenic mimicry formation via VE-cadherin regulation by Bcl-2. Med Oncol. 2012;29:3599–607. doi: 10.1007/s12032-012-0245-5. [DOI] [PubMed] [Google Scholar]
- 18.Tang N-N, Zhu H, Zhang H-J, Zhang W-F, Jin H-L, Wang L, Wang P, He G-J, Hao B, Shi R-H. HIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World J Gastroenterol. 2014;20:17894. doi: 10.3748/wjg.v20.i47.17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Sun B, Zhao X, Gu Q, Dong X, Yao Z, Zhao N, Chi J, Liu N, Sun R. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med. 2013;17:116–22. doi: 10.1111/j.1582-4934.2012.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavard J, Gutkind JS. VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol. 2008;10:883–5. doi: 10.1038/ncb0808-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean C, Chen XL, Nam JO, Tancioni I, Uryu S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J Cell Biol. 2014;204:247–63. doi: 10.1083/jcb.201307067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014;15:223–30. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 24.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 25.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, Saab KR, Osherov V, Widlund HR, Gasser M. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 27.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/S1097-2765(00)80221-X. [DOI] [PubMed] [Google Scholar]
- 28.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–51. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinella F, Caprara V, Di Castro V, Rosano L, Cianfrocca R, Natali PG, Bagnato A. Endothelin-1 induces the transactivation of vascular endothelial growth factor receptor-3 and modulates cell migration and vasculogenic mimicry in melanoma cells. J Mol Med (Berl) 2013;91:395–405. doi: 10.1007/s00109-012-0956-2. [DOI] [PubMed] [Google Scholar]
- 32.Basu GD, Liang WS, Stephan DA, Wegener LT, Conley CR, Pockaj BA, Mukherjee P. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8:R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson FM, Simeone AM, Lucci A, McMurray JS, Ghosh S, Cristofanilli M. Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4. Cancer. 2010;116:2806–14. doi: 10.1002/cncr.25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnegg CI, Yang MH, Ghosh SK, Hsu M-Y. Induction of vasculogenic mimicry overrides VEGF-A silencing and enriches stem-like cancer cells in melanoma. Cancer Res. 2015;75:1682–90. doi: 10.1158/0008-5472.CAN-14-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, Hendrix MJ. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–5. [PubMed] [Google Scholar]
- 36.Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–33. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- 37.Hess AR, Margaryan NV, Seftor EA, Hendrix MJ. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors. Dev Dyn. 2007;236:3283–96. doi: 10.1002/dvdy.21190. [DOI] [PubMed] [Google Scholar]
- 38.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–24. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–7. [PubMed] [Google Scholar]
- 40.Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279–88. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu XS, Sun W, Ge CY, Zhang WZ, Fan YZ. Contribution of the PI3K/MMPs/Ln-5gamma2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int J Oncol. 2013;42:2103–15. doi: 10.3892/ijo.2013.1897. [DOI] [PubMed] [Google Scholar]
- 42.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–56. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 43.Hess AR, Postovit L-M, Margaryan NV, Seftor EA, Schneider GB, Seftor RE, Nickoloff BJ, Hendrix MJ. Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Res. 2005;65:9851–60. doi: 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- 44.Vartanian A, Gatsina G, Grigorieva I, Solomko E, Dombrovsky V, Baryshnikov A, Stepanova E. The involvement of Notch signaling in melanoma vasculogenic mimicry. Clin Exp Med. 2013;13:201–9. doi: 10.1007/s10238-012-0190-9. [DOI] [PubMed] [Google Scholar]
- 45.Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit L-M, Strizzi L, Hendrix MJ. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010;70:10340–50. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254:157–64. doi: 10.1016/j.canlet.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 47.Huang M, Ke Y, Sun X, Yu L, Yang Z, Zhang Y, Du M, Wang J, Liu X, Huang S. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1α. Oncol Rep. 2014;32:1973–80. doi: 10.3892/or.2014.3454. [DOI] [PubMed] [Google Scholar]
- 48.Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, Lv Y. Role of Twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686–91. doi: 10.1016/j.bbrc.2011.03.124. [DOI] [PubMed] [Google Scholar]
- 49.Comito G, Calvani M, Giannoni E, Bianchini F, Calorini L, Torre E, Migliore C, Giordano S, Chiarugi P. HIF-1alpha stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic Biol Med. 2011;51:893–904. doi: 10.1016/j.freeradbiomed.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 50.Sun B, Zhang D, Zhang S, Zhang W, Guo H, Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249:188–97. doi: 10.1016/j.canlet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Misra RM, Bajaj MS, Kale VP. Vasculogenic mimicry of HT1080 tumour cells in vivo: critical role of HIF-1alpha-neuropilin-1 axis. PLoS One. 2012;7:e50153. doi: 10.1371/journal.pone.0050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maes H, Van Eygen S, Krysko D, Vandenabeele P, Nys K, Rillaerts K, Garg A, Verfaillie T, Agostinis P. BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Bras A, Lionneton F, Mattot V, Lelievre E, Caetano B, Spruyt N, Soncin F. HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–9. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Cao Z, Sun B2, Zhao X, Zhang Y, Gu Q, Liang X, Dong X, Zhao N. The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial-Mesenchymal Transition. Int J Mol Sci. 2017;18:E500. [DOI] [PMC free article] [PubMed]
- 56.Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/S1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- 57.Mourad-Zeidan AA, Melnikova VO, Wang H, Raz A, Bar-Eli M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am J Pathol. 2008;173:1839–52. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319:1317–23. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol. 2014;229:1908–17. doi: 10.1002/jcp.24566. [DOI] [PubMed] [Google Scholar]
- 60.McDonald S, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–14. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y, Cheng R, Dong X. Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase Cα in epithelial ovarian cancer. Oncol Rep. 2014;32:771–9. doi: 10.3892/or.2014.3229. [DOI] [PubMed] [Google Scholar]
- 62.Qi L, Song W, Liu Z, Zhao X, Cao W, Sun B. Wnt3a Promotes the vasculogenic mimicry formation of colon cancer via wnt/β-catenin signaling. Int J Mol Sci. 2015;16:18564–79. doi: 10.3390/ijms160818564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X, Dong X, Chi J. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013. [DOI] [PubMed]
- 64.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 66.Labelle M, Schnittler HJ, Aust DE, Friedrich K, Baretton G, Vestweber D, Breier G. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor β signaling. Cancer Res. 2008;68:1388–97. doi: 10.1158/0008-5472.CAN-07-2706. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q, An J, Dong X, Liu F, Wang Y. ZEB2 promotes vasculogenic mimicry by TGF-β1 induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Exp Mol Pathol. 2015;98:352–9. doi: 10.1016/j.yexmp.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Q-Q, Ma C, Wang Q, Song Y, Lv T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biol. 2015;37:1–13. doi: 10.1007/s13277-015-4450-7. [DOI] [PubMed] [Google Scholar]
- 69.Lissitzky J-C, Parriaux D, Ristorcelli E, Vérine A, Lombardo D, Verrando P. Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Res. 2009;69:802–9. doi: 10.1158/0008-5472.CAN-08-2391. [DOI] [PubMed] [Google Scholar]
- 70.Huang B, Xiao E, Huang M. MEK/ERK pathway is positively involved in hypoxia-induced vasculogenic mimicry formation in hepatocellular carcinoma which is regulated negatively by protein kinase A. Med Oncol. 2015;32:408. doi: 10.1007/s12032-014-0408-7. [DOI] [PubMed] [Google Scholar]
- 71.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and EPAC-dependent signals dowstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–62. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 72.Almenar-Queralt A, Kim SN, Benner C, Herrera CM, Kang DE, Garcia-Bassets I, Goldstein LS. Presenilins regulate neurotrypsin gene expression and neurotrypsin-dependent agrin cleavage via cyclic AMP response element-binding protein (CREB) modulation. J Biol Chem. 2013;288:35222–36. doi: 10.1074/jbc.M113.513705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 75.Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35(Suppl):S199–223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendrix MJ, Seftor RE, Seftor EA, Gruman LM, Lee LM, Nickoloff BJ, Miele L, Sheriff DD, Schatteman GC. Transendothelial function of human metastatic melanoma cells: role of the microenvironment in cell-fate determination. Cancer Res. 2002;62:665–8. [PubMed] [Google Scholar]
- 77.Seftor EA, Meltzer PS, Kirschmann DA, Margaryan NV, Seftor RE, Hendrix MJ. The epigenetic reprogramming of poorly aggressive melanoma cells by a metastatic microenvironment. J Cell Mol Med. 2006;10:174–96. doi: 10.1111/j.1582-4934.2006.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaessmeyer S, Bhoola K, Baltic S, Thompson P, Plendl J. Lung cancer neovascularisation: cellular and molecular interaction between endothelial and lung cancer cells. Immunobiology. 2014;219:308–14. doi: 10.1016/j.imbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Vartanian A, Karshieva S, Dombrovsky V, Belyavsky A. Melanoma educates mesenchymal stromal cells towards vasculogenic mimicry. Oncol Lett. 2016;11:4264–8. doi: 10.3892/ol.2016.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J, Lu Y, Lin YY, Zheng ZY, Fang JH, He S, Zhuang SM. Vascular mimicry formation is promoted by paracrine TGF-beta and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016;383:18–27. doi: 10.1016/j.canlet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri F, Laurenzana A, Del Rosso M, Chiarugi P. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-beta1. Stem Cells. 2016;34:2536–47. doi: 10.1002/stem.2412. [DOI] [PubMed] [Google Scholar]
- 82.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–84. doi: 10.1161/01.RES.87.5.378. [DOI] [PubMed] [Google Scholar]
- 83.Barnett FH, Rosenfeld M, Wood M, Kiosses WB, Usui Y, Marchetti V, Aguilar E, Friedlander M. Macrophages form functional vascular mimicry channels in vivo. Sci Rep. 2016;6:36659. doi: 10.1038/srep36659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basu GD1, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. 2005;7:R422-35. [DOI] [PMC free article] [PubMed]
- 85.Su M, Fan C, Gao S, Shen A, Wang X, Zhang Y. An HCG-rich microenvironment contributes to ovarian cancer cell differentiation into endothelioid cells in a three-dimensional culture system. Oncol Rep. 2015;34:2395–402. doi: 10.3892/or.2015.4215. [DOI] [PubMed] [Google Scholar]
- 86.Caceres-Cortes J, Mindeni M, Patersoni B, Caligiuri MA. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:17. doi: 10.1038/367017a0. [DOI] [PubMed] [Google Scholar]
- 87.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 88.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 90.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 91.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 92.Kumar D, Kumar S, Gorain M, Tomar D, Patil HS, Radharani NN, Kumar TV, Patil TV, Thulasiram HV, Kundu GC. Notch1-MAPK signaling axis regulates CD133+ cancer stem cell-mediated melanoma growth and angiogenesis. J Invest Dermatol. 2016;136:2462–74. doi: 10.1016/j.jid.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 93.Kim CFB, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 94.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 96.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 97.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 99.Prince M, Sivanandan R, Kaczorowski A, Wolf G, Kaplan M, Dalerba P, Weissman I, Clarke M, Ailles L. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma S, Chan KW, Hu L, Lee TKW, Wo JYH, Ng IOL, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–56. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 101.Patel GK, Yee CL, Terunuma A, Telford WG, Voong N, Yuspa SH, Vogel JC. Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma. J Investig Dermatol. 2012;132:401–9. doi: 10.1038/jid.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 103.Islam F, Qiao B, Smith RA, Gopalan V, Lam AK-Y. Cancer stem cell: fundamental experimental pathological concepts and updates. Exp Mol Pathol. 2015;98:184–91. doi: 10.1016/j.yexmp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 105.Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–69. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ritchie KE, Nör JE. Perivascular stem cell niche in head and neck cancer. Cancer Lett. 2013;338:41–6. doi: 10.1016/j.canlet.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 108.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, Liu N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–53. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 109.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wan F, Zhang S, Xie R, Gao B, Campos B, Herold-Mende C, Lei T. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010;20:877–89. doi: 10.1111/j.1750-3639.2010.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–23. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai CY, Schwartz BE, Hsu MY. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72:5111–8. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM, Shelton SE, Irvin DM, Brings VE, Ollila DW, Brekken RA. Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat Commun. 2014;5. [DOI] [PMC free article] [PubMed]
- 114.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 115.Mao XG, Xue XY, Wang L, Zhang X, Yan M, Tu YY, Lin W, Jiang XF, Ren HG, Zhang W, Song SJ. CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro Oncol. 2013;15:865–79. doi: 10.1093/neuonc/not029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–82. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32:12950–60. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M, Gong W, Chen C, Niu Q, Guo D, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS One. 2013;8:e57188. doi: 10.1371/journal.pone.0057188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Chen L, Wang Q, Wang L, Wang H, Shen Y, Li X, Fu Y, Shen Y, Yu Y. Circulating endothelial progenitor cells are involved in VEGFR-2-related endothelial differentiation in glioma. Oncol Rep. 2014;32:2007–14. doi: 10.3892/or.2014.3467. [DOI] [PubMed] [Google Scholar]
- 120.Lirdprapamongkol K, Chiablaem K, Sila-Asna M, Surarit R, Bunyaratvej A, Svasti J. Exploring stemness gene expression and vasculogenic mimicry capacity in well- and poorly-differentiated hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2012;422:429–35. doi: 10.1016/j.bbrc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh S, Bora-Singhal N, Kroeger J, Laklai H, Chellappan SP. βArrestin-1 and Mcl-1 modulate self-renewal growth of cancer stem-like side-population cells in non-small cell lung cancer. PLoS One. 2013;8:e55982. doi: 10.1371/journal.pone.0055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bora-Singhal N, Perumal D, Nguyen J, Chellappan S. Gli1-mediated regulation of Sox2 facilitates self-renewal of stem-like cells and confers resistance to EGFR inhibitors in non–small cell lung cancer. Neoplasia. 2015;17:538–51. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bora‐Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. STEM CELLS. 2015;33:1705–18. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee C-H, Wu Y-T, Hsieh H-C, Yu Y, Alice LY, Chang W-W. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie. 2014;104:117–26. doi: 10.1016/j.biochi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 126.Liu T, Sun B, Zhao X, Li Y, Zhao X, Liu Y, Yao Z, Gu Q, Dong X, Shao B. USP44+ cancer stem cell subclones contribute to breast cancer aggressiveness by promoting vasculogenic mimicry. Mol Cancer Ther. 2015;14:2121–31. doi: 10.1158/1535-7163.MCT-15-0114-T. [DOI] [PubMed] [Google Scholar]
- 127.Rodriguez MI, Peralta-Leal A, O’Valle F, Rodriguez-Vargas JM, Gonzalez-Flores A, Majuelos-Melguizo J, Lopez L, Serrano S, de Herreros AG, Rodriguez-Manzaneque JC, et al. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013;9:e1003531. doi: 10.1371/journal.pgen.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xia Y, Cai XY, Fan JQ, Zhang LL, Ren JH, Chen J, Li ZY, Zhang RG, Zhu F, Wu G. Rho kinase inhibitor fasudil suppresses the vasculogenic mimicry of B16 mouse melanoma cells both in vitro and in vivo. Mol Cancer Ther. 2015;14:1582–90. doi: 10.1158/1535-7163.MCT-14-0523. [DOI] [PubMed] [Google Scholar]
- 129.Zhang JG, Zhang DD, Wu X, Wang YZ, Gu SY, Zhu GH, Li XY, Li Q, Liu GL. Incarvine C suppresses proliferation and vasculogenic mimicry of hepatocellular carcinoma cells via targeting ROCK inhibition. BMC Cancer. 2015;15:814. doi: 10.1186/s12885-015-1809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orecchia P, Conte R, Balza E, Pietra G, Mingari MC, Carnemolla B. Targeting Syndecan-1, a molecule implicated in the process of vasculogenic mimicry, enhances the therapeutic efficacy of the L19-IL2 immunocytokine in human melanoma xenografts. Oncotarget. 2015;6:37426–42. doi: 10.18632/oncotarget.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeng F, Ju RJ, Liu L, Xie HJ, Mu LM, Zhao Y, Yan Y, Hu YJ, Wu JS, Lu WL. Application of functional vincristine plus dasatinib liposomes to deletion of vasculogenic mimicry channels in triple-negative breast cancer. Oncotarget. 2015;6:36625–42. doi: 10.18632/oncotarget.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Y, Mei L, Yu Q, Xu C, Qiu Y, Yang Y, Shi K, Zhang Q, Gao H, Zhang Z, He Q. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7:16792–801. doi: 10.1021/acsami.5b04596. [DOI] [PubMed] [Google Scholar]
- 133.Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM, Shelton SE, Irvin DM, Brings VE, Ollila DW, Brekken RA, et al. Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat Commun. 2014;5:5200. doi: 10.1038/ncomms6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan LY, Mintoff C, Johan MZ, Ebert BW, Fedele C, Zhang YF, Szeto P, Sheppard KE, McArthur GA, Foster-Smith E, et al. Desmoglein 2 promotes vasculogenic mimicry in melanoma and is associated with poor clinical outcome. Oncotarget. 2016;7:46492–508. doi: 10.18632/oncotarget.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orgaz JL, Ladhani O, Hoek KS, Fernandez-Barral A, Mihic D, Aguilera O, Seftor EA, Bernad A, Rodriguez-Peralto JL, Hendrix MJ, et al. Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma. Oncogene. 2009;28:4147–61. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu X-S, Sun W, Ge C-Y, Zhang W-Z, Fan Y-Z. Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int J Oncol. 2013;42:2103–15. doi: 10.3892/ijo.2013.1897. [DOI] [PubMed] [Google Scholar]
- 137.Wu N, Zhao X, Liu M, Liu H, Yao W, Zhang Y, Cao S, Lin X. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PLoS One. 2011;6:e16264. doi: 10.1371/journal.pone.0016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun Q, Zou X, Zhang T, Shen J, Yin Y, Xiang J. The role of miR-200a in vasculogenic mimicry and its clinical significance in ovarian cancer. Gynecol Oncol. 2014;132:730–8. doi: 10.1016/j.ygyno.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 139.Gao R, Cai C, Gan J, Yang X, Shuang Z, Liu M, Li S, Tang H. miR-1236 down-regulates alpha-fetoprotein, thus causing PTEN accumulation, which inhibits the PI3K/Akt pathway and malignant phenotype in hepatoma cells. Oncotarget. 2015;6:6014–28. doi: 10.18632/oncotarget.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang Y, Gu Q, Dong X, Liu F, Zhang Y, Li X. miR-27a-3p suppresses tumor metastasis and VM by down-regulating VE-cadherin expression and inhibiting EMT: an essential role for Twist-1 in HCC. Sci Rep. 2016;6:23091. doi: 10.1038/srep23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao X, Wang Y, Deng R, Zhang H, Dou J, Yuan H, Hou G, Du Y, Chen Q, Yu J. miR186 suppresses prostate cancer progression by targeting Twist1. Oncotarget. 2016;7:33136–51. doi: 10.18632/oncotarget.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shevde LA, Metge BJ, Mitra A, Xi Y, Ju J, King JA, Samant RS. Spheroid-forming subpopulation of breast cancer cells demonstrates vasculogenic mimicry via hsa-miR-299-5p regulated de novo expression of osteopontin. J Cell Mol Med. 2010;14:1693–706. doi: 10.1111/j.1582-4934.2009.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weng C, Dong H, Chen G, Zhai Y, Bai R, Hu H, Lu L, Xu Z. miR-409-3p inhibits HT1080 cell proliferation, vascularization and metastasis by targeting angiogenin. Cancer Lett. 2012;323:171–9. doi: 10.1016/j.canlet.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 144.Wan HY, Li QQ, Zhang Y, Tian W, Li YN, Liu M, Li X, Tang H. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014;355:148–58. doi: 10.1016/j.canlet.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 145.Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y, Gu X, Meng F. MiR-124 exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015;282:4376–88. doi: 10.1111/febs.13502. [DOI] [PubMed] [Google Scholar]
- 146.Xue H, Gao X, Xu S, Zhang J, Guo X, Yan S, Li T, Guo X, Liu Q, Li G. MicroRNA-Let-7f reduces the vasculogenic mimicry of human glioma cells by regulating periostin-dependent migration. Oncol Rep. 2016;35:1771–7. doi: 10.3892/or.2016.4548. [DOI] [PubMed] [Google Scholar]
- 147.Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y, Sun B. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60. doi: 10.1186/1756-9966-27-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van de Wouw AJ, Jansen RL, Griffioen AW, Hillen HF. Clinical and immunohistochemical analysis of patients with unknown primary tumour. A search for prognostic factors in UPT. Anticancer Res. 2004;24:297–301. [PubMed] [Google Scholar]
- 149.van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96:1473–7. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- 150.Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ, Huang Y, Zhao YQ, Jiang H. Plasticity of ovarian cancer cell SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer. 2008;18:476–86. doi: 10.1111/j.1525-1438.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- 151.Chen LX, Sun BC, Zhang SW, He YJ, Li XR, He ZJ. The mechanisms of microenvironments influence on vasculogenic mimicry between intraocular and subcutaneous melanoma. Zhonghua Yan Ke Za Zhi. 2009;45:641–6. [PubMed] [Google Scholar]
- 152.Serwe A, Rudolph K, Anke T, Erkel G. Inhibition of TGF-beta signaling, vasculogenic mimicry and proinflammatory gene expression by isoxanthohumol. Invest New Drugs. 2012;30:898–915. doi: 10.1007/s10637-011-9643-3. [DOI] [PubMed] [Google Scholar]
- 153.Vartanian AA, Burova OS, Stepanova EV, Baryshnikov AY, Lichinitser MR. Melanoma vasculogenic mimicry is strongly related to reactive oxygen species level. Melanoma Res. 2007;17:370–9. doi: 10.1097/CMR.0b013e3282f1d2ec. [DOI] [PubMed] [Google Scholar]
- 154.Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VEcadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 2014;45:1065–72. doi: 10.3892/ijo.2014.2500. [DOI] [PubMed] [Google Scholar]


