Abstract
Leukocytapheresis has reemerged as a novel “nondrug” approach in the treatment of inflammatory bowel disease. The technique involves the extracorporeal passage of peripheral blood through a column of cellulose diacetate beads (Adacolumn) or a nonwoven polyester fiber filter (Cellsorba). The benefits accrued from the filtered extraction of granulocytes, monocytes (Adacolumn), and lymphocytes (Cellsorba) appear greater than the simple extraction of these cells. There appears to be an immunologic modulation of leukocytes and dendritic cells and a diminished response to proinflammatory cytokines. Unfortunately, blinded placebo-controlled trials are lacking. Nevertheless, the aggregate clinical experience detailed in this review suggests a relatively safe and attractive alternative to current inflammatory bowel disease therapies. Randomized, controlled sham trials are in progress.
Keywords: Leukocytapheresis, inflammatory cytokines, granulocytes, monocytes
Treatments for ulcerative colitis (UC) and Crohn’s disease (CD) appear to proliferate exponentially, but close scrutiny of their efficacy leads to the conclusion that a considerable portion of patients fail to benefit or are at risk for drug side effects or adverse events.1,2 One effort to avoid such drug toxicity and improve clinical benefit has focused on selective apheresis of leukocytes.3,4 Granulocytes, monocytes, and, to a lesser degree, lymphocytes, play a significant role in initiating and maintaining the inflammatory reaction of inflammatory bowel disease (IBD) by releasing proinflammatory cytokines, proteases, and other mediators of inflammation.5,6 Recognizing the significant concentrations of granulocytes and monocytes both in the circulation and inflamed tissues of IBD patients,7,8,9 selective leukocytapheresis (LCAP) has been employed to remove these cells from the host’s circulation before exiting into the inflamed tissue, with the aim of improving or modifying the cellular immune response.10,11
Although not a new concept, interest in extracorporeal LCAP has increased recently and stimulated several studies, primarily small uncontrolled studies. Although effectiveness has not as yet been substantiated by blinded placebo-controlled randomized clinical trials, the idea of a no-drug approach is appealing. The concept of taking something from the patient that is contributing to the disease and not giving a chemical or biologic therapy to the patient presents an attractive therapeutic alternative.
Leukocytapheresis Techniques
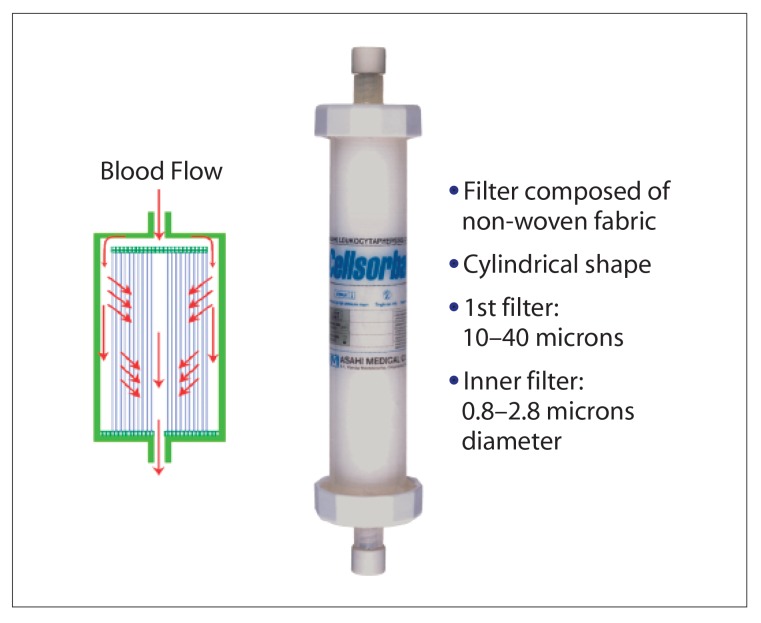
Most commonly with LCAP, peripheral blood is passed extracorporeally through a removable column or fiber filter that adsorbs leukocytes while the remaining blood is returned to the patient though a separate intravenous (IV) line. The two selective apheresis filters currently available are the Cellsorba system (Asahi Kasei Medical; Figure 1), which contains nonwoven polyester fibers that remove granulocytes and monocytes (90%), lymphocytes (70%), and some platelets,12 and the Adacolumn system (JIMRO; Figure 2), which contains cellulose diacetate beads that adhere to Fc gamma and complement receptors on granulocytes and monocytes, selectively removing them without significantly affecting lymphocytes or platelets (Figure 3).13 Alternatively, the centrifugal system uses one venous line to obtain blood and remove a buffy coat before reinfusing (multi-component system, Haemonetics).14 However, the fiber-and-column technique removes a 4-fold greater amount of leukocytes than centrifugation.15,16 A third, little-used technique is extracorporeal phototherapy in which blood removed from the patient is treated (with psoralen and ultraviolet irradiation) and then returned to the patient.17
Figure 1.
Cellsorba product design.
Figure 2.
Adacolumn product design.
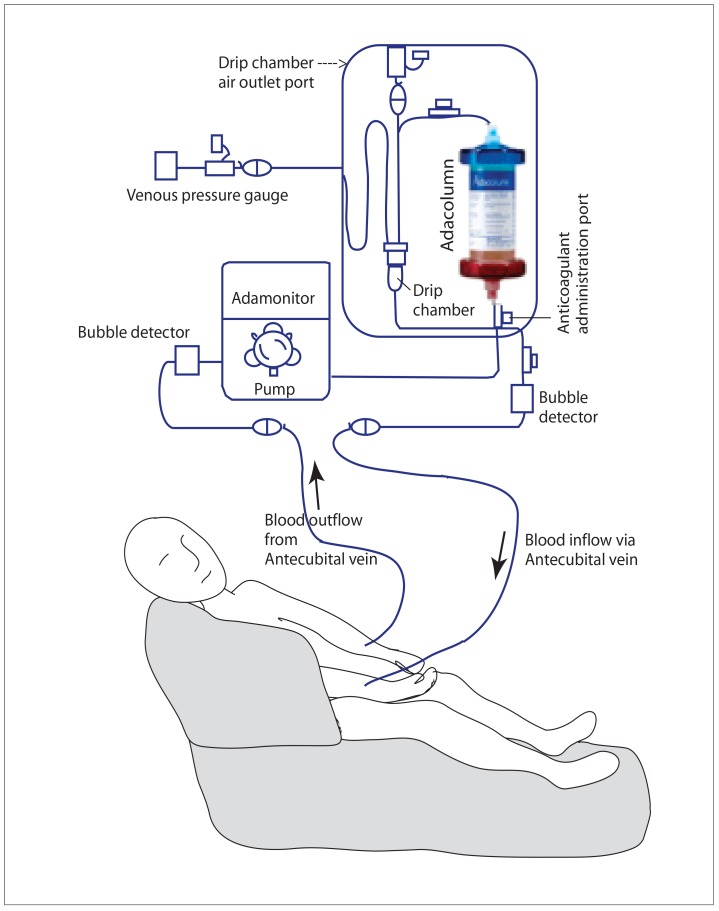
Figure 3.
Adacolumn circuit: an outline of the extra-corporeal circulation through the Adacolumn device.
How Does LCAP Work?
The benefits of LCAP are more than just the removal of activated white blood cells. The “quantitative” removal of activated circulating leukocytes by these filters presumably reduces the concentration of inflammatory cells in diseased tissue. These diverted leukocytes are replaced by naïve leukocytes from the bone marrow or peripheral pooling sites.18 As a result, there is a decrease in leukocyte expression of adhesion molecules (L-selectin, integrins)19 and reactive oxygen species.20 Other mechanisms at work include immunologic modulation of leukocytes and dendritic cells and reduced cytokine production, with an altered response to proinflammatory cytokines and endotoxins.21 See Table 1.
Table 1.
Immune Modulation Effects of Leukocytapheresis
|
IL=interleukin.
In addition, Adacolumn spares lymphocytes, which may be a benefit. Prior selective depletion of lymphocytes in CD patients not only showed no clinical advantage but the outcome was 21% poorer than in the control group.28 In both rheumatoid arthritis and CD there is an actual increase in lymphocytes, primarily CD4+ cells. These CD4+ cells, together with CD25+ T cells, inhibit T-cell activation and secrete an anti-inflammatory cytokine, interleukin (IL)-10, which aids in regulating a balanced immune response in the gut mucosa.29,30 The role of the circulatory immune complex in IBD is interrupted as well. Cellulose acetate beads in Adacolumn adsorb immunoglobulin (Ig)G and immune complex from plasma,31,32 which then bind to Fc gamma receptors on neutrophils and macrophages.33,34 The adsorbed IgG and immune complex generate complement activation fragments (C3a, C4a, C5a)7,31,33 as well as opsonins (C3b/C3bi, C4b/C4bi, C5), which then also adsorb to the column31,34,35 and bind leukocyte complement receptors CR1, CR2, and CR3 (MAC-1, CD11b, CD18) and CR4.36-38 Note that leukocyte adsorption is affected by complement opsonins, Fc gamma receptors, and complement receptors on leukocytes, especially neutrophils, monocytes, and macrophages. However, lymphocytes lack complement receptors except for B, T, and natural killer cells.37,39 Also, Fc gamma receptors are not usually expressed on lymphocytes, except for small segments of CD19+ B cells and CD56+ natural killer cells.40 This lack of expression might explain why Adacolumn selectively adsorbs granulocytes and not lymphocytes.
Ulcerative Colitis Experience and Disclaimer
The following chronology of experience with LCAP is plagued by the heterogeneity of the patient population, methodologic flaws of missing primary and secondary endpoints, inconsistent measures of disease activity varying from accepted validated instruments (eg, the Ulcerative Colitis Disease Activity Index [UCDAI] and the Inflammatory Bowel Disease Quality-of-Life index [IBDQ]) to simply the investigator’s subjective evaluation of improvement. Concomitant medications or changes in dosage or frequency of their use are frequently lacking in the record. There is only one sham (ie, placebo) study.41 Few studies are randomized and most trials are unblinded. Despite these drawbacks, the aggregate impression of the value of apheresis has earned its approval for use in UC by the Japanese ministry of health and a European commission of certification from a regulatory compliance organization (the TOV product service) in 1999.42
Adacolumn
The Adacolumn system employs a column of cellulose acetate beads that selectively adsorb 65% of granulocytes, 55% of monocytes, and 2% of lymphocytes; red blood cells and platelets are virtually unchanged, as are clotting factors.7 The following is a brief summary of the results of selected published studies on the use of Adacolumn (also referred to as granulocyte-monocyte adsorptive apheresis) for the treatment of UC; additional studies are listed in Table 2.
Table 2.
Selected Studies of Adacolumn for Treatment of UC
| Reference | N | Results |
|---|---|---|
| Shimoyama et al52 | 53 | 58.5% responded, 11 pts in remission at wk 7; prednisone dose reduced (24.2 mg to 14.2 mg mean dose) |
| Tomomasa et al53 | 12 | 8/12 pediatric pts improved over 5–10 sessions w/i 24 days; 4/5 relapsed in 3.5 mo; 4/8 in remission up to 22.8 mo |
| Sakuraba et al11 | 10 | 8/10 responses in 7.5 days with 3× weekly sessions vs 22.5 days if once weekly |
| Hanai et al54 | 46 | 42 in remission by week 20 after 10 sessions; increased IL-1RA |
| Yamamoto et al55 | 30 | 21/30 distal UC pts entered remission; mild AEs in 8 (headache, tenesmus, 1 fever, 1 LFT abnormal) |
| Sawada et al56 | 53 | 58.5% in remission at wk 7 (5 sessions in 5 weeks) vs 44% of prednisone pts |
| Hanai et al5 | 46 | 83% remission vs 65% with high-dose steroids at wk 12 |
AEs=adverse events; IL-1RA=interleukin-1 receptor antagonist; LFT=liver function test; pts=patients; UC=ulcerative colitis.
A combined Japanese and UK trial of granulocyte-monocyte adsorptive apheresis in 33 patients reported remission rates of 81% in steroid-refractory and 88% steroid-naive patients. Eleven cycles of apheresis over 11 weeks were used. Maintenance of remission occurred in 26 of 33 (78%) patients at 12 months and 14 of 25 patients became steroid-free with few adverse events.43
A disconcerting lack of benefit over a steroid comparator group occurred in 19 UC patients given prednisone and apheresis (4 with the Haemonetics centrifugal system, 15 with Adacolumn), with a clinical response of 68.4% with apheresis versus 75% with prednisone alone. Fifteen LCAP and 11 prednisone patients required colectomy. A problem with interpretation of these results exists because the apheresis group had a significantly higher initial prednisone requirement, indicating more severe disease. Clinical remission (Seo index) occurred in 6 of 19 apheresis patients (31.6%) versus 12 of 16 prednisone patients (75%). These results, although limited by a small sample size, bring into question the value of apheresis as initial therapy for severe UC.44
Maintenance of remission with intermittent every-2-week Adacolumn apheresis for 12 months was as effective in 7 of 10 UC patients as 6-mercaptopurine (6-MP) was in 6 of 10 patients. Three apheresis patients relapsed at 4, 5, and 12 months versus 3 6-MP patients at 4, 10, and 11 months; 1 6-MP patient was excluded after liver abnormalities. Apheresis appeared comparable to 6-MP in maintaining remission in this limited study.45
Twenty steroid-refractory and 10 steroid-dependent Japanese UC patients received 5 apheresis sessions with Adacolumn over 4 weeks. Twenty-four patients (55%) entered remission. Nine (20%) responded and 11 (25%) were unchanged. Only 2 of 10 “severe” steroid-refractory patients underwent remission but 7 of 10 “moderate” refractory patients did so. Steroids could be tapered in 9 of 10 steroid-dependent patients.46
Fifteen IBD patients intolerant or refractory to all prior IBD therapy were entered into an open-label pilot granulocyte/monocyte apheresis program with Adacolumn given in 5 sessions. Eleven of 15 UC patients completed the sessions; 4 patients responded (UCDAI reduction of >3 points), and 1 entered remission (UCDAI <2) with overall improvement in IBDQ scores. CD patient response was more impressive in 14 of 15 patients completing the 5 sessions. Eight CD patients (57%) responded and 5 entered remission. No device-related serious adverse events were noted. This initial US experience shows promise for refractory IBD patients.47
Twenty steroid-naïve active UC patients were treated with Adacolumn for an average of 6 to 10 sessions at 2 sessions per week. Seventeen patients (85%) entered remission with a decrease in C-reactive protein (CRP), total white blood cell count (WBC), polymorphonuclear leukocytes (PMNs), and monocytes. An elevated lymphocyte count and soluble tumor necrosis factor (TNF) receptors I and II were noted in the return blood flow to the patient after filtration. Sixty percent maintained in remission at 8 months.48
In a Scandinavian apheresis experience of 100 patients, remission occurred in 44 patients and a response was seen in 24 patients, for an overall response rate of 68%. Of the patients, 52 had UC, 44 had CD, and 4 had indeterminate colitis. The mean time to response was 7 weeks after 5 weekly sessions, and mean time to relapse was 5.5 months. A total of 27 of 50 patients discontinued steroids. Adverse events were minor but 1 patient had a pulmonary embolism using a central venous catheter.49
Semiweekly granulocyte and monocyte apheresis in place of weekly sessions resulted in a remarkable 73.1% remission rate (38/52 patients) with only 15.9 days to achieving remission versus a 46.7% remission rate (21/45 patients) and 28.1 days until remission in those utilizing a weekly schedule. Each group received a total of 10 treatments. If substantiated in future trials, the semiweekly program would immeasurably enhance compliance and overall patient acceptance.50
Five weeks (5 cycles) of Adacolumn apheresis in 12 patients with moderately active UC resulted in clinical remission in 8 patients, endoscopic and histologic improvement in 11 patients, and steroid withdrawal in 9. All 12 patients showed significant improvement in quality of life scores.51
Cellsorba
The Cellsorba system utilizes 2 filters. The unwoven fiber filter traps 90% of leukocytes (100% granulocytes, 60% lymphocytes, 35% platelets) but a “rebound” increase of 170% of initial leukocyte count occurs 20 minutes post-procedure, which then normalizes within 24 hours.35,41 The following is a brief summary of the results of selected published studies on the use of Cellsorba for the treatment of UC; additional studies are listed in Table 3.
Table 3.
Selected Studies of Cellsorba for treatment of UC
| Reference | N | Results |
|---|---|---|
| Sawada et al41 | 45 | “New” pts; 35 improved and maintained improvement |
| Sakata et al60 | 51 | 33 (64.7%) entered clinical and endoscopic remission; no AEs |
| Sawada et al6 | 39 | 74% benefited from 5 weekly then monthly sessions; AEs 24% vs 68% with high-dose prednisone |
| Matsumoto et al61 | 70 | 51% response in 6 sessions; 26% needed additional therapy, 6 to surgery |
AEs=adverse events; pts=patients; UC=ulcerative colitis.
Thirteen patients (8 with UC and 5 with CD) were treated with Cellsorba for 5 sessions then 5 monthly treatments (averaging 3 L filtration per session), resulting in 11 patients (84.6%) improving and 6 achieving remission at the completion of the 5 monthly sessions. Eight patients (61.5%) maintained their response without additional therapy.18
LCAP with Cellsorba improved the clinical and endoscopic picture of 2 steroid-resistant UC patients after 6 sessions. The same authors expanded on this experience, noting that 12 of 13 UC patients entered remission and 4 CD patients improved.57
In a multicenter open-label trial, 4 of 7 steroid-dependent or -resistant UC patients on a monthly apheresis program achieved remission for 12 months, and then remained steroid-free.58
Five patients with active UC received 6 sessions of LCAP with Cellsorba that resulted in clinical, histologic, and endoscopic improvement. An excess of CD83+ dendritic cells were found in the filtered buffy coats. LCAP also led to downregulation of IL-6 and IL-8.26
In the only placebo-controlled study with LCAP (Cellsorba), 10 treated patients and 9 patients in a sham group received 5 weekly sessions of apheresis followed by 2 more at 4-week intervals. Eight of 10 LCAP-treated patients showed significant improvement on the clinical activity index versus 3 of 9 sham-treated patients. Four sham-treated patients and 1 LCAP-treated patient reported adverse events, none of them severe. There was a statistically significant greater reduction in steroid use in the LCAP group.59
Other Techniques and Comparative Studies
Avoiding extracorporeal circulation (“off-line” leukapheresis), a 400 cc sample of peripheral blood was passed through a leukocyte “elimination” filter for 5 sessions (2000 cc treated) and reinfused, with subsequent clinical and endoscopic improvements in 1 patient. No follow-up series was reported, or adverse events.62
Fifty steroid-resistant UC patients received LCAP using a centrifugal cell separator (Haemonetics) for 5 weekly sessions. Twenty-six of 38 patients (68.4%) improved their stool frequency (<4/day), 17/30 (56.7%) “normalized” their CRP, 26/45 (57.7%) showed endoscopic remission, and 20/37 (54.1%) had histologic improvement. Overall, 74% (37/50) had improvement in their disease activity.58
A pilot study using an LCAP centrifugal procedure resulted in 13 of 14 corticosteroid-resistant patients entering remission within 4 weeks after apheresis and remaining in remission for 8 months without additional steroids. One of the 14 patients required a colectomy. Two adhesion molecules (L-selectin and VLA4a) on the surface decreased.19
Of 23 corticosteroid-resistant UC patients given centrifugal LCAP, 18 (78.3%) achieved remission within 4 weeks with confirmatory endoscopic and histologic benefit. No significant adverse events were reported.63
Honma and colleagues used both filtration and centrifugation to achieve remission in 23 of 25 steroid-resistant, moderate to severe UC patients for 5 weekly infusions. Details are lacking in this uncontrolled study regarding assessment of remission and outcome. A maintenance program of apheresis at 4-week intervals failed to improve a 50% relapse rate at two years. Steroids were tapered, 5-ASA/sulfasalazine were maintained, but no immunomodulating medications were reported. In addition, assessments of relapse were poorly described.64
Apheresis therapy in moderate to severe UC was studied with 3 different filter systems. Granulocyte apheresis (Adacolumn) showed 60% efficacy (3 remissions, 3 responses), LCAP (Cellsorba) was 70% effective (4 remissions, 3 responses), and mononuclear apheresis separating lymphocytes from monocytes was 90% effective (8 remissions, 1 response) with a longer lasting effect than other systems. Poor results were noted if the neutrophil count increased by 140% with decreased CD4 cells and a rise in inflammatory cytokines; this did not occur with the mononuclear apheresis system.65
Ogawa and associates conducted a comparator study of Cellsorba and Adacolumn. Seven of 13 patients (53.8%) using Cellsorba responded versus 9 of 13 (69%) using Adacolumn. A faster response was seen with Cellsorba and concomitant steroids (1.75 weeks) versus Adacolumn (2.5 weeks). Adverse events included headaches in 5 patients. The authors concluded that both systems are safe and useful in UC.66
Crohn’s Disease Experience
Considerably less experience has been reported in patients with CD and leukocytapheresis. See Table 4 for a summary of published studies.
Table 4.
Studies of Apheresis for Treatment of Crohn’s Disease
| Reference | N | System | Results |
|---|---|---|---|
| Kosaka et al67 | 18 | Cellsorba | 5 weekly sessions then 5 monthly sessions: 9 remissions, 14 improved CDAI, IOIBD |
| Matsui et al68 | 7 | Adacolumn | 5 entered remission |
| Kosaka et al69 | 6 | Adacolumn | 3 improved, 1 in remission after 5 weekly sessions then 2 sessions; IBDQ improvement |
| Fukuda et al70 | 21 | Adacolumn | “Improved” symptoms on CDAI, IBDQ, IOIBD at wk 7 |
| Sands et al47 | 15 | Adacolumn | 8 responded to 5 sessions, 5 entered remission; IBDQ improvement noted |
| Maiden et al71 | 13 | Adacolumn | 8/13 maintained remission after 5 sessions vs 14/17 controls; time to relapse: 181 days vs 104 days for controls |
CDAI=Crohn’s Disease Activity Index; IBDQ=Inflammatory Bowel Disease Questionnaire; IOIBD=International Organization for the Study of Inflammatory Bowel Disease.
Maintenance of Remission/Response
Difficulties abound in evaluating reports of maintenance efficacy. The literature often precludes evaluation of one device’s advantage over another when results are commingled.59,72 Control patients are usually nonexistent. Remission assessment and primary and secondary outcomes are not delineated. Most studies are unblinded. Maintenance studies are troubled by various assessments of relapse and concomitant medications are not recorded.
The literature is conflicted regarding a maintenance benefit. Some reports are highly suggestive of a prolonged remission or response in a maintenance experience,43,48,53,66 others are unfavorable.23,72 Honma’s initial success by inducing remission in 23 of 25 severe steroid-resistant UC patients was not sustained over 2 years despite monthly sessions compared to the steroid monotherapy group.64 The only controlled placebo (ie, sham) study with 5 of 10 UC patients entering remission after 5 weekly sessions was not extended beyond 8 weeks after the initial therapy.59
Questions remain unanswered as to whether a different patient population (eg, those who are not so severely ill or refractory to therapy), more frequent sessions, or concomitant immunomodulators or biological therapy would prolong remission rates.
Apheresis appears to play a promising role as an apparently safe modality in the induction of remission. Whether this holds true for maintenance of remission awaits further long-term studies.
Adverse Effects
Adverse effects have been remarkably few and, when encountered, extremely mild. In one study,73 a decline in WBC to 61.8% of baseline with Adacolumn occurred within 15 minutes. After 60 minutes, the WBC count returned to baseline. However, plasma C3a concentrations increased from 123±61 mg/mL at baseline to 417±96 mg/mL at 60 minutes, indicating activation of the complement system and anaphylatoxin production. This is of questionable clinical significance.
Nagase and colleagues reviewed 1,978 LCAP sessions between 1992 and 1997. Moderate reactions occurred in 31 sessions (1.6%) involving 15 patients (16%). These included nausea, fever, chills, nasal obstruction, palpitations, and respiratory or chest symptoms. There was prompt recovery without sequelae after a transient interruption of administration or medical therapy. Although a 46% incidence of clotting in the filter IV lines occurred, most were of little significance and this has not been reported in the subsequent experience with LCAP.74 There is only 1 report of a significant event, by Ljung and associates.49 A pulmonary embolus occurred in a patient in which a central venous catheter was used for apheresis.
Conclusion
Leukocytapheresis appears promising as a “no-drug” therapy in active IBD. Unfortunately, the accumulated IBD literature lacks placebo-controlled trials (often lacking primary or secondary endpoints) and is hampered by heterogenic populations with a mixture of concomitant medications. The data for maintenance of initial benefit are still scant and conflicted. Nevertheless, the impressive response and remission rates are enhanced by the extremely low incidence of adverse events, suggesting it is a safe therapy meriting further investigation. Selective apheresis given more frequently than once weekly may offer a more expeditious remission rate resulting in time and indirect cost savings. A randomized placebo-controlled trial is underway in the United States. Whether its role in combination with immunomodulators and/or biologic therapies would enhance its efficacy in maintaining remission awaits further study.
Acknowledgement
The author is grateful to Drs. Bruce Sands and Maria Abreu for their comments and to Gail Torodash for secretarial assistance.
References
- 1.Scott BB. Systematic review: how effective are the usual treatments for ulcerative colitis? Alimen Pharmacol Therap. 2004;20:143–149. doi: 10.1111/j.1365-2036.2004.02018.x. [DOI] [PubMed] [Google Scholar]
- 2.Bebb JR. Systematic review: how effective are the usual treatments for Crohn’s Disease? Alimen Pharmacol Therap. 2004;20:157–159. doi: 10.1111/j.1365-2036.2004.02019.x. [DOI] [PubMed] [Google Scholar]
- 3.Pullman WE, Elsebury S, Kobayashi M, et al. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992;102:529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- 4.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hanai H, Watanabe F, Yamada M, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36–44. doi: 10.1159/000080079. [DOI] [PubMed] [Google Scholar]
- 6.Sawada K, Muto T, Shimoyama T, et al. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Des. 2003;9:307–321. doi: 10.2174/1381612033391928. [DOI] [PubMed] [Google Scholar]
- 7.Saniabadi AR, Hanai H, Takeuchi K, et al. Adacolumn, an absorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Tabuchi T. Granulocytapheresis as a possible cancer treatment. Anticancer Res. 1995;15:985–990. [PubMed] [Google Scholar]
- 9.Tibble JA, Sigthorsson G, Bridger DS, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 10.Rembacken BJ, Newbould HE, Richards SJ, et al. Granulocyte apheresis in inflammatory bowel disease: possible mechanisms of effect. Ther Apher. 1998;2:93–96. doi: 10.1111/j.1744-9987.1998.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 11.Sakuraba A, Naganuma M, Hibi T, et al. Intensive therapy of granulocyte and monocyte adsorption apheresis induces rapid remission in patients with ulcerative colitis. Gastroenterology. 2003;124:A-522. [Google Scholar]
- 12.Sawada K, Shimoyama T. Therapeutic cytapheresis For inflammatory bowel disease. Ther Apher. 1998;2:90–92. doi: 10.1111/j.1744-9987.1998.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohara M, Saniabadi AR, Kokuma S, et al. Granuloctyapheresis in the treatment of patients with rheumatoid arthritis. Artif Organs. 1997;21:989–994. doi: 10.1111/j.1525-1594.1997.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 14.Kohgo Y, Itibi H, Chiba T, et al. Leukocytapheresis using a centrifugal cell separator in refractory ulcerative colitis: a multicenter open label trial. Ther Apher. 2002;6:255–260. doi: 10.1046/j.1526-0968.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- 15.Daibutsu M, Takagi T, Ogawa H, et al. Treatment of chronic rheumatoid arthritis by lymphocytapheresis. Ther Plasmapheresis. 1987;7:257–260. [Google Scholar]
- 16.Suemitsu J, Yoshida M, Yamawaki N, et al. Leukocytapheresis therapy by extra corporeal circulation using a leukocyte removal filter. Ther Apher. 1998;2:31–36. doi: 10.1111/j.1744-9987.1998.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 17.Reinisch W, Nahavandi H, Santella R, et al. Extracorporeal photochemotherapy in patients with steroid-dependent Crohn’s disease: a prospective pilot study. Aliment Pharmacol Therap. 2001;15:1313–1322. doi: 10.1046/j.1365-2036.2001.01054.x. [DOI] [PubMed] [Google Scholar]
- 18.Sawada K, Ohnishi K, Kosaka T, Fukui, et al. Leuocytapheresis therapy with leukocyte removal filter for inflammatory bowel disease. J Gastroenterol. 1995;30(8):124. [PubMed] [Google Scholar]
- 19.Ayabe T, Ashida T, Taniguchi M, et al. A pilot study of centrifugal leukocyte apheresis for corticosteroid-resistant active ulcerative colitis. Intern Med. 1997;36:322–326. doi: 10.2169/internalmedicine.36.322. comment 317-318. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama A, Nagase S, Ueda A, et al. Oxidative stress during leukocyte absorption apheresis. J Clin Apher. 2003;18:61–66. doi: 10.1002/jca.10054. [DOI] [PubMed] [Google Scholar]
- 21.Kohgo Y. Why is leukocytapheresis effective in inflammatory bowel disease? J Gastroenterol. 2004;39:1226–1227. doi: 10.1007/s00535-004-1509-4. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi M, Hiwatashi N, Hayakawa T, et al. Leukocyte removal filter-passed lymphocytes produce large amounts of interleukin-4 in immunotherapy for inflammatory bowel disease: role of bystander suppression. Ther Apher. 1998;2:109–114. doi: 10.1111/j.1744-9987.1998.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 23.Andoh A, Ogawa A, Kitamura K, et al. Suppression of interleukin-1B- and tumor necrosis factor-alpha-induced inflammatory responses by leukocytapheresis therapy in patients with ulcerative colitis. J Gastroenterol. 2004;39:1150–1157. doi: 10.1007/s00535-004-1464-0. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka T, Suzuki K, Kawakami, et al. Dynamic changes in cytokine levels in serum and synovial fluid following filtration leukocytapheresis therapy in patients with rheumatoid arthritis. J Clin Apher. 2001;16:74–81. doi: 10.1002/jca.1016. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda Y, Akbar SM, Matsui H, et al. Depletion and decreased function of antigen-presenting dendritic cells caused by lymphocytapheresis in ulcerative colitis. Dis Colon Rectum. 2003;46:521–528. doi: 10.1007/s10350-004-6593-2. [DOI] [PubMed] [Google Scholar]
- 26.Takeda Y, Shonohara N, Saniabdi AR, et al. Adhesion-dependent release of hepatocyte growth factor and interleukin-1 receptor antagonist from human blood granulocytes and monocytes: evidence for the involvement of plasma IgG, complement C3 and beta 2 integrin. Inflamm Res. 2004;53:277–283. doi: 10.1007/s00011-004-1253-5. [DOI] [PubMed] [Google Scholar]
- 27.Burt RK, Traynor A, Oyama Y, et al. High-dose immune suppression and autologous hematopoietic stem cell transplatation in refractory Crohn’s disease. Blood. 2003;101:2064–2066. doi: 10.1182/blood-2002-07-2122. [DOI] [PubMed] [Google Scholar]
- 28.Lerebours E, Bussel A, Modigliani R, et al. Treatment of Crohn’s disease by lymphocyte apheresis: a randomized controlled trial. Gastroenterolgy. 1994;107:357–361. doi: 10.1016/0016-5085(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 29.Van Montfrans C, Rodriguez Pena MS, Pronk I, et al. Prevention of colitis by interleukin 10-transduced T lymphocytes in the SCID mice transfer model. Gastroentrology. 2002;123:1865–1876. doi: 10.1053/gast.2002.37067. [DOI] [PubMed] [Google Scholar]
- 30.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 31.Hiraishi K, Takeda Y, Shiobara N, et al. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to access the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–340. doi: 10.1046/j.1526-0968.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Arrigo C, Candal-Couto JJ, Greer M, et al. Human neutrophil Fc receptor-mediated adhesion under flow: a hollow fibre model of intravascular arrest. Clin Exp Immunol. 1995;100:173–179. doi: 10.1111/j.1365-2249.1995.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay FC, Nineham LJ, Perumal R, et al. Intra-articular and circulating immune complexes and antiglobulins (IgG and IgM) in rheumatoid arthritis; correlation with clinical features. Ann Rheum Dis. 1979;38:1–7. doi: 10.1136/ard.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moldovan I, Galon J, Maridonneau-Parini I, et al. Regulation of production of soluble Fc gamma receptors type III in normal and pathological conditions. Immunol Lett. 1999;68:125–134. doi: 10.1016/s0165-2478(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 35.Shirokaze J. Leukocytapheresis using a leukocyte removal filter. Ther Apher. 2002;6:261–266. doi: 10.1046/j.1526-0968.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 36.Ross GD, Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myones BL, Dalzell JG, Hogg N, et al. Neutrophil and monocyte cell surface p150, 95 has C3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988;82:640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman SL, Devery-Pocius JE, Ross GD, et al. Phagocytosis by human monocyte-derived macrophages: independent function of receptors for C3b (CR1) and C3b (CR:3) Complement. 1984;1:213–227. [PubMed] [Google Scholar]
- 39.Muto S, Veticka V, Ross GD, et al. CR3 (CD11b/CD18) expressed by cytotoxic T cells and natural killer cells is upregulated in a manner similar to neutrophil CR3 following stimulation with various activating agents. J Clin Immunol. 1993;13:175–84. doi: 10.1007/BF00919970. [DOI] [PubMed] [Google Scholar]
- 40.Sulica A, Morel P, Metes D, et al. Ig binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001;20:371–414. doi: 10.3109/08830180109054414. [DOI] [PubMed] [Google Scholar]
- 41.Sawada K, Ohnishi K, Kosaka T, et al. Leukocytapheresis with leukocyte removal filter as new therapy for ulcerative colitis. Ther Apher. 1997;1:207–211. doi: 10.1111/j.1744-9987.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandborn WJ. Preliminary data on the use of apheresis in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:S15–S21. doi: 10.1097/01.mib.0000195387.26892.22. [DOI] [PubMed] [Google Scholar]
- 43.Hanai H, Watanabe F, Takeuchi K, et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35. doi: 10.1053/jcgh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 44.Jo Y, Matsumoto T, Miru R, et al. Addition of leukocytapheresis to steroid therapy: is it beneficial in recurrence of moderate to severe ulcerative colitis? Dis Colon Rectum. 2003;46(suppl):S3–S9. doi: 10.1097/01.DCR.0000088851.79497.9B. [DOI] [PubMed] [Google Scholar]
- 45.Sakuraba A, Sato T, Iawgami Y, et al. Intermittent therapy with granulocyte and monocyte apheresis maintains remission in ulcerative colitis. Gastroenterology. 2004;126:A466–A467. [Google Scholar]
- 46.Naganuma M, Funakoshi S, Sakuraba A, et al. Granulocytapheresis is useful as an alternative therapy in patients with steroid-refractory or –dependent ulcerative colitis. Inflamm Bowel Dis. 2004;10:251–257. doi: 10.1097/00054725-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Sands BE, Sandborn WJ, Wolf DC, et al. Pilot study of the safety and efficacy of granulocyte/monocyte adsorption apheresis with adacolumn in patients with inflammatory bowel disease. Am J Gastroenterol. 2004;99:S263–S264. [Google Scholar]
- 48.Suzuki Y, Yoshimura N, Saniabadi A, et al. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565–571. doi: 10.1023/b:ddas.0000026299.43792.ae. [DOI] [PubMed] [Google Scholar]
- 49.Ljung T, Thomsen OO, Vatn M, et al. Granulocyte, monocyte/macrophage apheresis for IBD in clinical practice: clinical results from the first 100 patients in Scandinavia. Gastroenterology. 2005;128:A-581. [Google Scholar]
- 50.Sakaruba A, Inoue N, Kohgo Y, et al. A multicenter, randomized, controlled trial between weekly and semiweekly treatment with granulocyte and monocyte adsorption apheresis for active ulcerative colitis. Am J Gastroenterol. 2005;100:S321. [Google Scholar]
- 51.D’Ovidio V, Arati A, Viscido A, et al. Quality of life in steroid-dependent/refractory ulcerative colitis after granuloctyapheresis. Inflamm Bowel Dis. 2006;12(2):S27. [Google Scholar]
- 52.Shimoyama T, Sawada K, Hiwatashi N, et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1–9. doi: 10.1002/jca.1000. [DOI] [PubMed] [Google Scholar]
- 53.Tomomasa T, Kobayashi A, Kaneko H, et al. Granulocyte adsorptive apheresis for pediatric patients with ulcerative colitis. Dig Dis Sci. 2003;48:750–754. doi: 10.1023/a:1022892927121. [DOI] [PubMed] [Google Scholar]
- 54.Hanai H, Takeuchi T, Iida T, et al. Evaluations of clinical efficacy and steroid-sparing effect of granulocyte and monocyte adsorptive apheresis in patients with corticosteroid-dependent ulcerative colitis. Gastroenterology. 2003;124:A518. [Google Scholar]
- 55.Yamamoto T, Umegae S, Kitagawa T, et al. Granulocyte and monocyte adsorptive apheresis in the treatment of active distal ulcerative colitis: a prospective, pilot study. Aliment Pharmacol Ther. 2004;20:783–792. doi: 10.1111/j.1365-2036.2004.02189.x. [DOI] [PubMed] [Google Scholar]
- 56.Sawada K, Hiwatashi N, Munakata A, et al. A multi-center randomized controlled study of safety and efficacy of adsorptive granulocyte and monocyte apheresis in patients with active ulcerative colitis. Gastroenterology. 2004;126:A462. [Google Scholar]
- 57.Kawamura A, Saitoh M, Yonekawa M, et al. New techniques of leukoctyapheresis by use of non-woven polyester fiber for inflammatory bowel disease. Ther Apher. 1999;3:334–337. doi: 10.1046/j.1526-0968.1999.00207.x. [DOI] [PubMed] [Google Scholar]
- 58.Kohgo Y, Hibi H, Chiba T, et al. Leukocyte apheresis using a centrifugal cell separator in refractory ulcerative colitis: a multicenter open label trial. Ther Apher. 2002;6:255–260. doi: 10.1046/j.1526-0968.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- 59.Sawada K, Kusugami K, Suzuki Y, et al. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am J Gastroenterol. 2005;100:1362–1369. doi: 10.1111/j.1572-0241.2005.41089.x. [DOI] [PubMed] [Google Scholar]
- 60.Sakata H, Kawamura N, Horie T, et al. Successful treatment of ulcerative colitis with leukocytapheresis using non-woven polyester filter. Ther Apher Dial. 2003;7:536–539. doi: 10.1046/j.1526-0968.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto T, Fujiyama Y, Okawa K, et al. Multicenter prospective open label study of leukocytapheresis to patients with intractable moderate to severe ulcerative colitis. Gastroenterology. 2005;128:A-582–3. [Google Scholar]
- 62.Endo Y, Tsuzuki H, Fujino M, et al. Off-line leukofiltration for active ulcerative colitis: a case report. Ther Apher. 2001;5:480–483. doi: 10.1046/j.1526-0968.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- 63.Ayabe T, Ashida T, Kohgo Y. Centrifugal leukocyte apheresis for ulcerative colitis. Ther Apher. 1998;2:125–128. doi: 10.1111/j.1744-9987.1998.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 64.Honma T, Sugimara J, Asakura H, et al. Leukoctyapheresis’s effective in inducing but not in maintaining remission in ulcerative colitis. J Clin Gastroenterol. 2005;39:886–890. doi: 10.1097/01.mcg.0000180638.59140.c5. [DOI] [PubMed] [Google Scholar]
- 65.Miyata M, Kasugai K, Ishikawa T, et al. Efficacy of mononuclear cell apheresis for ulcerative colitis comparison with removal filer leukocytaphereses. Gastroenterology. 2004;126:A630. [Google Scholar]
- 66.Ogawa M, Cho E, Yasuda K, et al. Clinical evaluation of leukocytapheresis and granulocyte/monocyte apheresis for active ulcerative colitis. Am J Gastroenterol. 2005;100:S291. [Google Scholar]
- 67.Kosaka T, Sawada K, Ohnishi K, et al. Effect of leukocytapheresis therapy using a leukocyte removal filter in Crohn’s disease. Intern Med. 1999;38:102–111. doi: 10.2169/internalmedicine.38.102. [DOI] [PubMed] [Google Scholar]
- 68.Matsui T, Nishimura T, Matake H, et al. Granulocytapheresis for Crohn’s disease: a report on seven refractory patients. Am J Gastroenterol. 2003;98:511–512. doi: 10.1111/j.1572-0241.2003.07251.x. [DOI] [PubMed] [Google Scholar]
- 69.Kosaka T, Fukunaga K, Ohnishi K, et al. Adsorptive monocyte-granulocytapheresis (M-GCAP) for refractory Crohn’s disease. J Clin Apher. 2004;19:168–173. doi: 10.1002/jca.20023. [DOI] [PubMed] [Google Scholar]
- 70.Fukuda Y, Matsui T, Suzuki Y, et al. Adsorptive granulocyte and monocyte apheresis for refractory Crohn’s disease: an open multicenter prospective study. J Gastroenterol. 2004;39:1158–1164. doi: 10.1007/s00535-004-1465-z. [DOI] [PubMed] [Google Scholar]
- 71.Maiden L, Baur R, Takeuchi K, et al. Adacolumn reduces relapse rates in patients with IBD at significant risk of clinical relapse. Gastroenterology. 2005;128:A-581. doi: 10.1002/ibd.20505. [DOI] [PubMed] [Google Scholar]
- 72.Kondo Y, Shimoda T, Yoshimoto H, et al. Effective maintenance leukocytapheresis for patients with steroid dependent or resistant ulcerative colitis. Ther Apher. 2001;5:462–465. doi: 10.1046/j.1526-0968.2001.00379.x. [DOI] [PubMed] [Google Scholar]
- 73.Yonemura K, Ohashi N, Kajimura M, et al. Transient leukopenia and anaphylatoxin production during granulocyte apheresis as treatment for ulcerative colitis. J Clin Apher. 2002;17:107–110. doi: 10.1002/jca.10033. [DOI] [PubMed] [Google Scholar]
- 74.Nagase K, Sawada K, Ohnishi K, et al. Complications of leukocytapheresis. Ther Apher. 1998;2:120–124. doi: 10.1111/j.1744-9987.1998.tb00088.x. [DOI] [PubMed] [Google Scholar]