Abstract
Background:
Valproic acid (VPA) is a widely used broad-spectrum antiepileptic drug for therapy of generalized and focal epilepsies. Cross-sectional studies have suggested that valproate treatment may be associated with hyperinsulinemia. We decided to investigate hyperinsulinemia as a health-threatening side effect of VPA in Iranian epileptic patients.
Materials and Methods:
Body mass index (BMI), lipid profile, fasting serum insulin, fasting blood glucose (FBS), and homeostatic model assessment-insulin resistance (HOMA-IR) were measured in 30 VPA-treated epileptic patients and 30 controls (CBZ-treated). The Chi-square test, t-test, and Pearson correlation test were used.
Results:
BMI was higher in VPA group than in control group (25.7 ± 3.5 > 21.7 ± 4.1) (0.000 < 0.05). Prevalence of obesity was 16.6% in VPA group that was almost the same and even lower than general Iranian population. Serum triglyceride (TG) (150 ± 77.2) was higher than CBZ group (114 ± 35.2) (P = 0.023 < 0.05). However, serum high-density lipoprotein level was lower in VPA group than controls (45.2 ± 11.7 < 54.4 ± 13.9) (P = 0.008 < 0.05). Serum insulin, FBS, HOMA-IR, cholesterol, and low-density lipoprotein did not demonstrate statistically significant differences between the two groups (P > 0.05).
Conclusion:
Despite the majority of previous studies that are against VPA and according to our study, VPA could be prescribed safely and it may not cause IR and its complications.
Keywords: Body mass index, carbamazepine, epilepsy, homeostatic model assessment insulin resistance, insulin resistance, lipid profile, sodium valproate
Introduction
Valproic acid (VPA) is a widely used broad-spectrum antiepileptic drug (AED) for therapy of generalized and focal epilepsies.[1,2,3] A significant weight gain in the course of treatment of epilepsy with VPA was described in several clinical studies.[4,5,6,7,8] Although several studies have analyzed this side effect of VPA treatment, the cause remained unknown.[9] Several mechanisms have been postulated, including increased appetite and food intake,[10,11,12] increased thirst and energy-rich beverages,[10,11] a direct effect of VPA, or a metabolite on the hypothalamus,[12] decreased capacity for luxury or facultative thermogenesis (minor temperature changes in hypothalamus can be an explanation for increased appetite),[10,11,13] and impaired metabolism of fatty acid.[10] Weight gain due to VPA treatment is usually observed during the first 3 months of therapy,[14,15,16,17,18] reaching its maximum amount after 6 months.[11,12,13,19] Rather than cosmetic adverse effects, obesity and its associated insulin resistance (IR) are leading cause of premature death. It is estimated that approximately 300,000 death per year happened due to obesity-related morbidity.[20] Obesity has also become one of the most important health problems all around the world and it is no longer the sole problem of the developed countries, as its prevalence is increasing all over the globe including South-East Asia, Middle East, and Iran.[21,22] Psychosocial consequences are substantial as well,[23] including a decline in health-related quality of life.[24,25] The overweight and obesity may soon become as much health-threatening as cigarette smoking. Obesity is known as the gateway disease that can lead to the metabolic syndrome and type 2 diabetes with increasing risk of cardiovascular and stroke diseases, hypertension, obstructive sleep apnea syndrome, obesity hypoventilation syndrome, gallbladder disease, and certain types of cancer. It should be considered that epilepsy is, after headache, the second most common neurological disorder.[26] In addition, because epileptic patients have to use AED for a long time and often lifelong, these complications, especially cardiovascular and cerebrovascular, are inevitable. On the other hand, weight gain can cause noncompliance or discontinuation of anticonvulsants. We chose carbamazepine (CBZ) for comparison due to its lower side effect on insulin, lipid levels, and weight gain.[27,4] As different studies reported controversial results and also there was not any definite survey in our population, we decided to investigate the IR in two groups of epileptic patients treated with sodium valproate (VPA) and CBZ. By determining the association between VPA and hyperinsulinemia, we could decrease related mortalities and morbidities by modifying this side effect.
Materials and Methods
The study was carried out in the outpatient clinic, Department of Neurology, Alzahra University Hospital. Epilepsy type was classified according to the recommendations of the international league against epilepsy.[28] Sixty patients, who were between 18 and 50 years old, suffered from idiopathic generalized or focal epilepsy and received monotherapy treatment with VPA or CBZ were selected randomly. This study was done without health-controlled group. Patients who had other medical problems including renal failure, hepatic failure, endocrinopathy, symptomatic epileptic patient like tumor, trauma, infection, neurodegenerative disorders, and those who did not agree to participate in the study were excluded. Drug dosage was 400–2000 mg daily for VPA and 400–1200 mg daily for CBZ. Duration of therapy was at least 6 months, and as we have mentioned before, the maximum effect of VPA on weight gain is after the 6th month of treatment.[11,12,13,19] The protocol was approved by the Ethical Committee of our Institution, and an informed consent was obtained from all the patients after a full informative session. Patients’ characteristics are explained in Table 1.
Table 1.
Characteristics of randomized subjects
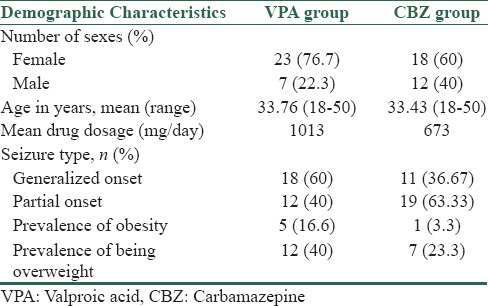
This study was a cross-sectional and descriptive one. At first, medical history of the patients was studied. Then they were clinically examined. The type of seizure, onset of seizure, kind of AED, and drug dosage, onset of treatment, and duration of treatment were registered. Body mass index (BMI) was measured ([weight/height2] [kg/m2]). According to the WHO and the National Heart, Lung, and Blood Institute, BMI results are classified as follows: Underweight: <18.5 kg/m2; normal weight: 18.5–24.9 kg/m2; overweight: 25–29.9 kg/m2; Class I obesity 30–34.9 kg/m2, Class II obesity 35–39.9 kg/m2, and Class III obesity >40 kg/m2.[29,30]
After an overnight fast, blood samples were obtained at 8:00 AM in order to determine serum fasting insulin, fasting blood glucose (FBS), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG).
Serum insulin and IR were measured with radioimmunoassay and homeostatic model assessment (HOMA) index, respectively.
HOMA is a method assessed to quantify IR and beta-cell function. HOMA-IR = fasting serum insulin (mU/L) × FBG (mol/l)/22.5.[31,32] Serum samples were analyzed in Hasht Behesht laboratory with radioimmunoassay method. The company that made glucose kitwas Pars Azmun (Tehran, Iran) and the one that made insulin kit was radiocel (Tehran, Iran).
Statistical analysis
Analysis was carried out using the statistical package of SPSS software (Version 16.0. Chicago, Inc) for personal computers. Chi-square test, t-test, and Pearson correlation test were used. P values less than 0.05 were considered significant.
Results
A total of 60 subjects were randomized to treatment (30 VPA and 30 CBZ). There were 23 female and 7 male in VPA group, 18 female and 12 male in CBZ group [Table 1]. Chi-square test noted that difference in number of sexes was not statistically significant (P-value = 0.15 > 0.05). The main results are summarized in Table 2. BMI was higher in VPA-treated patients in comparison to CBZ group (25.7 ± 3.5 > 21.7 ± 4.1). It was statistically significant due to P value of 0.000 (0.000 < 0.05).
Table 2.
Means of measured variables
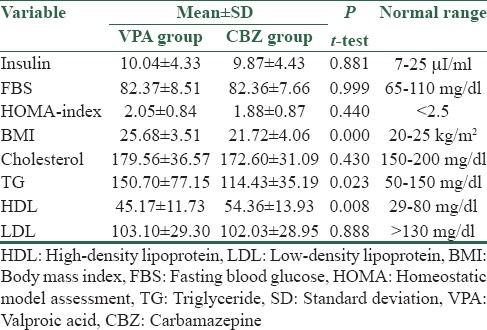
Serum TG levels were higher in VPA-treated patients than the other group (150 ± 77.2 > 114 ± 35.2, P = 0.023 < 0.05). VPA-treated patients had significantly lower serum HDL (45.2 ± 11.7 < 54.4 ± 13.9, P = 0.008 < 0.05).
There were no significant differences in serum LDL and total cholesterol between two groups (P = 0.888 > 0.05, P = 0.430 > 0.05). There were also no statistically significant differences between the groups concerning fasting serum insulin, FBS, and HOMA-IR index (P = 0.881 > 0.05, 0.999 > 0.05, 0.440 > 0.05).
Among VPA-treated patients, 40% (12 cases) had BMI between 25 and 29.9 (overweight) and 16.6% (5 cases) were obese (BMI > 30). However, the prevalence of being overweight and obesity in CBZ group was 23.3% and 3.3%, respectively.
Discussion
Obesity and dyslipidemia with other known risk factors are the major problems of public health. Psychosocial consequences are substantial as well,[23] including decline in health-related quality of life.[24,25]
In this study, VPA treatment was shown to be associated with higher BMI, TG, and lower HDL than CBZ group. Despite the majority of previous studies that have mentioned obesity as a common complication of VPA,[4,5,6,7,8,11,12,13,14,15,16,17,18,19] we have found a few articles without this effect.[27,29] In our study, 16.6% (5 cases) of VPA-treated patients and 3.3% (one case) of CBZ-treated were obese. Forty percent (12 cases) of main groups and 23.3% (7 cases) of controls were overweight. In addition, we could not find any relationship between drug dosage and BMI (P < 0.05). All our results did not show statistically significant difference in two sexes (P < 0.05). There are several surveys that have studied the prevalence of obesity and being overweight in Iran. In Tehran, Lipid and Glucose Study (TLGS), 40% of the adult study population (lived in Tehran, Iran) were overweight (BMI, 25–29.9 kg/m2) and 23.1% of them were obese (BMI ≥ 30 kg/m2).[22] Moreover, in a recent survey of blood donors in Tehran, 47% of the studied Iranian adult population were overweight and 24% of them were obese.[34] Another study in Iran noted the prevalence of 62.2% for overweight women and 28% for obese ones.[35] There are several other investigations that correlated with these statistics, respectively.[34,35] An important finding is that the overall prevalence of obesity in an Iranian population is quite comparable to the United States[36] and higher than in the United Kingdom, France, the Netherlands, and Italy.[37,38,39,40,41] These results are consistent with similar reports from Iran,[22,42,43] as well as other countries in the Middle East.[44,45,46,47,48,49,50,51] In one study in the Iranian population with 6246 participants, the mean TGs values were 190 and 162 mg/dl for males and females, respectively (P < 0.0001). The mean HDL-cholesterol was 39 in males and 45 mg/dl in females (P < 0.0001).[7] Hence, we must consider that although our results were statistically different in two groups, they were almost the same as the general population.
According to our results, we could mention that the prevalence of obesity and being overweight in VPA-treated patients is almost the same as the Iranian population and even lower for obesity, but in the control group (CBZ), it is less. It is controversial some studies showed that CBZ has no effects on the prevalence of metabolic syndrome in the epileptic patients.[52]
Due to this clue, something new might come to our mind that why these epileptic patients have even less BMI than the studied population? To find the answer, we must consider lots of factors, for example, neuropsychiatric, socioeconomic, genetic risks, and pregnancy,[53,54,55,56,57] which may interfere with our results. In addition, simultaneous study of other factors, for example, depression, nutrition in epileptic patients could help to find a true prevalence of this side effect. Statistically significant results for TG and HDL are related to BMI just the same as several previous studies.[8,16,33]
Different from the majority of previous surveys[6,9,16,27,33] and similar to a few of them[53,54,55] in our study not only FBS, serum insulin, and HOMA-IR did not show any statistically significant difference between two groups, but also all measurements were in normal range [Table 2]. Finally, IR did not happen in any of our patients. Our results could be in favor of VPA as an effective AED because as we mentioned hyperinsulinemia and IR are the major health problems for human beings. Reported IR might have other causes such as genetic rather than VPA side effect. In our study, we tried to find probable associations between different variables and we have found a direct relation between BMI and serum total cholesterol (r = 0.372, P = 0.003).
There was another statistically significant direct relation between HOMA-index and serum TG (r = 0.282, P = 0.029).
Conclusion
Almost the same prevalence of obesity and being overweight in comparison to the Iranian population might be a key to find more important factors such as nutritional, depression, and genetic that cause changes in BMI of epileptic patients. Despite the majority of previous studies that are against VPA and according to our study and a few related articles VPA could be prescribed safely and it may not cause IR and its complications. Similar studies with larger number of subjects for acquiring better insight about other causes of hyperinsulinemia and IR in this population are suggested.
Financial support and sponsorship
This study is the theses of Neurology resident which granted by Isfahan University of Medical Sciences (registered number: 388404), Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Davis R, Peters DH, McTavish D. Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;47:332–72. [PubMed] [Google Scholar]
- 2.Burea M, Genton B, Dravet C, Delgado-Escueta A, Tassinari CA, Thomas P, et al. Epileptic syndromes in infancy, childhood and adolescence - With Videos (English and French Edition) London: Libbey; 1992. pp. 313–27. [Google Scholar]
- 3.Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No 264 Group. N Engl J Med. 1992;327:765–71. doi: 10.1056/NEJM199209103271104. [DOI] [PubMed] [Google Scholar]
- 4.Rättyä J, Vainionpää L, Knip M, Lanning P, Isojärvi JI. The effects of valproate, carbamazepine, and oxcarbazepine on growth and sexual maturation in girls with epilepsy. Pediatrics. 1999;103:588–93. doi: 10.1542/peds.103.3.588. [DOI] [PubMed] [Google Scholar]
- 5.Jallon P, Picard F. Bodyweight gain and anticonvulsants: A comparative review. Drug Saf. 2001;24:969–78. doi: 10.2165/00002018-200124130-00004. [DOI] [PubMed] [Google Scholar]
- 6.Verrotti A, Basciani F, De Simone M, Trotta D, Morgese G, Chiarelli F. Insulin resistance in epileptic girls who gain weight after therapy with valproic acid. J Child Neurol. 2002;17:265–8. doi: 10.1177/088307380201700405. [DOI] [PubMed] [Google Scholar]
- 7.Klein KM, Hamer HM, Reis J, Schmidtke J, Oertel WH, Theisen FM, et al. Weight change in monozygotic twins treated with valproate. Obes Res. 2005;13:1330–4. doi: 10.1038/oby.2005.161. [DOI] [PubMed] [Google Scholar]
- 8.El-Khatib F, Rauchenzauner M, Lechleitner M, Hoppichler F, Naser A, Waldmann M, et al. Valproate, weight gain and carbohydrate craving: A gender study. Seizure. 2007;16:226–32. doi: 10.1016/j.seizure.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Pijl H, Meinders AE. Bodyweight change as an adverse effect of drug treatment. Mechanisms and management. Drug Saf. 1996;14:329–42. doi: 10.2165/00002018-199614050-00005. [DOI] [PubMed] [Google Scholar]
- 10.Breum L, Astrup A, Gram L, Andersen T, Stokholm KH, Christensen NJ, et al. Metabolic changes during treatment with valproate in humans: Implication for untoward weight gain. Metabolism. 1992;41:666–70. doi: 10.1016/0026-0495(92)90061-e. [DOI] [PubMed] [Google Scholar]
- 11.Isojärvi JI, Laatikainen TJ, Knip M, Pakarinen AJ, Juntunen KT, Myllylä VV. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol. 1996;39:579–84. doi: 10.1002/ana.410390506. [DOI] [PubMed] [Google Scholar]
- 12.Egger J, Brett EM. Effects of sodium valproate in 100 children with special reference to weight. Br Med J (Clin Res Ed) 1981;283:577–81. doi: 10.1136/bmj.283.6291.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman CL, Leung NM, Guberman AH. Weight gain in epileptic patients during treatment with valproic acid: A retrospective study. Can J Neurol Sci. 1997;24:240–4. doi: 10.1017/s0317167100021879. [DOI] [PubMed] [Google Scholar]
- 14.Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. 2001;56:172–7. doi: 10.1212/wnl.56.2.172. [DOI] [PubMed] [Google Scholar]
- 15.Caksen H, Deda G, Berberoglu M. Does long-term use of valproate cause weight gain in prepubertal epileptic children? Int J Neurosci. 2002;112:1183–9. doi: 10.1080/00207450290026148. [DOI] [PubMed] [Google Scholar]
- 16.Luef G, Abraham I, Haslinger M, Trinka E, Seppi K, Unterberger I, et al. Polycystic ovaries, obesity and insulin resistance in women with epilepsy. A comparative study of carbamazepine and valproic acid in 105 women. J Neurol. 2002;249:835–41. doi: 10.1007/s00415-002-0731-3. [DOI] [PubMed] [Google Scholar]
- 17.Morrell MJ, Isojärvi J, Taylor AE, Dam M, Ayala R, Gomez G, et al. Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Res. 2003;54:189–99. doi: 10.1016/s0920-1211(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 18.Wirrell EC. Valproic acid-associated weight gain in older children and teens with epilepsy. Pediatr Neurol. 2003;28:126–9. doi: 10.1016/s0887-8994(02)00505-2. [DOI] [PubMed] [Google Scholar]
- 19.Dinesen H, Gram L, Andersen T, Dam M. Weight gain during treatment with valproate. Acta Neurol Scand. 1984;70:65–9. doi: 10.1111/j.1600-0404.1984.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 21.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 22.Mohamadnejad M, Pourshams A, Malekzadeh R, Mohamadkhani A, Rajabiani A, Asgari AA, et al. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322–4. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill AJ, Williams J. Psychological health in a non-clinical sample of obese women. Int J Obes Relat Metab Disord. 1998;22:578–83. doi: 10.1038/sj.ijo.0800631. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine KR, Cheskin LJ, Barofsky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract. 1996;43:265–70. [PubMed] [Google Scholar]
- 25.Orzano AJ, Scott JG. Diagnosis and treatment of obesity in adults: An applied evidence-based review. J Am Board Fam Pract. 2004;17:359–69. doi: 10.3122/jabfm.17.5.359. [DOI] [PubMed] [Google Scholar]
- 26.Schachter SC, Schomer DL. The Comprehensive Evaluation and Treatment of Epilepsy: A Practical Guide: Illustrated. Son Diego: Academic Press; 1997. p. 53. [Google Scholar]
- 27.Pylvänen V, Knip M, Pakarinen AJ, Turkka J, Kotila M, Rättyä J, et al. Fasting serum insulin and lipid levels in men with epilepsy. Neurology. 2003;60:571–4. doi: 10.1212/01.wnl.0000048209.07526.86. [DOI] [PubMed] [Google Scholar]
- 28.Bradly WG. Neurology in Clinical Practice. 4th ed. Tavistock: Butterworth; 2004. pp. 1910–1. [Google Scholar]
- 29.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i. [PubMed] [Google Scholar]
- 30.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – The evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 33.Pylvänen V, Pakarinen A, Knip M, Isojärvi J. Insulin-related metabolic changes during treatment with valproate in patients with epilepsy. Epilepsy Behav. 2006;8:643–8. doi: 10.1016/j.yebeh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Azizi F. Tehran lipid and glucose study. Tehran: Endocrine Research Center; 2001. [Google Scholar]
- 35.Bahrami H, Sadatsafavi M, Pourshams A, Kamangar F, Nouraei M, Semnani S, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6:158. doi: 10.1186/1471-2458-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janghorbani M, Amini M, Willett WC, Mehdi Gouya M, Delavari A, Alikhani S, et al. First nationwide survey of prevalence of overweight, underweight, and abdominal obesity in Iranian adults. Obesity (Silver Spring) 2007;15:2797–808. doi: 10.1038/oby.2007.332. [DOI] [PubMed] [Google Scholar]
- 37.VanItallie TB. Prevalence of obesity. Endocrinol Metab Clin North Am. 1996;25:887–905. doi: 10.1016/s0889-8529(05)70360-1. [DOI] [PubMed] [Google Scholar]
- 38.Waine C. Nutrition and Physical Activity Task Forces. Obesity. Reversing the Increasing Problem of Obesity in England. London: Department of Health; 1995. [Google Scholar]
- 39.Rolland-Cachera MF, Cole TJ, Sempe M, Tichet J, Rossignol G, Charraud A. Obesity in Europe 91. London: John Libbey; 192. Variations of the body mass index in the French population from 0 to 87 years; p. 113. [Google Scholar]
- 40.Seidell JC, Verschuren WM, Kromhout D. Prevalence and trends of obesity in the Netherlands 1987-1991. Int J Obes. 1995;19:924–7. [PubMed] [Google Scholar]
- 41.Pagano R, Lavecchia C. Overweight and obesity in Italy, 1990-91. Int J Obes. 1994;18:665–9. [PubMed] [Google Scholar]
- 42.Malekzadeh R, Mohamadnejad M, Merat S, Pourshams A, Etmadi A. Obesity pandemic: An Iranian perspective. Arch Iran Med. 2005;8:1–7. [Google Scholar]
- 43.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2003;61:29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 44.Yumuk VD, Hatemi H, Tarakci T, Uyar N, Turan N, Bagriacik N, et al. High prevalence of obesity and diabetes mellitus in Konya, a central Anatolian city in Turkey. Diabetes Res Clin Pract. 2005;70:151–8. doi: 10.1016/j.diabres.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Erem C, Arslan C, Hacihasanoglu A, Deger O, Topbas M, Ukinc K, et al. Prevalence of obesity and associated risk factors in a Turkish population (Trabzon city, Turkey) Obes Res. 2004;12:1117–27. doi: 10.1038/oby.2004.140. [DOI] [PubMed] [Google Scholar]
- 46.Al-Mahroos F, Al-Roomi K. Overweight and obesity in the Arabian Peninsula: An overview. J R Soc Health. 1999;119:251–3. doi: 10.1177/146642409911900410. [DOI] [PubMed] [Google Scholar]
- 47.Al-Malki JS, Al-Jaser MH, Warsy AS. Overweight and obesity in Saudi females of childbearing age. Int J Obes Relat Metab Disord. 2003;27:134–9. doi: 10.1038/sj.ijo.0802181. [DOI] [PubMed] [Google Scholar]
- 48.Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, et al. Obesity in Saudi Arabia. Saudi Med J. 2005;26:824–9. [PubMed] [Google Scholar]
- 49.Kaluski DN, Berry EM. Prevalence of obesity in Israel. Obes Rev. 2005;6:115–6. doi: 10.1111/j.1467-789X.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 50.Galal OM. The nutrition transition in Egypt: Obesity, undernutrition and the food consumption context. Public Health Nutr. 2002;5:141–8. doi: 10.1079/PHN2001286. [DOI] [PubMed] [Google Scholar]
- 51.Al-Nsour M, Zindah M, Belbeisi A, Hadaddin R, Brown DW, Walke H. Prevalence of selected chronic, non-communicable disease risk factors in Jordan: Results of the Jordan Behavioural Risk Factor Surveillance Survey. Prev Chronic Dis. 2012;9:110077. [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmood IH, Mded ZK. Effects of carbamazepine on blood pressure, serum glucose concentration, lipid profile and prevalence of metabolic syndrome in epileptic patients. Tikrit J Pharm Sci. 2012;8:2. [Google Scholar]
- 53.Azizi F, Rahmani M, Ghanbarian A, Emami H, Salehi P, Mirmiran P, et al. Serum lipid levels in an Iranian adults population: Tehran lipid and glucose study. Eur J Epidemiol. 2003;18:311–9. doi: 10.1023/a:1023606524944. [DOI] [PubMed] [Google Scholar]
- 54.Kwan P, Yip FP, Hui AC, Leung H, Ng PW, Hui KF, et al. Effects of valproate or lamotrigine monotherapy on the reproductive endocrine and insulin-related metabolic profile in Chinese adults with epilepsy: A prospective randomized study. Epilepsy Behav. 2009;14:610–6. doi: 10.1016/j.yebeh.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Luef G, Abraham I, Hoppichler F, Trinka E, Unterberger I, Bauer G, et al. Increase in postprandial serum insulin levels in epileptic patients with valproic acid therapy. Metabolism. 2002;51:1274–8. doi: 10.1053/meta.2002.34708. [DOI] [PubMed] [Google Scholar]
- 56.Tolou-Ghamari Z, Najafi MR, Habibabadi JM, Zare M. Preliminarily analysis of carbamazepine (CBZ) C0 in patients visited Isfahan epileptic clinics. Int J Prev Med. 2013;4(Suppl 2):S343–6. [PMC free article] [PubMed] [Google Scholar]
- 57.Najafi MR, Sonbolestan F, Sonbolestan SA, Zare M, Mehvari J, Meshkati SN. The course and outcome of pregnancy and neonatal situation in epileptic women. Adv Biomed Res. 2012;1:4. doi: 10.4103/2277-9175.94426. [DOI] [PMC free article] [PubMed] [Google Scholar]


