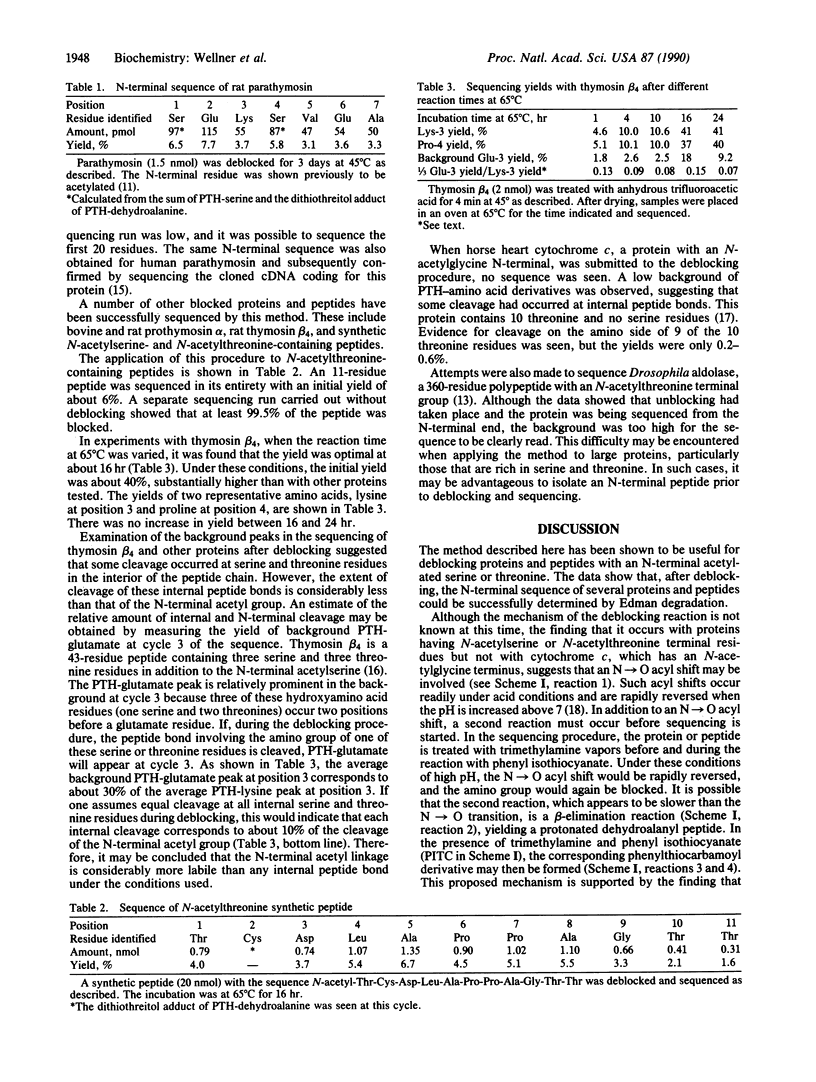
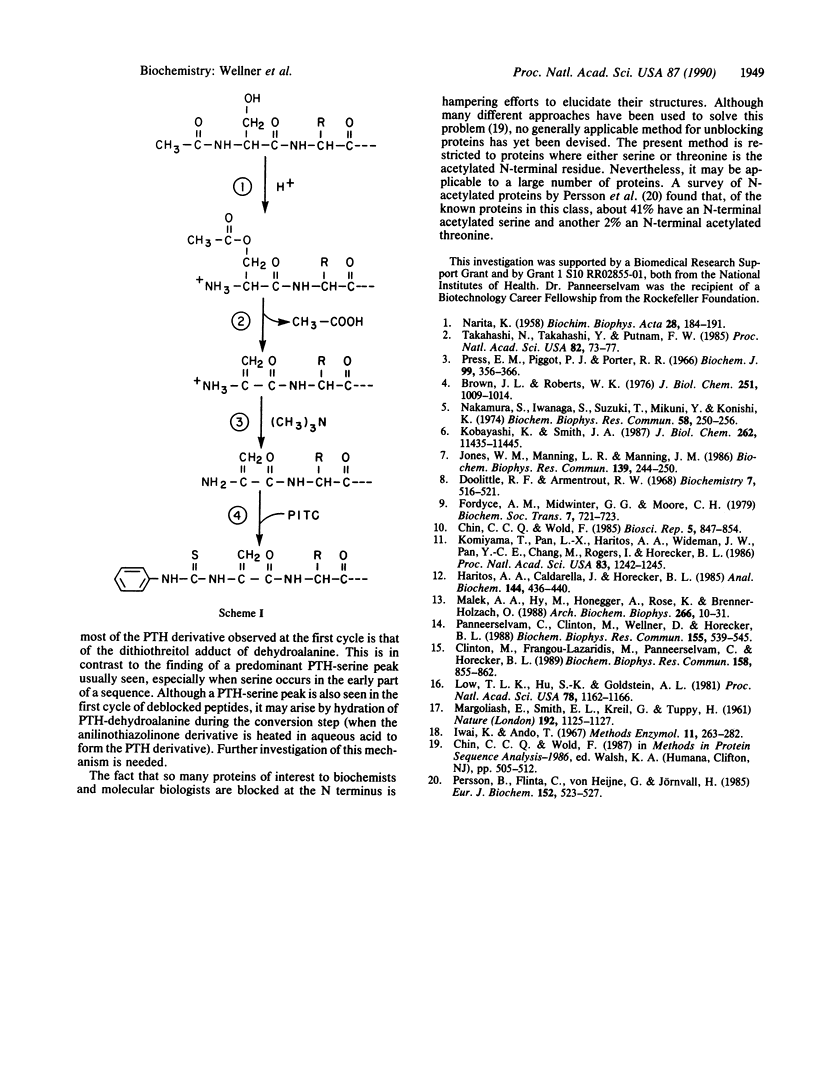
Abstract
Many proteins cannot be directly sequenced by Edman degradation because they have a blocked N-terminal residue. A method is presented for deblocking such proteins when the N-terminal residue is N-acetylserine (which occurs frequently in eukaryotic proteins) or N-acetylthreonine. The method has been applied successfully to the determination of the N-terminal amino acid sequence of human, bovine, and rat parathymosins. Prothymosin alpha and other blocked proteins and peptides were also readily deblocked and sequenced by this procedure. It is proposed that the mechanism of the deblocking reaction involves an acid-catalyzed N----O shift of the acetyl group followed by a beta-elimination.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. L., Roberts W. K. Evidence that approximately eighty per cent of the soluble proteins from Ehrlich ascites cells are Nalpha-acetylated. J Biol Chem. 1976 Feb 25;251(4):1009–1014. [PubMed] [Google Scholar]
- Chin C. C., Wold F. Studies on N alpha-acylated proteins: the N-terminal sequences of two muscle enolases. Biosci Rep. 1985 Oct-Nov;5(10-11):847–854. doi: 10.1007/BF01119896. [DOI] [PubMed] [Google Scholar]
- Clinton M., Frangou-Lazaridis M., Panneerselvam C., Horecker B. L. The sequence of human parathymosin deduced from a cloned human kidney cDNA. Biochem Biophys Res Commun. 1989 Feb 15;158(3):855–862. doi: 10.1016/0006-291x(89)92801-5. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Armentrout R. W. Pyrrolidonyl peptidase. An enzyme for selective removal of pyrrolidonecarboxylic acid residues from polypeptides. Biochemistry. 1968 Feb;7(2):516–521. doi: 10.1021/bi00842a005. [DOI] [PubMed] [Google Scholar]
- Fordyce A. M., Midwinter G. G., Moore C. H. The N-terminal amino acid sequence of sheep heart phosphofructokinase [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):721–723. doi: 10.1042/bst0070721. [DOI] [PubMed] [Google Scholar]
- Haritos A. A., Caldarella J., Horecker B. L. Simultaneous isolation and determination of prothymosin alpha, parathymosin alpha, thymosin beta 4, and thymosin beta 10. Anal Biochem. 1985 Feb 1;144(2):436–440. doi: 10.1016/0003-2697(85)90138-1. [DOI] [PubMed] [Google Scholar]
- Jones W. M., Manning L. R., Manning J. M. Enzymic cleavage of the blocked amino terminal residues of peptides. Biochem Biophys Res Commun. 1986 Aug 29;139(1):244–250. doi: 10.1016/s0006-291x(86)80105-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Smith J. A. Acyl-peptide hydrolase from rat liver. Characterization of enzyme reaction. J Biol Chem. 1987 Aug 25;262(24):11435–11445. [PubMed] [Google Scholar]
- Komiyama T., Pan L. X., Haritos A. A., Wideman J. W., Pan Y. C., Chang M., Rogers I., Horecker B. L. The primary structure of rat parathymosin. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1242–1245. doi: 10.1073/pnas.83.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Hu S. K., Goldstein A. L. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Malek A. A., Hy M., Honegger A., Rose K., Brenner-Holzach O. Fructose-1,6-bisphosphate aldolase from Drosophila melanogaster: primary structure analysis, secondary structure prediction, and comparison with vertebrate aldolases. Arch Biochem Biophys. 1988 Oct;266(1):10–31. doi: 10.1016/0003-9861(88)90232-9. [DOI] [PubMed] [Google Scholar]
- NARITA K. Isolation of acetylpeptide from enzymic digests of TMV-protein. Biochim Biophys Acta. 1958 Apr;28(1):184–191. doi: 10.1016/0006-3002(58)90445-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iwanaga S., Suzuki T., Mikuni Y., Konishi K. Amino acid sequence of the peptide released from bovine factor XIII following activation by thrombin. Biochem Biophys Res Commun. 1974 May 7;58(1):250–256. doi: 10.1016/0006-291x(74)90919-x. [DOI] [PubMed] [Google Scholar]
- Panneerselvam C., Clinton M., Wellner D., Horecker B. L. Bovine parathymosin: amino acid sequence and comparison with rat parathymosin. Biochem Biophys Res Commun. 1988 Sep 15;155(2):539–545. doi: 10.1016/s0006-291x(88)80528-x. [DOI] [PubMed] [Google Scholar]
- Persson B., Flinta C., von Heijne G., Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985 Nov 4;152(3):523–527. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Complete amino acid sequence of human hemopexin, the heme-binding protein of serum. Proc Natl Acad Sci U S A. 1985 Jan;82(1):73–77. doi: 10.1073/pnas.82.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]



