Abstract
Wide-ranging animals, such as birds, regularly traverse large areas of the landscape efficiently in the course of their local movement patterns, which raises fundamental questions about the cognitive mechanisms involved. By using precision global-positioning-system loggers, we show that homing pigeons (Columba livia) not only come to rely on highly stereotyped yet surprisingly inefficient routes within the local area but are attracted directly back to their individually preferred routes even when released from novel sites off-route. This precise route loyalty demonstrates a reliance on familiar landmarks throughout the flight, which was unexpected under current models of avian navigation. We discuss how visual landmarks may be encoded as waypoints within familiar route maps.
Keywords: global positioning system tracking, navigation, route map, waypoint, off-route release
The sensory basis of mechanisms that allow the homing pigeon (Columba livia) to navigate home from unfamiliar sites has been the subject of extensive study (1–4). In contrast, the problems of spatial cognition within the familiar area have received far less attention, and although such flights are thought to rely at least partly on visual features of the landscape (5–8), the exact nature of how visual landmarks are memorized, represented, and used for navigation remains unclear. Current theories of homing pigeon navigation emphasize the two-stage nature of the process: At the start of a homing journey, birds use magnetic, olfactory, or visual cues to position themselves with respect to home, then they recall a previously memorized compass bearing appropriate for their destination. The bearing is then assumed with the aid of the birds' directional sense [time-compensated sun compass (9) or magnetic compass (10, 11)]. For the remainder of the homing journey, this “mosaic map” model (2, 12, 13), much like the alternative “gradient map” (more relevant at unfamiliar sites) (12, 14), assigns little or no role to subsequent input from the landscape.
Evidence for the dominant role of the compass in determining initial orientation relies on findings that birds deviate predictably from the homeward course if they have been subjected to a clock-shift treatment, even after extensive experience with a site (15). Nevertheless, a few studies do support the existence of a possible alternative mechanism (16, 17) in which the spatial arrangement of landmarks near the release site is thought to provide directional information in lieu of an independent compass. Indeed, the reduced effect of clock-shift at familiar sites (18, 19) has been interpreted in terms of a conflict between orientational cues provided by the sun compass and those contained within the local visual landscape (20, 21). However, despite some compelling results that draw on pigeons' orientational performance at the release site, only indirect evidence is available to suggest that birds pay any attention to visual features along the remainder of the homing journey (6). Whether homing pigeons navigating within their familiar area ever perform true pilotage (3) remains an open question.
The recent development of miniature global positioning system (GPS) data logging devices (22, 23), which allow homing pigeons to be tracked along their homeward routes with extremely high resolution and precision, has opened up new possibilities for assessing the contribution of landscape features to familiar area navigation (24, 25). In this study, we used GPS tracking technology to elucidate the nature of the birds' map by conducting a fine-grained analysis of flights within the familiar area. To simulate the natural task of local area orientation as closely as possible, we specifically avoided interfering with the birds' navigational systems.
Methods
Subjects and Materials. Nine homing pigeons bred at the Oxford University Field Station at Wytham (51°46′58.34″N, 1°19′02.40″W) were used. All were at least 2 years old, weighed a minimum of 480 g, and had participated in several prior homing experiments but had never before visited any of the release sites used in the current study. They were familiarized with carrying miniature GPS logging devices attached to the back by a small Velcro strip glued to clipped feathers (for detailed methods, see ref. 26). GPS devices weighed 24–28 g and consisted of an integrated receiver and logger (μ-blox, Thalwil, Switzerland), ceramic patch antenna, and 3.7-V Li–polymer battery. Data fixes were logged by the device at 1-s intervals, with an accuracy of ±4 m in the longitude/latitudinal plane. Upon the birds' return to the home loft, data recorded by the device was downloaded by using the dedicated software μ-logger, and flight tracks were superimposed on British Ordnance Survey maps by using Fugawi moving map software (Northport Systems, Toronto).
Release Sites, Training, and Testing Procedure. Two release sites were used for training: Weston Wood (51°51′17.87″N, 1°12′55.46″W; distance from home, 10.7 km; direction from home, 41.4°) and High Cogges (51°46′59.38″N, 1°27′10.41″W; distance from home, 9.4 km; direction from home, 269.9°). During the initial training phase, birds were released from each of these sites 20 times consecutively over a period of ≈2 weeks. A maximum of three releases per day were conducted, restricted to times when the sun's disk was clearly visible. Each of the 20 releases was logged by the GPS device. Once birds had completed all 20 releases from a training site, the testing phase began. Subjects were released once each from four novel (“off-route”) release sites, the locations of which were chosen individually for each bird such that they lay ≈1,000–1,500 m (perpendicular distance) from a corridor defined by the birds' final three tracks recorded during the training phase. Two off-route sites were chosen on either side of the birds' corridor, at distances from home ranging between 7.2 and 10.3 km (Weston Wood) and 5.1 and 9.4 km (High Cogges). The birds' first attempts on the off-route test releases were logged by the GPS device. Both training and testing were completed at the first site (Weston Wood) before training at the second site began.
Results
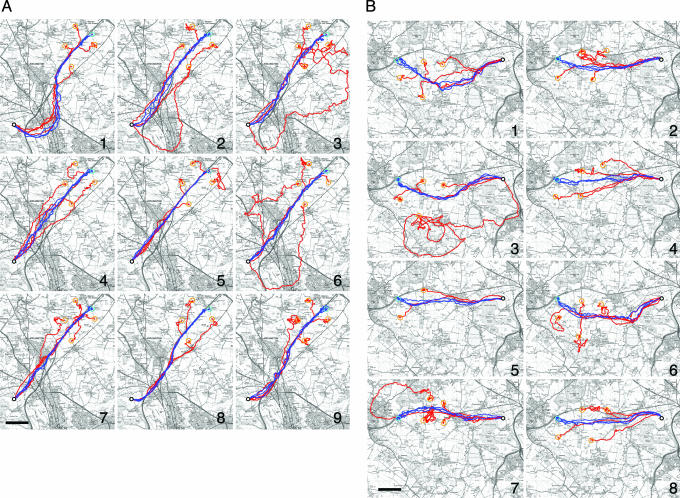
Fig. 1 illustrates the subjects' performance during the final stage of training from the two release sites. By the end of the training phase, birds had come to rely on narrow flight corridors, with mean widths (for the area enclosed by a given bird's last three training tracks) of 151 ± 60 m at Weston Wood and 176 ± 33 m at High Cogges. The subjects' tendency to recapitulate individually distinct routes was assessed by comparing degrees of track similarity within and between birds. We used the area enclosed between given pairs of tracks as an indicator of their similarity, with smaller areas corresponding to higher degrees of similarity. A randomization test using datasets consisting of the final three flights recorded from each subject at the two training sites was used to compare intraindividual and interindividual similarity in route choice. These 27 tracks at Weston Wood (9 birds × 3 tracks) and 24 tracks at High Cogges (8 birds × 3 tracks) were randomly assigned to groups of three, and areas enclosed by all possible paired comparisons within each group were calculated and summed to give cumulative areas. The analysis was reiterated 10,000 times to provide a test distribution of track similarity within groups of three tracks, with which particular birds' final three tracks could be compared. The mean cumulative area for groups of three randomly assigned tracks was 12.63 ± 7.80 km2 at Weston Wood and 20.09 ± 7.65 km2 at High Cogges. Cumulative areas enclosed by the final three training tracks belonging to individual birds were well below the mean at both sites, with all eight birds at High Cogges and five of nine birds at Weston Wood falling below the 2.5th percentile (with the remaining four birds close to this confidence boundary). The data thus demonstrate significantly higher intraindividual similarity than interindividual similarity, replicating our recent results found at shorter distances (27) and further confirming the route recapitulation phenomenon.
Fig. 1.
Flight tracks recorded from nine homing pigeons from a variety of release sites. (A) Shown are flights of individual subjects (birds 1–9) from Weston Wood and its vicinity. (B) Shown are flights by the same birds from High Cogges and its vicinity. Light blue circles indicate the location of the training release sites. Blue tracks correspond to subjects' final three training flights; red tracks show four subsequent off-route test releases (location of off-route sites marked by orange circles). Bird 9 failed to home on its second release from High Cogges and is thus missing from B; bird 5 disappeared after its second off-route test release from the High Cogges area. Location of home is indicated by a white dot. (Scale bars, 2 km.) [Map image copyright 2004, Crown Copyright Ordnance Survey, an EDINA Digimap/Joint Information Systems Committee (JISC) supplied service.]
Track efficiency (calculated as the aerial distance between release site and home divided by the distance traveled by subjects to reach home) during the final three training tracks for each bird averaged 0.85 ± 0.08 at Weston Wood and 0.80 ± 0.07 at High Cogges, indicating that even after extensive training through 20 releases in quick succession, subjects flew, on average, an extra 18–25% of the distance necessary to reach home. Average track efficiency during off-route test releases was 0.67 ± 0.13 at Weston Wood and 0.66 ± 0.15 at High Cogges, significantly lower than during the final stage of training (paired t test; Weston Wood, P < 0.001; High Cogges, P < 0.005).
To assess the influence of established routes on subsequent test releases, off-route tracks were analyzed in terms of their likelihood to approach and contact either any of the subject's final three training tracks or a mirror image of these reflected across a line connecting the off-route release site and home. Under the null hypothesis of no influence from the established route, birds were expected to be equally likely to make contact with the imaginary mirror-image tracks as with the actual tracks. In fact, as Fig. 1 shows, off-route tracks were deflected dramatically in the direction of the established route. In 76% of all off-route releases, birds made contact with their training tracks rather than the imaginary mirror image first, flying back to their established routes and rejoining these, recapitulating them from the point of contact (see below). Track efficiency was significantly higher in flights where contact with the established route was made (average 0.69 ± 0.16) than in those that reached the mirror-image track first (average, 0.56 ± 0.19; ANOVA, F1,55 = 7.60, P = 0.008). When comparing the distance at which birds first made contact with their preferred routes (defined as the straight line distance between the off-route release site and the first point of contact between the off-route track and any of the bird's final three training tracks) and the distance at which they would have been expected to hit their routes had they been flying on a straight course toward home (defined as the distance between the off-route release sites and the first point encountered along any of the final three tracks when a straight line was drawn between the off-route release site and home), we found that subjects reached their established routes significantly earlier than expected [on average 4.4 ± 3.8 km earlier at Weston Wood (one-sample t test; T = 6.84, P < 0.001) and 2.5 ± 1.9 km earlier at High Cogges (one-sample t test; T = 6.98, P < 0.001)].
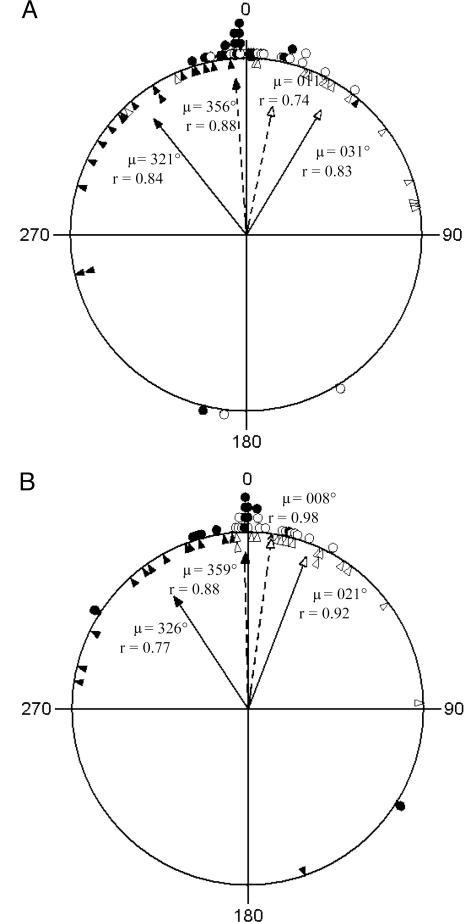
We explored changes in flight trajectory once birds came into contact with their established routes during off-route test releases. Preintercept trajectory was defined as the average direction over 500 m (≈25–30 s of flight) before the first off-route point that reached any of the same bird's final three training tracks, whereas postintercept trajectory was the average direction over the 500 m that followed this initial point of contact along the off-route track. Established route trajectory was also calculated as the average direction over the next 500 m of the same training track that the bird intercepted. We found that off-route test sites to the right of the established route produced tracks showing a significant rightwards deviation at the point of intercept with the established route (Fig. 2) (95% confidence interval for the mean at Weston Wood, 14° < μ < 48°; High Cogges, 8° < μ <34°), and those to the left showed a corresponding deviation leftwards (95% confidence interval at Weston Wood, 306° < μ < 337°; High Cogges, 302° < μ < 351°). These findings demonstrate that the point of intercept with the established route caused significant changes in heading. In addition, the postintercept trajectories were not significantly different from those of the established route (i.e., the angular differences between postintercept test trajectory and established route trajectory clustered around zero at both sites; see Fig. 2). Interestingly, there were only three outliers, and these all showed an angular difference of ≈180°, indicating that these subjects initially flew the wrong way up their previously established route. In sum, birds not only approached points along their familiar route but also used these points to effect changes in trajectory, bringing them in line with a previously flown route.
Fig. 2.
Circular diagrams showing changes in flight trajectory upon birds' first contact with their established routes during off-route test releases at Weston Wood (A) and High Cogges (B). Triangles indicate angular deviations in individual off-route trajectories before and after initial contact with the same bird's established route (open triangles correspond to tracks for which birds approached their established routes from the right, and filled triangles correspond to tracks for which they approached them from the left). Circles indicate angular differences between the established route and the off-route trajectory after initial contact (open and filled circles are distinguished as above according to direction of approach). Arrows show mean vectors for angular deviations in precontact versus postcontact off-route trajectories (solid arrows) and postcontact off-route versus established route trajectories (dashed arrows); filled arrowheads denote left approach and open arrowheads denote right approach. See text for further detail.
When off-route tracks rejoined the established route soon after release, the majority did so “downstream,” at a point closer to home than if the birds had been heading directly for the nearest point along their route. Of the 66 off-route tracks, 59 joined the familiar route downstream and only 7 joined the route upstream. Of the 39 cases in which rejoining the route occurred dramatically early (i.e., within 2 km of the point along the route nearest to the release site), 33 joined the route downstream.
Discussion
We report here that after extensive experience, pigeons assume stereotyped routes home and that these routes are neither the most direct paths home nor similar across subjects. What does such individual route recapitulation [which we have also found at shorter distances (27)] reveal about the mechanisms that underlie familiar area navigation? The currently prevalent mosaic map model (2, 12, 13) posits that, upon release, birds first use local cues to fix position, then recall and assume a previously memorized compass bearing to home. In contrast, precise route recapitulation suggests control by localized geocentric cues rather than compass commands that are not anchored to the landscape except at release.
In a more direct test of geocentric route control, we released birds from novel sites displaced perpendicularly off their established routes. Had navigation been compass controlled, the established route should have had no influence on the birds' new flight paths. However, the majority of such flights were deflected dramatically toward the established routes, with prominent changes in trajectory at the point of intercept. Birds rarely crossed their route without recapitulating it from there onwards. Thus, the recapitulated route clearly attracted birds from a distance and from novel directions and then controlled subsequent flight behavior.
It is difficult to account for these findings without invoking fundamental landscape influences, exerted throughout the homing journey. Both the precision of established route recapitulation and the striking deflections in the initial part of the off-route test releases suggest that birds were attending to localized geocentric cues that (i) were present at very high resolutions and (ii) could be perceived and accurately assessed from a distance of at least 1,500 m. The most likely candidate that fulfils both of these criteria is the local visual landscape.
We propose that, after extensive experience with a particular location, birds build a representation of the homeward route in the form of a “route map,” i.e., a series of memorized visual landmarks or “waypoints.” Route maps might operate in two distinct ways. In a compass-based route map, for each waypoint there would be an associated compass bearing (or even vector) that would direct the bird to the next waypoint, with the sequence of connected waypoints thus representing the home route. Each segment of the route would operate in a similar way to that proposed for the current mosaic map model's entire journey. Alternatively, consecutive waypoints may be within visual range of each other, such that birds can progress homeward through a form of pilotage known as steeple-chasing (28). Two aspects of our results argue in favor of pilotage. First, the off-route tracks demonstrate that landmark attraction can operate at distances of at least 1,500 m and at various points along the length of the route, suggesting that birds might well be capable of completing the entire journey by visual attraction alone, through approaching successive, intermediate, and directly perceived goals. Second, if consecutive waypoints were linked by compass bearings encoding the directional relationships between landmarks, it is surprising that birds persist with remarkably inefficient tracks even after 20 releases, when vector integration should have enabled them to take shortcuts. In contrast, the direct attraction to successive visual landmarks in steeple-chasing does not require that birds represent the directional relationships between successive landmarks, so a lack of shortcutting would be less surprising.
Further analysis of the off-route tracks revealed that when birds rejoined their learned route soon after release, they did so downstream. We propose three possible explanations. First, birds may have seen two or more waypoints simultaneously already at the release site and had a representation of the order in which they occurred along the homeward journey. By choosing to approach a waypoint nearer home, they were creating more efficient routes. Second, the route map could have been associated with a general compass direction, so that recognized waypoints were lined up in the homeward order with reference to the bird's internal compass. Third, waypoints downstream along the route would have resembled in appearance views encountered during previous flights, whereas points upstream had most likely never been seen from this, the opposite angle. Thus, recognition failure of upstream points may have accounted for local shortcuts.
Visual waypoints may not always be single discrete points in space. Consistent with our previous results (refs 25 and 29; see also ref. 30), birds sometimes followed linear landscape features. The first 4 km of tracks from Weston Wood (Fig. 1A) show extreme similarity both within and between birds, and this tightly packed band coincides with a major road running both close (300 m) to the release site and almost exactly in the direction of home (223° instead of 221°) for at least the first 4.2 km. Tracks diverge where the road curves south, although birds 8 and 9 abandon it only within the final 1–3 km, possibly already in sight of home. A roughly parallel railway line was also followed on off-route releases by birds 1, 2, 3, 4, and 8 for distances ranging from 1 to 5 km. In comparison, the trajectory of the nearest major linear feature at High Cogges (another road) is a less perfect match with the direction of home (65° instead of 90°). Only bird 7 followed this road. Pigeons may make use of linear landscape features to reduce the number of memorized waypoints required and therefore cognitive load. But it is also clear that they do so selectively, following only those whose trajectories orient homeward.
The precise details of route mapping mechanisms and the visual landmarks on which they are based, as well as their relationship with previous studies implying the use of compass orientation from longer distances, remain to be elucidated. Nevertheless, our data suggests that in short-distance, familiar-area orientation, pilotage rather than mosaic map-based navigation best describes pigeons' navigational strategies.
Acknowledgments
We thank H.-P. Lipp and G. Dell'Omo (University of Zürich, Zürich) for supplying GPS trackers and associated training; K.-K. Lau for help with GPS tracker development; A. Grafen, N. Meade, and D. Sumpter for statistical advice; the University of Bristol Speleological Society for discussion facilities; and two anonymous referees for helpful comments on the manuscript. This work was supported by Royal Society Equipment Grant RSRG22678 and Engineering and Physical Sciences Research Council Research Grant GR/R93261/01. J.M. was supported by the Biotechnology and Biological Sciences Research Council.
Author contributions: D.B. designed research; D.B. and J.M. performed research; D.B. and T.G. analyzed data; and D.B., J.M., and T.G. wrote the paper.
Abbreviation: GPS, global positioning system.
References
- 1.Wallraff, H. G. (2001) Ethol. Ecol. Evol. 13, 1–48. [Google Scholar]
- 2.Wiltschko, R. & Wiltschko, W. (2003) Anim. Behav. 65, 257–272. [Google Scholar]
- 3.Papi, F., ed. (1992) Animal Homing (Chapman & Hall, London).
- 4.Bingman, V. P. (1998) in Spatial Representation in Animals, ed. Healy, S. (Oxford Univ. Press, New York), pp. 69–85.
- 5.Wallraff, H. G., Kiepenheuer, J. & Streng, A. (1993) Behav. Ecol. Sociobiol. 32, 387–390. [Google Scholar]
- 6.Ulrich, C., Prior, H., Duka, T., Leshchins'ka, I., Valenti, P., Güntürkün, O. & Lipp, H.-P. (1999) Behav. Brain Res. 104, 169–178. [DOI] [PubMed] [Google Scholar]
- 7.Braithwaite, V. & Guilford, T. (1991) Proc. R. Soc. London Ser. B 245, 183–186. [Google Scholar]
- 8.Biro, D., Guilford, T. & Dawkins, M. (2003) Anim. Behav. 65, 115–122. [Google Scholar]
- 9.Schmidt-Koenig, K. (1958) Z. Tierpsychol. 15, 301–331. [Google Scholar]
- 10.Keeton, W. T. (1971) Proc. Natl. Acad. Sci. USA 68, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiltschko, R., Nohr, D. & Wiltschko, W. (1981) Science 214, 343–345. [DOI] [PubMed] [Google Scholar]
- 12.Wallraff, H. G. (1974) Das Navigationssystem der Vogel (Oldenbourg, Munich).
- 13.Wallraff, H. G. (1991) in Orientation in Birds, ed. Berthold, P. (Birkhäuser, Basel), pp. 128–165.
- 14.Wiltschko, W. & Wiltschko, R. (1978) Oikos 20, 177–187. [Google Scholar]
- 15.Füller, E., Kowalski, U. & Wiltschko, R. (1983) J. Comp. Physiol. A 153, 55–58. [Google Scholar]
- 16.Gagliardo, A., Ioalè, P. & Bingman, V. (1999) J. Neurosci. 19, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagliardo, A., Odetti, F., Ioalè, P., Bingman, V. P., Tuttle, S. & Vallortigara, G. (2002) Behav. Brain Res. 136, 201–209. [DOI] [PubMed] [Google Scholar]
- 18.Foà, A. & Albonetti, E. (1980) Z. Tierpsychol. 54, 327–338. [Google Scholar]
- 19.Bingman, V. & Ioalè, P. (1989) Behavior 110, 205–218. [Google Scholar]
- 20.Chappell, J. (1997) J. Exp. Biol. 200, 2269–2277. [DOI] [PubMed] [Google Scholar]
- 21.Wallraff, H. G., Chappell, J. & Guilford, T. (1999) J. Exp. Biol. 202, 2121–2126. [PubMed] [Google Scholar]
- 22.von Hünerbein, K., Hamann, H.-J., Rüter, E. & Wiltschko, W. (2000) Naturwissenschaften 87, 278–279. [DOI] [PubMed] [Google Scholar]
- 23.Steiner, I., Bürgi, C., Werffeli, S., Dell'Omo, G., Valenti, P., Tröster, G., Wolfer, D. P. & Lipp, H.-P. (2000) Physiol. Behav. 71, 589–596. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, S. J., Guilford, T., Rezek, I. & Biro, D. (2004) J. Theor. Biol. 227, 39–50. [DOI] [PubMed] [Google Scholar]
- 25.Guilford, T., Roberts, S. J., Biro, D. & Rezek, I. (2004) J. Theor. Biol. 227, 25–38. [DOI] [PubMed] [Google Scholar]
- 26.Biro, D., Guilford, T., Dell'Omo, G. & Lipp, H.-P. (2002) J. Exp. Biol. 205, 3833–3844. [DOI] [PubMed] [Google Scholar]
- 27.Meade, J., Biro, D. & Guilford, T. (2004) Proc. R. Soc. London Ser. B, in press.
- 28.Baker, R. R. (1984) Bird Navigation: The Solution of a Mystery? (Hodder & Stoughton, London).
- 29.Biro, D. (2002) Ph.D. thesis (Univ. of Oxford, Oxford).
- 30.Lipp, H.-P., Vyssotski, A. L., Wolfer, D. P., Renaudineau, S., Savini, M., Tröster, G. & Dell'Omo, G. (2004) Curr. Biol. 14, 1239–1249. [DOI] [PubMed] [Google Scholar]