Abstract
Objective
To examine the comparative antidepressant efficacy of S-adenosyl-L-methionine (SAMe) and escitalopram in a placebo-controlled, randomized, double-blind clinical trial.
Methods
189 outpatients (49.7% female, mean age 45 ± 15 years) with DSM-IV-diagnosed major depressive disorder (MDD) were recruited from 4/13/05-12/22/09 at the Massachusetts General Hospital and at Butler Hospital. Patients were randomized for 12 weeks to SAMe 1600-3200 mg/day, escitalopram 10-20 mg/day, or placebo. Doses were escalated at 6 weeks in the event of non-response. The main outcome measure was the Hamilton Depression Rating Scale (HAM-D-17). Tolerability was assessed by the Systematic Assessment for Treatment Emergent Effects-Specific Inquiry (SAFTEE-SI).
Results
All 3 treatment arms demonstrated a significant improvement of about 5-6 points in HAM-D-17 scores (p < 0.001 for all), and no significant differences were observed between the treatment arms (p > 0.05 for all). Response rates in the intent-to-treat (ITT) sample were 36% for SAMe, 34% for escitalopram, and 30% for placebo. Remission rates were 28% for SAMe, 28% for escitalopram, and 17% for placebo. No comparisons between treatment groups attained significance (p > 0.05 for all). Tolerability was good, with gastrointestinal side effects (19% for stomach discomfort and 20% for diarrhea) as the most common in the SAMe arm. No significant differences in side effects were observed between treatment groups (p > 0.05 for all).
Conclusions
The results fail to support an advantage over placebo for either the investigational treatment SAMe or the standard treatment escitalopram.
Keywords: S-adenosyl methionine, S-adenosyl-L-methionine SAMe, escitalopram, depression
Introduction
S-adenosyl-L-methionine (SAMe) is a natural substance synthesized from the amino acid L-methionine and adenosine triphosphate (ATP) through the one-carbon cycle1. In the brain, SAMe functions as a methyl donor, shifting its methyl group to various key neurotransmitters via methyl-transferase reactions2. Over the past 30 years, more than 45 randomized clinical trials have supported SAMe’s antidepressant efficacy as monotherapy against placebo and tricyclic antidepressants3,4. In a recent double-blind controlled study, SAMe augmentation was shown to be effective in nonresponders to selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs)5.
Thus far no published studies have compared SAMe against newer antidepressants such as the SSRIs, the standard first-line treatment for depression. We therefore carried out a three-armed, randomized, double-blind trial comparing SAMe against placebo and escitalopram in a sample of patients with major depressive disorder (MDD). Escitalopram is especially well-suited for comparison with SAMe because both have comparable tolerability and attrition rates to placebo5,6, thus minimizing the risk of unblinding patients.
A12-week treatment duration was selected to reduce transient improvement, increase treatment effect size, and provide statistical power equivalent to that of a larger sample7. Also, patients who obtain a true drug response within 6 weeks are likely to continue to respond after another 6 weeks of treatment, while those with a placebo-like response will most likely fail to sustain response7. The study included a crossover phase in which nonresponders to either escitalopram or SAMe received the combination of the two drugs, though this report will focus on the main outcome data for the first 12 weeks of double-blind treatment.
We assessed the acute effects of SAMe or escitalopram vs. placebo on clinical improvement, quality of life, and psychosocial functioning. We also assessed the tolerability of the three treatments. We hypothesized that we would obtain similar efficacy findings for SAMe and escitalopram, and that both would yield beneficial clinical effects significantly greater than placebo. Likewise, we expected differences in specific side effects between SAMe, escitalopram, and placebo, e.g. a greater incidence of sexual dysfunction or gastrointestinal upset with escitalopram.
Method
We recruited male and female subjects of ages 18-80, from April 13, 2005 to December 22, 2009, at the Massachusetts General Hospital in Boston, MA and at Butler Hospital in Providence, RI. The trial (NCT00101452) was conducted according to the U.S. Food and Drug Administration (FDA) guidelines and the Declaration of Helsinki. Subjects were outpatients with MDD, diagnosed by the Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P)8. After complete description of the study to the subjects, written informed consent was obtained. Patients were recruited through clinician referral and general advertisement in local newspapers, radio, and television. The study was approved by both sites’ Institutional Review Boards (IRBs).
Inclusion Criteria
In addition to the diagnostic criteria for MDD, scores of ≥25 on the Inventory of Depressive Symptomatology – Clinician-Rated (IDS-C)9 at the screen and baseline visits were required. The 17-item Hamilton Depression Rating Scale (HAM-D-17)10-12 was used as the main outcome instrument for antidepressant efficacy.
Exclusion Criteria
Pregnancy or women of child-bearing potential who were not using a medically accepted means of contraception; serious suicidality or homicidality; unstable medical illness, including cardiovascular, hepatic, renal, respiratory, endocrine, neurological, or hematological; organic mental disorders; substance use disorders, including alcohol, active within the preceding six months; schizophrenia and other psychotic disorders or psychotic features; bipolar disorder; acute bereavement; severe borderline or antisocial personality disorder; current primary diagnoses of panic disorder or obsessive-compulsive disorder; seizure disorder; concurrent use of other psychotropic drugs; hypothyroidism; a ≥6-week course of either escitalopram ≥10 mg/day or SAMe ≥1200 mg/day during the current depressive episode; intolerance to SAMe or escitalopram; having taken an investigational psychotropic drug within the last year; failure to respond to 2 or more antidepressant trials at adequate doses (e.g., fluoxetine ≥40 mg/day) and duration (≥6 weeks) during the current depressive episode; any depression-focused ongoing psychotherapy; history of bleeding diatheses, low platelet counts, gastrointestinal bleeding, or use of medications that alter bleeding risk; a Clinical Global Improvement13 scale score (CGI-I) of “much” or “very much improved” between the screen and baseline visits and/or an IDS-C score <25 at either the screen or the baseline visit.
Eligible subjects were randomized in a 1:1:1 manner for 12 weeks of double-blind treatment with SAMe, escitalopram, or placebo. Randomization numbers were assigned by a biostatistician, in consecutive order, stratified by site, and maintained by the research pharmacists at both sites. We used a double-dummy design to maintain the blind, since SAMe tablets differed in appearance from escitalopram tablets. Each patient took tablets from two bottles, with one bottle containing either SAMe or SAMe-placebo and the other containing either escitalopram or escitalopram-placebo, depending on the randomization. SAMe tosylate and matching placebo were supplied by Pharmavite LLC, CA. Escitalopram and matching placebo were purchased from Forest. Preparation of blinded compounds took place at each site’s research pharmacy. All patients, clinicians and research coordinators remained blinded to the intervention. Subjects were compensated $25 for each completed visit.
During the screen visit, patients were administered the SCID-I/P, the 28-item Hamilton Depression Rating Scale (HAM-D-28) (from which the HAM-D-17 score was derived), the CGI-Severity scale13, the IDS-C9, the IDS-Self-Report (IDS-SR)9, the Quality of Life Enjoyment and Satisfaction Scale (Q-LES-Q)14, the Medical Outcome Survey Short Form-36 (SF-36)15,16, and the Anger Attacks Questionnaire (AAQ)17.
During subsequent visits, patients were administered the Mood Module of the SCID, the HAM-D-28, the CGI-Severity and Improvement scales, the IDS-SR, and the Systematic Assessment for Treatment Emergent Effects-Specific Inquiry (SAFTEE-SI) Scale18. At baseline and Visit 7 (week 12), patients also were administered the IDS-C, Q-LES-Q, SF-36, AAQ, and Consumptive History (use of alcohol, tobacco, and caffeine). Side effects and adverse events were documented with the SAFTEE-SI.
Dosing
During the first 6 weeks, patients were randomly assigned to either SAMe 1600 mg/day, escitalopram 10 mg/day, or placebo. To maximize the probability of response, a dose increase was allowed for nonresponders (patients with a <50% HAM-D-17 reduction) at week 6; escitalopram could be increased to 20 mg/day and SAMe to 3200 mg/day for weeks 7-12. Patients who experienced intolerable side effects at the higher dose were allowed to decrease the dose to the previous level.
Statistical Analysis
The primary efficacy measure was the change in HAM-D-17 score over 12 weeks. Response was defined as a ≥50% decrease in the HAM-D-17 score, and remission as a final HAM-D-17 score of ≤7. Secondary measures of efficacy included changes in scores on the IDS-C, IDS-SR, and CGI ratings for Severity and Improvement over time.
Calculations for the originally proposed sample with n=100 patients per treatment group projected at least 80 percent power (β = 0.20) to detect a treatment difference with 0.05 probability of occurring by chance (2-tailed α = 0.05), assuming a modest effect size19 for continuous measures (d = 0.39) and for categorical measures (w = 0.25).
Analyses of treatment effectiveness were conducted for an intent-to-treat (ITT) sample, including all patients randomized to any of the three treatment arms (i.e. completed a baseline visit and accepted supply of medications). Mixed model regression analysis was employed for the change in HAM-D-17 score to adjust for the length of time actually spent in treatment. Chi-square analyses were used to compare response and remission rates.
Side effects documented on the SAFTEE-SI Scale were categorized by severity as: 0-none, 1-mild, 2-moderate, 3-severe. Because some SAFTEE items could be present at baseline, we defined treatment-emergent as any SAFTEE side effect for which severity increased by two or more levels from baseline. For example, a side effect with severity rating that changed from none to moderate or from mild to severe would be considered as treatment-emergent. Analysis was based on the number of patients in each arm reporting these side effects at any time during the 12-week treatment. Differences between treatment groups were compared by chi-square analyses.
Statistical analyses were carried out using SPSS version 17.0 (SPSS Inc, Chicago, Illinois) and SAS 9.2 software (SAS Institute Inc., 2001), by authors Baer, Ludington, and Mischoulon, with assistance from authors Clain, Walker, and. Durham. An alpha level of 0.05 was used to determine statistical significance.
Results
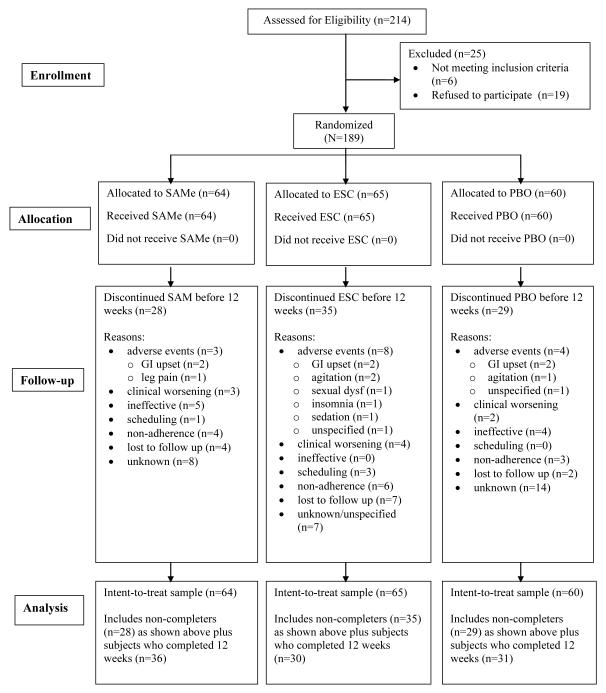
We screened 214 subjects, and randomized 189 (49.7% female, mean age 45 ± 15 years; Figure 1). Race distribution included Caucasian (n = 137; 72%), African-American (n = 33; 17%), Asian (n = 2; 1%), and Native American (n = 2; 1%). Eight subjects (4%) self-described as Hispanic. The remaining subjects did not self-identify a specific category (Table 1).
Figure 1.
CONSORT Statement Flow Diagram
Table 1.
Demographics for Intent-to-Treat Sample
| Study Site, n (%) | Mass. General Hospital Butler Hospital |
123 (65) 66 (35) |
|
| ||
| Age, y, mean (SD) [range] | 45 (15) [17-79] | |
|
| ||
| Gender, n (%) | Female | 94 (50) |
|
| ||
| Race/Ethnicity, n (%) | Caucasian | 137 (72) |
| African American | 33 (17) | |
| Native American/Alaskan | 2 (1) | |
| Asian | 2 (1) | |
| Hispanic/Latino | 8 (4) | |
| No Response | 7 (4) | |
|
| ||
| Education, n (%) | Did not graduate High School | 21 (11) |
| Graduated High School | 33 (18) | |
| Some College | 45 (24) | |
| Graduated 2 Year College | 16 (9) | |
| Graduated 4 Year College | 27 (14) | |
| Some Graduate School | 10 (5) | |
| Graduated Graduate School | 28 (15) | |
| No Response | 9 (5) | |
|
| ||
| Marital Status, n (%) | Never Married | 86 (46) |
| Married/Cohabitating | 42 (22) | |
| Separated/Divorced | 43 (23) | |
| Widowed | 8 (4) | |
| No Response | 10 (5) | |
|
| ||
| Employment Status, n (%) | Employed Full-Time | 46 (24) |
| Employed Part-Time | 32 (17) | |
| Student | 4 (2) | |
| Retired | 21 (11) | |
| Unemployed | 48 (25) | |
| Volunteer | 2 (1) | |
| Disabled | 20 (11) | |
| Homemaker | 1 (1) | |
| Other/No Response | 15 (8) | |
|
| ||
| Baseline IDS-SR, mean (SD) [range] | 36.6 (11.3) [10- 73] |
|
|
| ||
| Baseline HAM-D-17, mean (SD) [range] |
19.2 (4.7) [4-32] | |
Abbreviations:
IDS-SR: Inventory of Depressive Symptomatology – Self-Rated
HAM-D-17: Hamilton Depression Rating Scale – 17 item
Ninety-seven subjects (SAMe n = 36; ESC n = 30; PBO n = 31) completed the study. For the ITT analysis using last-observation-carried-forward (LOCF), we selected all 189 randomized patients (SAMe n = 64; ESC n = 65; PBO n = 60). For all treatment groups, mean HAM-D-17 scores, IDS-C scores, and CGI-S ratings decreased significantly over 12 weeks of treatment (p < 0.001 for all), but no comparisons between groups for these scales and CGI-I reached significance (Table 2).
Table 2.
Changes in Main Outcome Instruments, Response and Remission Rates for Intent-to-Treat (ITT) Sample
| INTENT-TO-TREAT (n = 189) | ||||||
|---|---|---|---|---|---|---|
| Instrument | SAMe (n = 64) | Escitalopram (n = 65) | Placebo (n = 60) | |||
| Mean | SD | Mean | SD | Mean | SD | |
| HAM-D-17 BSL | 18.98 | 5.09 | 19.25 | 4.88 | 19.43 | 4.07 |
| HAM-D-17 END | 12.79* | 7.38 | 12.94* | 6.98 | 14.32* | 6.92 |
| IDS-SR BSL | 34.87 | 9.74 | 37.54 | 12.35 | 37.44 | 11.56 |
| IDS-SR END | 23.29* | 12.53 | 26.84* | 15.28 | 28.57* | 14.21 |
| CGI-S BSL | 4.38 | 0.76 | 4.44 | 0.69 | 4.29 | 0.65 |
| CGI-S END | 3.08* | 1.46 | 3.14* | 1.44 | 3.28* | 1.37 |
| CGI-I END | 2.73 | 1.24 | 2.76 | 1.24 | 2.90 | 1.24 |
| n | % | n | % | n | % | |
| Responsea | 23 | 35.9 | 22 | 33.8 | 18 | 30.0 |
| Remissiona | 18 | 28.1 | 18 | 27.7 | 10 | 16.7 |
Abbreviations:
HAM-D-17: Hamilton Depression Rating Scale – 17 item
IDS-SR: Inventory of Depressive Symptomatology – Self-Rated
CGI-S: Clinical Global Improvement Scale – Severity
CGI-I: Clinical Global Improvement Scale – Improvement
BSL: Baseline visit
END: End visit
P < 0.001 for change from baseline to endpoint
There were no significant differences between the 3 treatment groups for response and remission rates based on ≥ 50% improvement in HAM-D-17 score from baseline to end (P > 0.05 for all 2-way and 3-way comparisons) for the ITT sample.
Mixed model random regression analysis (unstructured covariance matrix model with linear and quadratic terms for week) for change in HAM-D-17 score showed in the test of fixed effects a significant effect for time by study week (F = 51.50, p < 0.001) but not for treatment (F = 0.35, p = 0.705), agreeing with the LOCF analysis that all treatment groups improved over time. There was a significant interaction between treatment and baseline HAM-D-17 (F = 24.09, p < 0.001). Because these scores did not appear significantly different by inspection (Table 2), we ran a one-way Analysis of Variance (ANOVA) with baseline HAM-D-17 as dependent variable and treatment group as independent variable, showing no significant treatment group effect (F=0.142, p=0.87). The MMRM interaction effect between baseline HAM-D-17 and treatment group was significant only in the model containing visit number, visit number squared (quadratic), baseline HAMD-17, and all interactions with treatment group, suggesting that the significant difference occurred in the context of the covariates chosen. We also ran analyses using autoregressive covariance matrix and compound symmetry covariance matrix, but results were not substantially different.
Response rates were 35% for SAMe, 34% for escitalopram, and 30% for placebo. Remission rates were 28% for SAMe, 28% for escitalopram, and 17% for placebo. Differences between groups were not significant (Table 2).
We examined the time course of improvement for each treatment arm and outcome measure. The HAM-D-17 improvement course is illustrated in Figure 2. Differences in scores at individual time points illustrated trends to significance and a significant difference between SAMe and placebo and between SAMe and escitalopram (Figure 2, Supplementary eTable 1).
Figure 2. Time Course of HAM-D-17 Scores (Intent-to-Treat Sample).
* Significant difference in HAM-D-17 score between SAMe and PBO at Week 8 (p = 0.026) and Week 10 (p = 0.034). All other comparisons were non-significant.
Tolerability data were available for 166 subjects. The most common side effects reported were gastrointestinal, with rates of 19% for stomach discomfort and 20% for diarrhea in the SAMe group, though these did not separate from placebo or escitalopram (p>0.05 for all comparisons). SAMe had a significant advantage over escitalopram regarding anorgasmia; escitalopram had a significant advantage over placebo in diminished mental acuity/sharpness; and SAMe had a significant advantage over placebo in dizziness or faintness (Table 3). The large number of SAFTEE symptoms (total 55) would render all comparisons insignificant after Bonferroni correction. Since this is the first comparison of SAMe versus an SSRI, we erred on the side of false positives, to give a sense of what differences in adverse effects one should expect. Fifteen subjects (SAMe n = 3, ESC n = 8, PBO n = 4) discontinued from the study specifically due to adverse effects (Figure 1).
Table 3.
Adverse Effects in the Safety Sample (n = 166) (SAFTEE Scale) (Statistically significant differences only)
| # | Side Effect | Placebo (n = 52) |
SAMe (n = 59) |
Escitalopram (n = 55) |
|||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| 13. | Dizziness or faintnessa | 4 | 7.6 | 0 | 0.0 | 2 | 3.6 |
| 38. | Delayed or absent orgasmb | 4 | 7.6 | 2 | 3.4 | 10 | 18.2 |
| 46. | Diminished mental acuity/sharpnessc | 6 | 11.5 | 1 | 1.7 | 0 | 0.0 |
| 52. | Hot flashesd | 0 | 0.0 | 0 | 0.0 | 4 | 7.3 |
χ2 = 4.54; p = 0.049 (PBO vs SAMe)
χ2 = 8.43; p = 0.015 (3-way comparison); χ2 = 7.31; p = 0.011 (SAMe vs ESC)
χ2 = 9.95; p = 0.007 (3-way comparison); χ2 = 6.61; p = 0.012 (PBO vs ESC)
χ2 = 8.20; p = 0.017 (3-way comparison)
Discussion
This randomized placebo-controlled study comparing SAMe monotherapy against SSRI monotherapy must be considered a failed trial. Both active treatments, including an established, FDA-approved antidepressant, demonstrated comparable antidepressant efficacy, but neither separated from placebo at the end of 12 weeks. On the HAM-D-17 scale only, significant separation between SAMe and placebo was obtained at treatment weeks 8 (Visit 5) and 10 (Visit 6), with separation lost by week 12 (Visit 7) (Figure 2). This depressive worsening at the last visit likely also affected the findings in the MMRM analysis. Given that a treatment change awaited non-responders, week 12 may have caused uncertainty as to whether it was the final visit, and clouded issues of expectancy. However, the additional gain for the escitalopram group after week 10 does not suggest any general psychological effects contributing to this change.
Response and remission rates for both active treatments (30-36% response and 28% remission) were lower than expected, based on what is known about the efficacy of established antidepressants20. However most responders attained full remission, particularly with the active treatments (about 28% for SAMe and escitalopram versus 17% for placebo). This suggests that the active treatments produced a more robust or “true” effect compared to placebo, arguing in favor of their purported efficacy. Placebo response rates were in the range of 30% in the ITT sample, a rate not unusual in antidepressant studies. This may also reflect the fact that the greater the chance of receiving an active treatment (in this case a two in three chance), the greater the placebo response rate21. A recent meta-analysis by Iovieno and Papakostas22 found that placebo response rates of ≥ 30% correlated with a lower risk ratio of responding to antidepressant versus placebo and a greater number needed to treat (NNT) for response, which appears consistent with our findings. A meta-analysis from our group23 does not suggest a greater placebo-response rate for studies of CAM interventions. Conversely, a reanalysis of data from the Hypericum Depression Trial Study Group24, which compared Hypericum against sertraline, suggests that patient's beliefs that they are receiving a particular drug may be more important than the actual drug itself25.
Given the generally encouraging body of evidence for the efficacy of SAMe, as well as for escitalopram, our findings of equivalence with placebo were surprising. To examine whether baseline depressive severity may have impacted on the findings, we ran separate post hoc outcome analyses for changes in HAM-D-17, IDS-SR, CGI-S, and response/remission rates for patients with baseline HAM-D-17 scores ≥20 (n=80) and for those with HAM-D-17 scores <20 (n=109). The results were essentially the same for both groups as for the complete sample, suggesting that the findings were not influenced by depressive severity (data not shown).
Both active treatments were available to individuals without participating in a study if they were inclined to see a physician and had medical insurance or could afford to pay for the drugs. In addition, there may be unique characteristics about subjects who opt to participate in a study of a nutritional supplement, and this might explain a number of factors that could have contributed to the lack of separation from placebo: 1) Nearly 60% of the sample had pretreatment HAM-D scores of 19 or less, a level of severity for which it is difficult to show significant drug-placebo differences; 2) We had an unusually high rate of randomization of screened subjects (88%); 3) We enrolled only 63% of the projected sample; 4) Only 51% completed the study.
In addition to sample characteristics, some unique characteristics of this study may have contributed to the lack of separation from placebo. There are few 12-week placebo-controlled trials in MDD. In two trials pooled by Golden et al26, placebo response and remission rates were 41.5% and 20.5% after 6 weeks of treatment, and increased to 61.2% and 44.0% after 12 weeks. Placebo response and remission rates in that range can reduce the ability to detect active treatment effects because of a "ceiling" effect. As shown previously, there is a cumulative risk of spontaneous improvement, and such risk may be maximized in 12-week trials27. The decision to delay up-titration of escitalopram until after week 6 may have also compromised efficacy. Finally, the separation between SAMe and placebo at the penultimate visits suggests that this trial may have been subject to type II error, i.e. the “false negative” study that can occur based on chance. Other factors may include limitations of our conception of MDD and current trial methods.
Tolerability of all treatments was good, with GI complaints as the most commonly reported adverse effects for both active treatment arms, and surprisingly for the placebo arm as well. GI complaints are common with SSRIs and with SAMe, and our previous study of SAMe augmentation also resulted in high rates of GI side effects5. It is possible that suggestiveness on the part of patients who were informed of the most common side effects with these treatments may have resulted in higher rates of GI side effects.
Like all clinical trials, our study has limitations. The trial was originally powered for 300 subjects, and the actual sample size obtained, even after a one-year no-cost extension, was slightly under two-thirds of that expected, representing the study’s major limitation. While this would inevitably diminish power to detect a difference between active treatments and placebo, our sample is large enough to provide a conclusive statement about the efficacy of SAMe as a monotherapy for MDD. We did not discern a clinically meaningful difference with placebo, and it seems unlikely that a larger sample would have yielded a significant difference between treatments. Although every effort was made to ensure that ratings, recruitment practices, and inclusion/exclusion criteria were consistent between sites, there may have been site-related factors that impacted on outcomes.
To conclude, in this first head-to-head comparison of SAMe against an SSRI, neither SAMe nor escitalopram separated from placebo after 12 weeks, constituting a failed study. Despite the smaller than expected sample, this study was more rigorously designed than most previous trials. It is possible that SAMe may be better suited as an augmentation therapy5,28 than as a monotherapy; another interpretation of the findings is that SAMe possesses comparable antidepressant efficacy to escitalopram, but that an unusually high placebo response rate confounded our findings. The findings may not be generalizable to all antidepressants or even all SSRIs, and future comparisons with other agents will be needed to better clarify SAMe’s potential antidepressant effect and its place in the psychopharmacological armamentarium.
Supplementary Material
Supplementary eTable 1: Time course of Main Outcome Instruments for Intent-to-Treat Sample (N = 189)
Clinical Points.
In this clinical trial involving treatment of major depressive disorder (MDD), the natural product S-adenosyl-L-methionine (SAMe) and the SSRI escitalopram did not demonstrate a significant advantage over placebo.
While all 3 treatment groups experienced a significant clinical improvement, the abnormally high placebo response rate prevents any definitive statement about the efficacy of either of the antidepressants tested.
This study is the first comparison of SAMe against an SSRI, and therefore further comparisons between SAMe and established antidepressants are called for.
Funding and Acknowledgments
This study was supported by the National Institutes of Health (NIH) and the National Center for Complementary and Alternative Medicine (NCCAM), R01 grant R01AT001638. This manuscript reflects the views of the authors and may not reflect the opinions or views of all the study investigators, the NIH, or the NCCAM. The ClinicalTrials.gov Identifier is NCT00101452. The sponsors had no further role in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the paper for publication.
SAMe tosylate and matching placebo were supplied by Pharmavite LLC, CA
The authors thank Kelly Colombo, B.A. (Butler Hospital Mood Disorders Research Program) for her assistance with study procedures and data management.
Previous Presentation
Portions of the material in this manuscript were presented at the Annual Meeting of the American Psychiatric Association, San Francisco, CA, May 19 and 21, 2013.
Financial Disclosures
Dr Mischoulon has received research support from Nordic Naturals, the Bowman Family Foundation, Fisher Wallace, Methylation Sciences, Inc., and Ganeden. He has served as a consultant to Bristol-Meyers-Squibb Company. He has received writing honoraria from Pamlab and Nordic Naturals and speaking honoraria from Nordic Naturals. He has received royalties from Back Bay Scientific for PMS Escape, and royalties from Lippincott Williams & Wilkins, for textbook “Natural Medications for Psychiatric Disorders: Considering the Alternatives” (David Mischoulon and Jerrold F Rosenbaum, Eds.). He has received honoraria from Reed Medical Education (a company working as a logistics collaborator for the MGH Psychiatry Academy). The education programs conducted by the MGH Psychiatry Academy were supported through Independent Medical Education (IME) grants from pharmaceutical companies co-supporting programs along with participant tuition. Commercial entities currently supporting the MGH Psychiatry Academy are listed on the Academy's website www.mghcme.org.
Dr Price has received research support from Medtronic, Neuronetics, NIH, HRSA, and NeoSync. He has served on advisory panels for Abbott and AstraZeneca. He has served as a consultant to Gerson Lehrman, Wiley, Springer, Qatar National Research Fund, and Abbott.
Dr Carpenter has received research support from Medtronic, Neuronetics, NIH, and NeoSync. She has served on advisory panels or provided consulting services for Abbott, Corcept, Johnson&Johnson, and Takeda-Lundbeck.
Dr Tyrka has received research support from Medtronic, Neuronetics, NeoSync, and NIH.
Dr Papakostas has served as a consultant for Abbott Laboratories, AstraZeneca PLC, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., GlaxoSmithKline, Evotec AG, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc. He has received honoraria from Abbott Laboratories, Astra Zeneca PLC, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Lundbeck, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc. He has received research support from AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, PAMLAB LLC, Pfizer Inc., and Ridge Diagnostics (formerly known as Precision Human Biolaboratories). He has served (not currently) on the speaker’s bureau for BristolMyersSquibb Co and Pfizer, Inc.
Dr Ludington has received research support from Methylation Sciences, Inc., Neuraltus, Brain Cells, Ocera, and Halyzome.
Dr Fava has received research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra-Zeneca, BioResearch, BrainCells, Inc., Bristol-Myers Squibb Company, Cephalon, Clinical Trial Solutions, LLC, Eli Lilly & Company, EnVivo Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., Ganeden, GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, NARSAD, NCCAM, NIDA, NIMH, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite LLC, Roche, Sanofi-Aventis, Shire, Solvay Pharmaceuticals, Inc., Synthelabo, and Wyeth-Ayerst Laboratories. He has served as an advisor and consultant to Abbott Laboratories, Affectis Pharmaceuticals AG, Amarin, Aspect Medical Systems, Astra-Zeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, Inc, BioMarin Pharmaceuticals, Inc., Biovail Pharmaceuticals, Inc., BrainCells, Inc, Bristol-Myers Squibb Company, Cephalon, Clinical Trials Solutions, LLC, CNS Response, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eisai, Inc., Eli Lilly & Company, EPIX Pharmaceuticals, Euthymics Bioscience, Inc., Fabre-Kramer, Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithKline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante Inc., Merck, Methylation Sciences, Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc, PharmaStar, Pharmavite LLC, Precision Human Biolaboratory, Prexa Pharmaceuticals, Inc., PsychoGenics, Psylin Neurosciences, Inc., Ridge Diagnostics, Inc., Roche, Sanofi-Aventis, Sepracor, Schering-Plough, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Synthelabo, Takeda, Tetragenex, TransForm Pharmaceuticals, Inc., Transcept Pharmaceuticals, Vanda Pharmaceuticals Inc, Wyeth-Ayerst Laboratories. He has received speaking and publishing honoraria from Adamed, Co., Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, Astra-Zeneca, Belvoir, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithKline, Imedex, Novartis, Organon Inc., Pfizer Inc, PharmaStar, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed-Elsevier, UBC, and Wyeth-Ayerst Laboratories. He holds equity in Compellis. He currently holds a patent for SPCD and a patent application for a combination of azapirones and bupropion in MDD, and has received copyright royalties for the MGH CPFQ, SFI, ATRQ, DESS, and SAFER diagnostic instruments.
All other authors and Ms Colombo report no competing interests.
Clinical Trials Registration
ClinicalTrials.gov Identifier NCT00101452 (available at www.clinicaltrials.gov)
References
- 1.Spillmann M, Fava M. S-adenosyl-methionine (ademethionine) in psychiatric disorders. CNS Drugs. 1996;6(6):416–425. [Google Scholar]
- 2.Baldessarini RJ. The neuropharmacology of S-adenosyl-L-methionine. Am J Medicine. 1987;83(5A):95–103. doi: 10.1016/0002-9343(87)90860-6. [DOI] [PubMed] [Google Scholar]
- 3.Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Rep. 2003;5(6):460–466. doi: 10.1007/s11920-003-0085-2. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 5):18–22. doi: 10.4088/JCP.8157su1c.04. [DOI] [PubMed] [Google Scholar]
- 5.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl Methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors (SRIs) for SRI- Non-responders with Major Depressive Disorder: A Double-blind, Randomized Clinical Trial. American J Psychiatry. 2010;167(8):942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 6.Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63(4):331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- 7.Quitkin FM, Rabkin JG, Stewart JW, McGrath PJ, Harrison W. Study duration in antidepressant research: advantages of a 12-week trial. J Psychiatr Res. 1986;20(3):211–216. doi: 10.1016/0022-3956(86)90004-x. [DOI] [PubMed] [Google Scholar]
- 8.First BM, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID I/P) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 9.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton M. Development of a rating scale for primary depressive illness. Br J Social Clin Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 13.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology, revised. DHEW Pub. No. (ADM)76-338. National Institute of Mental Health; Rockville, MD: 1976. [Google Scholar]
- 14.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire (Q-LES-Q): a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 15.Sherbourne CD, Wells KB, Hays RD, Rogers W, Burnam MA, Judd LL. Subthreshold depression and depressive disorder: clinical characteristics of general medical and mental health specialty outpatients. Am J Psychiatry. 1994;151(12):1777–1784. doi: 10.1176/ajp.151.12.1777. [DOI] [PubMed] [Google Scholar]
- 16.Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA. 1989;262(7):914–919. [PubMed] [Google Scholar]
- 17.Fava M, Rosenbaum JF, McCarthy M, Pava J, Steingard R, Bless E. Anger attacks in depressed outpatients and their response to fluoxetine. Psychopharmacol Bull. 1991;27(3):275–279. [PubMed] [Google Scholar]
- 18.Rabkin JG, Markwoitz JS, Ocepek-Welikson K, Wagner SS. General versus systematic inquiry about emergent clinical events with SAFTEE: Implications for clinical research. J Clin Psychopharmacology. 1992;12(1):3–10. doi: 10.1097/00001573-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 20.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60(11):1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 21.Papakostas G, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73(10):1300–1306. doi: 10.4088/JCP.11r07485. [DOI] [PubMed] [Google Scholar]
- 23.Freeman MP, Mischoulon D, Tedeschini E, Goodness T, Cohen LS, Fava M, Papakostas GI. Complementary and alternative medicine for major depressive disorder: a meta-analysis of patient characteristics, placebo response rates, and treatment outcomes relative to standard antidepressants. J Clin Psych. 2010;71(6):682–688. doi: 10.4088/JCP.10r05976blu. [DOI] [PubMed] [Google Scholar]
- 24.Hypericum Depression Trial Study Group Effect of Hypericum perforatum (St John's wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807–1814. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- 25.Chen JA, Papakostas GI, Youn SJ, Baer L, Clain AJ, Fava M, Mischoulon D. Association between patients’ beliefs regarding assigned treatment and clinical response: Re-analysis of data from the Hypericum Depression Trial Study Group. J Clinical Psychiatry. 2011;72(12):1669–1676. doi: 10.4088/JCP.10m06453. [DOI] [PubMed] [Google Scholar]
- 26.Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dubé EM. Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. J Clinical Psychiatry. 2002;63(7):577–584. doi: 10.4088/jcp.v63n0707. [DOI] [PubMed] [Google Scholar]
- 27.Posternak MA, Zimmerman M. Short-term spontaneous improvement rates in depressed outpatients. J Nerv Ment Dis. 2000;188(12):799–804. doi: 10.1097/00005053-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Alpert JE, Papakostas G, Mischoulon D, Worthington JJ, 3rd, Petersen T, Mahal Y, Burns A, Bottiglieri T, Nierenberg AA, Fava M. S-Adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661–664. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary eTable 1: Time course of Main Outcome Instruments for Intent-to-Treat Sample (N = 189)