ABSTRACT
Allergen-specific IgG produced by immune mothers is associated with less predisposition to allergy development in their children. This finding has been described by several groups over the last few decades, but the mechanisms by which maternal IgG can inhibit allergy development are still not fully understood. With the purpose of summarizing past investigations, we review the literature on murine models of maternal immunization with allergens and on immune regulation in humans after passive therapy with purified IgG. Based on our review, a new hypothesis about these mechanisms is presented, which may provide a foundation for the future development of therapies to inhibit allergy development.
Keywords: allergy, B cells, IVIg, maternal IgG, T cells
Introduction
The passive transfer of maternal IgG antibodies (MatIgG) to offspring is predominantly mediated by the FcRn receptor.1 In humans, this transfer mainly occurs via placental transport and seems to start during the second trimester of pregnancy.2,3 The levels of MatIgG transferred to the fetus can increase until the end of pregnancy.4 Moreover, during lactation, transfer can also occur because FcRn receptors are expressed on the epithelial cells of newborns' intestines, with receptor interaction protecting MatIgG from catabolism.5
The maternal transfer of allergen-specific IgG, including IgGs against cat epithelium, pollen6 and dietary antigens such as OVA,7 was first shown many years ago and is associated with lower predisposition to allergy development during the first years of life. Our group has demonstrated that maternal immunization with allergens can inhibit type I hypersensitivity in offspring in murine models,8-13 and years ago, we suggested that this phenomenon may be related to MatIgG levels in offspring.14 Direct evidence that MatIgG is able to suppress IgE production in offspring was also obtained years ago in a murine model of OVA immunization.15
Although these findings have been described since the 1980s, our understanding of the exact mechanism of MatIgG interaction with the fetal immune system to inhibit allergy in children has not risen proportionally. Here, we intend to highlights several of the most important findings in this regard, with the goal of contributing a new perspective on the potential of IgG antibodies to control the development of allergies.
Given this purpose, we focus on 2 main topics in the literature that are relevant to our discussion: I- the knowledge resulting from murine models used to investigate the role of maternal IgG in inhibiting the hypersensitivity response in offspring and II- the knowledge resulting from passive therapy with purified IgG in humans to regulate immune responses.
Based on this discussion, we present a new hypothesis and certain considerations to provide a foundation for the future development of therapies capable of inhibiting allergy development.
Lessons from murine models of allergy regulation
The most obvious interpretation of the effect of MatIgG on the immunity of children is based on the fact that passively transferred MatIgG can neutralize inhaled or ingested allergens in offspring, particularly during the neonatal stage, reducing the need for processing and presentation and hence inhibiting IgE production.16
In more recent work, it has been demonstrated that maternal antibodies produced in response to maternal immunization with respiratory syncytial virus (RSV) are passively transferred to offspring and can neutralize RSV, preventing infection without inducing other cellular components.17
However, in the past, it has been demonstrated that antigen neutralization is not able to completely prevent the stimulation of an immune response in offspring after neonatal immunization against measles and tetanus.18 In this model, the presence of high levels of maternal antibodies could reduce the humoral response but did not prevent T cell-mediated immunity in the offspring, which were able to produce cytokines such as IFN-γ and IL-5 and which displayed cytotoxic activity at normal levels. In the context of allergen neutralization mediated by passive MatIgG transference, it has been shown that offspring derived from non-immunized mothers and subjected to passive transfer of purified IgG from OVA-immunized females exhibited blocked anaphylactic IgE production, without blockade of allergen-specific IgM production.11
This evidence shows that MatIgG is not only linked to antigen neutralization; rather, MatIgG can also mediate an immune-sensitizing process that is more related to immune regulation than to a hypersensitivity response.
Additionally, the interaction between MatIgG and the immune system of offspring may involve immune complexes composed of MatIgG and antigens; these complexes can directly interact with FcγRs expressed on the cells of offspring. In this context, FcγRIIb inhibitory receptors seem to be the most important FcγRs mediating maternal-fetal immune-adaptive interactions.
All mature murine B cells express the isoform FcγRIIb.19 The ITIM motifs of the FcγRIIb receptors are capable of inhibiting activation of B cells when near the ITAM motifs of the B cell receptors (BCRs).20 It has been demonstrated that FcγRIIb receptors can co-localize with BCRs, destabilizing the immunological synapse of the B cells that are necessary for isotype switching,21 consequently inhibiting the production of IgE in response to allergens.
It is unlikely that such a mechanism can occur in vivo, inhibiting the response of offspring to vaccines, because FcγRIIb-MatIgG binding is independent of antibody specificity and because increasing dose of a vaccine can induce an immune response in neonates.22 Mice genetically deficient (KO) for FcγRIIb receptors also exhibit inhibition of the immune response to vaccines in the presence of MatIgG23 and show an exacerbation of the production of IgG antibodies.24 Taken together, this evidence suggests a functional influence of FcγRIIb on the B cells of offspring that is not able to block the induction of immune responses but that again suggests an immunoregulatory state, thus supporting a possible application in the control of hypersensitivity reactions.
A third possibility in terms of maternal-fetal interaction is that MatIgG can directly and idiotypically interact with the immune system of offspring in the absence of antigen. In this regard, the inhibition of anti-phospholipase IgE antibody A2 (from bee venom) was demonstrated in the offspring of mothers that received anti-phospholipase A2 IgG.25 This phenomenon may be mediated by interactions between MatIgG idiotypes and both T cell receptors (TCRs) and BCRs, exerting a stimulating and/or regulatory effect on the cells.26
MatIgG can influence the formation of the clonal repertoire of offspring by anti-idiotypic interactions with BCRs, as evidenced in rabbits.27 These idiotypic interactions between MatIgG and the BCRs and TCRs of offspring occur during the fetal period28,29 and are able to select B and T cell repertoires in offspring.30
This “shaping” of the B cell repertoire of offspring results in long-term functional alterations that are B cell intrinsic and that can be evidenced up to adulthood.31 This phenomenon is termed “maternal imprinting.”
In 2003, the induction of nTreg lymphocytes in response to MatIgG transfer via preconceptional immunization with the dust mite antigen Dp was suggested, but at the time, whether MatIgG per se could be responsible for this induction was not investigated.14
Some years later, asthma inhibition in offspring mediated by preconceptional maternal oral tolerance induction was shown in a murine model using OVA allergen. In this model, it became evident that allergen-specific MatIgG plays a pivotal role in the inhibition of asthma in offspring and that this effect depends not only on neutralization but also on induction of IFN-γ production by memory T cells in offspring as a crucial event.32
In a similar murine model of preconceptional immunization, it was demonstrated that the passive transfer of MatIgG purified from OVA-immunized mothers to normal females during pregnancy could also induce phenotypic changes in the B cells of offspring, which could be detected at 3 d old.11 Although these alterations were induced in the absence of antigen and thus in the absence of immune complexes, it is likely that the effect of MatIgG is due to idiotypic interactions between MatIgG and the fetal immune system.
Taken together, experimental studies on the relationship between MatIgG and allergy inhibition in offspring have clarified that the mechanisms are mediated not only by allergen neutralization; rather, it also seems that an allergen-specific immunoregulatory status can be induced in offspring as a result of complex interactions of MatIgG with T and B cells in offspring, although these interactions are not fully understood.
Lessons from human IVIg therapy
Intravenous immunoglobulin (IVIg) is composed of a pool of purified human IgG antibodies that is routinely used to treat patients with primary immunodeficiency and as an immunomodulator for transplantation and autoimmune disorders.33 IVIg preparations have been derived from plasma from more than 3,000 donors in accordance with blood donation guidelines, which do not consider the donor's atopic background. These preparations represent a healthy IgG repertoire with mixed atopic background profiles since allergy can affect up to 40% of the population in developed countries. All commercial preparations have an IgG purity above 95%, with predominance of the IgG1 isotype (>56%).34
In the literature, in vitro IVIg has been described as capable of decreasing IFN-γ in the supernatant of peripheral blood mononuclear cell (PBMC) cultures from healthy individuals.35 In similar experiments with PBMC and umbilical cord cell cultures, decreased levels of IFN-γ, IL-10 and IL-12 in response to stimulation of the TCR with anti-CD3 have been demonstrated.36 Indeed, several authors have already described IVIg as influencing the production of cytokines in PBMC cultures.35,37-40 However, it has also been reported that IVIg is capable of suppressing the allogenic responses of T cells by Treg activation via ZAP-70,41 demonstrating that IVIg can interact with receptors expressed on the lymphocytes of treated subjects, modulating both activity and function.
Together, this evidence demonstrates that IgG can directly modulate cytokine production by T cells, possibly based on idiotypic interactions. These interactions are similar to those cited above in the context of MatIgG26 and can be mediated by the mutual recognition of variable regions between antibodies and clonal receptors, including TCRs. This phenomenon might also occur in vivo and, as it depends on variable region recognition, may vary according to IgG specificity.
In this context, in vivo human treatment with IVIg has provided certain important evidence about the modulatory potential of IgG. In particular, IVIg has been used to prevent recurrent spontaneous abortions (RSAs), as proposed years ago.42 RSAs are related to the production of anti-nuclear antibodies,43 anti-thyroid protein antibodies44 and anti-trophoblast antibody.45 The exact mechanism by which IVIg acts to prevent RSAs is still not well understood but is probably mediated by idiotypic interactions between transferred antibodies and treated subjects' B and T cell repertoires; these interactions result in the modulation of cytokine production, as evidenced in vitro, as well as other mechanisms that have not been fully elucidated.
In the context of IgE regulation, in 1991, Mazer BD and colleagues46 described a reduction of IgE levels in children with severe asthma who were treated monthly with a high dose of IVIg. Three years later, Jakobsson T and colleagues47 also suggested that 5 monthly infusions with a mean dose of 0.8 g/kg of IVIg treatment could reduce IgE levels in patients with severe bronchial asthma, but their results were not statistically significant. Moreover, several years ago, it was shown that monthly high-dose (2 g/kg) IVIg treatment in patients with atopic dermatitis (AD), which is characterized by high IgE production, could reduce the eczema skin score, but only 3 patients were evaluated.48 Five years later, another group of researchers compared the effect of 2 g/kg of IVIg as stat infusion treatment with that of cyclosporine treatment in 6 patients with severe AD. In this case, it is important to note that a single dose of IVIg treatment could also reduce the AD severity score (SCORAD), although not as well as cyclosporine, reinforcing the therapeutic potential of IVIg in regulating the effects of IgE.49
A few years later, 30 patients with AD were submitted to 3 months of therapy with 2 g/kg of IVIg, and a decrease in IL-5 serum levels, with no influence on the production of IFN-γ, was observed.50 None of the studies elucidated the mechanism by which IVIg could down-regulate AD, but interesting relevant evidence was obtained 16 y ago. In particular, Zhuang Q and Mazer B showed that in vitro inhibition of IgE production in purified human B cells was more pronounced following treatment with Fab’2 fragments than when using intact IVIg.51
Very recent in vivo evidence in pemphigus vulgaris patients also revealed that the complete clinical remission of this disease after therapy with 0.4 g/kg of IVIg for 5 d could possibly be related to the induction of regulatory B10 cells after long-term IVIg therapy.52
These observations strongly suggest that intense idiotypic interactions occur between IVIg and B cells, which can modulate B cell function, inhibiting IgE production and even inducing regulatory B cells. Taken together, these could be the mechanisms by which type I hypersensitivity development can also be inhibited.
It was also recently shown that human IVIg can penetrate mouse, monkey and human cells, reacting with intracellular molecules such as DNA, histone and tubulin, and that human IVIg exhibits regulatory potential in murine splenocytes.53 These effects are apparently more pronounced in CD4 T cells, with no influence observed in CD8 T cells.
These results elucidate the reason why a murine model of experimental autoimmune arthritis (EAA) could be regulated by human IVIg treatment. In this model, human IVIg reduced the maturation of Th17 cells, induced the production of IL-10 and augmented the expression of FcγRIIb receptors on mouse cells.54
Taken together, these results open a wide field of investigation, considering that IVIg can idiotypically interact with B and T cells at the membrane and cytoplasm levels, resulting mainly in cytokine modulation in T cells but also in functional modulation of B cells. As these interactions are influenced by IgG idiotypes, it is very possible that the specificity of produced or passively transferred IgG has a direct influence on the specificity of modulatory and regulatory cells of the immune system. In the case of in vivo IVIg treatment, we also suggest that the IgG repertoires of donors can differentially affect the immune functions of treated patients.
Furthermore, this effect possibly results from a complex repertoire diversity that is developed prior to transfer and in response to diverse ambient and infection-derived antigens. Therefore, we believe that this mechanism could not be induced by monoclonal antibodies (MAbs) that are already used for the management of allergy, such as omalizumab. This MAb has its effect mediated by direct IgE neutralization, without inducing immune regulation at the B and T cell levels.55
Hypothesis: Passively transferred IgG as a specific regulator of allergy development in offspring
Recently, the possible effect of maternal immunization with allergens on inducing regulatory cells in offspring was reviewed.56 Here, we want to further propose that MatIgG per se can influence the maturation and antigen presentation processes of the lymphocytes of offspring, generating a direct impact on the induction of regulatory B cells and the modulation of cytokine production by T cells. For this hypothesis, we suggest the following name: “MatIgG primary modulation theory.”
The fact that antibodies can reach primary and secondary lymphoid organs was described decades ago57-60; this means that we need to discuss the result of contact between T and B cells and MatIgG in 2 different contexts: cells in maturation (primary lymphoid organs) or matured cells (secondary lymphoid organs).
In primary lymphoid organs, during the maturation process, immature T and B cells already express clonal receptors and are programmed to develop effecter or regulatory functions, processes that possibly can be interrupted by MatIgG.
As a main event, we believe that in a homeostatic microenvironment, as in a normal primary lymphoid organ, anti-idiotypic MatIgG can idiotypically interact with the clonal receptors of immature cells, which can occur in the bone marrow for B cells and in the thymus mainly for αβT cells but also for γδT and B cells, resulting in modulation of the functional activities of these cells. As this mechanism is clone specific, it is possible that if the maternal immune system were sensitized and produced high levels of allergen-specific MatIgG, and consequently anti-idiotypic MatIgG, the passive transfer of this molecule would modulate the maturation of anti-allergen cells, yielding mature cells that could exert a modulatory or regulatory role in the periphery in offspring.
Based on the discussed considerations, we believe that idiotypic interactions between MatIgG and B and αβT cells in offspring can induce functional alterations that induce allergen-specific regulatory B cells and modulate cytokine production by allergen-specific αβT cells.
As a secondary event, it is possible that MatIgG directly permeates the membrane of developing cells in offspring and influences intracellular events in a way that is not fully understood but that can cooperate with the induced functional and modulatory alterations.
Aggeliki S and collaborators have shown that Fab’2 fragments are the main mediators responsible for the cell-regulatory effects of membrane-permeable human IgG.53 These results suggest that after membrane overlap, IgG can interact with intracellular molecules in an idiotype-specific manner. Therefore, it is possible that different IgG repertoires can influence different intracellular pathways, resulting in different effects. High levels of allergen-specific MatIgG can also favor the maturation of regulatory B or modulated αβT cells in offspring, which can be allergen specific if this maturation happens simultaneously with membrane idiotypic interactions, as cited above.
Briefly, we believe that a non-atopic MatIgG repertoire can exert a modulatory effect on immature cells in the primary lymphoid organs of offspring via membrane clonal receptors and intracellular interactions and that these events can stimulate allergen-specific regulatory B cells and modulate the secretion of cytokines by αβT cells, which can in turn inhibit allergy exacerbation (Fig. 1). Although no evidence of γδT cell modulation mediated by IgG is described in the literature, we cannot discard the idea that this population could also be modulated by MatIgG.
Figure 1.
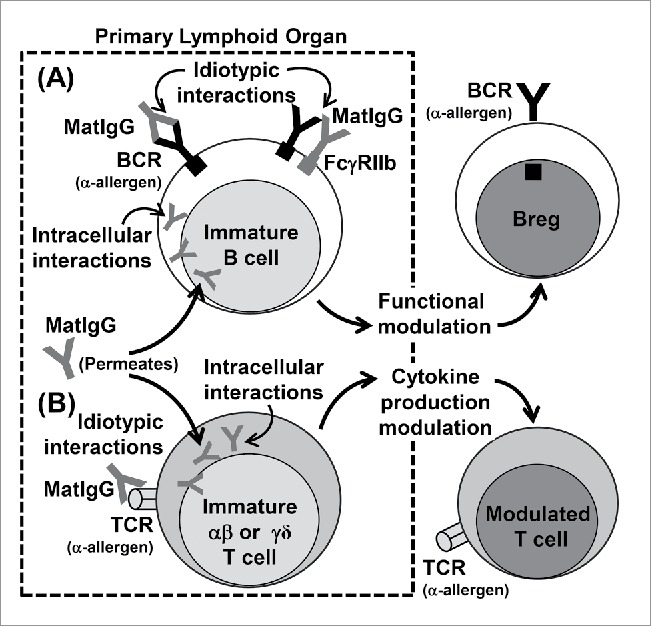
Possible effects of MatIgG on (B)and (T)cells in primary lymphoid organs in offspring. MatIgG can interact with immature B cells in offspring by membrane idiotypic interactions with allergen-specific BCRs and by intracellular interactions after permeating the membrane; as a result, B cells can acquire regulatory B function (A). Similarly, MatIgG can interact with immature αβT and γδT cells in offspring by membrane idiotypic interactions with allergen-specific TCRs and by intracellular interactions after permeating the membrane; as a result, T cells' cytokine production can be modulated (B).
In secondary lymphoid organs, there is a predominance of mature αβT and B cells that also express clonal receptors and that are already programmed with effector or regulatory properties, functions that can be influenced by extracellular and intracellular MatIgG interactions.
At this level we believe that as a main event, MatIgG possibly idiotypically interacts with clonal BCRs expressed in the surface of mature B cells in offspring, which can result in the internalization and processing of MatIgG-derived peptides and their presentation to αβT cells in a tolerogenic/regulatory context because MatIgG does not induce an inflammatory stimulus capable of up-regulating co-stimulatory molecules and inflammatory cytokines. This phenomenon can result in the induction of regulatory anti-allergen B cells and can also modulate cytokine secretion by αβT cells in the periphery in offspring.
Similar to what occurs in primary lymphoid organs, as a secondary event, it is possible that MatIgG directly permeates the membrane of mature B and T cells in offspring and influences intracellular events, consequently collaborating with the allergen-specific functional modulation induced by clonal receptor interactions (Fig. 2).
Figure 2.
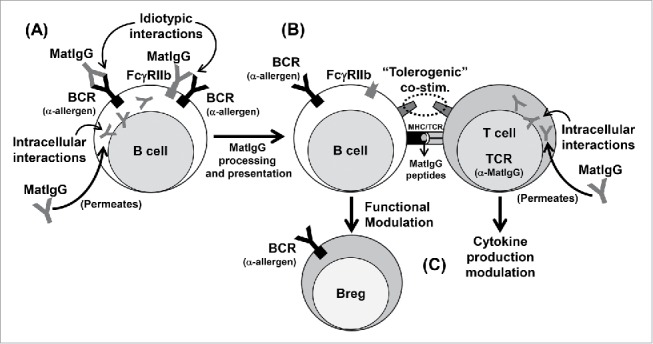
Possible effects of MatIgG on (B)and (T)cells in secondary lymphoid organs in offspring. MatIgG can interact with allergen-specific B cells in offspring by membrane idiotypic interactions and by intracellular interactions after permeating the membrane; as a result, B cells can process and present MatIgG peptides via MHC molecules (A). The presentation of MatIgG peptides without inflammatory signals can result in a tolerogenic/regulatory context (B). As a result of this process, B cells acquire regulatory function, and T cells' cytokine production can be modulated (C).
In practice, it is still difficult to obtain experimental evidence that non-atopic MatIgG can induce allergen-specific regulatory B cells and modulates T cells' cytokine production, inhibiting allergy development in offspring. In this context, experimental protocols for passive transference of MatIgG purified from allergen-immunized or non-immunized mothers followed by evaluation of the B and T lymphocytes in the primary organs of their offspring can be suggested. Purified MatIgG can also be used to evaluate the in vitro influence on B and T cell maturation using primary lymphoid organ cultures that have been previously standardized. Similar in vitro protocols can be adopted to evaluate this hypothesis in humans using IgG purified from atopic or non-atopic individuals. In this case, it is especially important to compare these results with those obtained with commercially available IVIg. We believe that further evidence elucidating this hypothesis will emerge in the literature in the next few years.
Considerations regarding future allergy prevention strategies
IVIg is produced from a pool of sera from hundreds of individual donors, and the exclusion criteria for donation only include possible infection and prior transfusions. The frequency of atopy in the populations of developed countries is approximately 40%,61 and this parameter is not an exclusion criterion, so IVIg used in the treatment of patients reflects the IgG profiles of both atopic and non-atopic individuals at an undetermined ratio.
Translated to in vivo conditions, the separation of IVIg from atopic and non-atopic donors might enable more efficient treatment in certain situations. Thus, hypothetically, the treatment of atopic women with IgG from non-atopic individuals could favor the induction of anti-allergen regulatory mechanisms in the fetus, without negatively affecting the maintenance of pregnancy and without causing significant side effects. Therefore, we suggest that assessment of the atopic background of IgG donors will probably facilitate understanding of the effects of IVIg and will open new possibilities for allergy prevention strategies.
Conclusion
In conclusion, our review yields a new hypothesis, suggesting that the anti-allergen IgG repertoire can mediate allergy inhibition by modulating B and T cell function and regulatory properties. This mechanism may induce long-term effects that may have implications for the future development of human therapies for allergy regulation.
Disclosure of potential conflicts of interest
The author declares no conflicts of interest related to this paper.
Funding
This study was funded through grants from the Laboratory of Medical Investigation-56, Medical School, University of Sao Paulo, Sao Paulo, Brazil (LIM-56 HC-FMUSP); the São Paulo Research Foundation (FAPESP – grant #2015/17256–3); and the National Council for Scientific and Technological Development (CNPq – grant #115603/2015-8).
References
- [1].Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7(9):715-25; PMID:17703228; http://dx.doi.org/ 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- [2].Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol 1996; 157(8):3317-22; PMID:8871627 [PubMed] [Google Scholar]
- [3].Simister NE. Placental transport of immunoglobulin G. Vaccine 2003; 21(24):3365-9; PMID:12850341; http://dx.doi.org/ 10.1016/S0264-410X(03)00334-7 [DOI] [PubMed] [Google Scholar]
- [4].Malek A, Sager R, Schneider H. Maternal-fetal transport of immunoglobulin G and its subclasses during the third trimester of human pregnancy. Am J Reprod Immunol 1994; 32(1):8-14; PMID:7945815; http://dx.doi.org/ 10.1111/j.1600-0897.1994.tb00873.x [DOI] [PubMed] [Google Scholar]
- [5].Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 1996; 93(11):5512-16; PMID:8643606; http://dx.doi.org/ 10.1073/pnas.93.11.5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jenmalm MC, Björkstén B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin Exp Allergy 2000; 30(1):34-40; PMID:10606928; http://dx.doi.org/ 10.1046/j.1365-2222.2000.00771.x [DOI] [PubMed] [Google Scholar]
- [7].Vance GH, Grimshaw KE, Briggs R, Lewis SA, Mullee MA, Thornton CA, Warner JO. Serum ovalbumin-specific immunoglobulin G responses during pregnancy reflect maternal intake of dietary egg and relate to the development of allergy in early infancy. Clin Exp Allergy 2004; 34(12):1855-61; PMID:15663559; http://dx.doi.org/ 10.1111/j.1365-2222.2004.02111.x [DOI] [PubMed] [Google Scholar]
- [8].Fusaro A, Maciel M, Victor J, Oliveira C, Duarte A, Sato M. Influence of maternal murine immunization with Dermatophagoides pteronyssinus extract on the type I hypersensitivity response in offspring. Int Arch Allergy Immunol 2002; 127(3):208-16; ; http://dx.doi.org/ 10.1159/000053865 [DOI] [PubMed] [Google Scholar]
- [9].Fusaro AE, Brito CA, Victor JR, Rigato PO, Goldoni AL, Duarte AJS, Sato MN. Maternal-fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology 2007; 122(1):107-15; PMID:17608811; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fusaro AE, de Brito CA, Taniguchi EF, Muniz BP, Victor JR, Orii NM, da Silva Duarte AJ, Sato MN. Balance between early life tolerance and sensitization in allergy: dependence on the timing and intensity of prenatal and postnatal allergen exposure of the mother. Immunology 2009; 128(1):e541-50; PMID:19740315; http://dx.doi.org/ 10.1111/j.1365-2567.2008.03028.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Victor JR, Muniz BP, Fusaro AE, de Brito CA, Taniguchi EF, Duarte AJS, Sato MN. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol 2010; 11:11; PMID:20222978; http://dx.doi.org/ 10.1186/1471-2172-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lira AADL, Oliveira MGD, Oliveira LMD, Duarte AJDS, Sato MN, Victor JR. Opposite effect on offspring Fc gamma RIIb B cells expression on dependency of maternal immunisation with OVA or Dermatophagoides pteronyssinus: different mechanisms for different allergens? Allergy 2014; 69:344. [Google Scholar]
- [13].Muniz BP, Victor JR, Oliveira LdM, de Lima Lira AA, Perini A, Olivo CR, Arantes-Costa FM, Martins MA, da Silva Duarte AJ, Sato MN. Tolerogenic microenvironment in neonatal period induced by maternal immunization with ovalbumin. Immunobiology 2014; 219(5):377-84; PMID:24582301; http://dx.doi.org/ 10.1016/j.imbio.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [14].Victor J, Fusaro A, Duarte A, Sato M. Preconception maternal immunization to dust mite inhibits the type I hypersensitivity response of offspring. J Allergy Clin Immunol 2003; 111(2):269-77; PMID:12589344; http://dx.doi.org/ 10.1067/mai.2003.39 [DOI] [PubMed] [Google Scholar]
- [15].Jarrett EE, Hall E. IgE suppression by maternal IgG. Immunology 1983; 48(1):49-58; PMID:6848454 [PMC free article] [PubMed] [Google Scholar]
- [16].Siegrist CA. Neonatal and early life vaccinology. Vaccine 2001; 19(25–26):3331-46; PMID:11348697; http://dx.doi.org/ 10.1016/S0264-410X(01)00028-7 [DOI] [PubMed] [Google Scholar]
- [17].Kwon YM, Hwang HS, Lee JS, Ko EJ, Yoo SE, Kim MC, Lee YN, Kim KH, Song JM, Lee S, et al.. Maternal antibodies by passive immunization with formalin inactivated respiratory syncytial virus confer protection without vaccine-enhanced disease. Antiviral Res 2014; 104:1-6; PMID:24462695; http://dx.doi.org/ 10.1016/j.antiviral.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Siegrist CA, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert PH. Induction of neonatal TH1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine 1998; 16(14–15):1473-8; PMID:9711791; http://dx.doi.org/ 10.1016/S0264-410X(98)00111-X [DOI] [PubMed] [Google Scholar]
- [19].Rabinovitch N, Gelfand EW. Expression of functional activating and inhibitory Fcgamma receptors on human B cells. Int Arch Allergy Immunol 2004; 133(3):285-94; PMID:14976398; http://dx.doi.org/ 10.1159/000076836 [DOI] [PubMed] [Google Scholar]
- [20].Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature 1994; 369(6478):340; PMID:8183374 [DOI] [PubMed] [Google Scholar]
- [21].Sohn HW, Pierce SK, Tzeng SJ. Live cell imaging reveals that the inhibitory FcgammaRIIB destabilizes B cell receptor membrane-lipid interactions and blocks immune synapse formation. J Immunol 2008; 180(2):793-9; PMID:18178817; http://dx.doi.org/ 10.4049/jimmunol.180.2.793 [DOI] [PubMed] [Google Scholar]
- [22].Heyman B, Dahlström J, Diaz De Ståhl T, Getahun A, Wernersson S, Karlsson MC. No evidence for a role of FcgammaRIIB in suppression of in vivo antibody responses to erythrocytes by passively administered IgG. Scand J Immunol 2001; 53(4):331-4; discussion 339–345; PMID:11285111; http://dx.doi.org/ 10.1046/j.1365-3083.2001.00890.x [DOI] [PubMed] [Google Scholar]
- [23].Karlsson MC, Wernersson S, Diaz de Ståhl T, Gustavsson S, Heyman B. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proc Natl Acad Sci U S A 1999; 96(5):2244-9; PMID:10051626; http://dx.doi.org/ 10.1073/pnas.96.5.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wernersson S, Karlsson MC, Dahlström J, Mattsson R, Verbeek JS, Heyman B. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J Immunol 1999; 163(2):618-22; PMID:10395649 [PubMed] [Google Scholar]
- [25].Seeger M, Thierse HJ, Lange H, Shaw L, Hansen H, Lemke H. Antigen-independent suppression of the IgE immune response to bee venom phospholipase A2 by maternally derived monoclonal IgG antibodies. Eur J Immunol 1998; 28(7):2124-30; PMID:9692881; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199807)28:07%3c2124::AID-IMMU2124%3e3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- [26].Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev 1984; 79:5-24; PMID:6378763; http://dx.doi.org/ 10.1111/j.1600-065X.1984.tb00484.x [DOI] [PubMed] [Google Scholar]
- [27].Borghesi C, Nicoletti C. Autologous anti-idiotypic antibody response is regulated by the level of circulating complementary idiotype. Immunology 1996; 89(2):172-7; PMID:8943710; http://dx.doi.org/ 10.1046/j.1365-2567.1996.d01-724.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vakil M, Sauter H, Paige C, Kearney JF. In vivo suppression of perinatal multispecific B cells results in a distortion of the adult B cell repertoire. Eur J Immunol 1986; 16(9):1159-65; PMID:2428628; http://dx.doi.org/ 10.1002/eji.1830160921 [DOI] [PubMed] [Google Scholar]
- [29].Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J 1993; 12(1):357-63; PMID:8428591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ghosh SK, Chakrabarti D. Immunoregulation by processed immunoglobulin on B-cells. Indian J Biochem Biophys 1993; 30(6):414-21; PMID:7911781 [PubMed] [Google Scholar]
- [31].Fink K, Zellweger R, Weber J, Manjarrez-Orduno N, Holdener M, Senn BM, Hengartner H, Zinkernagel RM, Macpherson AJ. Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur J Immunol 2008; 38(1):90-101; PMID:18081043; http://dx.doi.org/ 10.1002/eji.200737872 [DOI] [PubMed] [Google Scholar]
- [32].Polte T, Hennig C, Hansen G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J Allergy Clin Immunol 2008; 122(5):1022-30.e1025; http://dx.doi.org/ 10.1016/j.jaci.2008.09.014 [DOI] [PubMed] [Google Scholar]
- [33].Lemieux R, Bazin R, Néron S. Therapeutic intravenous immunoglobulins. Mol Immunol 2005; 42(7):839-48; PMID:15829272; http://dx.doi.org/ 10.1016/j.molimm.2004.07.046 [DOI] [PubMed] [Google Scholar]
- [34].Seite JF, Shoenfeld Y, Youinou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev 2008; 7(6):435-9; PMID:18558358; http://dx.doi.org/ 10.1016/j.autrev.2008.04.012 [DOI] [PubMed] [Google Scholar]
- [35].Sticherling M, Trawinski H. Effects of intravenous immunoglobulins on peripheral blood mononuclear cell activation in vitro. Ann N Y Acad Sci 2007; 1110:694-708; PMID:17911484; http://dx.doi.org/ 10.1196/annals.1423.072 [DOI] [PubMed] [Google Scholar]
- [36].Tawfik DS, Cowan KR, Walsh AM, Hamilton WS, Goldman FD. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol 2012; 23(1):88-95; PMID:21265884; http://dx.doi.org/ 10.1111/j.1399-3038.2010.01129.x [DOI] [PubMed] [Google Scholar]
- [37].Takata Y, Seki S, Dobashi H, Takeshita S, Nakatani K, Kamezawa Y, Hiraide H, Sekine I, Yoshioka S. Inhibition of IL-12 synthesis of peripheral blood mononuclear cells (PBMC) stimulated with a bacterial superantigen by pooled human immunoglobulin: implications for its effect on Kawasaki disease (KD). Clin Exp Immunol 1998; 114(2):311-9; PMID:9822292; http://dx.doi.org/ 10.1046/j.1365-2249.1998.00712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu KH, Wu WM, Lu MY, Chiang BL. Inhibitory effect of pooled human immunoglobulin on cytokine production in peripheral blood mononuclear cells. Pediatr Allergy Immunol 2006; 17(1):60-8; PMID:16426257; http://dx.doi.org/ 10.1111/j.1399-3038.2005.00344.x [DOI] [PubMed] [Google Scholar]
- [39].Siedlar M, Strach M, Bukowska-Strakova K, Lenart M, Szaflarska A, Węglarczyk K, Rutkowska M, Baj-Krzyworzeka M, Pituch-Noworolska A, Kowalczyk D, et al.. Preparations of intravenous immunoglobulins diminish the number and proinflammatory response of CD14+CD16++ monocytes in common variable immunodeficiency (CVID) patients. Clin Immunol 2011; 139(2):122-32; PMID:21300572; http://dx.doi.org/ 10.1016/j.clim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- [40].Gille C, Dreschers S, Spring B, Tárnok A, Bocsi J, Poets CF, Orlikowsky TW. Differential modulation of cord blood and peripheral blood monocytes by intravenous immunoglobulin. Cytometry B Clin Cytom 2012; 82(1):26-34; PMID:21812105; http://dx.doi.org/ 10.1002/cyto.b.20609 [DOI] [PubMed] [Google Scholar]
- [41].Tha-In T, Metselaar HJ, Bushell AR, Kwekkeboom J, Wood KJ. Intravenous immunoglobulins promote skin allograft acceptance by triggering functional activation of CD4+Foxp3+ T cells. Transplantation 2010; 89(12):1446-55; PMID:20463648; http://dx.doi.org/ 10.1097/TP.0b013e3181dd6bf1 [DOI] [PubMed] [Google Scholar]
- [42].Stricker RB, Winger EE. Update on treatment of immunologic abortion with low-dose intravenous immunoglobulin. Am J Reprod Immunol 2005; 54(6):390-6; PMID:16305665; http://dx.doi.org/ 10.1111/j.1600-0897.2005.00335.x [DOI] [PubMed] [Google Scholar]
- [43].Xu L, Chang V, Murphy A, Rock JA, Damewood M, Schlaff W, Zacur HA. Antinuclear antibodies in sera of patients with recurrent pregnancy wastage. Am J Obstet Gynecol 1990; 163(5 Pt 1):1493-7; PMID:2240094; http://dx.doi.org/ 10.1016/0002-9378(90)90612-B [DOI] [PubMed] [Google Scholar]
- [44].Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990; 264(11):1422-5; PMID:2118190; http://dx.doi.org/ 10.1001/jama.1990.03450110068029 [DOI] [PubMed] [Google Scholar]
- [45].Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA 1995; 273(24):1933-6; PMID:7783303; http://dx.doi.org/ 10.1001/jama.1995.03520480053039 [DOI] [PubMed] [Google Scholar]
- [46].Mazer BD, Gelfand EW. An open-label study of high-dose intravenous immunoglobulin in severe childhood asthma. J Allergy Clin Immunol 1991; 87(5):976-83; PMID:2026848; http://dx.doi.org/ 10.1016/0091-6749(91)90420-S [DOI] [PubMed] [Google Scholar]
- [47].Jakobsson T, Croner S, Kjellman NI, Pettersson A, Vassella C, Björkstén B. Slight steroid-sparing effect of intravenous immunoglobulin in children and adolescents with moderately severe bronchial asthma. Allergy 1994; 49(6):413-20; PMID:8074263; http://dx.doi.org/ 10.1111/j.1398-9995.1994.tb00833.x [DOI] [PubMed] [Google Scholar]
- [48].Jolles S, Hughes J, Rustin M. The treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literature. Br J Dermatol 2000; 142(3):551-4; PMID:10735971; http://dx.doi.org/ 10.1046/j.1365-2133.2000.03377.x [DOI] [PubMed] [Google Scholar]
- [49].Bemanian MH, Movahedi M, Farhoudi A, Gharagozlou M, Seraj MH, Pourpak Z, Nabavi M, Aghamohammadi A, Shirkhoda Z. High doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitis. Iran J Allergy Asthma Immunol 2005; 4(3):139-43; PMID:17301437 [PubMed] [Google Scholar]
- [50].Jee SJ, Kim JH, Baek HS, Lee HB, Oh JW. Long-term efficacy of intravenous immunoglobulin therapy for moderate to severe childhood atopic dermatitis. Allergy Asthma Immunol Res 2011; 3(2):89-95; PMID:21461247; http://dx.doi.org/ 10.4168/aair.2011.3.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhuang Q, Mazer B. Inhibition of IgE production in vitro by intact and fragmented intravenous immunoglobulin. J Allergy Clin Immunol 2001; 108(2):229-34; PMID:11496239; http://dx.doi.org/ 10.1067/mai.2001.116291 [DOI] [PubMed] [Google Scholar]
- [52].Kabuto M, Fujimoto N, Tanaka T. Increase of interleukin-10-producing B cells associated with long-term remission after i.v. immunoglobulin treatment for pemphigus. J Dermatol 2016; 43(7):815-8; PMID:26871259; http://dx.doi.org/ 10.1111/1346-8138.13295 [DOI] [PubMed] [Google Scholar]
- [53].Sali AD, Karakasiliotis I, Evangelidou M, Avrameas S, Lymberi P. Immunological evidence and regulatory potential for cell-penetrating antibodies in intravenous immunoglobulin. Clin Transl Immunology 2015; 4(10):e42; PMID:26682050; http://dx.doi.org/ 10.1038/cti.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee SY, Jung YO, Ryu JG, Kang CM, Kim EK, Son HJ, Yang EJ, Ju JH, Kang YS, Park SH, et al.. Intravenous immunoglobulin attenuates experimental autoimmune arthritis by inducing reciprocal regulation of Th17 and Treg cells in an interleukin-10-dependent manner. Arthritis Rheumatol 2014; 66(7):1768-78; PMID:24644005; http://dx.doi.org/ 10.1002/art.38627 [DOI] [PubMed] [Google Scholar]
- [55].Lin CH, Cheng SL. A review of omalizumab for the management of severe asthma. Drug Des Devel Ther 2016; 10:2369-78; PMID:27528798; http://dx.doi.org/ 10.2147/DDDT.S112208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Victor JR. Influence of maternal immunization with allergens on the thymic maturation of lymphocytes with regulatory potential in children: a broad field for further exploration. J Immunol Res 2014; 2014:780386; PMID:25009823; http://dx.doi.org/ 10.1155/2014/780386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen ST. Cellular sites of immunoglobulins. I. Distribution of light polypeptide chains in human lymphoid tissue. Acta Pathol Jpn 1970; 20(4):487-503; PMID:4101698 [PubMed] [Google Scholar]
- [58].Chen ST, Izui S. Cellular sites of immunoglobulins. III. Localization of IgG-, IgA-, IgM- and their kappa and lambda light chain-containing cells in human palatine tonsils. Acta Pathol Jpn 1971; 21(1):85-92; PMID:4938482 [PubMed] [Google Scholar]
- [59].Chen ST. Cellular sites of immunoglobulins. II. The relative proportions of mucosal cells containing IgG, IgA, and IgM, and light polypeptide chains of kappa and lambda immunoglobulin in human appendices. Acta Pathol Jpn 1971; 21(1):67-83; PMID:4938481 [PubMed] [Google Scholar]
- [60].Chen ST, Tobe T, Iosobe Y, Chiu AF. Cellular sites of immunoglobulins. VI. Localization of immunoglobulins in the human thymus. Acta Pathol Jpn 1975; 25(1):69-73; PMID:1094798 [DOI] [PubMed] [Google Scholar]
- [61].Weinberger MM. What is the problem with asthma care for children? Arch Pediatr Adolesc Med 2011; 165(5):473-5; PMID:21536964; http://dx.doi.org/ 10.1001/archpediatrics.2011.46 [DOI] [PubMed] [Google Scholar]


