Abstract
Inwardly rectifying potassium channels (Kir channels) control cell membrane K+ fluxes and electrical signaling in diverse cell types. Heterozygous mutations in the human Kir6.2 gene (KCNJ11), the pore-forming subunit of the ATP-sensitive (KATP) channel, cause permanent neonatal diabetes mellitus (PNDM). For some mutations, PNDM is accompanied by marked developmental delay, muscle weakness, and epilepsy (severe disease). To determine the molecular basis of these different phenotypes, we expressed wild-type or mutant (R201C, Q52R, or V59G) Kir6.2/sulfonylurea receptor 1 channels in Xenopus oocytes. All mutations increased resting whole-cell KATP currents by reducing channel inhibition by ATP, but, in the simulated heterozygous state, mutations causing PNDM alone (R201C) produced smaller KATP currents and less change in ATP sensitivity than mutations associated with severe disease (Q52R and V59G). This finding suggests that increased KATP currents hyperpolarize pancreatic beta cells and impair insulin secretion, whereas larger KATP currents are required to influence extrapancreatic cell function. We found that mutations causing PNDM alone impair ATP sensitivity directly (at the binding site), whereas those associated with severe disease act indirectly by biasing the channel conformation toward the open state. The effect of the mutation on ATP sensitivity in the heterozygous state reflects the different contributions of a single subunit in the Kir6.2 tetramer to ATP inhibition and to the energy of the open state. Our results also show that mutations in the slide helix of Kir6.2 (V59G) influence the channel kinetics, providing evidence that this domain is involved in Kir channel gating, and suggest that the efficacy of sulfonylurea therapy in PNDM may vary with genotype.
Keywords: potassium channel, KATP channel, KCNJ11, ATP-binding site
ATP-sensitive potassium channels (KATP channels) control electrical signaling in diverse cell types by coupling cellular metabolism to potassium movement across cell membranes. In pancreatic beta cells, KATP channels link changes in blood glucose concentration to insulin secretion (1); in brain, they contribute to glucose sensing and seizure protection, in cardiac muscle they protect against ischemic stress; and in skeletal muscle, they influence tone (1). KATP channels comprise four pore-forming inwardly rectifying potassium channel (Kir channel) 6.2 subunits and four regulatory sulfonylurea receptor (SUR) subunits (2). The SUR1 isoform is found in beta cells. ATP binding to Kir6.2 closes the channel, whereas interaction of Mg-nucleotides with SUR1 opens it (3). Consequently, increased metabolism leads to channel closure, membrane depolarization, and electrical activity and, conversely, metabolic inhibition opens KATP channels and suppresses electrical activity (see Fig. 1 A).
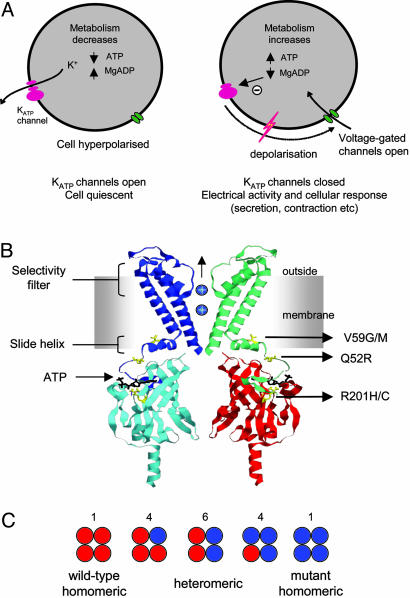
Fig. 1.
Functional role of KATP channels and location of mutations. (A) Schematic of how metabolic regulation of KATP channel activity sets the cell membrane potential. (B) Homology model of Kir6.2 (7), indicating the location of residues R201, Q52R, and V59G associated with neonatal diabetes. For clarity, only two subunits are shown, and the intracellular and transmembrane domains are from separate subunits. ATP (black) is shown in its binding site. (C) Schematic of the mixture of channels with the different subunit compositions expected when wild-type and mutant Kir6.2 are coexpressed (as in the heterozygous state). The relative numbers of the channel types expected if wild-type and mutant subunits segregate independently (i.e., follow a binomial distribution) are indicated above the figure.
Mutations in the human Kir6.2 gene (KCNJ11) were recently identified that severely impair insulin secretion and lead to permanent neonatal diabetes mellitus (PNDM) (4–6). One class of mutations produces only PNDM, and we refer to this as “mild” disease. The other is associated with a more severe phenotype in which PNDM is accompanied by marked developmental delay, muscle weakness, and epilepsy (4–6). We refer to this as severe disease. Of the 27 patients with Kir6.2 mutations studied to date, ∼30% had neurological problems.
Fig. 1B maps some of the mutations associated with PNDM onto a homology model of Kir6.2 (7). Two mutations in the same residue (R201H and R201C), which lies in the putative ATP-binding site (7, 8), cause mild disease. In contrast, the mutations Q52R, V59M, and V59G, which are associated with severe disease, lie some distance from the ATP-binding site, within the N-terminal region of the protein; moreover, V59 lies within the “slide helix,” a domain postulated to be involved in the opening and closing (gating) of Kir channels (7).
Previous work has shown that mutation of R201 to histidine decreases the ATP sensitivity of the KATP channel (4). However, all patients identified to date are heterozygotes, and no obvious loss of ATP sensitivity was observed when the heterozygous state was simulated by coinjection of R201H and wild-type Kir6.2 (4). The cause of PNDM was therefore unclear. Furthermore, the reason why some mutations are associated with muscle weakness, developmental delay, and epilepsy has not been addressed. In this paper, we compare the functional effects of mutations associated with severe disease (Q52R and V59G) with those that cause mild disease (R201C and R201H).
Materials and Methods
Mutagenesis and RNA Preparation. Human Kir6.2 (GenBank accession no. NM000525) and rat SUR1 (GenBank accession no. L40624) were used in this study. Because human Kir6.2 contains two common polymorphisms, E23K and I377V, we used the most common allele at these residues (i.e., E at position 23 and I at position 377). Site-directed mutagenesis of Kir6.2 was performed with the QuikChange XL system (Stratagene). Wild-type and mutant mRNAs were expressed in Xenopus laevis oocytes as described in ref. 9. Currents were recorded 1–3 days after injection with 0.1 ng of wild-type or mutant Kir6.2 mRNA and ∼2 ng of SUR1 mRNA (giving a 1:20 ratio). For each batch of oocytes, all mutations were injected to enable direct comparison of their effects.
Two-Electrode Voltage-Clamp Studies. Whole-cell currents were recorded from intact oocytes in response to voltage steps of ±20 mV from a holding potential of –10 mV, and they were filtered at 1 kHz and digitized at 4 kHz. Oocytes were constantly perfused at 20–24°C with solution containing 90 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM Hepes, pH 7.4 with KOH. Metabolic inhibition was produced by 3 mM Na-azide. Tolbutamide (0.5 mM) was tested on all oocytes to confirm that the observed current flowed through KATP channels.
Patch-Clamp Studies. Macroscopic currents were recorded from giant excised inside-out patches by using the patch-clamp technique. Currents were filtered at 0.15 kHz and digitized at 0.5 kHz. The pipette solution contained 140 mM KCl, 1.2 mM MgCl2, 2.6 mM CaCl2, and 10 mM Hepes, pH 7.4 with KOH. The internal (bath) solution contained 107 mM KCl, 1 mM K2SO4, 10 mM EGTA, and 10 mM Hepes, pH 7.2 with KOH, and nucleotides as indicated). All experiments were carried out in the absence of Mg2+ to prevent the activating effects of nucleotides mediated by SUR1 (3). Rapid exchange of internal solutions was achieved with a sewer pipe system. Experiments were conducted at 20–24°C. The macroscopic slope conductance, G, was measured between –20 and –100 mV (9). ATP concentration–response curves were fit with the Hill equation: G/Gc = 1/(1 + ([ATP]/IC50)h), where [ATP] is the ATP concentration, IC50 is the ATP concentration at which inhibition is half maximal, and h is the slope factor (Hill coefficient). To control for possible rundown, Gc was taken as the mean of the conductance in control solution before and after ATP application.
Single-channel currents were recorded from small inside-out membrane patches at –60 mV, filtered at 5 kHz, and sampled at 20 kHz. Open probability was determined (in the absence of ATP) from current records of ∼1-min duration shortly after patch excision as I/iN, where I is the mean macroscopic current, i is the single-channel current, and N is the number of channels (taken as the maximum number of open levels). For heterozygous channels (Fig. 1C), the mean PO represents the average of all types of channels (〈PO〉).
Data Analysis of Heterozygous Channels. For analysis of heterozygous channels, we assumed independent mixing of wild-type and mutant subunits. Thus, the relative numbers of KATP channels with different subunit compositions (Fig. 1C) were expected to follow the binomial distribution as described in refs. 2 and 10. The values of IC50,H and PO,H (the intrinsic open probability; i.e., the open probability in the unliganded state) for the heterozygous channel population can then be obtained from the following equations:
 |
[1] |
and
 |
[2] |
where the index, i, denotes the number of mutant subunits (0 < i < 4) and IC50,i and PO,i stand for the IC50 and PO of channels containing i mutant subunits. Assuming that the energy of the channel open state, O, is simply the sum of the contributions of the four individual subunits in the channel (GO,i):
 |
[3] |
The values of IC50,i and PO,i were estimated from the following equations (for further details see Supporting Text, which is published as supporting information on the PNAS web site):
 |
[4] |
and
 |
[5] |
where PMAX denotes the maximum (intraburst) open probability, AS denotes the contribution of interburst closures to the channel open probability, and the parameter λ is the difference in the contribution of the individual channel subunit j (1 < j < 4) to the energy of the open state (GO,j) between the mutant (GO,M,j) and the wild-type (GO,WT,j) channel:
 |
[6] |
The values of λ (0.62), PMAX (0.86), and AS (0.62) were calculated from Eqs. 4–6 by using measured parameters for wild-type and homomeric mutant channels; the values of IC50 and PO for all individual species of heteromeric channels were determined from Eqs. 4 and 5 and for the heterozygous channel population from Eqs. 1 and 2.
Results and Discussion
To examine the functional differences between the two classes of mutations, we expressed wild-type or mutant human Kir6.2, together with SUR1, in Xenopus oocytes (9). We first compared the effect of mutations on the properties of homomeric channels composed of a single type of Kir6.2 subunit. We then simulated heterozygosity by coxpressing a 1:1 mixture of wild-type and mutant Kir6.2 mRNAs with SUR1, which is expected to result in a mixed population of channels containing different ratios of wild-type and mutant subunits (Fig. 1C): We refer to this population as heterozygous channels.
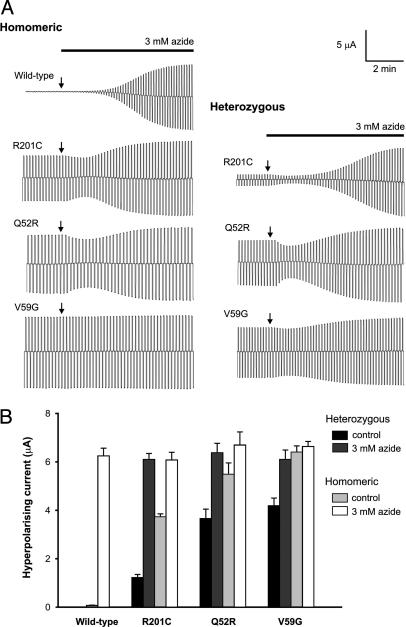
Unlike Wild-Type Channels, Mutant KATP Channels Are Not Closed by Resting ATP Levels. When expressed in Xenopus oocytes, wild-type KATP channels are normally closed but they are activated by metabolic inhibitors, such as azide, which lower intracellular ATP (Fig. 2). In contrast, significant resting whole-cell K+ currents are present in oocytes expressing homomeric Q52R, V59G, or R201C (homQ52R, homV59G, and homR201C, respectively) (Fig. 2 and Table 1). This result suggests that metabolism causes less block of mutant KATP channels than wild-type channels. Whole-cell homV59G and homQ52R currents were not increased further by azide application, as if these channels are fully activated at rest and insensitive to metabolically induced changes in nucleotides. However, homR201C channels were enhanced by azide, suggesting that they are more ATP-sensitive than homV59G and homQ52R channels. For heterozygous channels (hetV59G, hetQ52R, and hetR201C), hetV59G and hetQ52R resting whole-cell currents were of similar amplitude, constituting ∼65% of their homomeric values, but were far larger than wild-type currents (Fig. 2B). These resting currents were further activated by azide, reaching a final amplitude close to that of both homomeric and wild-type channels. Resting hetR201C currents were smaller than hetQ52R and hetV59G currents but still significantly larger than wild-type (Fig. 2 and Table 1). These differences in resting current probably account for the difference between the mild and severe forms of the disease because they may be expected to hyperpolarize the patients' cells and thereby influence electrical activity and cell function to differing extents.
Fig. 2.
Effects of mutations on whole-cell KATP currents. (A) Whole-cell currents recorded from Xenopus oocytes coexpressing SUR1 and either wild-type or mutant Kir6.2, as indicated, in response to voltage steps of ±20 mV from a holding potential of –10 mV in 90 mM K+. The start of azide application is indicated by arrows. The initial decrease in KATP current is due to a small block by azide that is mediated by an interaction with SUR1. (B) Mean steady-state whole-cell currents evoked by a voltage step from –10 to –30 mV before (control) and after application of 3 mM azide. The number of oocytes is 8 –12 in each case.
Table 1. Macroscopic and single-channel properties of PNDM mutations.
| Mutations | Irest, μA (n) | IC50 ATP, μM (n) | PO(0) (n) | i, pA (n) |
|---|---|---|---|---|
| Wild-type | 0.07 ± 0.01 (8) | 7.0 ± 1.1 (6) | 0.53 ± 0.02 (8) | 4.1 ± 0.2 (3) |
| homQ52R | 5.49 ± 0.47 (10)* | 84 ± 12 (6)* | 0.83 ± 0.01 (8)* | 4.2 ± 0.2 (6)† |
| homV59G | 6.42 ± 0.24 (10)* | 7,400 ± 1,500 (6)* | 0.83 ± 0.01 (8)* | 4.0 ± 0.1 (6)† |
| homR201C | 3.73 ± 0.12 (12)* | 106 ± 12 (6)* | 0.60 ± 0.03 (9)† | 3.9 ± 0.3 (6)† |
| hetQ52R | 3.66 ± 0.39 (9)* | 23 ± 3 (6)* | 0.70 ± 0.03 (9)* | ND |
| hetV59G | 4.19 ± 0.32 (9)* | 26 ± 6 (6)‡ | 0.70 ± 0.03 (9)* | ND |
| hetR201C | 1.22 ± 0.13 (12)* | 11 ± 2 (5)§ | ND | ND |
Mean values of resting whole-cell KATP current (Irest), ATP concentration producing half-maximal block of the channel (IC50), intrinsic open probability (i.e., in the absence of ATP) PO(0), and single channel current at -60 mV (i). Statistical significance against wild-type is indicated: *, P < 0.001; ‡, P < 0.01; §, P < 0.05; †, not significant. ND, not determined.
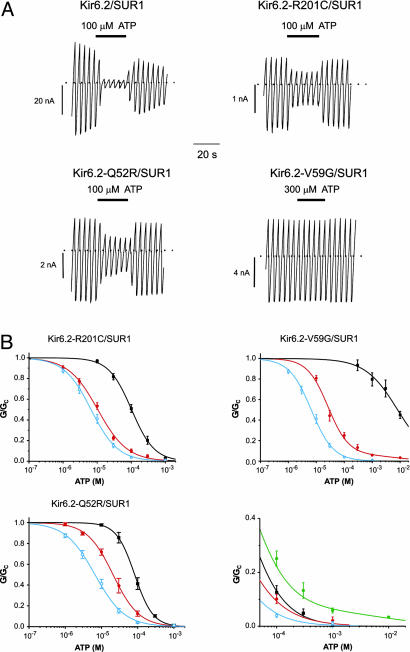
The Molecular Basis of Disease Severity. To explore the molecular basis of the different metabolic sensitivities, we examined the ability of ATP to block wild-type and mutant channels in inside-out patches (Fig. 3). All homomeric mutant channels were substantially less sensitive to intracellular ATP than wild type, the order of potency being WT > Q52R > R201C > V59G (Table 1). The reduced ATP sensitivity is expected to contribute to the larger resting whole-cell KATP currents. Although all homomeric mutant channels showed markedly reduced ATP sensitivity, significant differences were observed for heterozygous channels (Fig. 3B). In particular, hetQ52R and hetV59G channels were half-maximally blocked by ATP concentrations of 20–30 μM, whereas the ATP sensitivity of hetR201C channels was higher (11 μM) (Table 1). The different ATP sensitivities of the heterozygous channels parallel the variation in resting whole-cell currents observed for these channels in oocytes and underlie the difference between the mild and severe forms of disease.
Fig. 3.
Effects of mutations on KATP channel ATP sensitivity. (A) KATP current recorded in response a succession of voltage ramps from –110 to +100 mV to an inside-out patch excised from a Xenopus oocyte coexpressing SUR1 and either wild-type or mutant Kir6.2, as indicated. The dotted line indicates the zero current level. ATP was applied to the intracellular membrane face as indicated. (B) (Upper Left) Mean relationship between [ATP] and KATP conductance, G, expressed relative to the conductance in the absence of nucleotide, Gc, for Kir6.2/SUR1 (open blue circles, n = 6), and heterozygous (filled red circles, n = 6) and homomeric (filled black squares, n = 6) Kir6.2-R201C/SUR1 channels. The smooth curves are the best fit to the Hill equation. For wild-type, IC50 = 6.6 μM and h = 1.1. For heterozygous R201C, IC50 = 10.4 μM and h = 1.0. For homomeric R201C, IC50 = 102 μM and h = 1.3. (Upper Right) Mean relationship between [ATP] and KATP conductance expressed relative to the conductance in the absence of nucleotide for Kir6.2/SUR1 (open blue circles, n = 6) and heterozygous (red filled circles, n = 6) and homomeric (black filled squares, n = 6) Kir6.2-V59G/SUR1 channels. The smooth curves are the best fit to a modified Hill equation containing 1/16 of homomeric channels. For wild-type, IC50 = 6.6 μM and h = 1.1. For heterozygous V59G, IC50 = 26 μM and h =1.18. For homomeric V59G, IC50 = 8.1 mM and h = 0.75. (Lower Left) Mean relationship between [ATP] and KATP conductance expressed relative to the conductance in the absence of nucleotide for Kir6.2/SUR1 (open blue circles, n = 6) and heterozygous (filled red circles, n = 6) and homomeric (filled black squares, n = 6) Kir6.2-Q52R/SUR1 channels. The smooth curves are the best fit to the Hill equation. For wild-type, IC50 = 6.6 μM and h = 1.1. For heterozygous Q52, IC50 = 21 μM and h = 1.2. For homomeric Q52R, IC50 = 83 μM and h = 1.7. (Lower Right) Mean relationship between [ATP] and KATP conductance expressed relative to the conductance in the absence of nucleotide for wild type (open blue circles) and hetR201C (filled red circles), hetQ52R (filled black squares), and hetV59G (filled green hexagons) channels.
In previous studies using the mouse Kir6.2 clone, we were unable to detect a significant change in the ATP sensitivity of the heterozygous channel population with the R201H mutation (4). We therefore tested the effect of the R201H mutation in the human Kir6.2 clone. We found that ATP produced a half-maximal block of homomeric R201H channels at 298 ± 25 μM (n = 5), and of the heterozygous channel population at 12.5 ± 1.1 μM (n = 5). The latter value is close to that found for hetR201C, which also causes mild disease, and significantly less than that found for mutations associated with severe disease.
In pancreatic beta cells, an increase in KATP current will hyperpolarize the plasma membrane and suppress electrical activity, which will lead to a reduction in insulin secretion and, thereby, diabetes (11). Although the ATP concentration causing half-maximal block of hetR201C channels is only slightly greater than that found for wild-type channels (11 μM vs. 7 μM) (Table 1), the magnitude of the relative current at 0.1 mM ATP is ∼2.5-fold larger (0.1 ± 0.02, n = 5, compared with 0.04 ± 0.01, n = 6, respectively) (Fig. 3B). Because [ATP]i is unlikely to fall below 0.1 mM in beta cells, even when extracellular glucose is low (12), the difference in wild-type and mutant KATP current at this (and higher) ATP concentration probably explains why the R201C and R201H mutations result in neonatal diabetes.
Accumulating evidence implicates small changes in KATP current with impaired insulin secretion (11). In particular, a 4-fold reduction in the ATP sensitivity of Kir6.2 produces severe neonatal diabetes in transgenic mice (13). Mutations in metabolic genes, such as glucokinase (GCK), also result in a larger KATP current (14), reduced insulin release (15) at a given glucose concentration, and maturity onset diabetes of the young in humans (16, 17). It is also pertinent that a common polymorphism (E23K) in KCNJ11 is associated with an increased risk of type 2 diabetes in humans (18). The functional effects of this polymorphism remain controversial (19, 20), but our finding that neonatal diabetes (a far more dramatic phenotype) is produced by only a subtle shift in the ATP concentration–inhibition curve suggests that the effects of a Kir6.2 gene variant predisposing to diabetes in later life may be hard to discern experimentally, particularly in the heterozygous state.
All mutations cause neonatal diabetes, but only some of them are also associated with developmental delay, muscle weakness, and epilepsy (4–6). Our results show that disease severity is correlated with the extent of KATP channel block by ATP and with the magnitude of the whole-cell KATP current under resting conditions. Kir6.2 is not expressed only in the beta cell; it is also found in other endocrine cells, skeletal muscle, cardiac muscle, and neurones throughout the brain (21–23). In many of these tissues, KATP channels are normally silent and open only under metabolic stress. Thus, a greater reduction in ATP sensitivity is required to increase the resting KATP current sufficiently to influence electrical activity. This may explain why mutations that produce a small decrease in the ATP sensitivity of heterozygous channels (R201C and R201H) result in neonatal diabetes alone, whereas those causing a greater reduction (Q52R and V59G) are associated with severe disease.
Kir6.2 is strongly expressed within the hippocampus, cerebellum, and basal ganglia and at lower levels in cortex (23, 24). Further studies at the organ/whole-animal level are needed to explain precisely how the severe mutations in Kir6.2 lead to epilepsy, developmental delay, muscle weakness, and dysmorphic features. However, clonic-tonic epilepsy, like that found in severe disease, is usually associated with enhanced cortical or hippocampal activity (25). In our case, it is most simply explained as a result of overactivity of KATP channels in GABAergic interneurones (26), which results in a reduced inhibitory tone and a predisposition to seizure. Consistent with this idea, KATP channels in the hippocampus are expressed in most inhibitory interneurones but only in a minority of excitatory pyramidal neurones (27), and the KATP channel opener diazoxide inhibits firing of interneurones but not pyramidal cells (28). The observed muscle weakness could be of neurological or muscle origin, because Kir6.2 is expressed in both skeletal muscle and peripheral nerve. Interestingly, although Kir6.2 is expressed in the heart, no marked effects were observed in the ECG (4), which agrees with studies on transgenic mice expressing Kir6.2 with reduced ATP sensitivity, which also have a normal ECG (29).
Our results suggest that, in addition to their neurological features, patients with severe mutations might have greater impairment of beta cell function and thus more severe diabetes than those with mild mutations. Because subjects with mild mutations, such as R201H, do not show any insulin increment with i.v. glucose or glucagon (4), it would not be possible to detect a more severe defect. However, consistent with this idea, R201H subjects do produce insulin in response to sulfonylureas (4, 6), but no insulin increment was seen in either of the two patients with neurological features who have been tested (E. R. Pearson and A. T. Hattersley, personal communication).
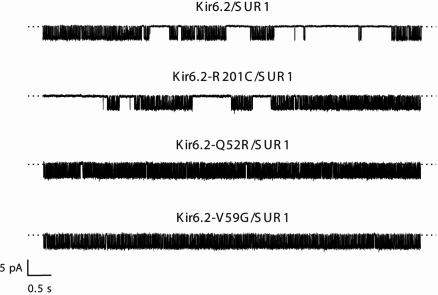
Effects of PNDM Mutations on Single-Channel Kinetics. Why does the Q52R mutation cause a greater shift in the ATP sensitivity of the heterozygous channel than the R201C mutation, despite the similar ATP sensitivities of the homomeric channels? To answer this question, we recorded single-channel currents in inside-out membrane patches in ATP-free solution, where the intrinsic gating of the channel can be assessed. Fig. 4 shows that the open probabilities of the mutant channels in the unliganded state, PO(0), were strikingly different. Thus, the PO(0) of homomeric channels containing the R201C mutation was not significantly different from wild-type, whereas that of homQ52R and homV59G channels was substantially greater (Table 1). It is well documented that an increase in PO(0) reduces the ability of ATP to close the KATP channel (30, 31). Thus, mutations at residue 201, which lies within the putative ATP-binding site (8), probably act by reducing ATP binding per se, whereas the Q52R and V59G mutations appear to decrease ATP sensitivity indirectly, by favoring the open conformation of the channel. Because V59G channels have a much lower ATP sensitivity than Q52R channels, despite a similar PO(0), the V59G mutation is also likely to affect ATP binding and/or the mechanism by which ATP binding is transduced into channel closure. The data are also consistent with a role for the slide helix, within which residue 59 resides, in Kir channel gating.
Fig. 4.
Effects of mutations on single-channel currents. Single KATP channel currents were recorded at –60mV from inside-out patches excised from oocytes coinjected with mRNAs encoding SUR1 plus either wild-type or mutant Kir6.2, as indicated.
Lipids, such as PIP2, increase the PO(0) and reduce the ATP sensitivity of the KATP channel (32). To determine whether the V59G mutation affects PO(0) directly or indirectly by means of an increase in PIP2 binding, we used neomycin, a polycation that binds to PIP2, closes KATP channels and has been used to evaluate PIP2 affinity (33). In 3/3 patches, 100 μM neomycin did not alter Kir6.2-V59G/SUR1 currents over 5–10 min (data not shown). This finding is consistent with the idea that the V59G mutation affects PO(0) directly, rendering gating insensitive to PIP2 modulation.
Given that the KATP channel pore is composed of four Kir6.2 subunits (2), in the heterozygous state most channels will contain a mixture of wild-type and mutant subunits (Fig. 1C). Because ATP binding to a single subunit is sufficient to close the channel (10), channels will exhibit a substantially reduced ATP sensitivity only when all four subunits are mutant (i.e., in ∼1/16th of channels in the heterozygous state, if the number of mutant subunits in the tetramer is binomially distributed). This finding explains why mutations in the ATP-binding site, like R201C, cause only a small shift in the ATP concentration–inhibition curve of heterozygous channels.
An empirical indication of the extent of intrinsic channel opening in the mixed population of channels that is expected when wild-type and mutant cDNAs are coexpressed is given by the mean PO(0) (〈PO(0)〉; see Materials and Methods). As Table 1 shows, 〈PO(0)〉 was significantly higher for hetQ52R and hetV59G channels than wild type. These mutations therefore reduce the free energy of the open state in the heterozygous channel population. We simulated an ensemble of Kir6.2 tetramers containing a binomial mix of wild-type and mutant subunits exhibiting free energy additivity in their effects on gating (see Materials and Methods and Supporting Text). The 〈PO(0)〉 of hetQ52R channels is predicted to be 0.73, in excellent agreement with the experimental observation (0.70) (Table 1), and the predicted half-maximal inhibitory [ATP] is 22 μM (observed 23 μM). Thus, the lower ATP sensitivity of hetQ52R channels is consistent with an additive energetic influence of mutant and wild-type subunits on channel gating and, thereby, ATP sensitivity. In the case of the V59G channels, simulations suggest that the mutation must affect both gating and ATP-binding/transduction and indicate that the latter effect is largely suppressed in heterozygous channels containing wild-type subunits (as is the case for hetR201C). These findings explain why hetQ52R and hetV59G channels have similar ATP sensitivities despite the very different ATP sensitivities of the homomeric channels.
The results we have described thus far predict that the second mutation at V59 (V59M), which is also associated with severe disease, will have an increased intrinsic open probability, whereas the R201H mutation, which, like R201C, causes PNDM alone, will not. Consistent with this idea, the PO(0) of homV59M channels was 0.83 ± 0.1 (n = 5), significantly different from wild-type (P > 0.001), whereas there was no significant difference in the PO(0) between wild-type and R201H channels.
Mutations May Differentially Alter Sulfonylurea Efficacy. Our finding that Kir6.2 mutations produce neonatal diabetes by different molecular mechanisms may have implications for therapy. We observed that 500 μM tolbutamide blocked azide-activated whole-cell currents by 89 ± 1% (n = 12), 65 ± 5% (n = 9), and 41 ± 2% (n = 9) for hetR201C, hetQ52R, and hetV59G channels, respectively. This difference is consistent with previous reports that mutations that enhance intrinsic KATP channel opening reduce the inhibitory efficacy of sulfonylureas (30). Thus, our results indicate that, whereas sulfonylureas may provide a valuable alternative to insulin injections for patients with mild mutations (like R201) (4, 6), those patients with mutations affecting intrinsic gating may require more intensive drug therapy or a combination of drug and insulin therapy.
Conclusions
Our results demonstrate that KCNJ11 mutations that cause a small decrease in the ATP sensitivity of heterozygous KATP channels result in neonatal diabetes alone, whereas those that produce a greater reduction in ATP-sensitivity are associated with additional symptoms. The molecular mechanism by which a mutation affects the channel ATP sensitivity determines the severity of its effect in the heterozygous state, with those mutations that influence gating producing larger effects on ATP sensitivity, and a more severe disease phenotype, than those that lie in the putative ATP-binding site. Our results also suggest that the efficacy of sulfonylurea therapy may vary with genotype.
Supplementary Material
Acknowledgments
We thank Chris Miller for helpful discussion. This work was supported by grants from the Royal Society, the Wellcome Trust, and Christ Church College, Oxford. F.M.A. is a Royal Society GlaxoSmithKline Research Professor. A.T.H. is a Wellcome Trust Research Leave Fellow.
Author contributions: P.P., J.F.A., J.L., A.L.G., A.T.H., and F.M.A. designed research; P.P., J.F.A., and J.L. performed research; P.P. and J.F.A. analyzed data; and P.P., J.F.A., J.L., A.L.G., A.T.H., and F.M.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Kir channel, inwardly rectifying potassium channel; SUR1, sulfonylurea receptor 1; KATP channel; ATP-sensitive potassium channel; PNDM, permanent neonatal diabetes mellitus.
References
- 1.Seino, S. & Miki, T. (2003) Prog. Biophys. Mol. Biol. 81, 133–176. [DOI] [PubMed] [Google Scholar]
- 2.Shyng, S. & Nichols, C. G. (1997) J. Gen. Physiol. 110, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker, S. J., Gribble, F. M., Zhao, C., Trapp, S. & Ashcroft, F. M. (1997) Nature 387, 179–183. [DOI] [PubMed] [Google Scholar]
- 4.Gloyn, A. L., Pearson, E. R., Antcliff, J. F., Proks, P., Bruining, G. J., Slingerland, A. S., Howard, N., Srinivasan, S., Silva, J. M., Molnes, J., et al. (2004) N. Engl. J. Med. 350, 1838–1849. [DOI] [PubMed] [Google Scholar]
- 5.Vaxillaire, M., Populaire, C., Busiah, K., Cave, H., Gloyn, A. L., Hattersley, A. T., Czernichow, P., Froguel, P. & Polak, M. (2004) Diabetes 53, 2719–2722. [DOI] [PubMed] [Google Scholar]
- 6.Sagen, J. V., Raeder, H., Hathout, E., Shehadeh, N., Gudmundsson, K., Baevre, H., Abuelo, D., Phornphutkul, C., Molnes, J., Bell, G. I., et al. (2004) Diabetes 53, 2713–2718. [DOI] [PubMed] [Google Scholar]
- 7.Antcliff, J. F., Haider, S., Proks, P., Sansom, M. S .P. & Ashcroft, F. M. (2004) EMBO J., in press. [DOI] [PMC free article] [PubMed]
- 8.John, S. A., Weiss, J. N., Xie, L. H. & Ribalet, B. (2003) J. Physiol. 552, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gribble, F. M., Ashfield, R., Ammala, C. & Ashcroft, F. M. (1997) J. Physiol. 498, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markworth, E., Schwanstecher, C. & Schwanstecher, M. (2000) Diabetes 49, 1413–1418. [DOI] [PubMed] [Google Scholar]
- 11.Ashcroft, F. & Rorsman, P. (2004) Hum. Mol. Genet. 13, R21–R31. [DOI] [PubMed] [Google Scholar]
- 12.Detimary, P., Dejonghe, S., Ling, Z., Pipeleers, D., Schuit, F. & Henquin, J. C. (1998) J. Biol. Chem. 273, 33905–33908. [DOI] [PubMed] [Google Scholar]
- 13.Koster, J. C., Marshall, B. A., Ensor, N., Corbett, J. A. & Nichols, C. G. (2000) Cell 100, 645–654. [DOI] [PubMed] [Google Scholar]
- 14.Sakura, H., Ashcroft, S. J., Terauchi, Y., Kadowaki, T. & Ashcroft, F. M. (1998) Diabetologia 41, 654–659. [DOI] [PubMed] [Google Scholar]
- 15.Terauchi, Y., Sakura, H., Yasuda, K., Iwamoto, K., Takahashi, N., Ito, K., Kasai, H., Suzuki, H., Ueda, O., Kamada, N., et al. (1995) J. Biol. Chem. 270, 30253–30256. [DOI] [PubMed] [Google Scholar]
- 16.Froguel, P., Vaxillaire, M., Sun, F., Velho, G., Zouali, H., Butel, M. O., Lesage, S., Vionnet, N., Clement, K., Fougerousse, F., et al. (1992) Nature 356, 162–164. [DOI] [PubMed] [Google Scholar]
- 17.Hattersley, A. T., Turner, R. C., Permutt, M. A., Patel, P., Tanizawa, Y., Chiu, K. C., O'Rahilly, S., Watkins, P. J. & Wainscoat, J. S. (1992) Lancet 339, 1307–1310. [DOI] [PubMed] [Google Scholar]
- 18.Gloyn, A. L., Weedon, M. N., Owen, K. R., Turner, M. J., Knight, B. A., Hitman, G., Walker, M., Levy, J. C., Sampson, M., Halford, S., et al. (2003) Diabetes 52, 568–572. [DOI] [PubMed] [Google Scholar]
- 19.Schwanstecher, C., Meyer, U. & Schwanstecher, M. (2002) Diabetes 51, 875–879. [DOI] [PubMed] [Google Scholar]
- 20.Riedel, M. J., Boora, P., Steckley, D., de Vries, G. & Light, P. E. (2003) Diabetes 52, 2630–2635. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki, N., Gonoi, T., Clement, J. P. T., Namba, N., Inazawa, J., Gonzalez, G., Aguilar-Bryan, L., Seino, S. & Bryan, J. (1995) Science 270, 1166–1170. [DOI] [PubMed] [Google Scholar]
- 22.Sakura, H., Ammala, C., Smith, P. A., Gribble, F. M. & Ashcroft, F. M. (1995) FEBS Lett. 377, 338–344. [DOI] [PubMed] [Google Scholar]
- 23.Karschin, C., Ecke, C., Ashcroft, F. M. & Karschin, A. (1997) FEBS Lett. 401, 59–64. [DOI] [PubMed] [Google Scholar]
- 24.Liss, B., Bruns, R. & Roeper, J. (1999) EMBO J. 18, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick, D. A. & Contreras, D. (2001) Annu. Rev. Physiol. 63, 815–846. [DOI] [PubMed] [Google Scholar]
- 26.Dunn-Meynell, A. A., Rawson, N. E. & Levin, B. E. (1998) Brain Res. 814, 41–54. [DOI] [PubMed] [Google Scholar]
- 27.Zawar, C., Plant, T. D., Schirra, C., Konnerth, A. & Neumcke, B. (1999) J. Physiol. 514, 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griesemer, D., Zawar, C. & Neumcke, B. (2002) Eur. Biophys. J. 31, 467–477. [DOI] [PubMed] [Google Scholar]
- 29.Koster, J. C., Knopp, A., Flagg, T. P., Markova, K. P., Sha, Q., Enkvetchakul, D., Betsuyaku, T., Yamada, K. A. & Nichols, C. G. (2001) Circ. Res. 89, 1022–1029. [DOI] [PubMed] [Google Scholar]
- 30.Trapp, S., Proks, P., Tucker, S. J. & Ashcroft, F. M. (1998) J. Gen. Physiol. 112, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enkvetchakul, D., Loussouarn, G., Makhina, E., Shyng, S. L. & Nichols, C. G. (2000) Biophys. J. 78, 2334–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shyng, S. L. & Nichols, C. G. (1998) Science 282, 1138–1141. [DOI] [PubMed] [Google Scholar]
- 33.Krauter, T., Ruppersberg, J. P. & Baukrowitz, T. (2001) Mol. Pharmacol. 59, 1086–1093. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.