Abstract
The impact of alternate routes of vaccine administration, subcutaneous (SC) or intramuscular (IM), on the safety and immunogenicity of herpes zoster subunit candidate vaccine (HZ/su) was assessed in Japanese adults aged ≥ 50 y. During this phase III open-label study, 60 subjects were randomized (1:1) to receive HZ/su through SC or IM routes in a 0, 2 month schedule. Vaccine response rates (VRRs) and geometric mean concentrations (GMCs) of varicella zoster virus glycoprotein E (gE)-specific antibodies were determined by ELISA. Solicited and unsolicited symptoms were recorded for 7 and 30 d after each vaccination and graded 1–3 in severity. Serious adverse events (SAEs) were recorded throughout the study. At one month post-dose 2, VRRs were 100% (95% Confidence Interval (CI): 88.1–100) in both groups; anti-gE antibody GMCs were 44126.1 mIU/ml (95% CI: 36326.1–53601.0) and 45521.5 mIU/ml (95% CI; 37549.5–55185.9) in the SC and IM groups, respectively. Injection site reactions (pain, swelling and redness) were common, and observed more frequently following SC administration. Grade 3 redness and swelling were more frequently observed after SC administration. Fatigue and headache were the most frequently reported general symptoms for both routes of administration. Ten and 7 unsolicited AEs were reported in the SC and IM group, respectively. Two unsolicited AEs (1 in SC; 1 in IM) were considered related to vaccination by the investigator. Three non-fatal SAEs considered unrelated to vaccination were reported during the study. Administration of the HZ/su vaccine candidate resulted in a substantial immune response that was comparable between SC and IM subjects, but local reactogenicity may be greater for SC.
KEYWORDS: adjuvant, adjuvanted vaccine, herpes zoster, HZ/su vaccine, immunogenicity, intramuscular, subcutaneous, reactogenicity
Introduction
Herpes Zoster (HZ) is caused by the reactivation of latent varicella zoster virus (VZV) in the dorsal root or cranial nerve ganglia.1 Primary VZV infection causes varicella (chickenpox) and usually occurs in childhood, after which the virus lays dormant in the affected ganglia, often for decades, until reactivation.2 HZ is typically characterized by a vesicular rash in a unilateral dermatomal distribution that is usually accompanied by radicular pain.1 The incidence of HZ increases with age,2-4 at least partly due to age-related decline in VZV-specific immunity.5 HZ risk begins to increase particularly in adults ≥ 50 y of age (YOA).6 In Japan, HZ incidence has been reported to be 4.15 per 1,000 person-years,7 comparable to the incidence rates reported in the US and Europe.8-11
The herpes zoster recombinant subunit candidate vaccine (HZ/su), consisting of VZV glycoprotein E (gE)12 and the proprietary AS01B adjuvant system,13 when administered intramuscularly (IM) has shown an age-independent vaccine efficacy of 97% in the prevention of HZ, with a clinically acceptable safety profile.14 In Japanese subjects, IM administration of 2 doses of HZ/su resulted in a substantial immune response,15 comparable to other study populations.16-18
In some countries, notably Japan, subcutaneous (SC) vaccine administration is preferred over IM vaccination.19 As previous investigations with other vaccines have suggested that both vaccine immunogenicity and reactogenicity may be impacted by the route of administration,20-21 this study was conducted to evaluate the safety and immunogenicity of the HZ/su candidate vaccine in Japanese adults 50 YOA or older when HZ/su was administered SC compared to IM.
Results
Demographics
A total of 60 subjects were enrolled in the study (30 subjects each in the SC and IM groups; Fig. 1). In both groups, 24 (40%) subjects were 50–59 YOA, 24 (40%) were 60–69 YOA and 12 (20%) were at least 70 YOA. The mean age of subjects was 61.9 y (standard deviation: 7.7), and 50% of subjects were female.
Figure 1.
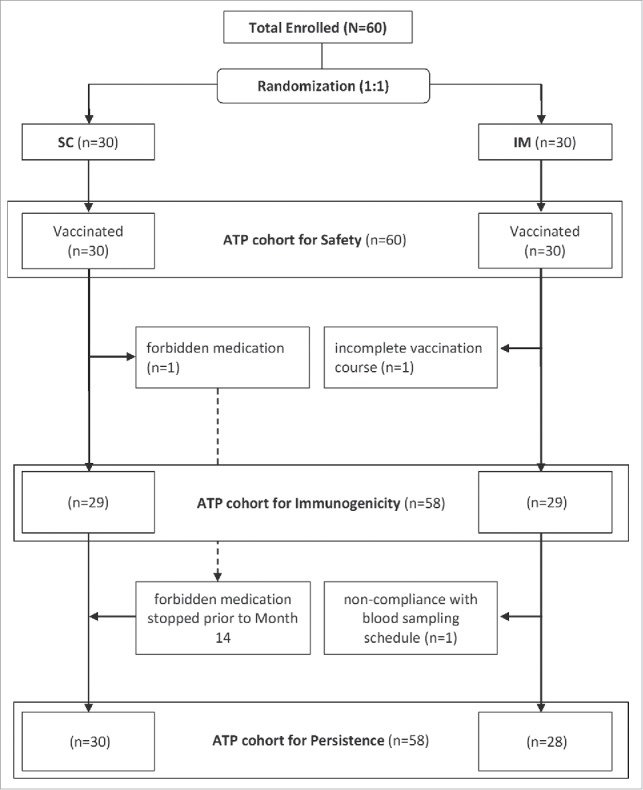
Disposition of study participants. SC: Subcutaneous IM: Intramuscular.
Immunogenicity
All subjects were seropositive for anti-gE antibodies at study entry. At one month post-dose 2, Vaccine Response Rates (VRRs) were 100% [95% Confidence Interval (CI): 88.1 – 100]) for both SC and IM groups. At 12 months post-dose 2, the percentage of subjects above the VRR cut-off declined, but was still comparable between groups (SC: 83.3% [95% CI: 65.3 – 94.4]; IM: 89.3% [95% CI: 71.8 – 97.7]; Fig. 2).
Figure 2.
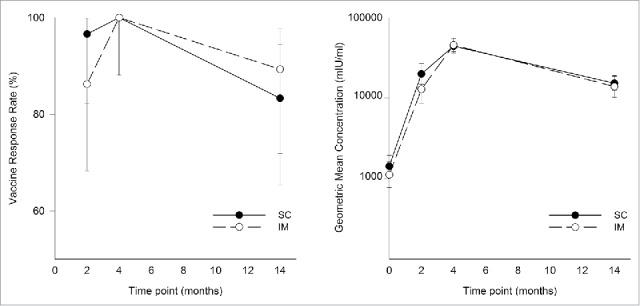
Vaccine response rate and geometric mean concentrations of anti-gE antibody (ATP cohort for immunogenicity). Samples were collected at the indicated time points (for both subcutaneous (SC) versus intramuscular (IM) groups) and anti-gE antibody concentrations were determined by ELISA. Data are vaccine response rates (VRRs) and geometric mean concentrations (GMCs) and error bars indicate 95% confidence interval.
The mean geometric increase (MGI) was 31-fold (SC group) and 40.8-fold (IM group) at one month post-dose 2, and 10.7-fold (SC group) and 11.6-fold (IM group) at 12 months post-dose 2 (Fig. 2). At one month post-dose 2, anti-gE antibody Geometric Mean Concentrations (GMCs) were 44126.1 mIU/ml (95% CI: 36326.1–53601.0) and 45521.5 mIU/ml (95% CI; 37549.5–55185.9) in the SC and IM groups, respectively. At 12 months post-dose 2, anti-gE antibody GMCs were 15250.9 mIU/ml (95% CI: 12464.0–18660.9) and 13870.2 (95% CI: 10184.2–18890.3), in the SC and IM groups, respectively.
Safety and reactogenicity
During the 7-days post-vaccination, at least one solicited or unsolicited symptom was reported after 100% of doses in the SC group and 91.5% of doses in the IM group. Solicited injection site reactions were common, and were reported after 98.3% of doses in the SC group and after 84.7% of doses in the IM group. Pain was the most frequently reported injection site reaction after both SC (88.3% of doses) and IM (79.7% of doses) administration. Redness and swelling were more frequent after SC (76.7% and 70% of doses) compared with IM (39% and 30.5% of doses) administration (Fig. 3).
Figure 3.
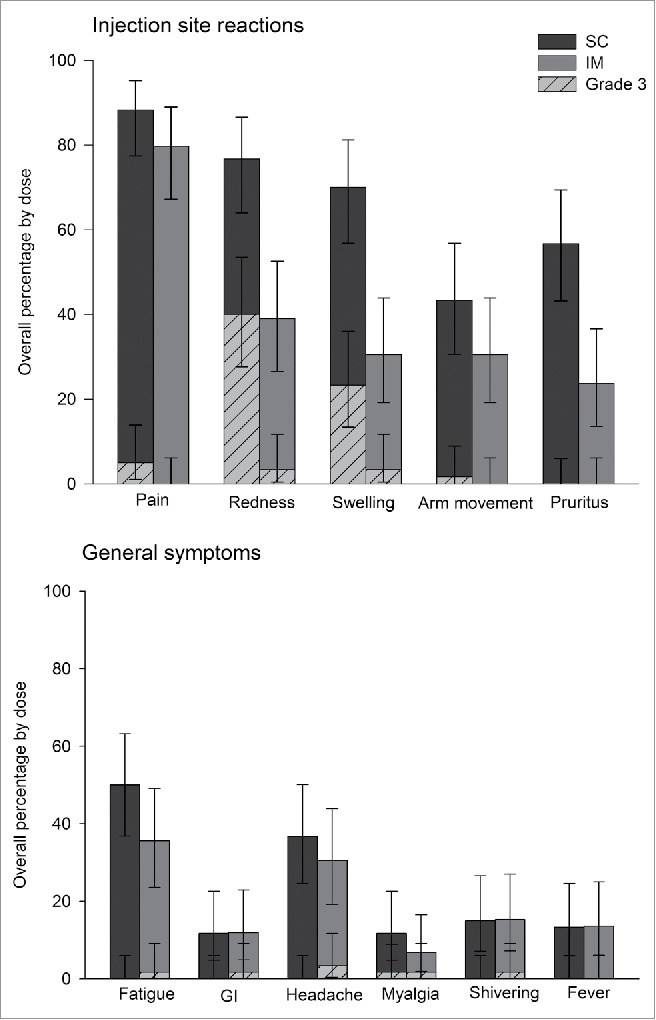
Overall occurrence of solicited injection site reactions and general symptoms during the 7-day vaccination follow-up period (Total vaccinated cohort).
Solicited systemic symptoms were reported after 60% of doses in the SC group and 50.8% of doses in the IM group. Fatigue was the most frequently reported general symptom for both SC and IM administration, observed after 50% of SC doses and 35.6% of IM doses. Grade 3 solicited systemic symptoms were uncommon, with Grade 3 myalgia reported most frequently in the SC group (after 1.7% of doses) and Grade 3 headache reported most frequently in the IM group (after 3.4% of doses) compared to SC group (0%) (Fig. 3).
During the 30 d following vaccination, 10 unsolicited adverse events (AEs) were reported by 9 subjects in the SC group, and 7 unsolicited AEs were reported by 6 subjects in the IM group. No specific AE was reported by more than one subject. Seven subjects in the SC group and 3 subjects in the IM group experienced at least one unsolicited symptom necessitating a medically attended visit. Nasopharyngitis [1 subject in SC group] and pollakiuria [1 subject in IM group] were the only 2 unsolicited AEs that were considered related to vaccination by the investigator.
Two subjects in the SC group and one subject in the IM group experienced a non-fatal serious adverse events (SAEs) during the study period (SC: chronic obstructive pulmonary disease exacerbation 6 d after dose 2, finger deformity after 271 d post-dose 2; IM: spinal compression fracture after 360 d post-dose 2). These SAEs were not considered to be causally related to the study vaccine by the investigator. No HZ cases or potential immune mediated diseases were reported during the study period.
Discussion
This study assessed the impact of alternative routes of vaccine administration on the immunogenicity and safety of the candidate HZ/su, in Japanese adults aged ≥50 years. Immune responses were comparable between SC and IM routes of administration. However, injection site reactions of any grade, and Grade 3 redness and swelling in particular, were observed more frequently after SC administration.
The HZ/su candidate vaccine has consistently been shown to elicit robust immune responses in humans including in adults aged 50 y and older.16-18 The current results are consistent with previous findings and further expand those findings by suggesting that immunogenicity of the vaccine is independent of the route of administration.
Although it has been suggested that IM administration contributes to a stronger immune response,22,23 our study would be in line with studies of other vaccines that report immune responses to be unaffected by the route of administration.24-27
Injection site reactions to HZ/su were observed more frequently after SC administration. This effect was limited to local symptoms; in particular, redness and swelling, and we found no evidence for a difference in systemic symptoms between routes of administration. This is also consistent with studies of other vaccines.20-24
Therefore, although the small sample size limits the extent to which these results can be extrapolated to the general population, in combination with the body of research already available, this study suggests that SC administration of the HZ/su candidate vaccine is unlikely to affect immunogenicity, but local reactogenicity may be greater.
Methods
Study design and subjects
This phase III, open-label, randomized (1:1) single-center trial (ClinicalTrials.gov identifier NCT01777321) was carried out in Japan, between 17 June 2013 and 11 November 2014. Healthy subjects of Japanese ethnic origin, aged 50 y or older, were enrolled. Subjects received 2 doses, with a 2 months interval, of the HZ/su vaccine in the deltoid region either by SC or IM route. As this was the first time the HZ/su candidate vaccine was administered SC in humans, a GSK Safety review committee (SRC) reviewed all safety and reactogenicity data accrued during a 7-day period following the first dose. The SRC review included consideration of prespecified criteria for acceptable frequency and severity of AEs, and the second dose was administered only after approval of the SRC.
Potential subjects were excluded from participation if they received any investigational or non-registered drug/vaccine within 30 days, or any immunosuppressants or immune-modifying drugs within 6 months before study start, were allergic to any vaccine component, had a history of HZ, or were previously vaccinated against HZ or varicella. Other reasons for subject exclusion were any underlying illness, pregnancy, or planning to get pregnant. Enrollment of potential subjects with a temperature of ≥37.5 was deferred until resolution of the fever.
The guidelines of the Declaration of Helsinki, Good Clinical Practice and other applicable regulatory requirements were followed while conducting the study. The study was approved by the Kyushu Clinical Pharmacology Research Clinic institutional review board, and all subjects provided written informed consents before being included in the trial.
Study vaccine
Each dose (0.5ml) of HZ/su contained 50 μg of recombinant VZV gE combined with the AS01B Adjuvant System (liposome, 50μg 3-O-desacyl-4′-monophosphoryl lipid A, 50μg of Quillaja saponaria Molina, fraction 21 (QS21, Licensed by GSK from Antigenics Inc., a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation). The reconstituted vaccine was administered within 6 hours of reconstitution.
Immunogenicity assessment
Blood samples were collected before vaccination, 2 months post-dose 1, and one and 12 months post-dose 2. Antibodies against gE were measured by ELISA. The assay cut-off was 18 mIU/ml for all time-points, except for the persistence time-point at 12 months post-dose 2, for which the assay cut-off was 97 mIU/ml.
Safety and reactogenicity assessment
Solicited injection site reactions (pain, swelling, redness, pruritus at the injection-site, and impaired movement/range of motion of the vaccinated arm) and solicited systemic symptoms (fatigue, fever, gastrointestinal symptoms [nausea, vomiting, diarrhea and abdominal pain], headache, myalgia, and shivering) were recorded for 7 d after each vaccination. All unsolicited AEs were recorded for 30 d after each vaccination. All SAEs were recorded throughout the study period. Study withdrawals and medical conditions occurring during the course of the trial were also recorded. The severity of symptoms was graded on a scale of 0–3. Grade 3 AEs were defined as “preventing normal daily activity,” with the exception of redness and swelling, for which Grade 3 was defined as a reaction with a diameter >100 mm, and fever, for which Grade 3 was defined as an axillary temperature >39°C.
Statistical analysis
The first and second co-primary objectives were the assessment of VRRs and GMCs of anti-gE antibodies one month after administration of the second vaccine dose. A vaccine response was defined as a 4-fold increase in post-vaccination antibody concentrations as compared to pre-vaccination antibody concentrations. GMCs were calculated by taking the anti-log of the mean of the log concentrations. A MGI was calculated as the geometric mean of the within-subject ratio of the post-vaccination antibody concentration to the pre-vaccination concentration.
The third co-primary objective was the assessment of the safety and reactogenicity of the vaccine in all subjects. The percentage of subjects/doses reporting solicited injection site reactions, solicited systemic symptoms, and unsolicited symptoms were calculated with exact 95% CI.
For each endpoint, no formal comparison between groups was made, and CI were used to suggest comparability between groups. All statistical analyses were performed using the Statistical Analysis Sytems software version 9.2 (Cary, NC, USA). Immunogenicity analyses were performed on the according-to-protocol cohort for immunogenicity, which included subjects who complied with all protocol-defined study procedures and for whom immunogenicity data were available at one month post-dose 2. In order to assess the persistence of anti-gE antibodies, VRRs and GMCs with 95% CI were calculated in the ATP cohort for persistence, consisting of subjects who complied with all protocol defined study procedures and for whom the immunogenicity data were available at 12 months post-dose 2. Safety and reactogenicity were analyzed on the total vaccinated cohort which included all subjects who received at least one study vaccine dose.
Abbreviations
- AEs
Adverse events
- CI
Confidence interval
- gE
Varicella zoster virus glycoprotein E
- GMCs
Geometric mean concentrations
- HZ
Herpes zoster
- HZ/su
Herpes zoster subunit vaccine
- IM
Intramuscular
- MGI
Mean geometric increase
- SAEs
Serious adverse events
- SC
Subcutaneous
- SRC
Safety review committee
- VRRs
Vaccine response rates
- VZV
Varicella zoster virus
- YOA
Years of age
Disclosure of potential conflicts of interest
Peter Vink and Martine Douha are employees of the GlaxoSmithKline group of companies and, as such, are compensated by GSK for work both related and unrelated to the submitted work. Peter Vink receives GSK stock equity as part of his compensation. Himal Lal and Thomas Heineman were employees of GSK and received salary and stock as compensation at the time of the study design, conduct, and interpretation of data and writing of manuscript. Himal Lal is currently an employee of Pfizer. Thomas Heineman is currently an employee of Genocea Biosciences. Masayuki Ogawa and Masahiro Eda are employees of the Japan Vaccine Company. Masanari Shiramoto declares having no potential conflicts of interest.
Acknowledgments
We thank study participants and staff at SOUSEKAI PS Clinic, Japan. We thank An Ranquin and Katherine Ward for writing the study reports for this study. Editorial assistance and coordination in the preparation of this manuscript was provided by Dr. Jarno Jansen and Dr. Bart van Heertum (XPE Pharma for GSK Vaccines). Writing assistance was provided by Dr. Sasi Taneja and Prachee Panda (GSK).
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals S.A., Rixensart, Belgium and Japan Vaccine Company, Tokyo, Japan. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript.
Clinical Trial Registration: www.clinicaltrials.gov, NCT01777321
References
- [1].Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010; 4 8(Suppl 1):S2-7; PMID:20510263; http://dx.doi.org/ 10.1016/S1386-6532(10)70002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol 2011; 6:341-55; PMID:21695042; http://dx.doi.org/ 10.2217/fvl.10.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract 2011; 11:397-402; PMID:21199312; http://dx.doi.org/ 10.1111/j.1533-2500.2010.00432.x [DOI] [PubMed] [Google Scholar]
- [4].Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1-30; quiz CE2-4 [PubMed] [Google Scholar]
- [5].McElhaney JE. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Expert Rev Vaccines 2009; 8:593-606; PMID:19397416; http://dx.doi.org/ 10.1586/erv.09.12 [DOI] [PubMed] [Google Scholar]
- [6].Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc 2009; 84:274-80; PMID:19252116; http://dx.doi.org/ 10.4065/84.3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Toyama N, Shiraki K. Epidemiology of herpes zoster and its relationship to varicella in Japan: A 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol 2009; 81:2053-8; PMID:19856466; http://dx.doi.org/ 10.1002/jmv.21599 [DOI] [PubMed] [Google Scholar]
- [8].Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology 2013; 81:928-30; PMID:23999562; http://dx.doi.org/ 10.1212/WNL.0b013e3182a3516e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chapman RS, Cross KW, Fleming DM. The incidence of shingles and its implications for vaccination policy. Vaccine 2003; 21:2541-7; PMID:12744889; http://dx.doi.org/ 10.1016/S0264-410X(03)00034-3 [DOI] [PubMed] [Google Scholar]
- [10].Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, Chosidow O, Blanchon T, Hanslik T. Herpes zoster: Burden of disease in France. Vaccine 2010; 28:7933-8; PMID:20946861; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.074 [DOI] [PubMed] [Google Scholar]
- [11].Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar Herpes Zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis 2013; 13:170; PMID:23574765; http://dx.doi.org/ 10.1186/1471-2334-13-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clark TG, Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev Biol (Basel) 2005; 121:153-63; PMID:15962478 [PubMed] [Google Scholar]
- [13].Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10:471-86; PMID:21506645; http://dx.doi.org/ 10.1586/erv.11.29 [DOI] [PubMed] [Google Scholar]
- [14].Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al.. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087-96; PMID:25916341; http://dx.doi.org/ 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- [15].Lal H, Zahaf T, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella zoster virus subunit candidate vaccine (HZ/su): a phase-I, open-label study in Japanese adults. Hum Vaccin Immunother 2013; 9:1425-9; PMID:23584252; http://dx.doi.org/ 10.4161/hv.24269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E, Heineman TC. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 2012; 206:1280-90; PMID:22872734; http://dx.doi.org/ 10.1093/infdis/jis497 [DOI] [PubMed] [Google Scholar]
- [17].Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mold JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis 2013; 208:1953-61; PMID:23904292; http://dx.doi.org/ 10.1093/infdis/jit365 [DOI] [PubMed] [Google Scholar]
- [18].Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, Plassmann G, Ledent E, Heineman TC. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32:1745-53; PMID:24508036; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.019 [DOI] [PubMed] [Google Scholar]
- [19].Saitoh A, Okabe N. Current issues with the immunization program in Japan: can we fill the “vaccine gap”? Vaccine 2012; 30:4752-6; PMID:22521841; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.026 [DOI] [PubMed] [Google Scholar]
- [20].Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ 2000; 321:1237-8; http://dx.doi.org/ 10.1136/bmj.321.7271.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ajana F, Sana C, Caulin E. [Are there differences in immunogenicity and safety of vaccines according to the injection method?]. Med Mal Infect 2008; 38:648-57; PMID:18986787; http://dx.doi.org/ 10.1016/j.medmal.2008.09.012 [DOI] [PubMed] [Google Scholar]
- [22].Cook IF, Barr I, Hartel G, Pond D, Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 2006; 24(13):2395-402; PMID:16406171; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.057 [DOI] [PubMed] [Google Scholar]
- [23].de Lalla F, Rinaldi E, Santoro D, Pravettoni G. Immune response to hepatitis B vaccine given at different injection sites and by different routes: a controlled randomized study. Eur J Epidemiol 1988; 4(2):256-8; PMID:2969825; http://dx.doi.org/ 10.1007/BF00144763 [DOI] [PubMed] [Google Scholar]
- [24].Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, Thomas S, Sadorge C. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged ≥50 years: a randomised non-inferiority clinical trial. Vaccine 2015; 33:789-95; PMID:25555381; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.024 [DOI] [PubMed] [Google Scholar]
- [25].Ruben FL, Froeschle JE, Meschievitz C, Chen K, George J, Reeves-Hoché MK, Pietrobon P, Bybel M, Livingood WC, Woodhouse L. Choosing a route of administration for quadrivalent meningococcal polysaccharide vaccine: intramuscular versus subcutaneous. Clin Infect Dis 2001; 32(1):170-2; PMID:11118395; http://dx.doi.org/ 10.1086/317553 [DOI] [PubMed] [Google Scholar]
- [26].Mark A, Carlsson RM, Granstöm M. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine 1999; 17(15–16):2067-72; PMID:10217608 [DOI] [PubMed] [Google Scholar]
- [27].Rothstein EP, Kamiya H, Nii R, Matsuda T, Bernstein HH, Long SS, Hosbach PH, Meschievitz CK. Comparison of diphtheria-tetanus-two component acellular pertussis vaccines in United States and Japanese infants at 2, 4, and 6 months of age. Pediatrics 1996; 97(2):236-42; PMID:8584384 [PubMed] [Google Scholar]


