Abstract
Targeting aberrant tyrosine kinase activity may impact clinical outcome in acute myeloid leukemia (AML). We conducted a phase I study of the tyrosine kinase inhibitor midostaurin with bortezomib alone and in combination with chemotherapy in patients with AML. Patients on dose levels 1 and 2 (DL1 & 2) received midostaurin 50 mg bid and escalating doses of bortezomib (1 to 1.3 mg/m2). Patients on DL3 or higher received midostaurin and bortezomib following chemotherapy with mitoxantrone, etoposide, cytarabine (MEC). None of the patients enrolled to DL1 & 2 had dose-limiting toxicities (DLTs) or a clinical response. Among patients enrolled to DL3 or higher, DLTs were peripheral neuropathy, decrease in ejection fraction and diarrhea. A 56.5% CR rate and 82.5% overall response rate (CR + CR with incomplete neutrophil or platelet count recovery) were observed. The midostaurin/bortezomib/MEC combination is active in refractory/relapsed AML, but is associated with expected drug-related toxicities. (NCT01174888)
Keywords: Bortezomib, midostaurin, AML, tyrosine kinase, chemotherapy
Introduction
Acute myeloid leukemia (AML) is a biologically and clinically heterogeneous disease characterized by clonal accumulation and expansion of immature myeloid cells in the blood and bone marrow (BM).[1] Despite advances in understanding the biology of the disease [2] and characterization of prognostic genetic and epigenetic aberrations [3], the overall outcome remains poor. [4] Primary refractoriness to chemotherapy and failure to maintain a first complete remission (CR1) are responsible for these poor outcomes. Therefore, novel strategies are needed to improve the outcome of AML patients.
The discovery of molecular abnormalities that contribute to leukemogenesis creates an opportunity for designing molecular targeting compounds. Among the most common targetable aberrations are the mutated tyrosine kinase proteins. An internal tandem duplication (ITD) of the juxtamembrane domain of the FMS-like tyrosine kinase 3 receptor (FLT3) gene is found in approximately 25% of patients [5,6] and encodes a protein with aberrant kinase activity that confers a proliferative and survival advantage.[7,8] Overexpression of FLT3 wild-type and FLT3 ligand proteins may also contribute to the aggressiveness of the disease.[9,10] Therefore, compounds that inhibit FLT3 and other aberrant kinases represent a rational therapeutic approach.
Tyrosine kinase inhibitors (TKIs) are designed to target the activated FLT3 receptor, alone or in combination with chemotherapy. Until recently, TKIs had not been associated with improved outcome; however, a randomized trial of untreated patients with AML and the multikinase inhibitor sorafenib in combination with chemotherapy showed a 3 year event free survival (EFS) of 40% in those who received sorafenib, versus 22% in the placebo group.[11] In a larger study of patients with untreated FLT3 mutated AML and the multi-targeted FLT3 inhibitor midostaurin or placebo in combination with chemotherapy followed by maintenance therapy, those who received midostaurin had a significant improvement in EFS and overall survival when compared to those in the placebo group.[12] These data support continued efforts to evaluate TKIs in the treatment of AML.
We reported that tyrosine kinase activity in AML blasts may be modulated indirectly by targeting the transcription of genes encoding receptor tyrosine kinases (RTKs; i.e., FLT3 and KIT).[13] The transcriptional factors Sp1 and NF-κB form a transactivating complex that binds to the promoter regions of RTKs, inducing gene transcription and protein expression.[13] We showed that the proteasome inhibitor bortezomib (Velcade®) disrupts the Sp1/NF-κB transactivating complex and decreases the expression of genes encoding for RTKs.[13,14] Although initial studies found bortezomib to be relatively inactive as a single agent,[15] the addition of bortezomib to standard chemotherapy resulted in an encouraging CR rate in older patients with AML. [16]
Herein, we hypothesize that the combination of a TKI, which inhibits the enzymatic activity of the TK protein, with bortezomib, which interferes with tyrosine kinase coding genes’ transcription, is an effective strategy for dual targeting of aberrant kinase activity in AML.
We developed a phase I trial that combines bortezomib with midostaurin to determine the safety and clinical response of these two agents and MEC (mitoxantrone, etoposide, cytarabine) chemotherapy in patients with relapsed or refractory AML.
Materials and Methods
Study design and patient enrollment
Patients with primary refractory, relapsed or relapsed refractory AML were eligible. Enrollment to dose levels (DL) 1 and 2 (bortezomib and midostaurin only) was open to all patients. Enrollment to DL3 or higher (bortezomib, midostaurin and MEC) was open to patients ≤ 70 years. Once DL1 and 2 were deemed tolerable, patients > 70 accrued to DL1 or DL2, for a maximum of 6 patients at each DL. These additional patients would confirm tolerability and facilitate analysis of clinical response. Informed written consent approved by The Ohio State University Human Studies Committee was obtained prior to study entry. This trial was registered with the NCI clinical trials network (NCT01174888).
Patients were required to have a total bilirubin <2.0 mg/dL or ≤1.5 X upper limit normal, creatinine <1.7 mg/dL, ALT/AST ≤2.5 X upper limit of normal, ejection fraction ≥ 50%, and Eastern Cooperative Oncology Group performance status ≤2. Patients with a pre-existing grade 2 or higher neuropathy or other neurologic toxicity were excluded.
To determine the maximum tolerated dose (MTD) a 3+3 dose escalation design was utilized. Patients enrolled on DL1 and 2 received midostaurin 50 mg orally twice a day on days 1–14 and bortezomib at doses of 1 mg/m2 (DL1) or 1.3 mg/m2 (DL2) on days 1,8,15,22 every 28 days for up to three cycles. Patients on DL3 received MEC: mitoxantrone 4 mg/m2/d, etoposide 40 mg/m2/d and cytarabine 1 gm/m2/d intravenously (IV) days 1–6 followed by midostaurin 50mg PO bid days 8–21 and bortezomib 1.3 mg/m2 IV days 8,11,15,18. Due to the development of dose limiting sensory and autonomic neuropathy in DL3, first the route of administration and then the dose and schedule of bortezomib were changed in DL3* and DL3A, respectively. Patients on DL3A received bortezomib 1 mg/m2 SQ days 8 and 15 only. Patients on DL4 received standard dose MEC, mitoxantrone 6 mg/m2/d, etoposide 80 mg/m2/d, and cytarabine 1 gm/m2/d IV days 1–6 followed by midostaurin 50 mg PO bid days 8–21 and bortezomib 1.3 mg/m2 SQ days 8 and 15. See table V for details of the treatment schedule. Patients enrolled to DL3, 3*, 3A and 4 received one cycle of therapy with bortezomib, midostaurin and MEC.
Table V.
Treatment dose and schedule
| Dose level | Mitoxantrone | Etoposide | Cytarabine | Midostaurin | Bortezomib | Number Treated | Number Replaced | Number of DLTs |
|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | 50 mg BID PO d 1–14 | 1.0 mg/m2 IV d 1,4,8,11 | 6 | 0 | 0 |
| 2 | - | - | - | 50 mg BID PO d 1–14 | 1.3 mg/m2 IV d 1,4,8,11 | 5 | 2A | 0 |
| 3 | 4 mg/m2 IV d 1–6 | 40 mg/m2 IV d 1–6 | 1 g/m2 IV d 1–6 | 50 mg BID PO d 8–21 | 1.3 mg/m2 IV d 8,11,15,18 | 3 | 1 | |
| 3* | 4 mg/m2 IV d 1–6 | 40 mg/m2 IV d 1–6 | 1 g/m2 IV d 1–6 | 50 mg BID PO d 8–21 | 1.3 mg/m2 SQ d 8,11,15,18 | 6 | 3 | |
| 3A | 4mg/m2 IV d 1–6 | 40mg/m2 IV d 1–6 | 1 g/m2 IV d 1–6 | 50mg BID PO d 8–21 | 1.0 mg/m2 SQ d 8 and 15 | 8 | 1B | 2 |
| 4 | 6 mg/m2 IV d 1–6 | 80 mg/m2 IV d 1–6 | 1 g/m2 IV d 1–6 | 50 mg BID PO d 8–21 | 1.3 mg/m2 SQ d 8 and 15 | 6 | 3C | 2 |
One patient developed sepsis on day 10 of cycle 1 and treatment was held. One patient withdrew consent on day 2 of therapy to pursue supportive care.
The patient withdrew consent on day 14 of therapy for non-treatment related reasons.
One patient developed sepsis on day 12 of therapy and treatment was held. One patient had doses held beginning on day 18 while undergoing evaluation for a possible ileus. One patient refused to continue treatment on day 19 of therapy because of grade 2 nausea.
Disease response was determined using International Working Group criteria. [17] For patients enrolled to DL3, 3*, 3A and 4, BM aspirates and biopsies were performed at absolute neutrophil count (ANC ≥1000/μL) and platelet count recovery (PLT ≥100,000/μL), or 35 days after initiation of treatment, whichever came first. For patients on DL1 and 2, BM aspirates and biopsies were done between days 26–28 of each cycle. Patients at any dose level who achieved a CR or a CR with incomplete count recovery (CRi) could proceed to alloSCT or receive 3 cycles of midostaurin and bortezomib only at the ongoing doses.
Patients who did not complete all study therapy were replaced. Hydroxyurea was permitted during the first cycle to maintain a white blood cell (WBC) count less than 40,000/μL, but no other antileukemic therapies were permitted. Patients requiring treatment with hydroxyurea after completion of cycle 1 were considered to have refractory disease.
Definition of Dose Limiting Toxicity (DLT)
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. DLT was defined during cycle 1. Bortezomib or midostaurin-related non-hematologic grade 3 or 4 toxicities were considered DLT. Given the frequency of infectious complications associated with cytotoxic chemotherapy, these were not considered DLT unless the severity or duration was longer than expected. Hematologic DLT was defined as a failure to recover ANC or PLT count by day 42 in patients with <5% blasts in the BM, absence of myelodysplastic changes, and/or absence of evidence of disease by flow cytometry in BM.
Pharmacodynamic Analysis
BM mononuclear cells (MNCs) were isolated from samples procured pre- (day 0) and post-treatment (days 3 and 8 of cycle 1) in patients who consented to participate in the correlative studies. Total RNA was extracted by Trizol reagent (Invitrogen, Carlsbad, CA), reverse-transcribed and amplified using Taqman Gene Expression Assay or Taqman microRNA Assay (Applied Biosystems, Foster City, CA). Reactions were carried out in triplicate in the ABI Prism 7900HT Real-time PCR system. The expression levels were normalized to internal controls (GAPDH for FLT3 and primary miR-29b; and RNU44 for miR-155) and the data were analyzed using the Delta Ct method, as previously reported. [18–20]
Statistical methods
Standard dose escalation rules were used in the context of a 3+3 phase I trial design, and the MTD was defined as the highest DL where at most one of 6 patients experiences DLT. Although initially planned, sequential patient sampling results in too few of procured samples prevented a meaningful molecular analysis.
Results
Patients
Patient characteristics for all dose levels are shown in Table I. The median number of treatments received prior to enrollment were 2 (range 1–4). Thirteen (38%) patients had primary refractory disease and 12 (35%) had a short (<12 months) CR1 duration. Only 9 (26%) had a long CR1 (>12 months) prior to enrollment. Thus, 73% of the patients enrolled had relatively chemotherapy-resistant (i.e., primary refractory or short CR1) disease.
Table I.
| Patient Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | CR1 Duration | Prior AML Therapy | Cytogenetics | FLT3 | NPM1 | CEBPA | ELN | Dose Level |
Response | Transplant |
| 67/M | 3mo | Decitabine | 46,XY | ITD+ | Neg | Neg | Int-I | 1 | NR | N |
| 65/M | 16mo | Decitabine, Anti-FLT3 antibody, lenalidomide+ ARAC/Idarubicin, 5+2 | 46,XY,del(2)(p22)[5]/46,sl,t(15;19)(q15;q13.1)[4]/46,sl,t(1;6)(q21;q23)[3]/46,sl,t(7;10)(q11.2;p11.2)[2] | TKD+ | Pos | Neg | Adverse | 1 | NR | N |
| 72/M | 6mo | 7+3, HIDAC, Imatinib | 48,XY,+13,+19[15]/46,XY[5] | Neg | Neg | Neg | Int-II | 1 | NR | N |
| 72/F | PR | Decitabine | 46,XX,del(5)(q22q35)[1]/46,XX[19] | Neg | Neg | Neg | Int-II | 1 | NR | N |
| 76/M | 21mo | Decitabine | 46,XY | Neg | Pos | Neg | Fav | 1 | NR | N |
| 75/F | 9mo | Decitabine | 46,XX | Neg | Neg | Neg | Int-I | 1 | NR | N |
| 72/M | PR | Decitabine | 46,XY | Neg | Neg | Neg | Int-I | 2 | NR | N |
| 64/F | PR | 7+3, 5+2, MEC | 46,XX | ITD+ | Pos | Neg | Int-I | 2 | NR | N |
| 56/F | PR | 5-azacytidine, 7+3, HIDAC | 46,XX,del(9)(q13q22),del(21)(q22)[12]/46,XX,t(5;12)(q31;q22),del(21)(q22) | UK | UK | UK | Adverse | 2 | NR | N |
| 65/M | 9mo; RR | 7+3, IDAC, allograft, lenalidomide | 43,X,-Y,dup(1)(q41q24),−3,del(5)(q13q31),del(6)(q13q23), −7,add(8)(p11.2),ad d(15)(q11.2),psu dic(16:?)(p11.2;?), −17,+mar1,+mar2[1 | Neg | Neg | Neg | Adverse | 2 | NR | N |
| 67/F | 10mo | 7+3, HIDAC, allograft | 46,XX | ITD+ | Pos | Neg | Int-I | 2 | NR | N |
| 34/M | 9mo | ADE, HIDACx2 | 46,XY,inv(16)(p13.1q22)[3]/46,idem,del(7)(q21q36)[9] | Neg | Neg | Neg | Favorable | 3 | CR | Yes |
| 19/M | 2mo | 7+3, HIDACx1 | 46,XY,t(6;9)(p23;q34)[5]/46,sl,del(3)(p13p21)[8]/47,sl,+8 | ITD+ | Neg | Neg | Adverse | 3 | CR | Yes |
| 60/F | PR | 7+3+panobinostat | 46,XX | ITD+ | Neg | Neg | Int-I | 3 | CRi | No |
| 19/F | PR | 7+3 | 46,XX | ITD+ | Neg | Neg | Int-I | 3* | CR | Yes |
| 53/F | PR | 7+3 | 46,XX | Neg | Neg | Neg | Int-I | 3* | CR | Yes |
| 59/M | 17mo | 7+3, HIDACx3 | 46,XY | Neg | Neg | Neg | Int-I | 3* | CR | No |
| 69/F | PR | Vidaza | 41–45,XX,del(5)(q13q33), −7,add(7)(q11.2),add(12)(p11.2),add(13)(q12),psu dic(14;9)(q32;p24)hsr(14)(q32), −17,add(18)(p11.2),+mar[cp8]/44,idem,add(5)( q31)[cp4,one is 4n]/46,XX[13]/ | Neg | Neg | Neg | Adverse | 3* | CR | No |
| 55/M | PR | 7+3, 5+2, Azacytidine+MEC | 44,XY,add(3)(p21),del(5)(q15q33), −7,add(12)(p13), −16, −20,+mar1[1]/45–47,sl, 3,-add(3),hsr(11)(q23),+16,+20,der(21)(11qter->11q23::hsr MLL::11q23->11q13::hsrMLL::11q23->?::21p13->21qter)-mar1,+mar2[cp11]/42–45,sdl1, −9,+add(11)(q23),-hsr(11), −16,-der(21),+mar3[cp3]/44–45,sl,-add(3),+mar2 | Neg | Neg | Neg | Adverse | 3* | NR | No |
| 70/M | 9mo | 7+3, HIDACx2, SGI-110 | 46,XY | Neg | Pos | Neg | Favorable | 3* | CRi/CR | No |
| 23/M | 16mo | 7+3, Autologous Tx | 46,XY,del(9)(q13q22)[13]/46,XY[7] | Neg | Neg | Neg | Int-II | 3A | CR | Yes |
| 27/F | 12mo | 7+3, HIDACx4 | 46,XX | Neg | Pos | Neg | Favorable | 3A | CR | Yes |
| 49/M | 9mo | 7+3, HIDAC, 5-azacytidine+MEC | 46,Y,t(X;6)(p11.2;p25)[11]/46,XY,t(2;3)(p23;q21)[9] | ITD+ | Pos | Pos | Int-II | 3A | NR | No |
| 60/M | 23mo | 7+3,HIDAC | 46,XY | Neg | Neg | Neg | Int-I | 3A | CRi | Yes |
| 31/M | 13mo | 7+3+lenalidomide, HIDACx4 | 46,XY | Neg | Neg | Pos | Fav | 3A | CRi | Yes |
| 57/M | 12mo | 7+3, HIDAC x1 | 46,XY[20] | Neg | Neg | Pos | Fav | 3A | CR | Yes |
| 70/M | PR | Decitabine + Sorafenib | 46,XY[20] | Neg | Neg | Neg | Int-I | 3A | CRi | No |
| 58/M | PR | 7+3 (twice) | Complex | Neg | Neg | Neg | Adverse | 3A | NR | No |
| 48/M | 7mo | 7+3+lenalidomide, HIDAC, allograft | 46,XY | Neg | Neg | Neg | Int-I | 4 | CR | DLI |
| 48/M | PR | 7+3 | 47,XY,+8[13] | ITD+ | Neg | Neg | Int-II | 4 | CR | No |
| 53/F | PR | Decitabine+7+3 | 46,XX,t(2;3)(p25;p11.2) | ITD+ | Pos | Pos | Int-II | 4 | NR | No |
| 59/M | 5mo | 7+3 | 44–47,XY,dic(3;17)(q11.1;q11.2), −5,der(5)inv(5)(q13q34)ins(5;?)(q13;?), −6, −7,+8,ins(16;?)(q12;?),add(17)(p11.2), add(20)(p13),+der(?)t(?;17)(?;q11.2),+r1,+r2,+mar[cp2]/46,XY[18] | Neg | Neg | Neg | Adverse | 4 | CR | Yes |
| 37/F | 5mo | 7+3,HIDACx2, lenalidomide+ARAC+ Idarubicin | 46,XX,inv(16)(p13.1q22)[18]/ | Neg | Neg | Neg | Favorable | 4 | CRi | Yes |
| 62/F | 33mo | 7+3,HIDACx2 | 47,XX,+4[10]/46,XX[10] | Neg | Pos | Pos | Int-II | 4 | CR | Yes |
M=male, F=female, mo=month, CR1= 1st complete remission, AML=acute myeloid leukemia, ELN= European LeukemiaNet, ITD+=internal tandem duplication mutation positive;TKD=tyrosine kinase domain mutation positive, Neg=negative, Int-1=Intermediate-I, Int-II=Intermediate-II, Fav=favorable, NR=No response, CR=Complete remission, CRi=Complete remission with incomplete count recovery, CRi=Complete remission with incomplete platelet or neutrophil recovery, UK=unknown, Pos=positive, HIDAC=high dose cytarabine, allograft-allogeneic stem cell transplant, IDAC=intermediate dose cytarabine, 7+3=daunorubicin and cytarabine, 5+2=daunorubicin and cytarabine, Autologous tx= autologous transplant, ADE=cytarabine, daunorubicin and etoposide, PR=primary refractory
Treatment and Toxicity
For DL1 and DL2, grade 3 or higher febrile neutropenia and other infections occurred in 5 of 11 patients. Although the most common non-hematologic toxicities were fatigue, nausea and diarrhea, none met criteria for DLT. See Table III for a list of non-hematologic toxicities that occurred at DLs1 and 2.
Table III.
Non-hematologic toxicities regardless of attribution* (Dose Levels 1 and 2)
| CDUS Toxicity Type Code | All Grades (No. of events) | Grade 3 or higher (No. of events) |
|---|---|---|
| Infection | ||
| Febrile neutropenia | 6 | 6 |
| Catheter related infection | 2 | 2 |
| Pneumonia | 4 | 3 |
| Sinusitis | 1 | 1 |
| Skin infection | 2 | 2 |
| Sepsis | 1 | 1 |
| Urinary tract infection | 1 | 1 |
| Gastrointestinal | ||
| Diarrhea | 14 | 4 |
| Vomiting | 7 | 1 |
| Oral hemorrhage | 3 | 1 |
| Cardiovascular | ||
| Hypotension | 3 | 1 |
| Pulmonary | ||
| Dyspnea | 5 | 1 |
| Hypoxia | 4 | 3 |
| Respiratory failure | 1 | 1 |
| Laryngitis | 1 | 1 |
| Nasal congestion | 4 | 2 |
| Stridor | 2 | 2 |
| Neurologic | ||
| Anxiety | 2 | 2 |
| Delirium | 2 | 2 |
| Generalized muscle weakness | 3 | 1 |
| Metabolic | ||
| Acidosis | 1 | 1 |
| Hyperkalemia | 1 | 1 |
| Other | ||
| Fatigue | 12 | 2 |
| Multi-organ failure | 1 | 1 |
Clinically insignificant and correctable electrolyte abnormalities are not included in this list.
For DL3 and higher, grade 3 or higher febrile neutropenia was highly prevalent and occurred at least once in 15 of 23 patients. Episodes of febrile neutropenia and infection were not more severe or prolonged than expected and did not meet the criteria for DLT.
One of the first three patients on DL3 (n=3) developed grade 3 peripheral (sensory) and autonomic neuropathies that were dose-limiting. Following this event the route of administration of bortezomib was changed from IV to SQ and three additional patients enrolled (DL3*), as previous studies reported a better neuropathic toxicity profile with this administration route.[21,22] Of these, one with a history of anthracycline exposure had a grade 3 decrease in ejection fraction that was considered DLT. Therefore three additional patients were enrolled to DL3*. Of these, one developed grade 3 diarrhea and one developed grade 3 peripheral sensory neuropathy, both dose-limiting. After these events, the dose and frequency of bortezomib was changed to 1 mg/m2 on days 8 and 15 only, while doses of MEC and midostaurin remained the same (DL3A). Of the four patients enrolled on DL3A (3 completed treatment; one withdrew consent on day 14 and was replaced), none had DLTs.
Six patients enrolled on DL4 where bortezomib was escalated to 1.3 mg/m2 and mitoxantrone and etoposide were increased to 6 mg/m2/d and 80 mg/m2/d, respectively. In the first cohort of 3 patients no DLTs were observed, but two patients did not receive all doses of midostaurin therapy due to midostaurin-unrelated events (one had doses held during an episode of sepsis and the other had doses held during evaluation for a possible ileus) and were not evaluable and were replaced. In the second 3-patient cohort, two developed grade 3 diarrhea that was dose limiting, and one refused to complete midostaurin treatment because of grade 2 nausea. Given that 2 patients experienced a DLT on DL4, dose escalation was stopped, and four additional patients were enrolled to DL3A. Of these, one did not receive all doses of midostaurin because of the need to start voriconazole for presumed fungal pneumonia and was replaced. Concurrent administration of midostaurin and triazole compounds was contraindicated due to the risk of QT interval prolongation. This patient was subsequently diagnosed with grade 3 left ventricular systolic dysfunction during an episode of sepsis that was dose limiting. The fourth patient enrolled to DL3A prior to determination of this DLT and developed grade 3 diarrhea, thus meeting DLT criteria. Given that there were 2 DLTs at DL3A we could not determine the MTD of the regimen. See Table IV for a list of non-hematologic toxicities that occurred at DLs 3 and higher.
Table IV.
| Toxicity | All Grades (No. events) | Grade 3 or higher (No. events) |
|---|---|---|
| Infection | ||
| Febrile neutropenia | 26 | 26 |
| Catheter related infection | 10 | 18 |
| C. Difficle Colitis | 2 | 2 |
| Sepsis | 2 | 2 |
| Pneumonia | 8 | 6 |
| Typhlitis | 2 | 2 |
| Gastrointestinal disorders | ||
| Diarrhea | 53 | 8 |
| Renal | ||
| Decreased urine output | 2 | 2 |
| Acute kidney injury | 4 | 3 |
| Dehydration | 3 | 3 |
| Cardiovascular | ||
| Ejection fraction decreased | 1 | 1 |
| Electrocardiogram QT corrected interval prolonged | 13 | 2 |
| Left ventricular systolic dysfunction | 1 | 1 |
| Hypertension | 8 | 6 |
| Hypotension | 16 | 2 |
| Atrial fibrillation | 1 | 1 |
| Edema | 23 | 2 |
| Pulmonary | ||
| Hypoxia | 12 | 5 |
| Respiratory failure | 3 | 3 |
| Atelectasis | 2 | 2 |
| Pulmonary edema | 3 | 2 |
| Neurologic | ||
| Peripheral motor neuropathy | 1 | 1 |
| Peripheral sensory neuropathy | 10 | 3 |
Clinically insignificant and correctable electrolyte abnormalities are not included in this list.
Clinical Responses
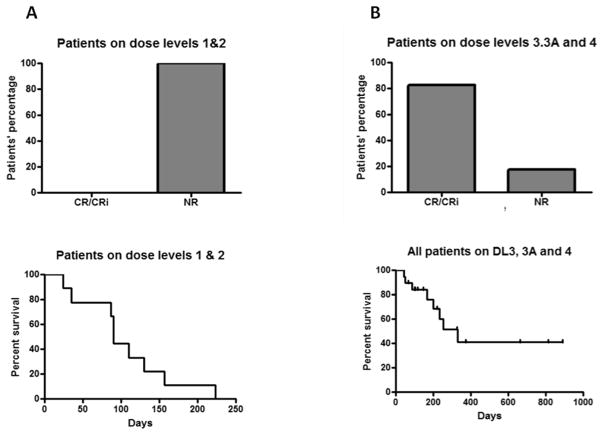
Of the 11 patients enrolled to DL1 (n=6) and DL2 (n=5), none achieved a CR/CRi, regardless of whether they were FLT3-ITD positive (n=3) or not. Three received 2 cycles of treatment while 8 patients completed only 1 cycle - 6 because of progressive disease and two withdrew consent to proceed with hospice. These patients were older (median age = 67; n=10) and had previously received intensive treatments (n=6). The remaining patients had refractory (n=2) or relapsed (n=3) disease after treatment with decitabine (see Table I).
Twenty-three patients enrolled on DL3 (n=3), DL3* (n=6), DL3A (n=8) and DL4 (n=6). By design these patients were younger with a median age of 53, and 21 of the 23 patients received induction chemotherapy with “7+3” based treatments. Two received either decitabine or 5-azacitidine as induction therapy. Of the 14 patients with relapsed disease, one relapsed after alloSCT. Two patients had relapsed refractory AML. The ORR was surprisingly high at 82.6% (95% CI: 61% to 95%), where only four patients did not respond to treatment. Thirteen achieved a CR (56.5%) and 6 achieved a CR with incomplete neutrophil and/or platelet recovery (26%).
Remissions occurred without any trend in relation to DL. Of those with relapsed disease and a CR1 duration of <12 months (n=9), 6 achieved a CR (66.7%) and 2 achieved a CRi (22.2%), an ORR of 88.8%. All five patients (100%) with a CR1 duration of >12 months achieved a CR (n=3) or CRi (n=2). Of those with primary refractory AML (n=9), 6 (66.7%) achieved a CR (n=4), or CRi (n=2). Of FLT3-ITD positive patients (n=6), four achieved a CR/CRi (66.7%); three of them had primary refractory disease following 7+3 based induction chemotherapy. Of FLT3-ITD negative patients (n=17), 15 (88.2%) achieved a CR/CRi.
Twelve of the 19 patients who achieved a CR/CRi proceeded to alloSCT. One received a donor lymphocyte infusion. Reasons for not proceeding to transplantation (n=6) included: performance status (n=1), early relapse (n=3), patient refusal (n=1) and acute renal failure requiring ongoing hemodialysis (n=1).
With a median follow-up of 131 days for patients who were alive, the median OS for those who received midostaurin, bortezomib and MEC was 330 days for all patients, 234 days for patients who did not proceed to alloSCT and was not reached for those who proceeded to an alloSCT (Figure 1).
Figure 1.
Disease response (upper panels) and survival (lower panels) A. Patients who received midostaurin and bortezomib (DLs 1 and 2); B. Patients who received midostaurin and bortezomib and MEC (DLs 3, 3*, 3A and 4). CR, complete remission; CRi, CR with incomplete count recovery (neutrophil or platelet); NR, non- responders
Correlative Studies
Only a minority of patients consented for correlative studies to assess miR-29b (measured as primary miR transcript), FLT3 and miR-155 expression levels at sequential time-points; in addition, some of the patients who did consent had inaspirable BM. Therefore, only 9 patients (4 on DL1/DL2 and 5 on DL3/DL3*/DL3A/DL4) had a full complement of the planned sequential samples (days 0, 3 and 8) available for RT-PCR analysis. As a result, any analyses of these data are felt to be exploratory and hypothesis-generating in nature.
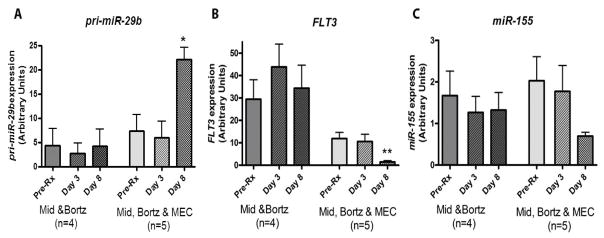
In patients on DLs1 and 2, no significant changes in the expression of FLT3 and miR-155 were noted. In patients enrolled on DLs 3, 3*, 3A and 4, there was a trend for increase of miR-29b (p=0.095) and significant decrease of FLT3 (p<0.001) and miR-155 (p=0.028) on day 8 compared to pretreatment baseline (Figure 2), likely related to the effect of the cytotoxic chemotherapy.
Figure 2.
Midostaurin, bortezomib combined with MEC upregulates pri-miR-29b, and downregulates FLT3 and miR-155 expression in AML patients. BM samples were collected prior treatment (Day 0) or posttreatment on Days 3 or 8 from patients receiving only midostaurin and bortezomib (Mid & Bortz) and those treated with midostaurin, bortezomib and MEC (Mid, Bortz & MEC). Samples were analyzed by quantitative RT-PCR for expression levels of (A) pri-miR-29b; (B) FLT3 mRNA; (C) miR-155. Data are normalized by the internal controls and presented as mean ± SE for each group of patients. *p<0.05; **p<0.01, when compared with the pretreatment basal level of each group.
Discussion
We report here a phase I trial of bortezomib, midostaurin and MEC chemotherapy in patients with relapsed or refractory AML. The trial was divided into two parts. In the first part patients received midostaurin and bortezomib to establish the safety of the combination. No DLTs were observed, thereby supporting that the combination of the TKI and proteasome inhibitor is feasible. However, meaningful clinical responses (CR or CRi) were not observed.
We then proceeded to the second part of the study where MEC chemotherapy was added. Patients enrolled to DL3 experienced dose-limiting peripheral and sensory neuropathy, therefore we changed the administration route of bortezomib from IV to SQ, and the number of doses decreased from 4 to 2 per cycle. Patients treated at DL3A and 4 did not experience neuropathy symptoms; however, we observed diarrhea possibly related to midostaurin and/or chemotherapy, as well as a decrease in ejection fraction in one patient that we deemed dose-limiting. Interestingly, DLTs experienced by the two cohorts were markedly different. Specifically, grade 3 peripheral neuropathy and diarrhea symptoms were observed only at DL3 and higher, despite the similar dose of midostaurin and bortezomib. This suggests that the potential toxicity of bortezomib and midostaurin was increased with the addition of cytotoxic chemotherapy.
Interestingly, however, we noted a surprisingly high ORR in this cohort with 82.5% of the patients achieving a CR (56.5%) or CRi (26%). Indeed, of 23 treated patients, only 4 failed to achieve a meaningful clinical response. The responses were seen both in younger (<60 years) and older (≥60 years) patients, and the vast majority of the responders (73%) had either prior refractory disease or a short CR1 duration. All patients with CR1 >12 months achieved CR/CRi. No preferential responses of FLT3-ITD-positive patients over FLT3-ITD-negative patients were noted. Interestingly, of four non-responders, 2 presented with the rare t(2;3)(q23;q21) either as sole abnormality or as part of a complex karyotype. Little is known about this translocation and how it can relate to treatment resistance.
Our initial hypothesis that bortezomib could enhance the pharmacologic activity of midostaurin and chemotherapy stemmed from data supporting bortezomib-dependent decrease in FLT3 and miR-155 expression. The limited number of samples available prevented us from reaching a definitive conclusion and were primarily hypothesis-generating. Nevertheless, we noted that bortezomib and midostaurin alone were ineffective in achieving these molecular endpoints. This could be related to a lack of sensitivity of refractory and relapsed disease in a subset of patients that were mainly older and highly pretreated. Interestingly, the molecular endpoints appeared to be achieved on day 8, when patients had already completed chemotherapy and bortezomib and midostaurin were either just administered or about to be started. Thus, based on the available data, the observed molecular changes were likely due to a chemotherapy-induced decrease in disease burden rather than the initial dosing with midostaurin and bortezomib.
Although preliminary, our results compare very well with those reported for MEC. Feldman et al. conducted a phase III trial of patients with relapsed or primary resistant AML randomized to MEC plus the anti-CD33 monocolonal antibody lintuzumab or MEC alone. [23] The ORR for the two arms combined was 32% and the median OS was 156 days. Here the CR and CRi rate (82.5%) and the median OS (330 days) in patients who received MEC with bortezomib and midostaurin was more than double those results. In a phase I trial of escalating doses of bortezomib in combination with MEC for patients with relapsed or refractory AML Advani et al observed a CR/CRi rate of 52%. [24]
Although our data are from a small number of patients, the clinical response is encouraging and it is tempting to speculate that the addition of bortezomib and/or midostaurin may have improved the clinical activity of MEC. Given the results from the RATIFY trial[12], it may be possible that midostaurin played more of a role in the clinical responses that were observed than bortezomib, although it is not possible to know this with certainty.
Despite the clinical activity of the regimen, the occurrence of expected toxicities may be challenging. While peripheral neuropathy could be virtually eliminated by changing the route and schedule of bortezomib or utilizing a new generation of proteasome inhibitors, diarrhea may be multifactorial (autonomic neuropathy, TKI, infections, chemo-induced enteritis) in this patient population and difficult to completely overcome. Nevertheless, it did not preclude 63% (n=12) of patients who achieved a CR or CRi and were optimal candidates from undergoing alloSCT.
In view of our results in this high-risk patient population, we concluded that bortezomib, midostaurin and MEC is an effective salvage regimen for patients with refractory/relapsed AML, but it may need to be optimized to reduce the incidence of diarrhea and peripheral neuropathy. Further studies with MEC and midostaurin alone may be explored in the future to evaluate this regimen.
Table II.
Clinical Responses in Dose Levels 3, 3*,3A, 4
| Dose Level | Number of Patients | Response |
|---|---|---|
| 3 | 3 | 2 CR 1 CRi |
| 3* | 6 | 4 CR 1 CRi 1 NR |
| 3A | 8 | 3 CR 3 CRi 2 NR |
| 4 | 6 | 4 CR 1 CRi 1 NR |
CR = Complete response; CRi = Complete response with incomplete count recovery; NR = No response
Acknowledgments
Midostaurin and bortezomib were provided by Novartis Pharmaceuticals and Millennium, respectively. Financial support for this study was provided by Novartis Pharmaceuticals.
This work was supported in part by the National Cancer Institute Grants No. CA101140 (GM), CA102031 (GM), CA140158 (GM, WB, AW), K12CA133250 (AW). AW is a scholar of the American Society of Hematology-Harold Amos Medical Faculty Development Program.
Footnotes
Conflict of Interest: None of the authors report a conflict of interest.
References
- 1.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29:475–486. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 3.Marcucci G, Yan P, Maharry K, et al. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32:548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 6.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19:1345–1349. doi: 10.1038/sj.leu.2403838. [DOI] [PubMed] [Google Scholar]
- 7.Fenski R, Flesch K, Serve S, et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol. 2000;108:322–330. doi: 10.1046/j.1365-2141.2000.01831.x. [DOI] [PubMed] [Google Scholar]
- 8.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 9.Ozeki K, Kiyoi H, Hirose Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 10.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–274. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 11.Rollig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 12.Stone RMS, Sanford B, et al. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (ind), High-Dose C Consolidation (consol), and As Maintenance (maint) Therapy in Newly Diganosed Acute Myeloid Leukemia (AML) Patients (pts) Age 18–60 with FLT3 Mutations (muts): An International Prospective Randomized (rand) P-Controlled Double-Blind Trial (CALGB 1063/RATIFY [Alliance]) Blood (ASH Annual Meeting Abstracts) 2015:6. [Google Scholar]
- 13.Liu S, Wu LC, Pang J, et al. Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell. 2010;17:333–347. doi: 10.1016/j.ccr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Liu Z, Xie Z, et al. Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood. 2008;111:2364–2373. doi: 10.1182/blood-2007-08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 16.Attar EC, Johnson JL, Amrein PC, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol. 2013;31:923–929. doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Aimiuwu J, Wang H, Chen P, et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood. 2012;119:5229–5238. doi: 10.1182/blood-2011-11-382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum W, Schwind S, Tarighat SS, et al. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood. 2012 doi: 10.1182/blood-2012-03-413898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mims A, Walker AR, Huang X, et al. Increased anti-leukemic activity of decitabine via AR-42-induced upregulation of miR-29b: a novel epigenetic-targeting approach in acute myeloid leukemia. Leukemia. 2013;27:871–878. doi: 10.1038/leu.2012.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau P, Coiteux V, Hulin C, et al. Prospective comparison of subcutaneous versus intravenous administration of bortezomib in patients with multiple myeloma. Haematologica. 2008;93:1908–1911. doi: 10.3324/haematol.13285. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 23.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 24.Advani AS, Elson P, Kalaycio ME, et al. Bortezomib + MEC (Mitoxantrone, Etoposide, Cytarabine) for Relapsed/Refractory Acute Myeloid Leukemia: Final Results of an Expanded Phase 1 Trial. 2014:978–978. [Google Scholar]