Abstract
Infection is the most common cause of mortality in early life, and immunization is the most promising biomedical intervention to reduce this burden. However, newborns fail to respond optimally to most vaccines. Adjuvantation is a key approach to enhancing vaccine immunogenicity, but responses of human newborn leukocytes to most candidate adjuvants, including most TLR agonists, are functionally distinct. Herein, we demonstrate that 3M-052 is a locally acting lipidated imidazoquinoline TLR7/8 agonist adjuvant in mice, which, when properly formulated, can induce robust Th1 cytokine production by human newborn leukocytes in vitro, both alone and in synergy with the alum-adjuvanted pneumococcal conjugate vaccine 13 (PCV13). When admixed with PCV13 and administered i.m. on the first day of life to rhesus macaques, 3M-052 dramatically enhanced generation of Th1 CRM-197–specific neonatal CD4+ cells, activation of newborn and infant Streptococcus pneumoniae polysaccharide–specific (PnPS-specific) B cells as well as serotype-specific antibody titers, and opsonophagocytic killing. Remarkably, a single dose at birth of PCV13 plus 0.1 mg/kg 3M-052 induced PnPS-specific IgG responses that were approximately 10–100 times greater than a single birth dose of PCV13 alone, rapidly exceeding the serologic correlate of protection, as early as 28 days of life. This potent immunization strategy, potentially effective with one birth dose, could represent a new paradigm in early life vaccine development.
The TLR7/8 adjuvant, 3M-052, which activates neonatal Th1 cytokine production, dramatically enhanced neonatal immunity to Streptococcus pneumoniae when combined with the alum-adjuvanted pneumococcal conjugate vaccine.
Introduction
Early life immunization is desirable, but vaccine-induced responses of newborns and young infants demonstrate slow initiation, low immunogenicity, and reduced persistence of functional antibodies (Abs) and cell-mediated responses (1). Although the majority of global immunization schedules are focused on the pediatric age group, development of early life vaccines has been hampered by this distinct immunity and an ad hoc approach in developing vaccines for adults prior to infant trials (2). By comparison to initiation of immunization in infancy, accelerated neonatal immunization strategies may be highly advantageous (3, 4) because (a) newborn vaccines achieve relatively high population penetration, as birth is the most reliable point of health care contact worldwide (5); (b) there is high risk of severe infection after very early life colonization; and (c) reduced vaccine responses can occur after bacterial colonization (6, 7). Adjuvantation is a key tool to enhance vaccine-induced immunity. Adjuvants can enhance, prolong, and modulate immune responses to vaccinal antigens to maximize protective immunity (8) and may potentially enable effective immunization in the very young (1). However, responses of human newborn leukocytes to most adjuvants, including most TLR agonists (TLRAs), are functionally distinct (2).
Considerations in selecting a clinically relevant adjuvanted vaccine formulation include (a) minimizing systemic inflammation (9), which can occur with TLRAs included in soluble or, to a lesser extent, with TLRA adjuvant-conjugated nanoparticle-based formulations (10, 11), and (b) ensuring activity toward the target population — not a forgone conclusion in newborns, given age-specific soluble and cellular factors (1) that shape distinct Th-mediated immunity (12), potentially limiting immune responses to vaccines and pathogens (13, 14). Our prior in vitro studies have suggested that, among the TLRAs, those that most effectively activate human newborn leukocytes are agonists of TLR7 and TLR8, a subfamily of endosomal leukocyte pattern recognition receptors (PRRs) that recognize uridine-rich single-stranded RNA molecules, as are found in viral RNA, and synthetic imidazoquinolines (IMQs) (15–17). The activity of TLR7/8As toward neonatal leukocytes suggests possible utility as neonatal vaccine adjuvants. For example, when conjugated to particulate vaccinal antigen, free resiquimod (R848) can have adjuvant activity in nonhuman primates (NHPs) immunized in the first month of life, but with noticeable systemic inflammation (10). In addition, to our knowledge, no studies have addressed whether TLRA adjuvantation of common aluminum salt–adjuvanted (alum-adjuvanted) conjugate vaccines — key to the pediatric immunization schedule — is feasible and effective at birth (i.e., the first 24 hours of life), a key point of global healthcare contact (18), during which the immune system is most distinct (1). To test the hypothesis that agents activating human neonatal leukocytes in vitro would also be active in newborns at birth in vivo, we undertook a rational vaccine design approach, employing a TLR7/8A adjuvant. We examined 3M-052, a locally acting lipidated IMQ TLR7/8A that can induce tumor-specific immunity by forming agonist depots for a gradual sustained release (19).
Immunization approaches that lead to more rapid and early protection against pneumococcus would be highly advantageous (20). Pneumococcus is an important pediatric pathogen comprising ≥92 different capsular polysaccharide serotypes that cause serious invasive disease, including meningitis, sepsis, otitis media, and pneumonia; it is responsible for approximately 10% of worldwide deaths in children less than 5 years of age (21). The poor efficacy of plain polysaccharide vaccines in young children prompted the development of pneumococcal conjugate vaccines (PCVs) that induce T cell–dependent mechanisms (22), with a recommended 3- to 4-dose schedule starting at 2 months of age (23). However, PCV-induced protection may not be fully achieved until completion of the recommended vaccination schedule (12–18 months of life) (20), and the inclusion of alum, though safe and effective, appears to be Th2 polarizing (24) and results in a formulation that requires multiple doses prior to achieving protective Ab titers. In this context, we have selected PCV13, which protects against Streptococcus pneumoniae, as a model vaccine to adjuvant, because (a) PCV13 is a well-studied vaccine with known correlates of protection, which allows clear and unambiguous evaluation of our adjuvantation strategy; (b) current PCVs can prevent severe disease in older children and offer newborns some indirect herd protection, but newborns are not directly protected; (c) pneumococcal diseases strikes in early life, particularly in resource-poor countries, such as Papua New Guinea, making a vaccine that provides rapid protection in early life desirable (3, 4); and (d) although studies of PCV7 immunization, with a 3-dose schedule starting at birth, induced protective serum Ab concentrations in human infants as early as 18 weeks (4.5 months), neonatal hyporesponsiveness was noted for several vaccine serotypes, as compared with infants starting a 3-dose schedule of PCV7 at 2 months of life (24, 25).
Here, 3M-052 induced robust Th1 cytokine production by human newborn and adult leukocytes in vitro, both alone and in synergy with alum-adjuvanted PCV13. Using a potentially clinically relevant neonatal rhesus macaque model, we demonstrate that, when admixed with PCV13 and administered i.m., 3M-052 dramatically accelerated and enhanced neonatal B and T cell immune responses, rapidly amplifying functional serotype-specific Ab titers to concentrations that correlate with protection after the first dose of a 3-dose series (day of life 0 [DOL0], DOL28, and DOL56), without serious adverse effects. This rational design approach to identify adjuvants active toward distinct populations may be broadly applicable, potentially closing the window of vulnerability to infections in early life.
Results
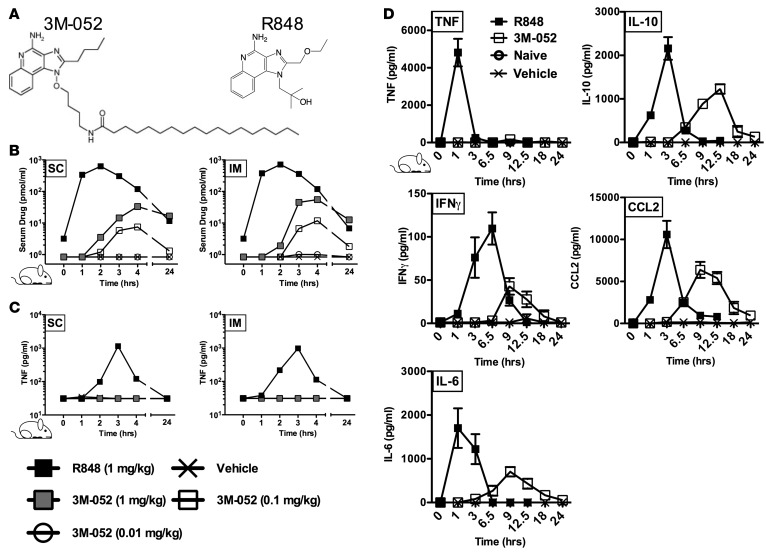
3M-052 is a locally acting TLR7/8 agonist.
Key to preventing excessive adjuvant reactogenicity, including fever and malaise, is avoiding systemic adjuvant distribution and consequent peripheral cytokine induction (9). We therefore selected 3M-052, a TLR7/8A that bears a C18 lipid moiety (11) that serves to localize its action. We conducted rodent pharmacokinetic (PK) and pharmacodynamic (PD) studies to compare the IMQ TLR7/8A R848 and its lipidated congener 3M-052 (Figure 1A), which can be formulated in an oil-in-water (O/W) emulsion vehicle (Supplemental Table 1; supplemental material available online with this article; doi:10.1172/jci.insight.91020DS1). Distinct PK differences were observable by measurement of R848 or 3M-052 serum drug levels determined by liquid chromatography–mass spectrometry (LC-MS/MS) before and after a single i.m. (into quadriceps) or s.c. (into scruff of neck) administration (Figure 1B). One hour after i.m. or s.c. injection in rats (both 1 mg/kg), serum R848 concentrations were >1,000 pmol/ml, while only a 4-hour peak of approximately 50 pmol/ml of 3M-052 was detected at equal treatment dosages. After a 1 mg/kg dose, less than 1 pmol/ml of 3M-052 was detectable in serum at 24 hours after dose, while a 100-fold lower dose of 3M-052 (0.01 mg/kg) was not detectable in serum up at any time. Blood TNF concentrations mirrored drug PK patterns, with a peak of approximately 1,000 pg/ml detectable in the R848-treated group and no induced systemic TNF detectable in any of the 3M-052–treated groups (Figure 1C). The relative PKs of R848 and 3M-052 were further characterized in mice that received a single s.c. dose of R848 or 3M-052 (each ~0.1 mg/kg) (Figure 1D). These studies confirmed the serum TNF PK observations in rats and demonstrated distinct serum and inducible mRNA expression kinetics between R848 and 3M-052 for IL-6, IL-10, IFN-γ, and CCL2 (Figure 1D) in lymph node tissue (Supplemental Figure 1A) and splenocytes (Supplemental Figure 1B). In all cases, cytokine mRNA expression peaked at 1–3 hours after R848 administration and at 6 hours after 3M-052 administration. s.c. administration of 3M-052 induced little if any cytokine mRNA expression but robust IFN-related and TLR7 gene expression in the spleens of treated mice (Supplemental Figure 1, A and B). Compared with R848, 3M-052 thus demonstrated reduced systemic distribution to the blood/serum, with induction of responses in the spleen suggesting confinement of this adjuvant to local and lymphatic leukocytes. Overall, although focused on adult and not newborn rodents, these data support the concept that 3M-052 has distinct advantages over R848 by being a locally acting adjuvant in vivo that avoids extensive systemic distribution and consequent induction of systemic inflammation.
Figure 1. Unlike R848, the lipidated, locally acting TLR7/8 agonist 3M-052 demonstrates low serum distribution and little systemic cytokine induction.
(A) The structure of R848 (resiquimod) and 3M-052, a TLR7/8 activating imidazoquinoline bearing a C18 lipid moiety and designed for slow dissemination from the site of injection. (B and C) Rodent pharmacokinetic and pharmacodynamic studies. Serum drug levels were measured by LC-MS/MS at the indicated times before or after dose in rats following a single i.m. (into quadriceps) or s.c. (into scruff of neck) administration of 3M-052 or R848 formulated in oil-in-water (O/W) emulsion (vehicle). The results represent median serum drug levels and induced TNF at each time point for each dose (n = 5). (D) Evaluation of s.c. mouse serum cytokine kinetics after a single dose of 3M-052 or R848 (both 1 mg/kg, 20 μg/mouse) formulated in O/W emulsion (vehicle) (n = 3).
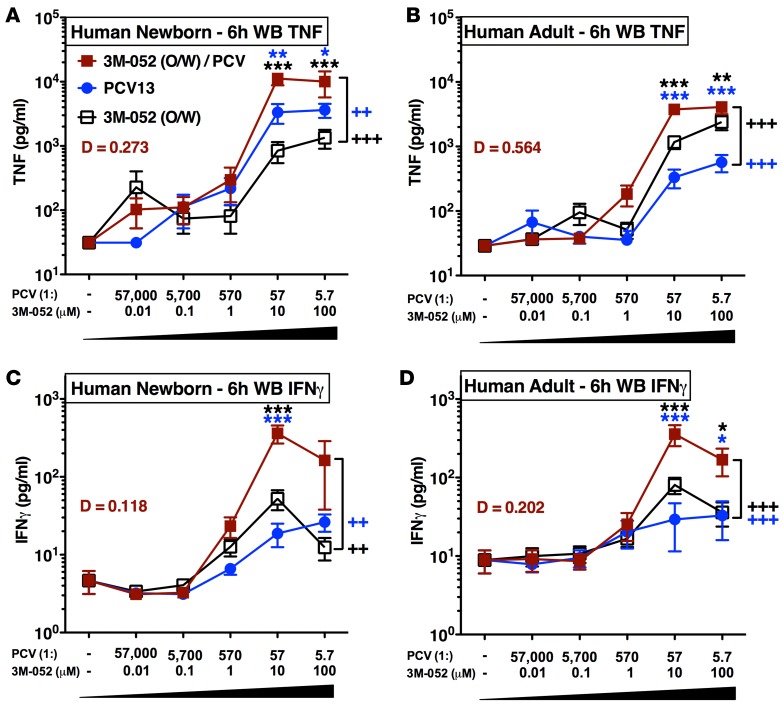
3M-052 enhances type 1 immunity in vitro.
To confirm activity of our adjuvanted vaccine formulation toward neonatal leukocytes, we characterized cytokine-inducing activity of 3M-052 alone or in combination with the FDA-approved alum-containing 13-valent PCV (PCV13; Prevnar 13, Pfizer subsidiary, Wyeth Pharmaceuticals) in vitro. We tested the ability of the alum-containing PCV13, 3M-052, and the admixed formulation 3M-052 + PCV13 to induce concentration-dependent cytokine production in human neonatal and adult blood (Figure 2). As combining adjuvants such as alum (in the PCV13 formulation) and 3M-052 may have antagonistic, additive, or synergistic effects, we tested combinations of PCV13 + O/W (no 3M-052) or PCV13 + O/W formulated with 3M-052 (i.e., PCV13 + 3M-052) at multiple concentrations. Both PCV13 and O/W-formulated 3M-052 alone activated neonatal and adult blood in a concentration-dependent manner, significantly inducing production of TNF over baseline (n = 12, P < 0.001). The vehicle control did not induce TNF or IFN-γ production at any concentration tested (data not shown). Of note, 3M-052 + PCV13 synergistically induced TNF (n = 12) and IFN-γ (n = 10) in both newborn cord and adult peripheral blood (Figure 1 and Supplemental Figure 2, A and B). Synergy between 3M-052 and the alum-adjuvanted PCV13 was of greatest magnitude in neonatal blood (TNF, with a synergy measure D = 0.273; IFN-γ D = 0.118). The synergistic effects of 3M-052 + PCV13 were mainly restricted to Th1-polarizing cytokines TNF and IFN-γ, with some evidence of enhanced neonatal IL-12p70 and IL-6 as well (Supplemental Figures 3 and 4). These human in vitro whole blood data were consistent with the ability of 3M-052 to act in combination with alum to induce a mixed Th1/Th2-response and enhance influenza hemagglutinin-specific IgG2a production when administered to mice in vivo (Supplemental Figure 5 and 6).
Figure 2. 3M-052 synergistically enhances type 1 immunity from newborn leukocytes when combined with pneumococcal conjugate vaccine in vitro.
Human neonatal and adult blood cultured in vitro for 6 hours with buffer control (RPMI), oil-in-water (O/W) vehicle, PCV13 alone (1:5.7 to 1:57,000 v/v), 3M-052 alone (0.01, 0.1, 1, 10, 100 μM), or combinations of each. The dashed line indicates the vehicle control. Supernatants were collected for ELISA and multiplex assay, TNF (A and B, n = 12) and IFN-γ (C and D, n = 10). For comparisons between overall groups [e.g., (PCV13 + 3M-052) vs. PCV13], 2-way repeated-measures ANOVA for nonparametric sample populations were applied, ++P < 0.01, +++P < 0.001. For comparison at individual concentrations, unpaired Mann-Whitney test was applied at each time point, *P < 0.05, **P < 0.01, ***P < 0.001. Results represent mean ± SEM. Level of synergy was calculated using an adapted Loewe definition of additivity (D > 1: antagonism, D = 1: additivity, D < 1: synergy).
Addition of a TLR7/8A accelerates neonatal serotype-specific Ab responses to PCV13.
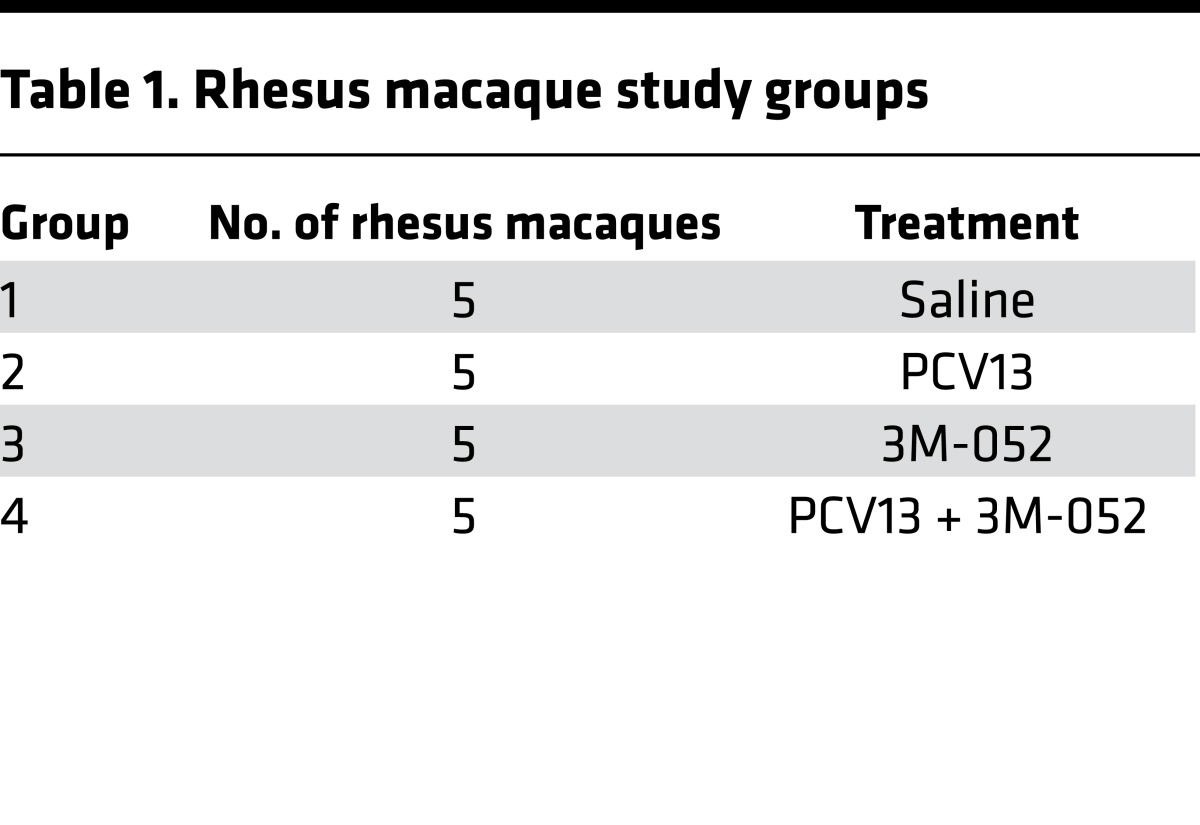
Having demonstrated a low reactogenicity potential of 3M-052 in vivo as well as a high Th1/Th2-polarizing activity toward human neonatal leukocytes in vitro and mice in vivo, we next assessed the effect of 3M-052 on PCV13 immunogenicity in neonatal animals. As human TLR8 is structurally and functionally divergent from murine TLR8, but is closely similar to monkey TLR8 (26), we conducted our study in NHPs (Indian origin rhesus macaques, Macaca mulatta). We have also previously demonstrated that compared with agonists of other TLRs, TLR8 agonists induce robust production of TNF and IL-1β in whole blood of neonatal and infant rhesus macaques assayed in vitro (15). Most studies to date investigating immunogenicity of PCVs in human or NHP neonates have employed half of the recommended human infant dose (24, 25, 27). Therefore, 4 cohorts of 5 rhesus macaques per cohort (Table 1) were immunized i.m. with saline (control), 3M-052 adjuvant alone (0.1 mg/kg 3M-052, or 40 μg per animal), a half dose of PCV13 alone, or a half dose of PCV13 admixed with 3M-052 PCV13 + 0.1 mg/kg 3M-052. All treatments began with a birth dose (DOL0), followed by booster doses at 1 (DOL28) and 2 months (DOL56) of life (Figure 3A). Peripheral blood was collected at the indicated time points to obtain plasma for an assay of antipneumococcal serotype Ab titers by polysaccharide-IgG binding microarray (Supplemental Table 2).
Table 1. Rhesus macaque study groups.
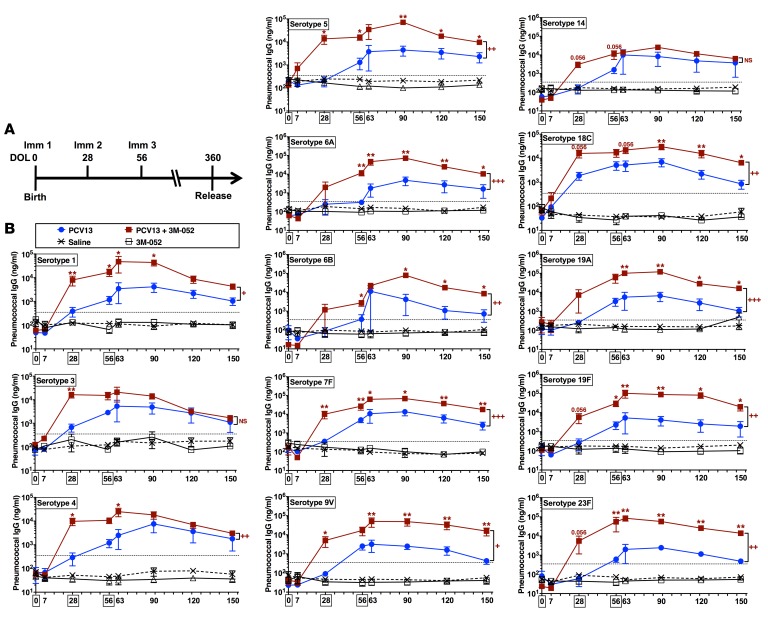
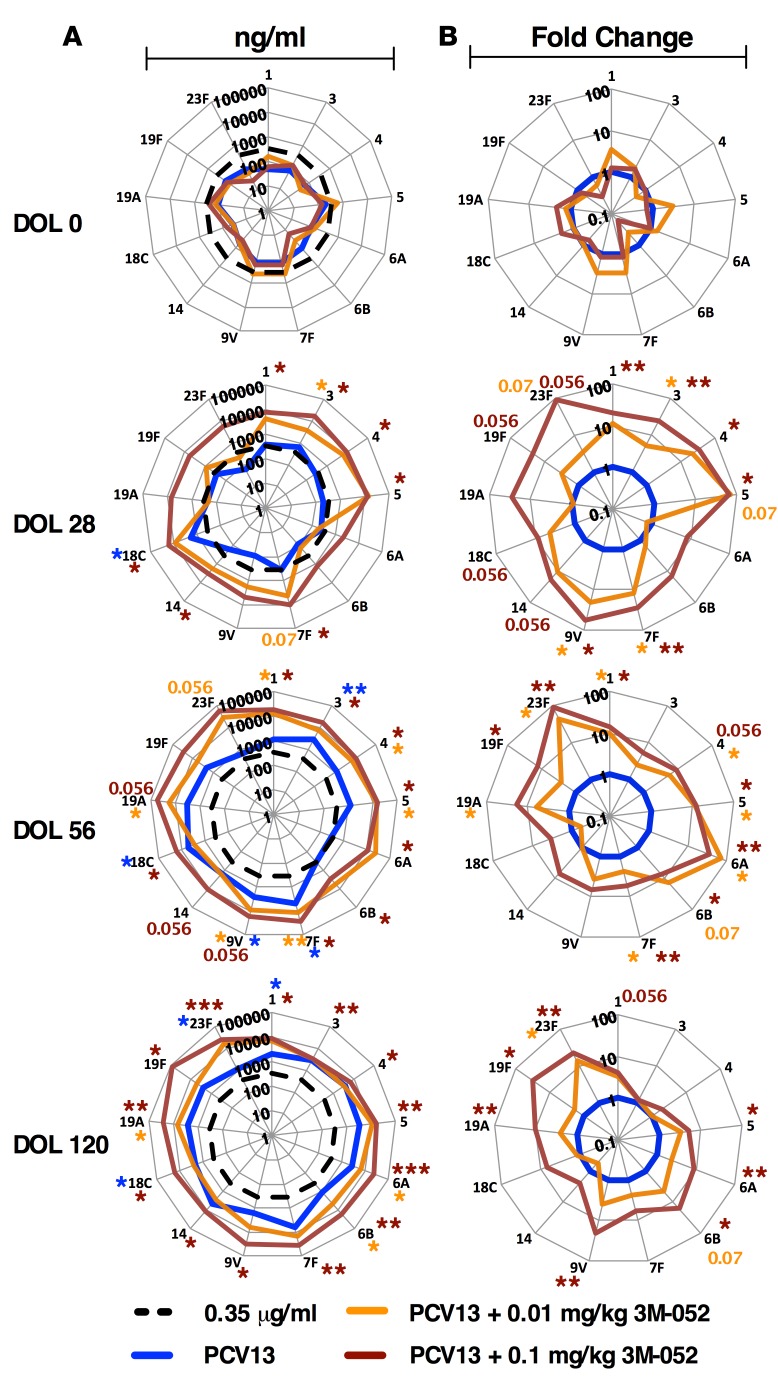
Figure 3. Addition of a TLR7/8 agonist accelerates neonatal serotype-specific antibody responses to PCV13.
(A) Rhesus macaque enrollment/immunization timeline. Neonatal and infant rhesus macaques were immunized at day of life 0 (DOL0), DOL28, and DOL56 (the 3 immunization time points are indicated by boxes) with either PCV13 alone or PCV13 coadministered with 3M-052 (a lipidated TLR7/8A). Peripheral blood was collected at the indicated time points for measurement of antipneumococcal serotype titers by polysaccharide-IgG binding microarray. (B) A total of 13 tested serotypes; n = 5 infants per group. Horizontal broken line indicates the WHO-recommended reference Ab concentration of IgG used as a correlate of protection levels in humans (0.35 μg/ml). Colored numbers refer to P values approaching significant for that group. For comparisons between overall groups [e.g., PCV13 vs. (PCV13 + 3M-052)], 2-way repeated-measures ANOVA for nonparametric sample populations were applied, +P < 0.05, ++P < 0.01, +++P < 0.001, or NS (not significant). For comparison at individual time points [e.g., PCV13 vs. (PCV13 + 3M-052) at DOL28], unpaired Mann-Whitney test was applied at each time point, *P < 0.05, **P < 0.01. Results represent mean ± SEM.
After a single immunization, PCV13 alone failed to induce antipneumococcal serotype Ab responses above 0.35 μg/ml (Figure 3B), the WHO’s reference IgG Ab concentration that is a correlate of protection in humans (28). In marked contrast, PCV13 adjuvanted with 3M-052 dramatically induced robust Ab responses as early as DOL28 (Figure 3B). Remarkably, a single dose at birth of PCV13 + 0.1 mg/kg 3M-052 induced pneumococcal polysaccharide–specific (PnPS-specific) IgG responses that were approximately 10–100 times greater than a single dose at birth of PCV13 alone (Figure 3B and Figure 4). These Ab responses significantly surpassed the WHO-recommended minimal protective Ab concentrations (0.35 μg/ml) for 7 serotypes (Figure 4A) and 6 serotypes as compared with PCV13 alone (Figure 4B).
Figure 4. In vivo adjuvanticity of 3M-052 is dose dependent.
Radar plot analysis of all 13 serotypes tested, including raw ng/ml (A) and fold-change analysis (B) at day of life 0 (DOL0), DOL28, DOL56, and DOL120. After 3 doses of (PCV13 + 3M-052), all immunized infants significantly exceeded protection level for all 13 serotypes tested. Broken black lines indicate the WHO-recommended reference. Colored asterisks refer to P values approaching significant for that group. n = 5 per infants per group for PCV13 and PCV13 + 0.1 mg/kg 3M-052, n = 3 infants per group for PCV13 + 0.01 mg/kg 3M-052. For comparison at individual time points [e.g., PCV13 vs. (PCV13 + 3M-052) at DOL28], unpaired Mann-Whitney test was applied at each time point, *P < 0.05, **P < 0.01, ***P < 0.001. Results represent mean ± SEM.
In vivo adjuvanticity of 3M-052 is dose dependent.
Next, we evaluated the PD immunogenicity range of 3M-052 by repeating our immunization schedule, but with a 10-fold reduced dose. Two additional cohorts of 3 rhesus macaques per cohort were immunized i.m. with the lower dose of 3M-052 adjuvant alone (0.01 mg/kg 3M-052, or 4 μg per animal) or PCV13 admixed with the lower dose of 3M-052 (PCV13 + 0.01 mg/kg 3M-052). As before, all treatments began with a birth dose (DOL0), followed by booster doses at 1 (DOL28) and 2 months (DOL56) of life and peripheral blood collection for downstream analysis (Supplemental Table 2). DOL28 Ab responses to PCV13 adjuvanted with the lower dose of 3M-052 significantly surpassed the WHO-recommended minimal protective Ab concentrations (0.35 μg/ml) for serotype 3 (Figure 4A) and serotypes 3, 7F, and 9V as compared with PCV13 alone (Figure 4B). Subsequent boosting immunizations on the second and third months enhanced the Ab responses in all groups receiving PCV13. However, while PCV13 + 0.01 mg/kg 3M-052 showed significantly enhanced responses to serotypes 6A, 6B, and 19A, only the PCV13 + 0.1 mg/kg 3M-052 group demonstrated significantly elevated anti-PnPs Ab responses to all 13 serotypes by DOL120 (Figure 4A and Supplemental Figure 7), demonstrating that TLR7/8A adjuvant dosage can be used to determine a therapeutic window of enhanced immunogenicity.
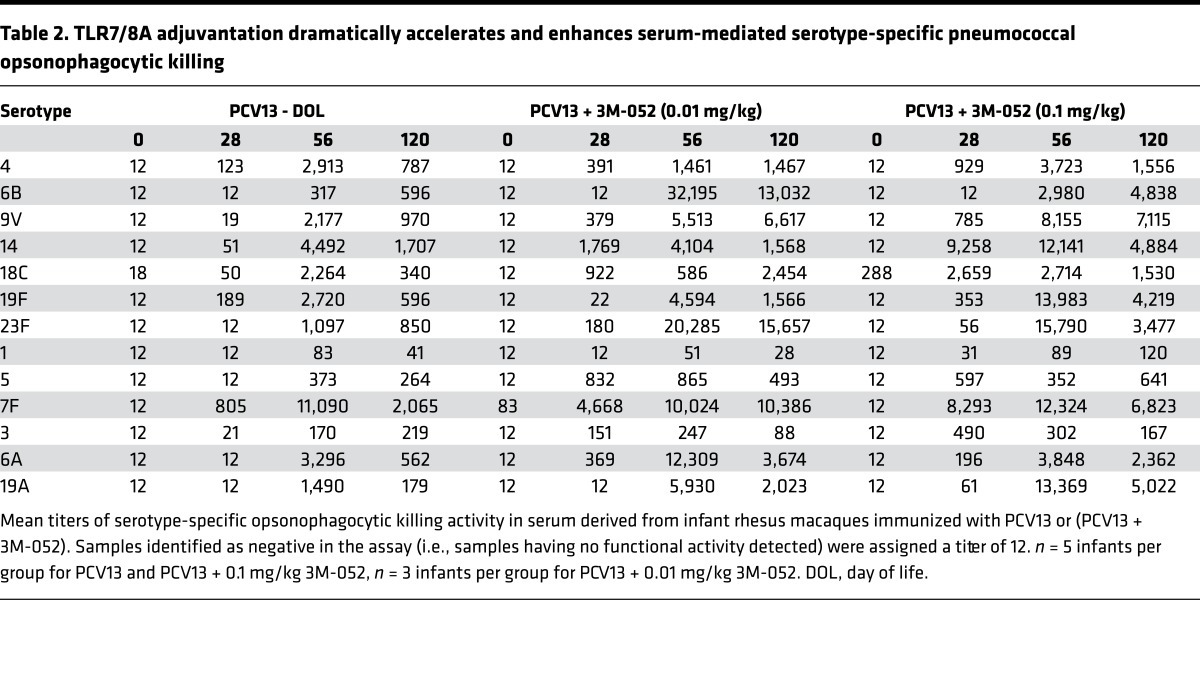
TLR7/8A adjuvantation dramatically accelerates and enhances serotype-specific pneumococcal opsonophagocytic killing.
To further characterize humoral immunity to the vaccine formulation, DOL0, DOL28, DOL56, and DOL120 sera from neonatal and infant rhesus macaques immunized with PCV13 alone, PCV13 + 0.01 mg/kg 3M-052 or PCV13 + 0.1 mg/kg 3M-052 were examined in a pneumococcal opsonophagocytosis assay. As expected, all rhesus macaque serum samples had no opsonic activity toward all 13 pneumococcal serotypes evaluated at birth (Table 2 and Supplemental Figure 8), with a single outlier animal having a slightly increased baseline over undetectable levels for serotype 18C. All PCV13 + 0.1 mg/kg 3M-052–immunized rhesus macaques demonstrated functional Ab responses to all 13 PS serotypes contained in PCV13. Consistent with the striking observations seen for PnPS-specific IgG titers, functional Ab-mediated responses were dramatically accelerated in animals receiving a single dose of (PCV13 + 3M-052), i.e., PCV13 adjuvanted with either dose of adjuvant. All animals receiving PCV13 + 0.1 mg/kg 3M-052 demonstrated a robust functional Ab activity to 11 of the 13 serotypes tested by DOL28, with opsonization indices approximately 10–100 greater than PCV13 alone (Table 2). In addition, for animals receiving PCV13 + 0.1 mg/kg 3M-052, serum opsonophagocytic activity at DOL120 (after immunization 3) was 2-fold (for serotypes 4, 14, 1, 5, and 7F), 4-fold (for serotypes 18C and 6A), or 10-fold (for serotypes 6B, 9V, 19F, 23F, and 19A) greater than that of animals receiving PCV13 alone (Supplemental Figures 8 and 9). Opsonophagocytic killing activity correlated with accelerated serotype-specific Ab responses to (PCV13 + 3M-052) (Supplemental Figure 10–12).
Table 2. TLR7/8A adjuvantation dramatically accelerates and enhances serum-mediated serotype-specific pneumococcal opsonophagocytic killing.
3M-052 has limited systemic activity in neonatal and infant primates.
Our study included assessments of the general health of enrolled animals as well as detailed monitoring for potential local and systemic reactogenicity. During the entire study period through DOL360, no serious adverse effects (29) were observed in any of the enrolled animals. Weight curves were documented throughout the course of the study, as weight is a sensitive indicator of infant well-being. Weight gains were similar in all treatment groups (Supplemental Figure 13A). Similarly, fever was not reported up to DOL150 in all treatment groups, with the only significantly minor change in body temperature (a 0.8°C increase [n = 5, P = 0.05]) observed in the PCV13 + 0.1 mg/kg 3M-052 treatment group 48 hours after the third immunization (Supplemental Figure 13, B and C). During the second of two birthing/enrollment seasons, 3 cohoused 3M-052– or (PCV13 + 3M-052)–treated infant animals presented with a transient and mild maculopapular rash. Complete blood counts are shown in Supplemental Table 3. When present, erythema (redness) at the site of injection was localized and mild to moderate. For the second immunization, the diameter of injection site erythema at 48 hours after i.m. injection was significantly greater in the (PCV13 + 3M-052) treatment groups (P = 0.05; Supplemental Figure 14, A and B) but not significant for any of the other treatment conditions or time points evaluated to DOL63. In all animals with localized erythema, this erythema resolved fully with no visible sequelae. In line with our murine studies, and in accordance with its chemical design as a hydrophobic/locally acting adjuvant, 3M-052 administration with or without PCV13 did not induce systemic cytokine induction in neonatal/infant rhesus macaques over the first 63 days of life (Supplemental Figure 15A) or 48 hours after a single dose of PCV13 + 0.01 mg/kg 3M-052 (DOL30) (Supplemental Figure 15, B–D).
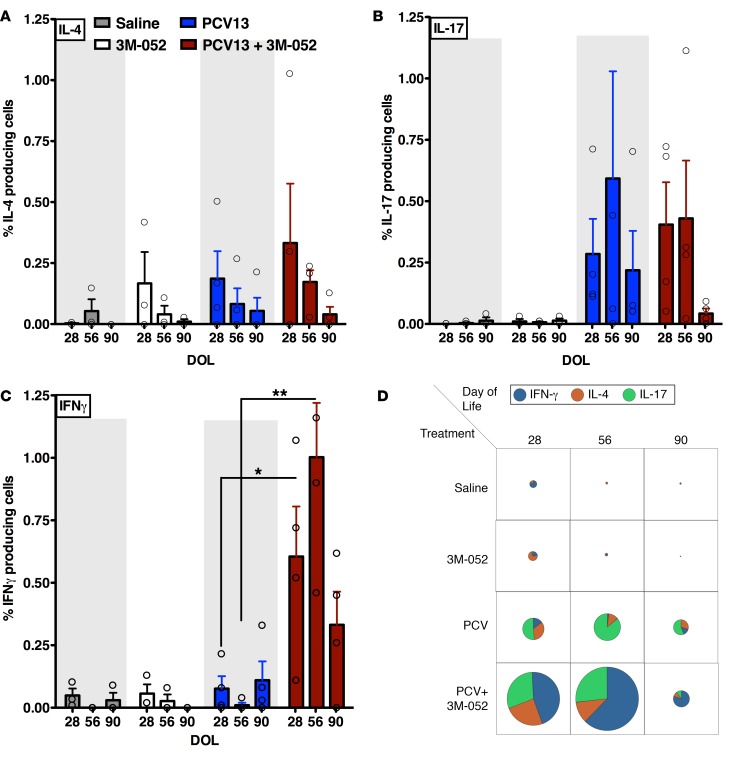
Unlike PCV13, (PCV13 + 3M-052) markedly enhanced Th1 CRM-197–specific neonatal CD4+ cells.
A methodology for the evaluation of vaccine-specific B and T lymphocytes from peripheral blood was developed for this study, driven by the fact that only limited volumes of blood can be obtained from infant macaques. Briefly, peripheral blood mononuclear cells (PBMCs) were sorted to obtain highly pure populations of B cells, T cells, and monocytes that were used for the evaluation of antigen-specific vaccine-induced B and T cells in infant rhesus macaques by means of enzyme-linked immunospot (ELISPOT) and intracellular cytokine staining assays, respectively. Cryopreserved PBMCs obtained at DOL28, DOL56, and DOL90 were used for restimulation in vitro, as outlined in Supplemental Figure 16. Autologous monocytes were differentiated to monocyte-derived dendritic cells (MoDCs), and after treatment of MoDCs with CRM197 (the protein component of PCV13), cells were cocultured with neonatal and infant CD4+ T cells for 10 days (Supplemental Figure 16A). CRM197-specific CD4+ T cells were quantified to determine the proportion of memory cells producing IL-4, IL-17, or IFN-γ (Figure 5, A–C). We observed similar numbers of CRM197-specific IL-17 memory CD4+ T cells, associated with reduced pneumococcal carriage, in response to PCV13 and PCV13 + 0.1 mg/kg 3M-052 (Figure 5, B and D). Remarkably, by DOL28, animals that received PCV13 + 0.1 mg/kg 3M-052 at birth had significantly higher proportions of CRM197-specific memory CD4+ T cells producing IFN-γ than those who received PCV13 alone (~0.61% vs. ~0.08%, P = 0.04) (Figure 5C). Indeed, the IL-17+/IL-4+/IFN-γ+ ratio in the (PCV13 + 3M-052) group was approximately 1:0.82:1.49 vs. approximately 1:0.65:0.27 for PCV13 (Figure 5D). We also observed a significant response for DOL56 (~1.01% vs. ~0.01%, ratio ~1:0.40:2.33 vs. ~1:0.14:0.02, P = 0.018), and a similar trend for DOL90 (~0.33% vs. ~0.11%, ratio ~1:0.25:0.50 vs. ~1:0.9:7.81, P = 0.08) (Figure 5, C and D).
Figure 5. (PCV13 + 3M-052) activates both Th17 and Th1 CRM-197–specific CD4+ cells.
(A–C) Percentage of IL-4–, IL-17–, and IFN-γ–producing CRM197-specific CD4+ T cells after ex vivo recall assays with CRM197-pulsed autologous rhesus DCs. (D) Pie charts representing scale and frequencies of cytokine-producing CRM197-specific CD4+ T cells, indicating that neonatal PCV13 alone treatment enhances CRM197-specific Th17 responses, while (PCV13 + 3M-052) enhances and accelerates a mixed Th1/Th17 response. For comparison at individual time points, the unpaired Mann-Whitney test was applied, *P < 0.05, **P < 0.01. Results represent mean ± SEM (n = 3–4).
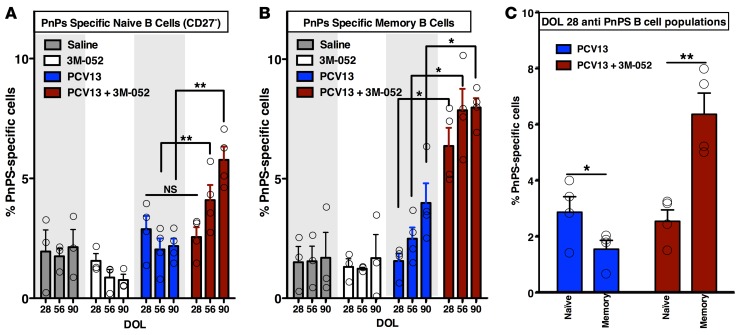
3M-052 enhances and accelerates activation of early life PnPS-specific B cells.
Mirroring its enhancement of PCV-specific Ab and T cell responses, when compared with PCV13 alone, inclusion of 3M-052 also significantly enhanced frequencies of PnPs-specific CD20+CD27– naive B cells (Figure 6A and Supplemental Figure 16B) and CD20+CD27+ memory B cells (Figure 6B) on DOL56 and DOL90. Of note, the switch from naive to a memory phenotype occurred much earlier in life for the (PCV13 + 3M-052)–immunized animals (DOL28) than for the PCV13 alone group (DOL56). In fact, DOL28 PnPs-specific memory cells were significantly elevated in the (PCV13 + 3M-052) treatment group and significantly lower for PCV13 alone (Figure 6C). Both the quantity and quality (avidity) of 4, 6B, 14, and 23F serotype-specific (Supplemental Figure 17, A and B) and total (Supplemental Figure 17C) anti–S. pneumoniae capsular polysaccharide Abs by ELISA and electrochemiluminescence support these observations. These data not only confirm the earlier increase in PnPs-specific Ab titer in the (PCV13 + 3M-052) treatment groups, but also demonstrate a trend of increased Ab avidity, which, together with an earlier switch in B cell memory phenotype observed by ELISPOT, indicates that 3M-052 accelerated and enhanced B cell activation (Supplemental Figure 17D). Indeed, addition of 3M-052 to PCV13 was associated with increased infiltration of CD68+ cells (i.e., monocytes/macrophages) into the injection site muscle (Supplemental Figure 18).
Figure 6. 3M-052 enhances and accelerates activation of early life PnPS-specific B cells.
(A and B) Antipneumococcal polysaccharide (PnPS) IgG/IgM-producing rhesus naive and memory B cell quantification. Higher frequencies of PnPS-specific B cells were noted in the (PCV13 + 3M-052) animals vs. the PCV13 group (n = 3–4). (C) Coadministration of 3M-052 with PCV13 to newborn rhesus macaques dramatically accelerated the transition of anti-PnPS B cells from naive to memory phenotype. Antipneumococcal polysaccharide (PnPS) IgG-producing naive (CD27–) and memory (CD27+) B cells were measured by flow cytometry. At day of life 28 (DOL28), the switch from naive to memory phenotype occurred earlier in (PCV13 + 3M-052)– vs. PCV13 only–immunized animals. Nonspecific polysaccharide in vitro activation in the control conditions (i.e., saline or 3M-052 alone) or specific CRM-197 treatment of B cells from all treatment groups never exceeded approximately 1.0%. For comparison at individual time points, the unpaired Mann-Whitney test was applied, *P < 0.05, **P < 0.01. Results represent mean ± SEM (n = 4 per group).
Discussion
Until the end of the 20th century, vaccine adjuvantation was largely limited to the use of alum (30). Over the past 20 years there has been explosive growth of information regarding PRRs that can activate leukocytes and thereby enhance immune responses. In parallel, a growing menu of adjuvants is now becoming available to immunologists and vaccinologists (31). Newborns and young infants demonstrate distinct immune responses, are at the greatest risk of infection of any age group, and receive most vaccines; yet to date, adjuvanted vaccine development programs have usually not systematically selected or optimized adjuvants for use in early life. Therefore, many vaccine formulations produce distinct and potentially suboptimal responses in the very young. A number of adjuvanted, including live (self-adjuvanted), vaccines induce relatively robust immunogenicity in early life: (a) in mice, measles vaccines employing DNA (32) and a live-replicating attenuated strain of Listeria monocytogenes (33) induced early protection; (b) in infant rhesus macaques, liposome adjuvant/replicon particles induce anti-measles immunity (34); and (c) in human newborns, Bacille Calmette Guérin, a live-attenuated Mycobacterium bovis vaccine that activates multiple PRRs induces robust Th1 responses at birth (35). However, to our knowledge, a molecular adjuvant that can help induce adult-level immunogenicity/protection in newborn primates had yet to be described.
An increased appreciation of immune ontogeny may inform future research and design of age-specific vaccine formulations. Accordingly, and as neither rodent models nor adult human leukocytes accurately model human newborn and infant responses (1), we focused on a candidate early life TLR7/8 adjuvantation system that was active toward human newborn leukocytes in vitro coupled with in vivo evaluation in an animal species (Macaca mulatta) that expresses TLR8 that is structurally and functionally similar to its human counterpart (15). In vitro modeling identified 3M-052 as a lipidated TLR7/8A adjuvant that both alone and in synergy with alum induced Th1 cytokine responses at birth and that when administered with alum-adjuvanted PCV13 in vivo dramatically accelerated and enhanced neonatal antigen-specific immunogenicity after a single immunization. Moreover, 3M-052 synergistically enhanced type II IFN and Th1-polarizing human cord blood cytokine production in response to PCV13 in vitro and dramatically accelerated S. pneumoniae antigen-specific neonatal rhesus macaque B and Th1 cell responses ex vivo. The ability of 3M-052 to enhance and accelerate activation of anti–PnPs-IgG (PnPS-specific) B cells, Th1-polarized CRM197-specific CD4+ T cells, and synergistically activated type II IFN responses in vitro shares similarity with the immune-polarizing effects of systemic viral infection (36) and signatures of bacterial viability (37), highlighting the potential of TLR7/8-triggered pathways to fundamentally shape immune responses (38, 39), especially vaccinal antigen-specific IFN-γ–producing T cells in early life (10). In light of the ability of 3M-052 to prime at a high dose (0.1 mg/kg) and boost at a lower dose (0.01 mg/kg), it is possible that (PCV13 + 3M-052) may enhance induction of extrafollicular B cell responses (40). Indeed, we speculate that vaccine adjuvantation with agonists of TLR7/8, PRRs key to detecting microbial RNAs, may induce a response that more closely resembles natural infection, with live pneumococci inducing Th1/Th17-polarized cell-mediated immunity (41) and supporting Tfh cell differentiation circumventing the neonatal inhibitory milieu and T cell–intrinsic factors and thereby enabling early life germinal center B cell responses (42). We hypothesize that the trend toward reduced numbers of blood circulating CRM197-specific IL-17 memory CD4+ T cells at day 90 in the (PCV13 + 3M-052) immunized animals may reflect migration of pneumococcal-specific IL-17 cells to mucosal sites where they may play roles in reducing pneumococcal carriage (43). Of note, 3M-052 + PCV13 expanded atypical PnPS-specific CD27– B cells, also known as “naive-mature B cells,” thought to represent direct precursors to multiple phenotypically distinct memory B cells, including germinal center–dependent Ab-secreting memory B cells with relatively high proliferation and somatic hypermutation (44). As 3M-052 is a dual TLR7/8A, direct activation of naive B cells through TLR7 may also contribute to the observed effects of this adjuvant (45).
A key concern regarding adjuvanted vaccine development is reactogenicity, the propensity of a formulation to cause acute inflammatory events either locally (e.g., erythema, tenderness) or systemically as fever. Of note, vaccine adjuvants are not licensed separately; rather, the adjuvant is a constituent of the licensed vaccine formulation. Therefore, as demonstrated in our assays, adjuvants must be evaluated both alone and as a component of a vaccine formulation. To the extent that they reflect activity in vivo, development of reliable platforms for in vitro modeling may help exclude adjuvants with high potential to induce unacceptable reactogenicity in the very young (1, 4). Through our adjuvantation approach, not only was the systemic inflammation associated with TLR7/8 stimulation reduced, but adjuvant efficacy was also maintained in newborns. Thus, we demonstrate that a rationally designed adjuvanted vaccine approach taking both age and species specificity into account could enable effective early life immunization.
Even though our study breaks ground in the area of early life immunization, as with any research effort, it has some limitations. Although NHPs are a helpful model, vaccine-induced reactogenicity and efficacy in infant NHPs can diverge from those observed in human infants (46). In addition, as 3M-052 likely exerts its adjuvant effects at local sites of injection (not the systemic bloodstream), our knowledge of it’s PD (i.e., the concentration required to achieve an adjuvant effect) is limited by the fact that (a) our in vitro whole blood assay system may not reflect the concentration needed at the injection site (e.g., i.m.) and (b) in vivo drug concentration data are often based on sampling the bloodstream and not the likely site of adjuvant action. Although the higher concentration in cord blood of monocytes, the major producers of TLR-mediated TNF in blood, may contribute to robust 3M-052 activation of newborn blood, we have previously demonstrated that agonists of other TLRs do not activate robust Th1 cytokines from newborn blood (16). Moreover, direct comparison of purified human newborn and adult monocytes at equal concentrations demonstrates a similar pattern of substantially greater responses to TLR8As that are refractory to inhibitory neonatal adenosine pathways (15–17).
The persistently high global burden of infections in the very young (47) provides a compelling rationale for developing additional safe and effective early life vaccines. Overall, four key aspects of our findings deserve particular emphasis: (a) human in vitro systems may be able to predict age-specific adjuvanticity, (b) chemical modification of adjuvants can help limit systemic reactogenicity, (c) newborn primates are not inherently incapable of robust immune responses at birth but can mount robust Th1 and Th2 cell and humoral responses when stimulated with an appropriately adjuvanted vaccine formulation, and (d) TLR7/8As, such as 3M-052, may offer substantial advantages for adjuvantation of PCV13 and other vaccines. To our knowledge, ours is the first report of employing medicinal chemistry and human in vitro modeling for development of a locally targeted, age-specific adjuvanted neonatal vaccine formulation with robust in vivo activity at birth, a key point of healthcare contact (18). Another translational feature of our approach is that it builds upon a traditional alum-adjuvanted vaccine formulation, providing a potentially practical path to modify common pediatric conjugate vaccines for greater efficacy. Overall, study of individual and combined adjuvantation systems with activity toward specific age groups may open new paths to develop adjuvanted vaccines for distinct vulnerable populations, such as the young and elderly. Prior to human clinical trials, further evaluation and optimization of this adjuvant approach, including potential dose-sparing effects, reactogenicity, safety, and efficacy, are warranted.
Methods
In vivo rodent vaccination studies.
To evaluate drug PK (serum drug levels) and PD (serum TNF), female crl:CD(SD) rats (~350–400 g) (Charles River Laboratories) received a single s.c. (scruff of neck) or two i.m. (quadriceps) administrations of 3M-052 or R848 formulated in O/W emulsion (vehicle). Rat serum was collected 5 minutes, 30 minutes, 2 hours, 4 hours, and 24 hours after dose. 3M-052 and R848 serum drug levels were determined by LC-MS/MS before or after dose, with a lower limit of quantification (LLQ) of 0.84 and 3.2 pmol/ml, respectively. Similarly, serum TNF concentrations were measured by ELISA at the indicated times before or after dose, with a LLQ of 31 pg/ml. To determine systemic cytokine response and IFN-inducible gene expression following free or lipidated TLR7/8 IMQ administration, 6- to 8-week-old female C57BL/6J mice (The Jackson Laboratory), weighing approximately 18 g each, were administered a single s.c. dose of 3M-052 or R848 formulated (both 1 mg/kg, 20 μg/mouse) in O/W emulsion (vehicle). After administration, whole blood was collected 1, 3, 6, 9, 18, and 24 hours after dose, while draining lymph nodes (brachial and axillary) and spleen were collected 1, 3, 6, and 18 hours after dose. TNF, IL-6, IL-10, IFN-γ, and CCL2 serum cytokine kinetics were evaluated by flow cytometry cytometric bead array (BD Biosciences). mRNA expression in draining lymph nodes and spleen after administration were determined by quantitative RT-PCR (Applied Biosystems), as described previously (11) and represented as relative fold-change expression (i.e., treatment relative expression/naive relative expression). For rodent studies, 6- to 8-week-old male BALB/c mice were immunized by s.c. injection (scruff of neck) with recombinant influenza A hemagglutinin (10 μg) alone or in combination with 0.01, 0.03, 0.1, 0.3, or 1 mg/kg 3M-052 or in combination with alum, 3 times (prime, boost, boost), 14 days apart. Hemagglutinin-specific serum Ig levels were measured by ELISA on day 77, 21 days after final immunization (11).
Human blood sample processing and in vitro stimulation.
Peripheral blood was collected from healthy adult volunteers, while human newborn cord blood was collected immediately after Cesarean section delivery of the placenta. Births to known HIV-positive mothers were excluded. Human blood was anticoagulated with 20 units/ml pyrogen-free sodium heparin (American Pharmaceutical Partners Inc.). All blood products were kept at room temperature and processed within 4 hours from collection. Human whole blood assays were completed as previously described (48). Briefly, neonatal cord blood or adult whole blood was mixed 1:1 with sterile prewarmed (37°C) RPMI 1640 medium (Invitrogen), and 180–225 μl of the 1:1 suspension was added to each well of a 96-well U-bottom plate (Becton Dickinson) containing 20–25 μl freshly prepared specific TLRAs at 10 times the final concentration. Suspensions containing 200–250 μl/well were gently mixed by pipetting and incubated for 6 hours at 37°C in a humidified incubator at 5%, CO2. After culture, plates were centrifuged at 500 g, and approximately 100–150 μl supernatant was carefully removed by pipetting without disturbing the cell pellet. Supernatants derived from human leukocyte stimulations were assayed by ELISA for TNF (BD Biosciences) and IL-1β (eBiosciences). Additionally, whole blood assay supernatants were analyzed by multiplex cytokine assays (Millipore). The minimum threshold for each analyte was set at the minimum detectable concentration for a given assay, defined as 3 standard deviations above the mean background.
TLRAs and multianalyte assays.
R848 (TLR7/8) was purchased from InvivoGen. All TLR7/8As and emulsions used in both in vitro and in vivo studies were verified to be free of endotoxin (<1 EU/ml) by the Limulus amoebocyte lysate assay per the manufacturer’s instructions (Charles River Laboratories). Cytokine and chemokine expression profiles in cell culture supernatants and peripheral blood plasma were measured using customized Milliplex human and NHP cytokine/chemokine magnetic bead panels (Millipore), respectively. Assays were analyzed on the Luminex 100/200 System employing xPOTENT software (Luminex) and Millipore Milliplex Analyst (version 3.5.5.0).
Vaccine formulation.
The point-of-use mixed vaccine formulation consisted of 2 components. The first component consisted of an O/W emulsion, made up of a pH 6 citrate buffer, soybean oil, and surfactants that contains 0.04–0.4 mg/ml of N-[4- [(4-amino-2-butyl-1H-imidazo[4,5-c]quinolin-1-yl)oxy]butyl]octadecanamide (3M-052) (11) (3M Drug Delivery Systems Division, 3M Center). Concentrations of 3M-052 O/W emulsion preparations were confirmed by high-performance liquid chromatography (HPLC). The 3M-052 O/W emulsion formulations were sterile filtered, aliquoted into sterile 2-ml serum vials sealed with rubber septa, and stored at 2°C–8°C until use. Dual agonist activity of 3M-052 was confirmed using HEK293 cells stably expressing either human TLR7 or TLR8 (11). The dosing range of 3M-052 was approximately 4–40 μg (0.01–0.1 mg/kg; 400 g birth weight) (Supplemental Table 1). The second component consisted of one-half of the recommended human infant dose of the Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein) (Pfizer), which included the 13 pneumococcal conjugates (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F). Each PCV13-containing administration totaled 1.1 μg per dose of saccharide per serotype, except for serotype 6B, which totaled 2.2 μg per dose. Each of the polysaccharides was conjugated separately to CRM197 and adsorbed on aluminum phosphate (0.0625 mg aluminum). Sodium chloride, succinic acid, polysorbate 80, and water for injection were also included in PCV13. Where possible, components were verified to be free of endotoxin (<1 EU/ml), as measured by the Limulus amebocyte lysate assay per the manufacturer’s instructions (Charles River Laboratories).
For the injection of animals, all preparations were made within 1 hour of planned injection using sterile techniques. Briefly, a premade PCV13 vial was gently mixed by hand several times. The total volume (500 μl) of the PCV13 vial was sterilely injected into the prealiquoted adjuvant vials containing 3M-052 adjuvant O/W emulsion (making a combined total volume of 700 μl). The combined (PCV13 + 3M-052) vial was then vortexed for 20 to 30 seconds. Using a 1 ml pyrogen-free syringe 22- to 25-gauge needle, 350 μl of the formulation was removed and injected i.m. into the quadriceps muscle. The vial containing the remaining 350 μl of the formulation was discarded. Both the adjuvant alone and half recommended human infant dose of PCV13 alone were treated similarly, replacing equal volumes of saline for O/W emulsion. 0.35 ml clinical-grade saline (sterile/pyrogen-free 0.9% NaCl solution for injection) was used as a placebo control. Oil droplet particle size was determined by dynamic light scattering using a Zetasizer nanoseries instrument (Malvern Nano-ZS, l 1⁄4 532 nm) as previously described (49). The particle size data refer to scattering intensity distributions (z-average) with accompanying polydispersity/heterogeneity index (PDI), with a PDI < 0.2 considered monodisperse.
Selection of rhesus macaque model.
Murine TLR8 is divergent from human and monkey TLR8, and mice mount distinct immune responses to TLR7/8As and TLR8As (50). Rhesus macaques are likely a relevant animal model for predicting TLR8 adjuvant responses in human infants (27, 51). To date, 10 TLR/TIR orthologs have been identified within the rhesus macaque (M. mulata) genome, with an overall mean amino acid identity of 96.7% to their corresponding human TLR/TIR sequences, compared with 87.4% to mouse TLR/TIR sequences (26). The most highly conserved TLR/TIR is TLR8, which demonstrates 98.6% amino acid identity to human TLR8. Moreover, TLR8 in rhesus macaques and humans is highly conserved in terms of its predicted distribution pattern of extracellular LRRs. Rhesus macaques are also well suited for our study because: (a) adult rhesus macaques have demonstrated human-like responses to TLR7/8As in vivo (52–54), (b) both infant and adult rhesus macaques demonstrate human-like TLR7/8A-induced cytokine responses in vitro (15), and (c) like humans, infant rhesus macaques respond immunologically to conjugated but not to unconjugated polysaccharides in vivo.
In vivo neonatal and infant rhesus macaque vaccination studies.
Rhesus macaques were obtained from the TNPRC specific pathogen–free breeding colony. Upon identification, pregnant dams were transferred to an indoor social group to allow for monitoring and delivery of infants. At birth, neonatal rhesus macaques (<24 hours of age) were enrolled into the longitudinal immunization study. Exclusion criteria were (a) maternal fever (≥40°C) during infant exam on DOL0, (b) birth weight <400g, (c) clinical signs of neonatal infection (e.g., infant temperature ≥36.5°C; nasal, ocular discharge; respiratory distress; cardiovascular instability), (d) congenital defects (e.g., abnormal digits, omphalocele), and/or (e) abnormal cling. Animals were group housed in dam/infant pairs with a maximum of 4 pairs (8 animals) together. All animals received standard environmental enrichment, including manipulanda in the cage, perches/swings, various food supplements, foraging or task-oriented feeding methods, and regular human interaction with caretakers. Animals were assigned to either the phlebotomy group or the biopsy/phlebotomy group in a predesigned random sequential order (Supplemental Table 2). As a per-protocol analysis method was employed, one enrolled animal that died of natural causes unrelated to the study treatments within 24 hours of birth was not included in the final analysis. A standardized procedure for tattooing; physical exam; assessment of local reactogenicity, including photographic documentation; and immunization of neonatal and infant rhesus macaques was employed. Briefly, on the day of immunization (DOL0, DOL28, and DOL56), the leg designated to receive treatment was clipped to remove hair and a tattoo was applied (at birth only) on the thorax. Standard physiological safety parameters, extrapolated from human infant clinical trials of PCV13 (29), included temperature and weight (normalized to TNPRC reference standard for rhesus macaques), which were recorded at multiple time points at observation/sample acquisition, as well as local signs of reactogenicity, such as leg circumference before/after immunization and photographic documentation of erythema (Supplemental Table 2). A standardized physical exam and assessment of local reactogenicity, including photographic documentation, were repeated 48 hours after immunization through DOL70, according to the schedule in Supplemental Table 2. For photography, animals were positioned in a standardized way and 9 sequential photographs obtained. These included photograph 1, the tattoo number (to avoid false attribution of pictures to animals); photograph 2, both ventral thighs; photographs 3 and 4, each ventral thigh individually; and photographs 5–8: each thigh individually from medial and lateral aspects. If local erythema (redness) or swelling were noted, a higher magnification photograph was taken of the area. Finally, the newborn underwent i.m. vaccination as outlined above. After DOL70, physical exam alone (i.e., without photography) was conducted according to the same schedule, up to 1 year of life. For biopsy samples, animals were anesthetized via i.m. ketamine hydrochloride (10 mg/kg) and dexmedetomidine (7.5–15 μg/kg i.m.) or i.m. tiletimine/zolazepam (8 mg/kg). i.m. buprenorphine (0.01 mg/kg) was also administered for analgesia when indicated, and atipamezole was administered i.m. as a reversal agent when dexmedetomidine was used. The cranial aspect of the rear limb distal to the coxofemoral joint and proximal to the stifle were surgically prepped, a sterile fenestrated drape was placed on the cranial aspect of the rear limb, and a no. 15 scalpel blade was used to make a 3-mm incision through the skin. Skin adjacent to the incision was undermined, and muscle tissue was exteriorized using sterile rat tooth forceps. Curved scissors were used to excise a 2-mm length of superficial musculature. Once completed, sterile gauze was placed over the skin incision if hemorrhage occurred. Finally, the skin incision was closed with a single interrupted suture or skin glue. The 2-mm cube muscle biopsies were obtained from the injection site (quadriceps muscle) prior to and 48 hours after each immunization (one in each thigh) and obtained in an alternating pattern (e.g., DOL0 left leg, DOL2 right leg, DOL30 left leg, DOL58 right leg). Lymph node biopsies were obtained on DOL7 and 63 and followed a similar pattern of alternation. Peripheral blood samples were drawn from each group at multiple time points, per Supplemental Table 2, including at DOL0 (preimmunization), DOL7, DOL28, DOL30, DOL35, DOL56, DOL63, DOL90, DOL150, DOL180, DOL240, and DOL360. Serum and plasma samples were stored at –80°C for subsequent immunogenicity assays. PBMCs were isolated and stored in liquid nitrogen. For select peripheral blood samples, standard hematology, serum chemistry, and urinalysis assays were conducted at the Clinical Laboratory Improvement Amendments–certified Department of Laboratory Medicine, Boston Children’s Hospital. Biomarkers evaluated included serum chemistry (electrolytes, creatinine, ALT, and AST to monitor for renal or hepatic damage). Macroscopic and microscopic urinalysis were employed to assess possible renal damage and/or inflammation. Complete blood counts (to detect dyscrasias) were measured at TNPRC within 2 hours of phlebotomy.
Multiplexing electrochemiluminescence and opsonophagocytosis assays.
Ab response in infant monkey sera was measured in a 96-well electrochemiluminescence multiplex assay employing Meso Scale Discovery (MSD) technology, as previously described (55, 56). Two 10-spot (per single microtiter well) 96-well plates were used to monitor Ab responses to all 13 pneumococcal serotypes. PnPSs were obtained from ATCC or Statens Serum Institut (SSI) for Danish serotype designations 3, 4, 6B, 9V, 14, 18C, 19F, and 23F. These were individually spotted in each well (100 μg/ml coating concentration, 5 ng/spot for all types) on one plate, while polysaccharides 1, 5, 6A, 7F, 19A, 22F, and 33F were individually spotted in each well on the second plate. Before addition to the plate, primate serum samples were combined with an absorbent containing C-polysaccharide (C-PS), 25A, and 45 capsular PS (from SSI) to neutralize Ab binding to C-PS and other common contaminants present in the PnPS-coating antigens. A Sulfo-tag–labeled goat anti-human IgG that emits light upon electrochemical stimulation was used as a secondary Ab. The total IgG concentration in rhesus serum was calculated with MSD Workbench v. 3 software using the human antipneumococcal reference serum, lot 89SF-2 (Lederle-Praxis Biologicals) (56), and 007SP (57), as controls. Pneumococcal opsonophagocytosis assays were conducted at the Laboratory of Moon Nahm (University of Alabama; Birmingham, Alabama, USA). The multiplexed opsonophagocytic killing assay, MOPA4, was used to test the infant rhesus macaque sera, as previously described (58, 59). Opsonization titers were defined as the serum dilution that kills 50% of bacteria. The lowest detectable titer in the MOPA was 24, and therefore, samples identified as negative in the assay (i.e., samples having no functional activity detected) were assigned a titer of 12 (i.e., half the lowest limit of detection).
Characterization of PnPS-specific B cells.
Rhesus macaque PBMCs were labeled with anti-CD14-PE (clone M5E2), CD20-V450 (clone L27), CD27-PE.Cy7 (clone M-T271), CD4-FITC (clone SK3) Abs (BD Biosciences). CD14+ monocytes, CD20+CD27– naive B cells, CD20+CD27+ memory B cells, and CD4+ Th cells were sorted with a FACSAria II cell sorter. Antipneumococcal B cells in peripheral blood were enumerated in sorted B cell populations. Sorted B cell populations were cultured at a concentration of 5 × 106/ml for 5 days at 37°C, 5% CO2, in RPMI media supplemented with penicillin/streptomycin, 10% FBS (Invitrogen), 1 μg/ml R848 (Invivogen), 10 IU/ml IL-2 (R&D Systems), and 8,000 U/ml IFN-α (Abcam). ELISpot plates (Millipore) were coated either with a combination of 10 μg/ml anti-rhesus IgG-Fc and anti-rhesus IgM-Fc (Nordic Immunological Laboratories) in PBS or with a 10 μg/ml pool of the following PnPSs: Danish designations 1, 3, 4, 5, 6A, 6B, 9V, 14, 18C, 19A, and 23F (ATCC) as well as 7F and 19F (SSI) in PBS. Plates were coated overnight at 4°C and blocked with RPMI 1640/10% FBS for 1 hour prior to plating of cells. After 5 days of culture, B cells were incubated for 16 hours on the coated and blocked ELISpot plate in RPMI supplemented with penicillin/streptomycin and 10% FBS. Unless indicated otherwise, 10% of each culture (~50,000 cells) was plated in anti-rhesus IgG/IgM-coated wells and 90% of each culture (~450,000 cells) was plated in wells coated with polysaccharides. Secreted immunoglobulins were detected using horseradish peroxidase–conjugated goat anti-rhesus immunoglobulin (Nordic Immunological Laboratories). Spots were developed using 3,3’,5,5’-Tetramethylbenzidine (TMB) (Mabtech) and visualized and counted using a series 5 ELISpot analyzer (Cellular Technology Limited). The fraction of PnPS-specific B cells was quantified as the ratio of the spots detected in polysaccharide-coated wells to spots detected in immunoglobulin-coated wells after correction for dilution.
Characterization of CRM197-specific CD4+ T cells.
Monocytes and T cells were sorted as described above. Sorted CD4+ T cells were nonspecifically maintained by culturing in RPMI 1640 media supplemented with penicillin/streptomycin, 10% fetal bovine albumin, and 100 ng/ml Concanavalin A (Sigma-Aldrich) during the generation of MoDCs from sorted monocytes. Monocytes were cultured at a concentration of 0.75 × 106/ml to 1 × 106/ml for 5 days in RPMI supplemented with penicillin/streptomycin, 10% FBS, 100 ng/ml granulocyte-macrophage colony-stimulating factor, and 50 ng IL-4 (R&D Systems). On day 5, MoDCs were harvested and incubated in the absence or presence of 5 μg/ml CRM197 (Sigma-Aldrich) for 5 hours in RPMI without FBS. After 5 hours, FBS was added to 10% (v/v), and 100 ng/ml lipopolysaccharide (ultrapure from List Biological Laboratories) was added for an additional 18 hours. MoDCs were subsequently harvested, washed, and cocultured at 5,000 cells per well with 50,000 T cells for 7 days. On day 7, cells producing IFN-γ, IL-4, or IL-17 were analyzed by intracellular cytokine staining after the addition of BD Golgi-plug (BD Biosciences) during the final 6 hours of culture. T cells were made permeable with Cytofix/Cytoperm reagents (BD Biosciences). Cells were stained with anti–IFN-γ-PE.Cy7 (clone B27, BD Biosciences), anti–IL-17-APC (clone 41802, R&D Systems), and anti–IL-4-V450 (clone 8D4-8, BD Biosciences). Cells were analyzed for production of these 3 cytokines by flow cytometry (LSRFortessa flow cytometer, Becton Dickinson) and analyzed with Flowjo software version 10 (Tree Star Inc.).
ELISAs.
Quantitation of total S. pneumoniae and capsular polysaccharides serotype 4-, 6B-, 14-, and 23F-specific IgG were determined by use of an adapted WHO-recommended ELISA protocol, as outlined by the Bacterial Respiratory Pathogen Reference Laboratory at the University of Alabama at Birmingham (60). Serum Ab concentrations were calculated by comparing the optical density of each unknown well at 405 nm and 690 nm (reference) and to the optical density of the standard (human antipneumococcal reference serum, lot 89-SF). For avidity determination, assessment of the overall strength of binding between Ab and antigen, a 0- to 4-M NaSCN gradient was used to determine the NaSCN concentration that competes off approximately 50% of the bound rhesus immunoglobulins (61).
Statistics.
Statistical significance and graphs were generated using Prism v. 5.0b (GraphPad Software) and Microsoft Excel (Microsoft Corporation). For data analyzed by normalization to control values (vehicle), column statistics were conducted using the 2-tailed Wilcoxon signed-rank test or unpaired Mann-Whitney test as appropriate. Gaussian sample distributions were assessed by Shapiro-Wilk normality test. Group comparisons employed 1-way ANOVA with Dunnett’s multiple comparison post-test or 2-way repeated-measures ANOVA comparing column and row effects. Results were considered significant at P < 0.05. Level of synergy was calculated using the Loewe definition of additivity (62), with D > 1 indicating antagonism, D = 1 additivity, and D < 1 synergy.
Study approval.
All experiments were conducted in accordance with relevant institutional and national guidelines, regulations, and approvals. All rodents were obtained from Charles River Laboratories, and studies were approved by the 3M Drug Delivery Systems IACUC. Nonidentifiable human cord blood samples were collected with approval from the Ethics Committees of Brigham and Women’s Hospital (protocol 2000-P-000117) and Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA (protocol 2011P-000118). Blood samples from adult volunteers were collected after written informed consent, with approval from the Ethics Committee of Boston Children’s Hospital (protocol X07-05-0223). Human experimentation guidelines of the US Department of Health and Human Services were observed. The longitudinal rhesus monkey experimental protocol (P0184) was approved by the IACUC at Tulane University (New Orleans, Louisiana, USA) and performed at TNPRC. Additionally, peripheral blood samples from rhesus macaques were derived from New England Primate Research Center (Southborough, Massachusetts, USA) and used under Harvard University IACUC approval (protocol 04936).
Author contributions
DJD and OL codesigned and supervised the study. DJD analyzed the data and wrote the manuscript with editorial input from OL. DJD, SDVH, AS, IB, CJM, MHG, JLB, PPA, and OL conceived and designed the NHP experiments. LF, TBT, CCM, MHG, and XA conducted the rhesus macaque experiments. MHG, JLB, and PPA supervised the NHP clinical trial. AS, WF, and DJD designed the NHP safety and reactogenicity methodologies and evaluated the readouts. SDVH conducted the ex vivo recall studies. DJD, IB, DK, AS, and SDVH conducted the human in vitro experiments and analyzed data from rodent and NHP trials. DS, JPV, JMB, and MAT provided adjuvanted materials and conducted rodent studies. RNF, AAL, and AO provided expert technical and statistical assistance. All authors commented on the manuscript.
Supplementary Material
Acknowledgments
We thank the members of the Levy laboratory for assistance with phlebotomy as well as helpful discussions. We also thank Amy Weiner, Geert vanden Bossche, and Chris Wilson of the Bill & Melinda Gates Foundation (BMGF) for their support and feedback; members of the Fichorova Laboratory; staff at the TNPRC and the New England Primate Research Center and Robert L. Burton and Moon H. Nahm at the Bacterial Respiratory Pathogen Reference Laboratory at the University of Alabama at Birmingham; and the Labor and Delivery staff at both Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center for their assistance. We are grateful for the mentorship and support of Michael Wessels, Richard Malley, and Gary Fleisher. The BMGF was the major funder for this study via Grand Challenges Explorations (award OPP1035192). OL’s laboratory is supported by US National Institutes of Health National Institutes of Allergy and Infectious Diseases Infant Immunity program grant 1R01AI100135-01, Molecular Mechanisms of Combination Adjuvants U01AI124284-01, and Adjuvant Discovery Program contract HHSN272201400052C and an internal Boston Children’s Hospital award to the Precision Vaccines Program, in addition to sponsored support from 3M Drug Delivery Systems, VentiRx Pharmaceuticals, and Crucell (Johnson & Johnson).
Footnotes
AS’s present address is: Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Conflict of interest: The Levy laboratory has received sponsored research support from VentiRx Pharmaceuticals, 3M Drug Delivery Systems, MedImmune, Crucell (Johnson & Johnson), and Shire.
Reference information:JCI Insight. 2017;2(2):e91020. doi:10.1172/jci.insight.91020.
Contributor Information
Annette Scheid, Email: Annette.Scheid@childrens.harvard.edu.
Ilana Bergelson, Email: ilana.bergelson@gmail.com.
Dhohyung Kim, Email: Dhohyung.Kim@gmail.com.
Willemina Foppen, Email: willekefoppen@gmail.com.
Al Ozonoff, Email: Al.Ozonoff@childrens.harvard.edu.
Lynn Fresh, Email: lfresh@tulane.edu.
Dmitri Smirnov, Email: dsmirnov@gmail.com.
Xavier Alvarez, Email: xavier@tulane.edu.
Ofer Levy, Email: ofer.levy@childrens.harvard.edu.
References
- 1.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35(7):299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowling DJ, Levy O. Pediatric Vaccine Adjuvants: Components of the Modern Vaccinologist’s Toolbox. Pediatr Infect Dis J. 2015;34(12):1395–1398. doi: 10.1097/INF.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Biggelaar AH, Pomat WS. Immunization of newborns with bacterial conjugate vaccines. Vaccine. 2013;31(21):2525–2530. doi: 10.1016/j.vaccine.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3(90):90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29(46):8215–8221. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 6.Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis. 2010;201(10):1570–1579. doi: 10.1086/652006. [DOI] [PubMed] [Google Scholar]
- 7.van den Biggelaar AH, et al. Effect of early carriage of Streptococcus pneumoniae on the development of pneumococcal protein-specific cellular immune responses in infancy. Pediatr Infect Dis J. 2012;31(3):243–248. doi: 10.1097/INF.0b013e318245a5a8. [DOI] [PubMed] [Google Scholar]
- 8.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 9.Mastelic B, et al. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biologicals. 2013;41(6):458–468. doi: 10.1016/j.biologicals.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Holbrook BC, et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J Immunol. 2016;197(2):555–564. doi: 10.4049/jimmunol.1600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29(33):5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Corbett NP, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS ONE. 2010;5(11):e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 14.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 15.Philbin VJ, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130(1):195–204.e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173(7):4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 17.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108(4):1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur J Immunol. 2009;39(1):36–46. doi: 10.1002/eji.200838620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh M, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193(9):4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien KL, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 22.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17(12):1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadzinowski J, et al. Phase 3 trial evaluating the immunogenicity, safety, and tolerability of manufacturing scale 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29(16):2947–2955. doi: 10.1016/j.vaccine.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 24.van den Biggelaar AH, et al. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: a randomized controlled trial in Papua New Guinea. Vaccine. 2009;27(9):1340–1347. doi: 10.1016/j.vaccine.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JA, Ojal J, Ashton L, Muhoro A, Burbidge P, Goldblatt D. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clin Infect Dis. 2011;53(7):663–670. doi: 10.1093/cid/cir444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanghavi SK, Shankarappa R, Reinhart TA. Genetic analysis of Toll/Interleukin-1 Receptor (TIR) domain sequences from rhesus macaque Toll-like receptors (TLRs) 1-10 reveals high homology to human TLR/TIR sequences. Immunogenetics. 2004;56(9):667–674. doi: 10.1007/s00251-004-0734-6. [DOI] [PubMed] [Google Scholar]
- 27.Skinner JM, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine. 2011;29(48):8870–8876. doi: 10.1016/j.vaccine.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Annex 3 Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. https://www.who.int/biologicals/vaccines/TRS_977_Annex_3.pdf Accessed January, 13, 2017.
- 29.Yeh SH, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126(3):e493–e505. doi: 10.1542/peds.2009-3027. [DOI] [PubMed] [Google Scholar]
- 30.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11(12):865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capozzo AV, et al. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J Immunol. 2006;176(9):5671–5681. doi: 10.4049/jimmunol.176.9.5671. [DOI] [PubMed] [Google Scholar]
- 33.Reikie BA, et al. A single immunization near birth elicits immediate and lifelong protective immunity. Vaccine. 2010;29(1):83–90. doi: 10.1016/j.vaccine.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Pan CH, et al. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J Virol. 2010;84(8):3798–3807. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith KC, Ormem IM, Starke JR. Tuberculosis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia, PA: Saunders; 2012: 789–811. [Google Scholar]
- 36.Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206(7):1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sander LE, et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474(7351):385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Haren SD, et al. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J Immunol. 2016;197(11):4413–4424. doi: 10.4049/jimmunol.1600282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acha-Orbea H, et al. Interplays between mouse mammary tumor virus and the cellular and humoral immune response. Immunol Rev. 1999;168:287–303. doi: 10.1111/j.1600-065X.1999.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 41.Olliver M, Hiew J, Mellroth P, Henriques-Normark B, Bergman P. Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect Immun. 2011;79(10):4210–4217. doi: 10.1128/IAI.05286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastelic B, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 43.Lu YJ, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkowska MA, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118(8):2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettengill MA, et al. Distinct TLR-mediated cytokine production and immunoglobulin secretion in human newborn naïve B cells. Innate Immun. 2016;22(6):433–443. doi: 10.1177/1753425916651985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polack FP, et al. Poor immune responses of newborn rhesus macaques to measles virus DNA vaccines expressing the hemagglutinin and fusion glycoproteins. Clin Vaccine Immunol. 2013;20(2):205–210. doi: 10.1128/CVI.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 48.Dowling DJ, et al. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS ONE. 2013;8(3):e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer CD, et al. The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells. Biomaterials. 2014;35(31):8876–8886. doi: 10.1016/j.biomaterials.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 50.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 51.Shen C, Xu H, Liu D, Veazey RS, Wang X. Development of serum antibodies during early infancy in rhesus macaques: implications for humoral immune responses to vaccination at birth. Vaccine. 2014;32(41):5337–5342. doi: 10.1016/j.vaccine.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wille-Reece U, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102(42):15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wille-Reece U, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203(5):1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174(12):7676–7683. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 55.Marchese RD, et al. Optimization and validation of a multiplex, electrochemiluminescence-based detection assay for the quantitation of immunoglobulin G serotype-specific antipneumococcal antibodies in human serum. Clin Vaccine Immunol. 2009;16(3):387–396. doi: 10.1128/CVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldblatt D, Ashton L, Zhang Y, Antonello J, Marchese RD. Comparison of a new multiplex binding assay versus the enzyme-linked immunosorbent assay for measurement of serotype-specific pneumococcal capsular polysaccharide IgG. Clin Vaccine Immunol. 2011;18(10):1744–1751. doi: 10.1128/CVI.05158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldblatt D, et al. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2011;18(10):1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13(9):1004–1009. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burton RL, Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol. 2012;19(6):835–841. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organization Pneumococcal Serology Reference Laboratories. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA). http://www.vaccine.uab.edu/ELISA Protocol.pdf Accessed December 7, 2016.
- 61.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86(1):83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 62.Berenbaum MC. Correlations between methods for measurement of synergy. J Infect Dis. 1980;142(3):476–480. doi: 10.1093/infdis/142.3.476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.