Abstract
Parkinson's disease (PD) is characterized by a progressive loss of midbrain dopamine neurons and the presence of cytoplasmic inclusions called Lewy bodies. Mutations in several genes including α-synuclein and parkin have been linked to familial PD. The loss of parkin's E3-ligase activity leads to dopaminergic neuronal degeneration in early-onset autosomal recessive juvenile parkinsonism, suggesting a key role of parkin for dopamine neuron survival. To evaluate the potential neuroprotective role of parkin in the pathogenesis of PD, we tested whether overexpression of wild-type rat parkin could protect against the toxicity of mutated human A30P α-synuclein in a rat lentiviral model of PD. Animals overexpressing parkin showed significant reductions in α-synuclein-induced neuropathology, including preservation of tyrosine hydroxylase-positive cell bodies in the substantia nigra and sparing of tyrosine hydroxylase-positive nerve terminals in the striatum. The parkin-mediated neuroprotection was associated with an increase in hyperphosphorylated α-synuclein inclusions, suggesting a key role for parkin in the genesis of Lewy bodies. These results indicate that parkin gene therapy may represent a promising candidate treatment for PD.
Keywords: gene therapy, lentivirus, neurodegenerative disease, Lewy body, neuroprotection
Parkinson's disease (PD) is one of the most common neurodegenerative disorders, affecting ≈2% of the population over the age of 60. Loss of dopaminergic neurons in the substantia nigra pars compacta and subsequent striatal dopamine depletion causes motor impairments including akinesia, resting tremor, muscle rigidity, and gait and postural deficits. The neuropathological hallmark of PD is the appearance of proteinaceous intracellular deposits identified as Lewy bodies and Lewy neurites. Although the mechanism leading to the selective degeneration of nigral dopamine neurons in sporadic PD remains unknown, clues about the pathogenesis of familial forms of PD are emerging because of the discovery of various gene mutations. Two missense mutations in α-synuclein (A53T and A30P) were the first to be identified, and these are responsible for early-onset autosomal dominant PD (1, 2). The subsequent findings that α-synuclein is a major component of Lewy bodies in sporadic PD (3, 4) and that α-synuclein locus triplication causes autosomal dominant PD (5), suggest that accumulation of wild-type α-synuclein is sufficient to cause PD. Other PD-linked mutations in genes encoding for parkin, UCH-L1, DJ-1, and PINK1 have also been identified (6, 7).
Mutations in the parkin gene are associated with autosomal recessive juvenile parkinsonism (AR-JP), a disease characterized by juvenile onset of typical parkinsonian symptoms and pathology (8). Parkin is an E3 ubiquitin ligase, and parkin mutations found in AR-JP patients lead to partial or complete loss of this activity (9). Several substrates of parkin have been identified such as CDCrel-1, synphilin-1, and o-glycosylated forms of α-synuclein (αSp22) and Pael-R (10). Both αSp22 and Pael-R have been found to accumulate in AR-JP brains (11). The absence of detectable parkin in the brain of AR-JP patients with exon 3 or 4 deletions in the parkin gene (12) and the recessive mode of inheritance indicate that a loss of function of parkin is likely to be responsible for AR-JP. Conversely, several reports have described neuroprotective effects of parkin in vitro against endoplasmic reticular stress (13, 14), α-synuclein or Pael-R overexpression (15–17), proteasomal inhibition (15), excitotoxicity (18), and polyglutamine toxicity (19). Similarly, parkin prevents dopaminergic cell loss in both α-synuclein and Pael-R transgenic flies (20). These findings support an essential role of parkin in the survival of dopaminergic neurons. In contrast to sporadic and dominant familial PD, Lewy bodies are generally absent in parkin mutation carriers (12, 21–23), suggesting that parkin may also be involved in the genesis of Lewy bodies. Despite previous evidence that parkin might be neuroprotective, this property has never been tested in a mammalian model of PD. Thus, we have evaluated the lentiviral delivery of parkin in an α-synuclein rat model of PD (24). In contrast to α-synuclein transgenic mouse models, expression of human α-synuclein with lentiviral or adeno-associated viral vectors induces a progressive degeneration of dopamine neurons in the substantia nigra (24–28). In the present study, we report that lentiviral-mediated expression of parkin in the substantia nigra protects dopamine neurons against A30P α-synuclein-induced neurotoxicity. Using a specific Ab for Ser-129-phosphorylated α-synuclein, Pser129, we also show that overexpression of parkin promotes the formation of inclusions containing phosphorylated α-synuclein reminiscent of Lewy bodies in PD brain (29). Thus, parkin may play a central role in mitigating the pathogenesis of PD by promoting the survival of dopaminergic neurons through the detoxification of misfolded proteins in both soluble and aggregated forms.
Methods
Lentiviral Vector Production. The cDNAs encoding nuclear-localized yellow fluorescent protein (YFP) (BD Biosciences Clontech), A30P human α-synuclein, and rat parkin were cloned into the SIN-W-PGK lentiviral transfer vector, and the viral particles (lenti-YFP, lenti-A30P, and lenti-parkin) were produced as described in refs. 24 and 30. The viral suspensions lenti-A30P/lenti-YFP and lenti-A30P/lenti-parkin were prepared by mixing viruses at 1:1 ratios. Viral particle content was normalized to 360,000 ng of p24 per ml for each lentiviral suspension.
Stereotaxic Injection. Lentiviral vectors were stereotaxically injected in the right substantia nigra of adult female Wistar rats (Iffa-Credo, Charles River Laboratories) weighing ≈200 g. Viral suspensions (2.5 μl volumes) were injected with a 10-μl Hamilton syringe at a speed of 0.2 μl/min with an automatic injector (Stoelting), and the needle was left in place for an additional 10 min before being withdrawn. Stereotaxic injections were delivered to two sites within the substantia nigra with the following coordinates in millimeters: anterior, lateral, and ventral, 4.8, 2, and 7.7 and 5.5, 1.7, and 7.7 for the first site and second site, respectively. The anterior and lateral coordinates were calculated from the Bregma, and the ventral coordinates were calculated from the skull surface. The experiments were carried out in accordance with European Community Council Directive 86/609/EEC for the care and use of laboratory animals.
Immunohistochemistry. Six weeks after the injection of lentiviral vector suspensions, animals were killed with a sodium pentobarbital overdose and transcardially perfused with saline and 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde for ≈24 h, cryoprotected in 25% sucrose/0.1 M phosphate buffer for 48 h, and processed as described in ref. 24.
The following primary Abs were used as described previously: a tyrosine hydroxylase (TH) sheep Ab (1:500) (Pel-Freez Biologicals), an α-synuclein polyclonal rabbit Ab (24), the LB509 human α-synuclein-specific monoclonal Ab (1:500) (Zymed), the Pser129 Ab specifically recognizing phospho-Ser-129 of α-synuclein (29) (1:100), and a rabbit Ab to the C terminus of parkin (1:500) (Cell Signaling Technology, Beverly, MA). For light microscopy, sections were stained by the classic avidin–biotin complex method as described in ref. 24. For fluorescent multiple labeling, the secondary Abs conjugated to Cy2, Cy3, and Cy5 were purchased from Jackson ImmunoResearch. TOPRO-3 (Molecular Probes) was used as a nucleus marker. Sections were then analyzed by confocal microscopy (TCS SP2 AOBS, Leica, Heidelberg).
The FD NeuroSilver kit (FD Neuro-Technologies, Baltimore) was used according to the manufacturer's protocol to detect degenerating neurons (23).
Quantification of TH-Positive Neurons and Phosphorylated Inclusions. The percentage of TH-immunoreactive (TH-IR) neurons relative to the contralateral side was determined by fluorescence microscopy in a blind manner as described in refs. 24 and 30. To determine the density of TH-IR terminals, striatal fibers were stained for TH with the ABC kit (Vector Laboratories), and the corresponding tissue optical densities were evaluated with nih 1.4 software as described in ref. 24. To determine the numbers of neurons containing phosphorylated α-synuclein inclusions, five sections throughout the substantia nigra were stained with the Pser129 Ab with the avidin–biotin complex method, and adjacent sections were stained for α-synuclein.
Statistical analysis was performed by one-way ANOVA followed by a Scheffé's probable least-squares difference post hoc test (statistica 5.1, StatSoft). The significance level was set at P < 0.05.
Results
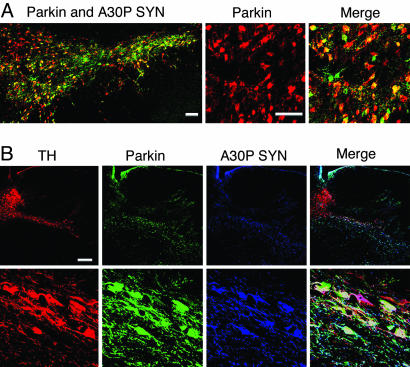
Lentiviral-Mediated Expression of Parkin and Human A30P α-Synuclein. To evaluate the neuroprotective effect of parkin on α-synuclein-induced nigrostriatal neurodegeneration, a 1:1 mixtures of lentiviral vector suspensions encoding A30P human α-synuclein and wild-type rat parkin (lenti-A30P/lenti-parkin) or A30P α-synuclein and YFP (lenti-A30P/lenti-YFP) were injected in the substantia nigra of rats. Control animals received either lenti-parkin alone or a 2-fold higher dose of lenti-YFP (lenti-YFP2X) (to match the total amount of viral particles relative to dual-virus treatments). As an initial step, we evaluated the substantia nigra transduction efficiencies of lentiviral vector mixes encoding A30P α-synuclein and parkin. Double staining specific for human α-synuclein and parkin (Fig. 1A) showed that the majority of transduced cells overexpressed both A30P α-synuclein and parkin in the injected side. No staining for endogenous α-synuclein and parkin was observed in the noninjected side (data not shown). Both proteins were localized in the cell bodies and axons of transduced neurons. The percentage of double-transduced cells was determined by confocal microscopy on four coronal sections of four animals and was revealed to be 72 ± 3%. Almost all transduced cells have a typical neuronal morphology confirming the strong tropism of lentiviral vectors for neuronal cells (31). Thus, coinjection of two lentiviral vectors represented a feasible strategy to investigate the relationship between these two proteins.
Fig. 1.
Lentiviral-mediated expression of human A30P α-synuclein (green) and parkin (red) in the rat substantia nigra. (A) A large proportion of double-infected neurons overexpresses both A30P α-synuclein and parkin. Quantification of double-immunostained cells revealed 72 ± 3% coinfected cells. (B) Confocal images of triple labeling for TH (red), parkin (green), and A30P human α-synuclein (blue) in the rat substantia nigra injected with lenti-A30P and lenti-parkin. Higher magnification (Lower) reveals the presence of numerous TH neurons still expressing A30P human α-synuclein and parkin at 6 weeks after lentiviral injection. (Scale bars: A, 100 μm; B, 250 μm.)
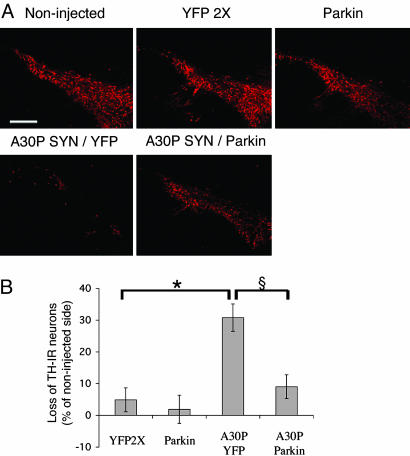
Expression of Parkin Protects Dopamine Neurons. Lentiviral-mediated expression of either wild-type or mutated human α-synuclein was described previously to induce a selective dopaminergic cell death in the rat (24). Animals expressing A30P α-synuclein showed a 33% loss of TH-IR neurons in the substantia nigra, and almost all dopaminergic neurons expressing human α-synuclein died within 6 weeks of postviral injection (24). Strikingly, confocal microscopy analysis of triple labeling for TH, human α-synuclein, and parkin revealed that animals injected with lenti-A30P/lenti-parkin retained numerous dopaminergic neurons still expressing A30P α-synuclein and overexpressing rat parkin at 6 weeks after lentiviral injection (Fig. 1B). Higher magnification of the substantia nigra pars compacta indicates that both viruses homogeneously diffused in the substantia nigra region. Animals injected with lenti-YFP2X or lenti-parkin showed no dopaminergic nigrostriatal lesion (Fig. 2A). In contrast, animals expressing A30P/YFP revealed a significant loss of nigral TH-IR cells. Interestingly, overexpression of parkin rescued TH-IR neurons from the A30P α-synuclein neurotoxicity. Quantification of the percent of nigral TH-IR neuron loss compared to the contralateral noninjected side showed that parkin significantly decreases the TH-IR cell loss from 31% (A30P/YFP) to 9% (A30P/parkin) (Fig. 2B). Parkin expression also prevented α-synuclein-induced loss of striatal TH-IR fibers (Fig. 3A). Quantification of TH-positive nerve terminal densities showed that parkin significantly decreased the loss of dopaminergic terminals from 16% (A30P/YFP) to 4% (A30P/parkin) (Fig. 3B). No reduction in TH staining was observed in the striatum of animals injected with lenti-YFP2X or lenti-parkin. A similar protective effect of parkin was observed with striatal sections stained for the vesicular monoamine transporter type 2 (data not shown).
Fig. 2.
Overexpression of parkin reduces A30P α-synuclein-induced dopamine nigral neuron loss. (A) TH expression at 6 weeks in the substantia nigra of rats injected with different mixes of lentiviral vectors encoding for YFP (YFP2×), rat parkin (Parkin), and A30P human α-synuclein (A30P). (B) Histograms represent the loss of TH-IR nigral neurons at 6 weeks relative to the contralateral side in rats unilaterally injected with the different lentiviral constructs. Values refer to means ± SEM; n = 5 animals for YFP2× or parkin; n = 10 for A30P/YFP; n = 18 for A30P/parkin; *, P < 0.05, compared with lenti-YFP-injected animals; §, P < 0.005, compared with A30P/YFP-expressing animals. (Scale bar: 350 μm.)
Fig. 3.
Parkin prevents the α-synuclein-induced dopaminergic fiber loss in the striatum of rats. (A) Striatal sections stained for the TH marker from rats that received intranigral injection of rats injected with different solutions of lentiviral vectors encoding for YFP (YFP2×), rat parkin (Parkin), and A30P human α-synuclein (A30P). (B) Histograms represent the loss of TH-IR fibers neurons at 6 weeks relative to the contralateral side in rats unilaterally injected with the different lentiviral suspensions. Animals injected with lenti-A30P/lenti-YFP showed a considerable reduction of dopaminergic innervation on the ipsilateral side of the striatum. Values refer to means ± SEM; n = 5 animals for YFP2× or parkin; n = 12 for A30P/YFP; n = 13 for A30P/parkin; *, P < 0.05, compared with lenti-YFP2×-injected animals; §, P < 0.005, compared with A30P/YFP-expressing animals.
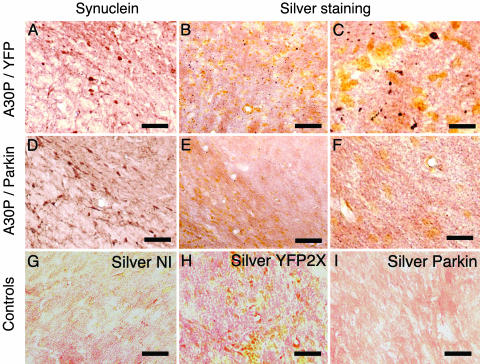
Parkin Prevents A30P-Induced Neurodegeneration. Neurons undergoing degeneration become argyrophilic and are therefore specifically detected with silver staining (32). Lentiviral-mediated expression of mutated human α-synuclein was shown to induce a strong neuritic and cellular pathology detected with silver staining (24). Animals expressing A30P/YFP showed the presence of silver-positive dark structures in both neurites and cell bodies (Fig. 4). A similar α-synuclein expression was observed in the brains of animals expressing A30P/YFP or A30P/parkin. Coexpression of parkin with A30P α-synuclein prevented the appearance of silver-positive degenerating neurons. No silver staining was detected on the noninjected side of animals expressing A30P/YFP or in animals injected with lenti-YFP or lenti-parkin. These results show that parkin prevents α-synuclein-induced neurodegeneration in the substantia nigra of rats expressing PD-linked mutated human α-synuclein.
Fig. 4.
Parkin prevents A30P α-synuclein-induced neurodegeneration. Brain sections from animals expressing A30P/YFP (A–C) or A30P/parkin (D–F) were stained for α-synuclein. A similar expression of A30P human α-synuclein was observed in both groups. No α-synuclein staining was observed for the noninjected side (NI), lenti-YFP-injected, or lenti-parkin-injected (data not shown) animals. Silver staining (B, C, and E–I) was performed on adjacent nigral sections to detect degenerating neurons. Higher magnification shows the presence of silver-positive dark structures in both cell body and axon of nigral neurons from animals expressing A30P/YFP (C). Coexpression of parkin with A30P α-synuclein prevents the appearance of silver-positive degenerating neurons (F). Noninjected side (NI) (G) and animals expressing YFP (H) or parkin (I) did not show any specific silver staining. (Scale bars: A, B, D, E, and G–I, 140 μm; C and F, 40 μm.)
Parkin Increases the Number of Phosphorylated α-Synuclein Inclusions. Lewy bodies are present in almost all forms of PD with the exception of AR-JP patients with parkin mutations, suggesting an essential role of the E3 ligase parkin in the formation of Lewy bodies (12, 21–23). Because of the difficulty of discriminating between pathological inclusions and accumulation of α-synuclein in subcellular regions associated with general α-synuclein staining, inclusions were detected with the Ab Pser129 specific for the phosphorylated form of α-synuclein at position 129 (29). This form of α-synuclein selectively and abundantly accumulates in Lewy bodies of synucleinopathy lesions (29), whereas only a small fraction of α-synuclein is phosphorylated in normal human and rat brains (29). A very low level of phosphorylated rat α-synuclein was observed in the substantia nigra of noninjected rats (Fig. 5A) and those overexpressing YFP (data not shown). In contrast, lentiviral-mediated expression of A30P α-synuclein led to the formation of round hyperphosphorylated Pser129-positive inclusions (Fig. 5B) and occasional phosphorylated neurites (Fig. 5C). Triple labeling with a nuclear marker, Pser129, and human α-synuclein-specific Abs showed that the intracytoplasmic phosphorylated inclusions are strongly immunopositive for human α-synuclein (Fig. 5D). To explore the effect of parkin in the formation of α-synuclein inclusions, the number of cells containing hyperphosphorylated inclusions was quantified in animals overexpressing A30P/YFP and A30P/parkin. Animals coexpressing parkin with A30P α-synuclein showed a 45% increase in the number of neurons containing hyperphosphorylated inclusions (Fig. 5E). A 41% increase in cells containing hyperphosphorylated inclusions was observed when only cells positive for α-synuclein were analyzed (data no shown). To determine whether other posttranslational modifications of synuclein aggregates might also correlate with the neuroprotection afforded by parkin, we also examined the brains of A30P/YFP- and A30P/parkin-overexpressing animals for the presence of ubiquitinated inclusions. These experiments showed no ubiquitinated inclusions in the brains of A30P-expressing animals either with or without parkin overexpression (data not shown). The finding that not all α-synuclein inclusions are immunoreactive for ubiquitin in patients with PD indicates that ubiquitin is not a prerequisite for the α-synuclein pathology (4).
Fig. 5.
Parkin increases the number of phosphorylated α-synuclein inclusions. Sections from the substantia nigra of rats expressing A30P α-synuclein and parkin were immunostained with the Pser129 Ab specific for phospho-Ser-129 of α-synuclein that selectively and extensively accumulates in human Lewy bodies. (A) A very weak Pser129 staining was observed in the noninjected side corresponding to the physiological level of phosphorylated rat α-synuclein. (B) The substantia nigra of animals overexpressing either A30P/YFP or A30P/parkin revealed the presence of numerous hyperphosphorylated inclusions reminiscent of Lewy bodies. (C) Pser129-positive neurites were occasionally observed in animals overexpressing A30P/parkin. (D) Triple staining with Pser129 Ab (green), LB509 human α-synuclein specific Ab (red), and TOPRO-3 nuclear marker (blue) shows that phosphorylated inclusions abundantly contain human α-synuclein. (E) The number of neurons containing Pser129-positive inclusions were quantified in the substantia nigra of rats expressing A30P/YFP or A30P/parkin. Values refer to means ± SEM; n = 6 animals per group; *, P < 0.05; **, P < 0.005. (Scale bars: A and B, 150 μm; C, 40 μm; D, 10 μm.)
Discussion
Abnormal accumulation of α-synuclein is considered to be a key pathological event in the process leading to selective dopaminergic degeneration in α-synuclein-linked and sporadic PD, but the neurotoxic role of inclusions in PD is highly debated (33). The major findings of this report are that gene therapy delivery of parkin efficiently prevents PD-linked mutant α-synuclein-induced dopaminergic cell loss in vivo and promotes the formation of hyperphosphorylated α-synuclein inclusions. Consistent with these results, inactivation of the gene encoding the E6-AP ubiquitin ligase leads to an increase in neurotoxicity and a decrease in the number of nuclear inclusions in a transgenic model of spinocerebellar ataxia type 1 (34). Recently, the E3-ligase CHIP (carboxyl terminus of the Hsc70-interacting protein) was reported to attenuate tau-induced cell death and also to facilitate hyperphosphorylated tau aggregation (35, 36). Parkin, CHIP and E6-AP may similarly enhance cell survival by eliminating soluble toxic proteins in favor of insoluble aggregates. Additionally, in vitro mammalian cell and transgenic fly studies also attributed a protective role to parkin in dopamine neuron survival (15, 20). Overexpression of parkin in the α-synuclein transgenic fly induces, on the contrary, a decrease in the number of nonphosphorylated α-synuclein inclusions. In the present study, however, we analyzed the effect of parkin in the formation of more mature posttranslationally modified α-synuclein-containing inclusions. The presence of phosphorylated α-synuclein was recently recognized as a pathological hallmark of α-synucleinopathy lesions (29, 37, 38). After 6 weeks of α-synuclein expression, not all α-synuclein-positive cells developed hyperphosphorylated inclusions. Because the formation of hyperphosphorylated α-synuclein inclusions is a progressive time- and dose-dependant process (37, 39), the majority of inclusions are likely nonphosphorylated in the short time period of 6 weeks. However, detecting these Pser129 inclusions has the advantage over classic α-synuclein staining of better differentiating true inclusions from nonaggregate subcellular accumulations of the protein (37). We observed that the neuroprotective effect of parkin is associated with a significant increase in the number of hyperphosphorylated α-synuclein inclusions. We therefore hypothesize that parkin helps dopamine neurons survive by promoting the sequestration of toxic prefibrillar oligomers in mature hyperphosphorylated inclusions. Interestingly, cytosolic dopamine has been shown to interact with α-synuclein to form adducts that stabilize the formation of toxic protofibrils, suggesting a potential mechanism for the selective degeneration of dopaminergic neurons (40). One toxic activity of these protofibrils appears to be the disruption of synaptic vesicles (41, 42).
Other findings also indicate that toxicity and aggregation are two distinct phenomena in α-synuclein-induced pathology. A recent study has reported behavioral impairments linked to neuronal dysfunction without aggregate formation in transgenic mice expressing A53T human α-synuclein (43). Additionally, toxicity induced by overexpression of human α-synuclein in primary midbrain cells is not associated with the presence of visible protein aggregates (15).
Brains of AR-JP patients with parkin mutations generally show dopaminergic neurodegeneration without Lewy bodies, the neuronal proteinaceous cytoplasmic inclusions that are typically found in PD. This finding suggests the requirement of parkin in the genesis of Lewy bodies. Our observation that coexpression of parkin with A30P human α-synuclein increases the number of neurons containing hyperphosphorylated inclusions is consistent with this hypothesis. Parkin may also act indirectly in the formation of inclusions by blocking the cellular death pathway triggered by A30P human α-synuclein and consequently compelling the resistant cells to accumulate α-synuclein in their cytoplasm and eventually form inclusions. Interestingly, parkin was shown to increase the formation of ubiquitinated inclusions when synphilin-1 and α-synuclein were coexpressed in vitro (44). Furthermore, the protective effect of parkin against α-synuclein-induced toxicity in cultured cells was also described to be associated with the appearance of higher-molecular-weight species of α-synuclein, suggesting that parkin promotes the aggregation of α-synuclein (16). We also recently investigated the ability of lentiviral vectors encoding glial cell line-derived neurotrophic factor to prevent nigral dopaminergic degeneration associated with the lentiviral-mediated expression of the A30P mutant human α-synuclein (45). Contrary to parkin, expression of this neurotrophic factor does not prevent α-synuclein-induced toxicity. This difference in neuroprotection may reflect a particular relationship between the two PD-linked proteins, α-synuclein and parkin, and their implication in a common cellular pathway. Dissecting the molecular mechanism of parkin's protection against α-synuclein toxicity should provide important clues about the unique vulnerability of dopamine neurons in PD. These results also indicate that gene therapy delivery of parkin or pharmacological agents increasing parkin expression may constitute a potential therapeutic strategy for PD. Interestingly, brain delivery of human α-synuclein with viral vectors has recently been scaled up to non-human primates, opening the potential to evaluate the neuroprotective property of parkin in a genetic primate model of PD (27, 46). The demonstration that parkin is able to overcome the considerable difficulty of improving the pathological phenotypes observed in genetic animal models of disease further strengthens the case for parkin-based strategies as promising treatments for patients with PD.
Acknowledgments
We thank Nicole Déglon, William Pralong, and Ruth-Luthi Carter for helpful comments; Philippe Colin, Christel Sadeghi, Anne Maillard, and Maria Rey for excellent technical help; and Dr. Michel Goedert for the A30P human α-synuclein cDNA. This work was supported by the Swiss National Science Foundation and the Michael J. Fox Foundation.
Author contributions: C.L. and P.A. designed research; C.L., B.L.S., A.B., and T.I. performed research; C.L., B.L.S., M.B., and A.S. analyzed data; and C.L. and P.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AR-JP, autosomal recessive juvenile parkinsonism; PD, Parkinson's disease; TH, tyrosine hydroxylase; TH-IR, TH-immunoreactive; YFP, yellow fluorescent protein.
References
- 1.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- 2.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18, 106–108. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. (1998) Proc. Natl. Acad. Sci. USA 95, 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., et al. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, T. M. & Dawson, V. L. (2003) Science 302, 819–822. [DOI] [PubMed] [Google Scholar]
- 7.Valente, E. M., Abou-Sleiman, P. M., Caputo, V., Muqit, M. M., Harvey, K., Gispert, S., Ali, Z., Del Turco, D., Bentivoglio, A. R., Healy, D. G., et al. (2004) Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- 8.Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y. & Shimizu, N. (1998) Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- 9.Shimura, H., Hattori, N., Kubo, S., Mizuno, Y., Asakawa, S., Minoshima, S., Shimizu, N., Iwai, K., Chiba, T., Tanaka, K. & Suzuki, T. (2000) Nat. Genet. 25, 302–305. [DOI] [PubMed] [Google Scholar]
- 10.Cookson, M. R. (2003) Neuron 37, 7–10. [DOI] [PubMed] [Google Scholar]
- 11.Cookson, M. R. (2003) Neuromol. Med. 3, 1–13. [DOI] [PubMed] [Google Scholar]
- 12.Shimura, H., Hattori, N., Kubo, S., Yoshikawa, M., Kitada, T., Matsumine, H., Asakawa, S., Minoshima, S., Yamamura, Y., Shimizu, N. & Mizuno, Y. (1999) Ann. Neurol. 45, 668–672. [DOI] [PubMed] [Google Scholar]
- 13.Imai, Y., Soda, M. & Takahashi, R. (2000) J. Biol. Chem. 275, 35661–35664. [DOI] [PubMed] [Google Scholar]
- 14.Darios, F., Corti, O., Lucking, C. B., Hampe, C., Muriel, M. P., Abbas, N., Gu, W. J., Hirsch, E. C., Rooney, T., Ruberg, M. & Brice, A. (2003) Hum. Mol. Genet. 12, 517–526. [DOI] [PubMed] [Google Scholar]
- 15.Petrucelli, L., O'Farrell, C., Lockhart, P. J., Baptista, M., Kehoe, K., Vink, L., Choi, P., Wolozin, B., Farrer, M., Hardy, J. & Cookson, M. R. (2002) Neuron 36, 1007–1019. [DOI] [PubMed] [Google Scholar]
- 16.Oluwatosin-Chigbu, Y., Robbins, A., Scott, C. W., Arriza, J. L., Reid, J. D. & Zysk, J. R. (2003) Biochem. Biophys. Res. Commun. 309, 679–684. [DOI] [PubMed] [Google Scholar]
- 17.Imai, Y., Soda, M., Inoue, H., Hattori, N., Mizuno, Y. & Takahashi, R. (2001) Cell 105, 891–902. [DOI] [PubMed] [Google Scholar]
- 18.Staropoli, J. F., McDermott, C., Martinat, C., Schulman, B., Demireva, E. & Abeliovich, A. (2003) Neuron 37, 735–749. [DOI] [PubMed] [Google Scholar]
- 19.Tsai, Y. C., Fishman, P. S., Thakor, N. V. & Oyler, G. A. (2003) J. Biol. Chem. 278, 22044–22055. [DOI] [PubMed] [Google Scholar]
- 20.Yang, Y., Nishimura, I., Imai, Y., Takahashi, R. & Lu, B. (2003) Neuron 37, 911–924. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi, H., Ohama, E., Suzuki, S., Horikawa, Y., Ishikawa, A., Morita, T., Tsuji, S. & Ikuta, F. (1994) Neurology 44, 437–441. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, S., Wakabayashi, K., Ishikawa, A., Nagai, H., Saito, M., Maruyama, M., Takahashi, T., Ozawa, T., Tsuji, S. & Takahashi, H. (2000) Movement Disorders 15, 884–888. [DOI] [PubMed] [Google Scholar]
- 23.van de Warrenburg, B. P., Lammens, M., Lucking, C. B., Denefle, P., Wesseling, P., Booij, J., Praamstra, P., Quinn, N., Brice, A. & Horstink, M. W. (2001) Neurology 56, 555–557. [DOI] [PubMed] [Google Scholar]
- 24.Lo Bianco, C., Ridet, J. L., Schneider, B. L., Deglon, N. & Aebischer, P. (2002) Proc. Natl. Acad. Sci. USA 99, 10813–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein, R. L., King, M. A., Hamby, M. E. & Meyer, E. M. (2002) Hum. Gene Ther. 13, 605–612. [DOI] [PubMed] [Google Scholar]
- 26.Kirik, D., Rosenblad, C., Burger, C., Lundberg, C., Johansen, T. E., Muzyczka, N., Mandel, R. J. & Bjorklund, A. (2002) J. Neurosci. 22, 2780–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirik, D., Annett, L. E., Burger, C., Muzyczka, N., Mandel, R. J. & Bjorklund, A. (2003) Proc. Natl. Acad. Sci. USA 100, 2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauwers, E., Debyser, Z., Van Dorpe, J., De Strooper, B., Nuttin, B. & Baekelandt, V. (2003) Brain Pathol. 13, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara, H., Hasegawa, M., Dohmae, N., Kawashima, A., Masliah, E., Goldberg, M. S., Shen, J., Takio, K. & Iwatsubo, T. (2002) Nat. Cell Biol. 4, 160–164. [DOI] [PubMed] [Google Scholar]
- 30.Deglon, N., Tseng, J. L., Bensadoun, J. C., Zurn, A. D., Arsenijevic, Y., Pereira de Almeida, L., Zufferey, R., Trono, D. & Aebischer, P. (2000) Hum. Gene Ther. 11, 179–190. [DOI] [PubMed] [Google Scholar]
- 31.Blomer, U., Naldini, L., Kafri, T., Trono, D., Verma, I. M. & Gage, F. H. (1997) J. Virol. 71, 6641–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltramino, C. A., de Olmos, J. S., Gallyas, F., Heimer, L. & Zaborszky, L. (1993) NIDA Res. Monogr. 136, 101–126; discussion 126–132. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg, M. S. & Lansbury, P. T., Jr. (2000) Nat. Cell Biol. 2, E115–E119. [DOI] [PubMed] [Google Scholar]
- 34.Cummings, C. J., Reinstein, E., Sun, Y., Antalffy, B., Jiang, Y., Ciechanover, A., Orr, H. T., Beaudet, A. L. & Zoghbi, H. Y. (1999) Neuron 24, 879–892. [DOI] [PubMed] [Google Scholar]
- 35.Shimura, H., Schwartz, D., Gygi, S. P. & Kosik, K. S. (2004) J. Biol. Chem. 279, 4869–4876. [DOI] [PubMed] [Google Scholar]
- 36.Petrucelli, L., Dickson, D., Kehoe, K., Taylor, J., Snyder, H., Grover, A., De Lucia, M., McGowan, E., Lewis, J., Prihar, G., et al. (2004) Hum. Mol. Genet. 13, 703–714. [DOI] [PubMed] [Google Scholar]
- 37.Neumann, M., Kahle, P. J., Giasson, B. I., Ozmen, L., Borroni, E., Spooren, W., Muller, V., Odoy, S., Fujiwara, H., Hasegawa, M., et al. (2002) J. Clin. Invest. 110, 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwatsubo, T. (2003) J. Neurol. 250, Suppl. 3, 11–14. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, M., Kanuka, H., Fujiwara, H., Koyama, A., Hasegawa, M., Miura, M. & Iwatsubo, T. (2003) Neurosci. Lett. 336, 155–158. [DOI] [PubMed] [Google Scholar]
- 40.Conway, K. A., Rochet, J. C., Bieganski, R. M. & Lansbury, P. T., Jr. (2001) Science 294, 1346–1349. [DOI] [PubMed] [Google Scholar]
- 41.Volles, M. J., Lee, S. J., Rochet, J. C., Shtilerman, M. D., Ding, T. T., Kessler, J. C. & Lansbury, P. T., Jr. (2001) Biochemistry 40, 7812–7819. [DOI] [PubMed] [Google Scholar]
- 42.Volles, M. J. & Lansbury, P. T., Jr. (2002) Biochemistry 41, 4595–4602. [DOI] [PubMed] [Google Scholar]
- 43.Gispert, S., Del Turco, D., Garrett, L., Chen, A., Bernard, D. J., Hamm-Clement, J., Korf, H. W., Deller, T., Braak, H., Auburger, G. & Nussbaum, R. L. (2003) Mol. Cell. Neurosci. 24, 419–429. [DOI] [PubMed] [Google Scholar]
- 44.Chung, K. K., Zhang, Y., Lim, K. L., Tanaka, Y., Huang, H., Gao, J., Ross, C. A., Dawson, V. L. & Dawson, T. M. (2001) Nat. Med. 7, 1144–1150. [DOI] [PubMed] [Google Scholar]
- 45.Lo Bianco, C., Déglon, N., Pralong, W. & Aebischer, P. (2004) Neurobiol. Dis., in press. [DOI] [PubMed]
- 46.Kirik, D. & Bjorklund, A. (2003) Trends Neurosci. 26, 386–392. [DOI] [PubMed] [Google Scholar]