Abstract
African NHPs are infected by over 40 different simian immunodeficiency viruses. These viruses have coevolved with their hosts for long periods of time and, unlike HIV in humans, infection does not generally lead to disease progression. Chronic viral replication is maintained for the natural lifespan of the host, without loss of overall immune function. Lack of disease progression is not correlated with transmission, as SIV infection is highly prevalent in many African NHP species in the wild. The exact mechanisms by which these natural hosts of SIV avoid disease progression are still unclear, but a number of factors might play a role, including: (i) avoidance of microbial translocation from the gut lumen by preventing or repairing damage to the gut epithelium; (ii) control of immune activation and apoptosis following infection; (iii) establishment of an anti-inflammatory response that resolves chronic inflammation; (iv) maintenance of homeostasis of various immune cell populations, including NK cells, monocytes/macrophages, dendritic cells, Tregs, Th17 T-cells, and γδ T-cells; (v) restriction of CCR5 availability at mucosal sites; (vi) preservation of T-cell function associated with down-regulation of CD4 receptor. Some of these mechanisms might also be involved in protection of natural hosts from mother-to-infant SIV transmission during breastfeeding. The difficulty of performing invasive studies in the wild has prohibited investigation of the exact events surrounding transmission in natural hosts. Increased understanding of the mechanisms of SIV transmission in natural hosts, and of the early events post-transmission which may contribute to avoidance of disease progression, along with better comprehension of the factors involved in protection from SIV breastfeeding transmission in the natural hosts, could prove invaluable for the development of new prevention strategies for HIV.
Keywords: SIV, natural hosts, African NHPs, pathogenesis, transmission, CCR5
Introduction
A family of retroviruses, collectively referred to as simian immunodeficiency viruses (SIVs), naturally infect a large number of African NHP species. Currently, over 40 different SIVs are known to be circulating in wild African NHPs. As natural hosts of SIVs, these NHPs generally do not progress to AIDS, despite chronic, highly active viral replication that is in same range as or even greater than what is typically reported in HIV patients (Ansari and Silvestri, 2014; Chahroudi et al., 2012; Locatelli et al., 2014; Pandrea and Apetrei, 2010; Pandrea et al., 2008a; VandeWoude and Apetrei, 2006). However, there have been some rare cases where AIDS or AIDS-like symptoms have been reported in natural hosts. These cases occurred primarily in NHPs in captivity that had outlived their normal lifespan of their species in the wild and had been infected and followed for decades (Apetrei et al., 2004; Ling et al., 2004; Pandrea et al., 2001, 2009). This clearly indicates that the lack of disease progression in the natural hosts of SIVs is not the result of infection by benign viruses, but rather that these species have adapted to avoid progression to AIDS. Although relatively sparse compared to the body of data on HIV, the research done on the pathogenesis of SIV-infection in natural hosts indicates that the lack of disease progression does not appear to be mediated by a more effective immune response by the host, but rather better management of the deleterious consequences of infection (Pandrea et al., 2008a).
The exact mechanisms by which natural hosts are able to avoid progression to AIDS in the face of a highly active infection are a subject of intensive research. Whatever they may be, the adaptations leading to control of the deleterious consequences of SIV infection in natural hosts are almost certainly the result of long-term coevolution between African hosts and their species-specific SIVs (Chahroudi et al., 2012; Fischer et al., 2012; Kobayashi et al., 2014; Ma et al., 2013; Pandrea and Apetrei, 2010). Here, we will review the current knowledge regarding SIV-infection in natural hosts, with an extra focus on the events surrounding transmission. The current research indicates that the key events involved in controlling and preventing disease progression appear to occur very early in infection, during the initial period following viral entry, and may be localized to the mucosal sites of infection. Understanding these mechanisms and comparing them to the mechanisms underlying progressive infections has the potential to unlock novel prevention strategies for HIV patients and potentially new paths to reaching a ‘functional cure’ for HIV.
1. Evolutionary History of SIVs in African NHPs
1.1. SIVs naturally infect only wild African NHPs
Before considering the exact features of SIV infection in African NHPs, it is important to understand the long evolutionary relationship that exists between these viruses and their hosts. SIVs have so far been found to infect over 45 species of African NHPs. This means that more than 40 genetically distinct SIVs are known to be circulating in the wild. While most natural hosts are infected by a single species-specific SIV, there are several examples of African NHP species, including mandrills, mustached monkeys, and mantled guerezas, which have multiple SIVs cocirculating in the wild (Ansari and Silvestri, 2014). No SIVs have been found yet that infect any wild Asian or New World NHPs. Therefore, Asian macaques are nonnatural hosts of SIV (Ansari and Silvestri, 2014; Lowenstine et al., 1986; Ohta et al., 1988). The various SIVmac variants that are used to experimentally infect rhesus macaques (RMs), pigtailed macaques, crab-eating macaques and stump-tailed macaques in captivity actually represent cross-species transmissions of SIVs from sooty mangabeys (SM), an African NHP species (Apetrei et al., 2005). In macaques and other Asian NHPs, SIV infections are pathogenic and lead to the development of AIDS, which is why the RM has become the primary model for HIV in humans.
From an evolutionary perspective, humans could also be considered to be a nonnatural host for HIV, as HIV only appeared in human populations within the last century, when the virus was acquired from chimpanzees/gorillas and sooty mangabeys. The progression to AIDS reflects the poor evolutionary adaption between HIV and humans. A similar situation exists in chimpanzees, which also acquired SIVcpz from various monkey species through cross-species transmissions and recombination events (Bailes et al., 2003), and then became the source of the SIVgor that infects gorillas (D’arc et al., 2015; Faria et al., 2014; Hirsch et al., 1989; Keele et al., 2006; Van Heuverswyn et al., 2006). Like HIV in humans, progression to AIDS has been shown to be a possible outcome of SIVcpz infection in chimpanzees, both in captivity and in the wild, though it is as of yet unclear how prevalent progression to AIDS is amongst wild chimps (Etienne et al., 2012; Keele et al., 2009a).
1.2. African NPHs and SIVs have coexisted for an extremely long period of time
Various studies incorporating geology, phylogeny and molecular genetics point to very ancient origin of SIV, though the exact estimates vary greatly. Some of the most concrete evidence for the origin of SIV infections comes from a study of NHPs isolated on Bioko Island. Located off the northern coast of Equatorial Guinea, Bioko Island was separated from the mainland around 10,000 years ago. Phylogenetic analysis of the SIVs endemic to the NHPs on the island revealed that each SIV was most genetically similar to SIVs infecting NHPs of the same genus on the mainland. This suggests that SIVs were already circulated in these species at the time of Bioko Island separation from the mainland, i.e., that SIVs are at the very least over 10,000 years old (Worobey et al., 2010). On the other end of the spectrum, an endogenous lentivirus infecting the gray mouse lemur in Madagascar was shown to be genetically related to mainland SIVs. As the NHPs on Madagascar have been separated from African NHPs for at least 14 million years, this study points to an extremely ancient origin of SIVs (Gifford et al., 2008; Perelman et al., 2011). Due to the discrepancy with the molecular clock data, it generally can be surmised that SIVs have been infecting African primates for anywhere between tens of thousands to millions of years (Compton and Emerman, 2013; Gifford et al., 2008; Ma et al., 2013). In fact, it is possible that SIVs have been infecting NHPs since the time of primate speciation, given the complete absence of SIVs in Asian and New World NHPs (VandeWoude and Apetrei, 2006).
While the precise age of SIV infections in their natural hosts may be uncertain, they clearly share a long evolutionary history together. One notable example illustrating this relationship can be found in the phylogeny of the various strains of SIVagm, which infect African green monkeys (AGMs). AGMs are NHPs belonging to the genus Chlorocebus. The genus encompasses four species of AGM: sabaeus, grivet, tantalus, and vervet (Ansari and Silvestri, 2014; Pandrea et al., 2008a), which were shown to be infected with a species-specific virus (Allan et al., 1991). The AGM subspecies are known to have diverged from their common ancestor between 1.5–3 million years ago and then spread out over sub-Saharan African, each settling into a different region (Xing et al., 2007). Given the geographical and temporal divergence of the subspecies, it is likely that their respective viruses also evolved in tandem with their hosts (Ma et al., 2013). This conclusion is supported by a recent study examining the diversity of SIVagm in AGMs geographical separated by the Drakensberg mountains along the coast of South Africa. Phylogenetic analysis demonstrated that the costal viruses and the inland viruses clustered separately and were genetically distinct from each other. As the Drakensberg mountains are around 280 million years old, the divergence between the two groups could have occurred anywhere between the aforementioned spread of AGMs 3 million years ago to around 100,000 years ago, during the mass migrations that occurred in the Plio-Pleistocene glacial period (Ma et al., 2013).
1.3. SIVs are genetically diverse and readily undergo cross-species transmission and recombination
The extensive evolutionary history of SIVs is also reflected in part by the extreme genetic diversity of SIVs and, subsequently, HIVs. Phylogenetic analysis of SIVs has revealed that there have been numerous cross-species transmissions and recombination events in the past, with many SIVs arising after crossing over between two sympatric species (Pandrea and Apetrei, 2010). There are also multiple examples of cross-species transmission subsequently followed by recombination, including: (i) SIVmnd-2, which is the result of a recombination between SIVmnd-1 and SIVrcm; (ii) SIVsab, which is an ancient recombinant of an ancestral SIVsab and SIVrcm; (iii) SIVmus, SIVmon, and SIVgsn, three viruses from three different NHP species (mustached monkeys, mona monkeys and greater spot-nosed monkeys) which share genetic and phylogenetic features, suggesting recombination (Jin et al., 1994a; Liégeois et al., 2014; Takemura and Hayami, 2004).
Another notable example is SIVcpz, which shares genetic similarity to both SIVrcm and SIVgsn. Chimpanzees regularly hunt other primates for food and the recombination of these two viruses almost certainly occurred in chimps (Bailes et al., 2003). This emergence of SIVcpz is most likely a relatively recent event, as is reflected by the reports of 10- to 16- fold increase in mortality and AIDS-like disorders in wild SIVcpz-infected chimpanzees (Etienne et al., 2011; Keele et al., 2009a; Rudicell et al., 2010). Additionally, expression levels of the SIV coreceptor CCR5 on the surface of chimpanzee CD4+ T-cells are intermediate between natural hosts and nonnatural hosts. As CCR5 expression levels are generally considered a marker of host adaptation to SIV (Pandrea et al., 2007a), this intermediary CCR5 expression on the CD4+ T-cells of chimps suggests a relatively recent cross-species transmission and consequently an incomplete adaptation of the chimpanzee host to the virus (Pandrea and Apetrei, 2010).
The propensity of SIVs to readily cross over between species is reflected by the fact that both HIV-1 and HIV-2 originated from multiple cross-species transmissions of SIVs to humans (Sharp and Hahn, 2011). HIV-1 arose from cross-species transmissions from two different primate sources. HIV Groups M and N are the result of SIVcpz transmission of from chimpanzees to humans (Keele et al., 2006). SIVgor, which is itself a cross-species transmission of SIVcpz from chimps to gorillas, went on to ultimately cross over to humans and generate HIV groups O and P (D’arc et al., 2015; Plantier et al., 2009; Takehisa et al., 2009; Van Heuverswyn et al., 2006). HIV-2, on the other hand, arose from multiple transmission of SIVsmm directly from SMs in Western Africa to humans (Ayouba et al., 2013; Hirsch et al., 1989; Sharp and Hahn, 2011). With increasing rates of interaction between African NHPs and humans due to poaching, hunting for bushmeat and deforestation (Cronin et al., 2015; Ziegler et al., 2016), it seems probable that further cross-species transmissions might occur and give rise to new HIVs. Consequently, it is important that further research be carried out to understand the evolutionary dynamics of SIVs in the wild (Aghokeng et al., 2010; Locatelli and Peeters, 2012).
2. General features of SIV transmission in natural hosts
2.1. Studying the natural history of SIV infection in African NHPs is extremely challenging
The information regarding the natural history of SIVs is relatively sparse. There are a variety of reasons for this: (i) lack of sufficient infrastructure to support field research; (ii) the endangered status of many African NHPs; (iii) reliance on alternative noninvasive sampling, including bushmeat and feces, which do not permit proper pathological or immunological studies. However, despite the intrinsic difficulty of field research, we carried out a number of studies that have been able to elucidate some of the characteristic features of SIV infection in the wild, including prevalence, viral loads (VLs) and routes of transmission.
2.2. Most features of SIV infection of natural hosts have been derived from studies on a limited number of species in captivity
Virtually all of the data regarding the characteristics of SIV infection of natural hosts have been derived from studies on a limited number of three animal models (AGMs, SMs and mandrills) in captivity (Ansari and Silvestri, 2014; Pandrea and Apetrei, 2010). There are several reasons for this. First, most SIVs are only known from their genetic sequences and have never been properly isolated or characterized. SIVagm, SIVsmm, and SIVmnd-1/SIVmnd-2 are among the minority of SIVs that have been studied in detail. Second, the majority of African NHPs are not widely available in primate facilities and importation of NHPs directly from Africa is virtually impossible. Third, as mentioned above, many NHPs are endangered and field work on wild animals is exceptionally difficult.
Our lab developed a new model of SIVsab infection in Caribbean AGMs that circumvents most of these problems. The difficulty in importation is averted, as AGMs (sabaeus) from the Caribbean are easy to import. Since the AGMs were brought from Africa to the Caribbean over 300 years ago during the 17th and 18th centuries, they have become well established, to the point of being considered a pest in some areas (Denham, 1981). These animals have been shown to be both free of SIVagm, and genetically indistinguishable from the sabaeus found in Africa, making them ideal for experimental studies (Daniel et al., 1988; Hendry et al., 1986; Pandrea et al., 2006a). Finally, neither African nor Caribbean AGMs are endangered, meaning that more invasive methods can be used for the study of SIV pathogenesis in AGMs, including experimental infections, blood draws and tissue sampling.
2.3. SIVs can be highly prevalent in natural hosts in the wild
In many African NHP species and subspecies, SIV infections are very prevalent in the wild. Some examples include: red colobus, AGMs, SMs, red-capped mangabeys, De Brazza monkeys and mandrills (Bibollet-Ruche et al., 2004; Estaquier et al., 1991; Fouchet et al., 2012; Leendertz et al., 2010; Locatelli and Peeters, 2012; Ma et al., 2013). The prevalence rates in these species can be uniformly high across the population, as is the case in AGMs, where 80–90% of adult females and 50–60% of adult males in the wild test positive for SIVagm infection (Ma et al., 2013, 2014; Otsyula et al., 1995). However, the picture is less well defined in some other African NHP species. In gorillas and chimpanzees, SIVs tend to be distributed heterogeneously, with high prevalence in certain populations and low prevalence or absence of infection in others (Sharp and Hahn, 2011). There are also several species where the occurrence of SIV infection is quite low, like the greater spot-nosed monkey or the mustached monkey (Aghokeng et al., 2010). Whether this is the result of a more pathogenic outcome of SIV infection in these species, as it is observed with the chimpanzees, or merely lower transmission rates is not currently known. It could also be that there simply has not been enough testing of these species to ascertain the true prevalence of infection. Or, it is possible that prevalence in these species is sporadic, with only particular populations harboring SIV, as is the case with gorillas (Neel et al., 2010). The disparity in SIV prevalence between different African NHP species further illustrates the need for more studies of SIV transmission and pathogenesis in wild natural hosts.
2.4. Baboons and other primates lack species-specific SIVs, despite being African NHPs
Despite the ubiquity of SIVs in the wild, it is important to note that not all African NHPs are in fact natural hosts for SIVs. A significant exception is represented by the different baboon species, which do not carry species-specific SIVs of their own. Instead, the SIVs that are found circulating in baboons are cross-species transmissions of SIVagm from the sympatric AGM populations (Jin et al., 1994b; van Rensburg et al., 1998). Very little is currently known about the pathogenesis of these SIVs in wild baboons. However, it has been shown that when experimentally infected with HIV-2, baboons will progress to AIDS like humans and macaques (Locher et al., 2001, 2003). Accordingly, CCR5 expression on CD4+ T-cells in baboons is similar to the levels expressed in humans and RMs (Pandrea et al., 2007a, 2009). Any other data on SIV-infection in baboons are extremely scarce, even when compared to the research done in other wild African NHPs. Since baboons have long cohabitated the same geographical locations as many SIV-infected NHP species, the lack of information regarding their place in the natural history of SIVs represents an important gap in the knowledge of the field.
Besides baboons, there are several other important African NHP species that have no species-specific SIV: (i) white crowned mangabeys (one study did report SIV infection, but that was in fact a cross species transmission of SIVagm that occurred in captivity) (Tomonaga et al., 1993); (ii) Patas monkeys (which were also reported to be infected with SIVagm, but have no species specific SIV) (Apetrei et al., 2010; Bibollet-Ruche et al., 1996); (iii) Bonobos and two species of chimps (Heuverswyn et al., 2007; Leendertz et al., 2011; Li et al., 2012; Prince et al., 2002). As with the baboons, the question remains how these animals can share habitats with other NHP species harboring SIV and not have their own species-specific virus, given the propensity of SIVs for cross-species transmissions and recombination.
2.5. Horizontal transmission of SIVs in natural hosts is common
Based on the prevalence of SIV-infection of natural hosts in the wild, it can be inferred that horizontal transmission of SIV from one animal to another must be a relatively common event. Horizontal transmission in NHPs has been reported to occur in a variety of ways, including: (i) sexual contact; (ii) biting; (iii) fighting; and (iv) grooming (Cooper et al., 1989; Estaquier et al., 1991; Etienne et al., 2012; Keele et al., 2009a; Ma et al., 2013; Otsyula et al., 1995; Phillips-Conroy et al., 1994; Rudicell et al., 2011; Santiago et al., 2005; Souquière et al., 2001). Compared to HIV in humans, where urogenital mucosal transmission constitutes the major route of horizontal transmission and where oral transmission is remarkably infrequent (apart from transmission during breastfeeding), in NHPs SIV transmission through oral exposure is fairly common (Baggaley et al., 2008; Chenine et al., 2010; Haase, 2011; Ruprecht et al., 1999). Consequently, transmission while grooming or cleaning wounds (i.e., oral exposure) most likely represents a significant route of infection in natural hosts. There have also been multiple descriptions of SIV transmission through biting in captive NHPs (Corbet et al., 2000; Lowenstine et al., 1992; Phillips-Conroy et al., 1994; Rey-Cuillé et al., 1998). Biting was also reported to constitute a route of transmission during fights for dominance between male mandrills in Gabon (Nerrienet et al., 1998). It is important to note that in these cases, whether through grooming or biting, NHPs are being exposed to blood, which typically has a significantly higher VL than breast milk or sexual secretions. This may explain in part why horizontal transmission via the oral mucosa is more frequent in NHPs than humans.
2.6. Vertical transmission of SIVs in natural hosts is rare
Unlike horizontal transmission, vertical transmission, also known as mother-to-infant transmission (MTIT), is exceedingly rare in natural hosts. In humans and macaques, exposure in utero or through breastfeeding represent the major routes of MTIT. The rates of MTIT via these routes can be quite high, with 35–40% of infants born to HIV-infected mothers becoming infected, especially if the mothers are acutely infected (Aldrovandi and Kuhn, 2010), and 40–70% of infant RM becoming infected when born to SIVmac-infected dams (Amedee et al., 2004). By comparison, in recent studies of wild vervets in South Africa and Gambia, the rates of MTIT were shown to be only 4–7% (Ma et al., 2013, 2014). Studies of natural hosts housed in various Primate Centers have also found a very low incidence of vertical transmission, supporting the findings of the surveys of animals in the wild (Chahroudi et al., 2011; Fouchet et al., 2012; Fultz et al., 1990; Ogino et al., 2013; Pandrea et al., 2008b).
One implication of the lack of MTIT in natural hosts is that there must be some evolutionary pressure against vertical transmission in the wild. While further research on this subject is still necessary, one possible explanation is that in the wild natural hosts normally live less than 20 years. Assuming that SIVs originally were pathogenic in their natural hosts and had a time frame for progression to AIDS similar to HIV patients (~10 years from infection until death) or perhaps an even more rapid progression, like in RMs (~2 years from infection until death), a delayed infection occurring only after reaching the age of sexual maturity might have conferred a significant evolutionary advantage. Indeed, given that all the cases of AIDS-like disease occurred in natural hosts in captivity in monkeys that have far exceeded their normal life expectancy, it is possible that such an evolutionary pressure still exists today (Chahroudi et al., 2012; Pandrea et al., 2009; Sodora et al., 2009; VandeWoude and Apetrei, 2006).
3. Horizontal SIV transmission in natural hosts
3.1. Mucosal transmission has only been described at a detailed level in nonnatural hosts
Studying the events following mucosal transmission through sexual contact in wild natural hosts is nearly impossible, for the reasons described above. Much of what is currently known about horizontal SIV transmission via the urogenital mucosa originated from studies of intravaginal transmission of SIVmac in RMs (Cohen et al., 2011; Haase, 2010, 2011). A very recent study also confirmed that the same general events outlined below also occur during penile transmission of SIVmac in RMs (Ma et al., 2016). While some aspects surrounding mucosal transmission have been shown to differ between natural and nonnatural hosts, the studies of mucosal transmission in RMs should provide a good approximation of the general events (Haase, 2011).
From the RM studies, it appears that the very first phase of transmission occurs when the virus crosses the mucosal epithelium and infects a small ‘founder population’ of target cells. This can occur at multiple locations throughout the mucosal site of entry, leading to establishment of ‘foci’ of early virus replication. These foci may appear preferentially where there are higher densities of target cells in the epithelium, though this has only been shown in the RM intravaginal model of transmission (Stieh et al., 2014). The founder CD4+ T-cell populations frequently exhibit markers indicating that they had been previously activated (i.e., still expressing enough CCR5 and transcriptional activators to support SIV infection) but are no longer active or proliferating (CD69neg CD25neg Ki67neg), and therefore are referred to as being in a ‘resting’ state (Li et al., 2005).
These ‘resting’ CD4+ T-cells were found to outnumber other resident immune cells types, such as dendritic cells and macrophages, at a ratio ranging between 4–5:1 (Zhang et al., 2004). However, mathematical modeling found that the number of these target cells normally present in the mucosa is not sufficient to sustain viral replication alone. In order to expand, the virus needs new target cells to be recruited to the early foci of replication by the innate inflammatory immune response stimulated by the virus. Following the recruitment of the target cells and viral expansion, the virus disseminates to the draining lymph nodes, where there is a high enough concentration of CD4+ T-cells to facilitate its rapid expansion. Traveling through the lymphatics, the virus is able to enter the bloodstream through the thoracic duct and spread systemically (Haase, 2011). One implication of this model is that there may be a window of opportunity for intervention prior to recruitment of additional target cells to the site of entry and viral dissemination, when infection is limited to a relatively small number of target cells. However, more research is necessary to test this hypothesis.
3.2. Target cell availability is reduced in the gut in natural hosts, but still correlates with rates of transmission
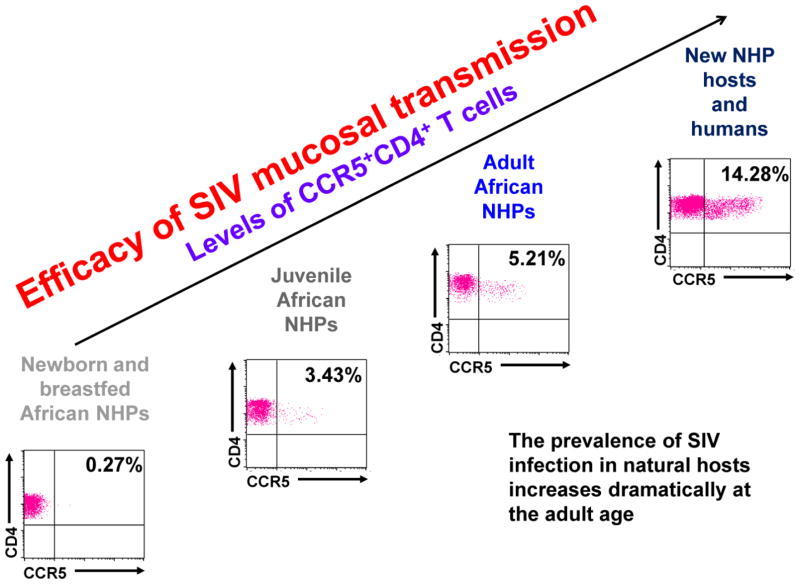
While most of the detailed knowledge on mucosal transmission comes from the RM intravaginal studies, there has also been significant research done on mucosal sites in natural hosts, particularly at the gastro-intestinal tract mucosa. A study of a large cohort of AGMs of varying ages demonstrated that the numbers of CD4+ CCR5+ cells are very low not only in the blood, but also in the gut and jejunum. A strong mathematical correlation was found between increases in levels of gut CD4+ CCR5+ T-cells and the rate of SIV mucosal transmission in these animals (Ma et al., 2014). Another experimental study established that a 10-fold higher dosage of SIVsab was needed to successfully intrarectally and intravaginally inoculate adult AGMs versus adult PTMs. This reduced susceptibly was correlated with the number of CD4+ CCR5+ T-cells in the rectum, which was shown to be 10-fold lower in the AGMs than PTMs (Pandrea et al., 2012a) (Figure 1).
Figure 1. Studies in natural hosts of SIVs lead to the hypothesis that susceptibility to SIV/HIV transmission is proportional to the levels of CD4+ T-cells expressing CCR5+ at the mucosal sites, which are higher in nonnatural hosts than natural hosts and are also correlated with age.
Each separate graphic represents flow cytometry gating for total gut CD4+ CCR5+ T-cells in the respective hosts, with the total percent population of the CD4+ CCR5+ T-cells given in the right hand corner.
Considering the extremely high prevalence of SIV infection in the wild, it is apparent that reduction in overall target cell availability in the gut does not coincide with protection of natural hosts from either transmission or CD4+ depletion in the gut associated lymphatic tissue (GALT, see 5.5), though it may render them more resistant to infection (Pandrea et al., 2012a). Therefore, it can be assumed that there may be an alternative effect of this reduction on the course of infection. One possible explanation is that, as CCR5 is a chemokine receptor, reduced expression of CCR5 by the CD4+ T-cells of natural hosts might decrease homing of effector memory cells to the gut, which in turn could reduce immune activation and inflammation (Pandrea and Apetrei, 2010).
3.3. A genetic bottleneck is imposed by the mucosal epithelium on the number of transmitted SIV variants
It has been demonstrated that the mucosal barrier actually acts as a significant obstacle to both SIV and HIV transmission, being difficult to cross, particularly in the vagina, where much of the mucosa is covered by durable stratified layers of squamous cell. However, a recent RM-based study demonstrated that transmission is possible at any location along the female reproductive tract (Stieh et al., 2014). On the other hand, the lower intestinal tract mucosa is formed by a simple columnar epithelium shaped into straight tubular crypts, being much thinner than vaginal mucosa and making the target cells more accessible to the virus. Due to these architectural features, the virus can cross the intestinal mucosa more easily than the vaginal mucosa.
There is also a stochastic barrier to infection at the mucosa, as the overall number of target cells initially available to infect is quite low in RMs and even lower in natural hosts. One consequence of these steep impediments to transmission is a ‘mucosal bottleneck’, where only a small number of transmitted viruses are actually able to go on to establish infection (Haase, 2011; Pandrea et al., 2007a, 2008b).
This genetic bottleneck imposed on transmitted viruses has been documented during mucosal transmission of HIV and SIVmac (Keele et al., 2008, 2009b). The same bottleneck was also reported in AGMs, both in the wild and in captivity (Ma et al., 2014; Pandrea et al., 2012a). In a study of AGMs in captivity, following both intravaginal and intrarectal inoculations with SIVagm, only 1–2 viruses were found to have been transmitted and then establish infection in each animal (Pandrea et al., 2012a). This agrees with the finding of a more recent study, which found that only a single transmitted viral variant established infection in multiple AGMs in the wild (Ma et al., 2014). The similarity of the mucosal bottleneck observed in both natural and nonnatural hosts implies that there is little difference in the way the mucosal epithelium restricts viral transmission.
4. Characteristics of horizontal and vertical transmission through the oral mucosa in natural hosts
4.1. Oral mucosal transmission is generally similar to transmission at other mucosal sites
Several studies in RMs have shown that SIV transmission across the oral mucosa is similar to transmission across other mucosal sites, such as the gut and the vagina (see 3.1). In these studies, both infant and adult RMs were shown to become infected after atraumatic oral inoculation (though a larger dosage of virus was necessary to infect adult RMs versus neonates) (Chenine et al., 2005; Milush et al., 2004). SIVmac was detectable in CD4+ T-cells and macrophages within 4 days post inoculation, with SIV replication being most concentrated in the oral mucosa, esophagus, tonsils and draining LNs. This approximates what was seen in the vaginal transmission studies, with establishment of viral replication at mucosal sites and virus dissemination into the draining lymphatics (Wood et al., 2013). Additionally, a pro-inflammatory response at the mucosa has been reported during oral SIVmac transmission in RMs, which may facilitate target cell recruitment to the site of infection to support early virus replication (Giavedoni et al., 2013).
4.2. Horizontal transmission through the oral mucosa is common in natural hosts, while vertical transmission by the same route is not
As previously mentioned, SIV transmission via the oral mucosa is a far more common route of transmission in wild NHPs than humans. Grooming and biting have been well documented as routes of horizontal transmission in natural hosts, both in captivity and in the wild (Cooper et al., 1989; Estaquier et al., 1991; Etienne et al., 2012; Keele et al., 2009a; Ma et al., 2013; Otsyula et al., 1995; Phillips-Conroy et al., 1994; Rudicell et al., 2011; Santiago et al., 2005; Souquière et al., 2001). This indicates that the oral mucosa is a portal of entry for SIV (through exposure to infectious blood, see 2.5), as well as an active site of viral replication and shedding in natural host. Nevertheless, MTIT during breastfeeding is very rare in natural hosts (see 2.6). Given the fact that the VLs in breast milk in chronically infected natural hosts are very high (between 104–106 SIV RNA copies/mL) (Gnanadurai et al., 2010; Ho et al., 2013; Wilks et al., 2011) significantly higher than what is typically seen in the breast milk of HIV-infected humans (Pillay et al., 2000), the very low levels of breastfeeding transmission may appear paradoxical (Permar et al., 2010a; Pillay et al., 2000; Salazar-Gonzalez et al., 2011). However, as discussed below, natural hosts have evolved a number of adaptions that protect them from MTIT, despite massive exposure of the oral mucosa to the virus through breast milk.
4.3. Protection from MTIT and oral transmission during breastfeeding correlates with age in natural hosts
Extensive research has revealed that apart from general resistance to disease progression, natural hosts are also characterized by even greater resistance to SIV infection until they reach the age of sexual maturity (Pandrea et al., 2012a) (Figure 1). This protection is observed both in utero, at delivery and during breastfeeding, which is one of the reasons that rates of MTIT remain so low in natural hosts (though high rates of miscarriage were observed in SIV-infected wild mandrills, which might be linked to SIV exposure in utero) (Pandrea, unpublished).
A correlation between age and susceptibility to infection was actually shown in one of the very first studies to examine prevalence and routes of transmission in natural hosts. Testing for SIV in wild grivets captured in the Awash National Park in Ethiopia established correlations between serological status for SIV antibodies, age and sex. Both male and female monkeys were seronegative for SIVgri until the age of sexual maturity (Phillips-Conroy et al., 1994). A follow-up study looked at samples collected over a period of two decades from a completely separate group of Ethiopian grivets and reported that, in that particular population, SIV was predominately transmitted by sexual contact, only occasionally by trauma and almost never by MTIT (Jolly et al., 1996). In a similar study examining the SIVgri serostatus in offspring born to SIVgri-infected dams, all the tested infants were seropositive for anti-SIV antibodies at birth, consistent with passive antibody transfer from the mother. However, postnatal antibody titers declined to low levels by 6 months and then to complete negativity by 1 year. As had been observed in the previous studies, the grivets remained seronegative until they reached the age of sexual maturity (Otsyula et al., 1995).
More recent studies looked at wild-caught vervets from multiple locations in South Africa and sabaeus from Gambia (Ma et al., 2013, 2014). These studies not only confirmed that prevalence of SIVagm-infection increased at the age of sexual maturity, but also found that the majority of acute infections (Fiebig Stage II, SIV seronegative animals with VLs above 106 RNA copies/mL plasma (Fiebig et al., 2003)) were in juvenile females at the age of the beginning of sexual activity. Of the infant AGMs tested, only 4–7% were found to be SIV-positive. This rate is very low, particularly in light of the fact that over 72% of lactating female dams tested SIVagm-positive and 44% of the infants had passively transferred anti-SIV antibodies (Ma et al., 2013). However, phylogenetic analysis showed that the viruses from the infected infants and juveniles were closely related to those found in adult females, strongly indicating vertical transmission through MTIT (Ma et al., 2013).
SIV prevalence has also been linked to age in wild populations of several other natural host species, including mandrills infected with SIVmnd-1 (Souquière et al., 2001) and SIVsmm-infected SMs (Chen et al., 1996; Fultz et al., 1990; Santiago et al., 2005; VandeWoude and Apetrei, 2006). These studies all demonstrated an age-related increase in SIV prevalence (VandeWoude and Apetrei, 2006). The same was found to hold true in captive animals, as studies of natural hosts housed in various Primate Centers confirmed a very low incidence of vertical transmission, supporting the findings of the surveys of animals in the wild (Chahroudi et al., 2011; Fouchet et al., 2012; Fultz et al., 1990; Ogino et al., 2013; Pandrea et al., 2008b).
Despite the numerous observational studies, to date, there has only been a single study that experimentally tested rates of SIV MTIT transmission in natural hosts (Pandrea et al., 2008b). In this study, mandrill dams were infected on the day of delivery and then allowed to nurse their offspring throughout the lactation period. Infecting the dams immediately after birth precluded any interference from passively transferred maternal antibodies. High VLs were confirmed in the milk and plasma of the dams, particularly during the acute phase of infection, yet none of the breastfed infants became infected. This observation is in contrast to similar studies of postnatal infection of RM dams, where 62–71% of infants became infected during breastfeeding (Amedee et al., 2003, 2004).
While it might be rare, MTIT does occur occasionally in natural hosts in the wild. As mentioned, the vervet study found 4–7% of infants to be SIV-positive. Also, observations of mandrills from Gabon revealed that SIVmnd transmission was more frequent within the same family of animals. While many infections were characteristic of horizontal transmission, possibly through grooming, wound care, or premastication of food, several were shown to be MTIT (Fouchet et al., 2012).
4.4. Oral transmission during breastfeeding in natural hosts does not correlate with virus shedding
It was initially suggested that a relatively low cell-associated VL in breast milk might be responsible for lower MTIT in natural hosts, but further studies in SMs showed little to no difference between SM and RM in milk VLs (Chahroudi et al., 2014; Wilks et al., 2011). It was later shown that while the VLs in breast milk in RMs and humans tends to be 1–2 log lower than plasma VLs, in AGMs plasma and milk VLs are similar (Permar et al., 2010a; Pillay et al., 2000; Salazar-Gonzalez et al., 2011). The aforementioned study of experimentally infected mandrill dames also demonstrated a lack of correlation between viral shedding in breast milk and rates of MTIT, despite the high VLs in breast milk (Pandrea et al., 2008b).
Interestingly, in nonnatural hosts (humans and RMs), plasma and milk virus are genetically similar, whereas in natural hosts milk virus is more compartmentalized and genetically distinct from plasma viral variants (Ho et al., 2013; Permar et al., 2010a; Salazar-Gonzalez et al., 2011; Wilks et al., 2011). This implies that the origin and replication of the virus in plasma vs. breast milk might be divergent in natural and nonnatural hosts; however, it is still unclear what implication this has for MTIT.
4.5. Factors in breast milk inhibit oral transmission, but are not unique to natural hosts
Human breast milk contains many factors that have been shown to inhibit HIV infection in vitro or are associated with lower rates of MTIT (Philippe Van de Perre, 2012). The long-standing question has been whether natural hosts exhibit additional factors in breast milk that could impair MTIT even more. Recent in vitro experiments with TZM-b1 cells showed that both SM and RM milk inhibits SIV infection, though the milk from SMs had a greater impact on the rate of infection. However, the results were somewhat confounded by the fact that SM breast milk contained more of the pro-inflammatory cytokine IL-8, which enhances cell-associated HIV infection. Additionally, there was no significant difference in the levels of antiviral factors, such as IFN-α, IFN-γ, TNF-α, MIP-1α, MIP-1β, RANTES and MDCs, in the milk of natural and nonnatural hosts (Chahroudi et al., 2014).
Besides cytokines and chemokines, antibodies are another factor postulated to play an important role in protecting natural hosts from MTIT. Anti-SIV IgG levels are 1–2 logs lower in the breast milk of SMs and AGMs than in macaques. While this might suggest that antibodies do not influence the rates of MTIT, breast milk IgA concentrations in the SMs and AGMs were shown to be roughly the same as the plasma IgA concentration, whereas milk IgA tends to be 0.5–1 log lower than plasma IgA in RMs and humans (Mabuka et al., 2012; Permar et al., 2010b; Rychert and Amedee, 2005). Interestingly, this trend parallels overall VLs in plasma vs. milk, suggesting that IgA levels might be correlated with virus levels. Furthermore, an SIV neutralizing IgG response was observed in the breast milk of AGMs that was not detected in RMs or humans (Amos et al., 2013; Fouda et al., 2011; Permar et al., 2010b; Wilks et al., 2011). As such, the impact of these milk factors (i.e., cytokines, chemokines and antibodies) on MTIT transmission in natural hosts remains uncertain.
4.6. Reduced target cell availability may protect natural hosts from oral transmission
As natural hosts have reduced availability of CD4+ CCR5+ T-cells (i.e., target cells) at the mucosal sites (see 5.4), target cell restriction at the oral mucosa has been proposed as a mechanism of protection from MTIT (Pandrea et al., 2008b). Infant RMs initially have low populations of CD4+ CCR5+ T-cells at their mucosal sites, but these levels increase dramatically to nearly the levels seen in adult RMs within the first 6 months postpartum (Pandrea et al., 2008b). This supports the observation that the virus challenge needed to infect newborn RM infants was 10-fold higher than the dose needed to infect older infants (Chenine et al., 2005). However, such an increase in CD4+ CCR5+ T-cells could not be observed in AGM infants or juveniles less than 3 years old (Ma et al., 2014; Pandrea et al., 2008b).
A recent study experimentally demonstrated the importance of CCR5+ availability to oral SIV transmission in AGMs. Infant AGMs were orally exposed to high concentrations of SIVsab, while juvenile and adult AGMs were intrarectally inoculated with the same dosage of virus. Following inoculation, none of the infant AGMs became infected, while half of the juveniles and all of the adults were infected. The juveniles that did not become infected were found to have lower levels of CD4+ CCR5+ T-cells in blood and mucosal sites than those that did become infected (Pandrea et al., 2012a).
No study has yet been able to measure the presence of CD4+ CCR5+ T-cells at the oral mucosa in wild natural hosts, given the difficulty of invasive sampling in the field. However, in the recent study of wild AGMs in Gambia, the highest frequency of circulating CD4+ T-cells expressing CCR5 among the infants tested was observed in the blood of the single infant AGM that was infected with SIVagm (Ma et al., 2014).
In the rare instances of MTIT in SMs, the SM infants infected through MTIT exhibited VLs 2 logs lower than those seen in adults, suggesting that only a low number of CD4+ CCR5+ T-cells support viral replication (Chahroudi et al., 2011). This is in contrast to what has been previously observed in human infants, where VLs are very high, sometimes exceeding those seen in adults (Chahroudi et al., 2011). Such an age-related difference in the levels of viral replication was not observed in AGMs (Ma et al., 2014). These results strongly suggest that target cell restriction at the oral mucosa does act to protect natural hosts from MTIT during breastfeeding and possibly until the age of sexual maturity, when the overall counts of mucosal CD4+ CCR5+ cells increase (Pandrea et al., 2008b) (Figure 1).
5. Post-transmission features that may impact SIV pathogenesis in natural hosts
5.1. Natural hosts sustain high levels of viral replication without disease progression
There are a number of features that distinguish natural, nonpathogenic hosts from nonnatural, pathogenic hosts, like macaques and humans (Table 1). Of these, the hallmark characteristic of all natural SIV infections are high levels of SIV replication during both the acute and chronic stages of infection (Brenchley et al., 2012; Diop et al., 2000a; Goldstein et al., 2006; Gordon et al., 2007; Gueye et al., 2004; Kouyos et al., 2010; Onanga et al., 2002, 2006; Pandrea et al., 2005, 2006a, 2008c, 2012a, 2007b; Silvestri et al., 2005; Souquière et al., 2009; Wilks et al., 2011). During the acute infection, the peaks of virus replication in natural hosts are comparable to those seen in untreated HIV-1 and SIVmac infections (Apetrei et al., 2007, 2011; Diop et al., 2000a; Goldstein et al., 2000; Gordon et al., 2007; Gueye et al., 2004; Ma et al., 2013, 2014; Milush et al., 2011; Mir et al., 2012, 2015; Onanga et al., 2002, 2006; Pandrea et al., 2003, 2006a, 2008c, 2012a, 2007b; Schmitz et al., 2012; Silvestri et al., 2005; Souquière et al., 2009; Vanderford et al., 2012). During the chronic infection, VLs in natural hosts tend to be as high or even slightly higher than the VLs seen in pathogenic infections (Pandrea et al., 2006b). Additionally, the chronic viral replication in natural hosts is remarkably stable, whereas it tends to vary widely and increase with disease progression in humans and macaques, thus being predictive of disease progression (Apetrei et al., 2007; Pandrea and Apetrei, 2010; Pandrea et al., 2003, 2008c; VandeWoude and Apetrei, 2006). As viral replication remains virtually unchanged throughout the course of chronic infection in natural hosts, it can be assumed that plasma viremia has no correlation with disease progression. However, in the rare instances where progression to AIDS has occurred in natural hosts, the levels of chronic viral replication were significantly higher (1–2 logs) than normally seen in the majority of naturally SIV-infected animals (Pandrea and Apetrei, 2010; Pandrea et al., 2009).
Table 1.
Comparative Pathogenesis Features with Impact on SIV/HIV Transmission in Natural versus Pathogenic Hosts
| Feature | Natural Hosts | Asian NHPs | Humans |
|---|---|---|---|
| Horizontal Transmission | Frequent | Frequent | Frequent |
| Vertical Transmission | Rare | Frequent | Frequent |
| Genetic bottleneck at transmission | Yes | Yes | Yes |
| Acute plasma viremia | +++ | +++ | +++ |
| Chronic plasma viremia | High, stable | Progressive | Progressive |
| Primary target cells | CCR5+ CD4+ T cells | CCR5+ CD4+ T cells | CCR5+ CD4+ T cells |
| CCR5 expression on CD4+ T cells (periphery) | + | +++ | +++ |
| CCR5 expression on CD4+ T cells (mucosal) | + | +++ | +++ |
| Maturation of CCR5 expression on CD4+ T cells | Adult age | 6–9 months | 5–6 years |
| Breast milk VLs | +++ | ++ | ++ |
| Inhibitory breast milk factors | Yes | Yes | Yes |
Though many studies have examined SIV replication in natural hosts in captivity, very limited information is available regarding the levels of viral replication in the wild. The most comprehensive studies to date on virus replication in the wild was performed using samples collected from several hundred wild-caught vervets from South Africa and sabaeus from Gambia (Ma et al., 2013, 2014). In these animals, plasma viremia ranged between 104–107 copies of SIVagm RNA copies/mL. Note that these numbers represents an average, as the VLs in bulk of the animals tested were fairly consistent, measuring between 104–105 RNA copies/mL. In both AGM species, approximately 10% of animals tested presented with VLs that were significantly higher (106–108 RNA copies/mL plasma). The plasma of these animals also tested seronegative for SIV-specific antibodies, suggesting that they were in the Fiebig Stage II of acute SIV infection (Fiebig et al., 2003), particularly since the levels of viral replication were in the same range of the VLs observed during acute infection in captive, experimentally infected AGMs (Ma et al., 2013).
Based on the findings of these studies and on data derived from studies of natural hosts in captivity, it can be reasonably inferred that there is no significant difference in viral replication between wild and captive natural hosts (Pandrea et al., 2006b). One may argue that high levels of viral replication observed may be due to a low virus turnover and not necessarily to high levels of virus replication. However, previous experimental studies have shown that virus turnover is high in SIV-infected AGMs and SMs, higher even than in SIVmac infection (Gordon et al., 2008; Pandrea et al., 2008c).
The higher levels of chronic viral replication observed in the wild AGMs compared to HIV-1 patients (Ma et al., 2013; Pandrea et al., 2003; Sharp and Hahn, 2011), may significantly impact viral transmission in the wild. A plasma viremia of around 1500 RNA copies/mL or higher is associated with significant increase in rates of sexual transmission in humans (Watkins et al., 2008). This is lower than either the average acute or chronic VLs in natural hosts. Assuming that similar virus shedding occurs in the body fluids of humans and NHPs, this would support mucosal exposure through sexual contact as the primary route of transmission in the wild (Ansari and Silvestri, 2014).
5.2. SIV primarily targets CD4+ CCR5+ T-cells during mucosal transmission in natural hosts
For most SIVs, the primary viral binding receptor is CD4, while chemokine receptors such as CCR5 serve as coreceptors (Chen et al., 1997; Gautam et al., 2007; Moore et al., 2004; Zhang et al., 2000). This shared receptor and coreceptor usage indicates that, like HIV, the primary target cells of SIV are CD4+ CCR5+ T-cells (Chen et al., 1997, 1998a; Moore et al., 2004; Pandrea et al., 2007a; VandeWoude and Apetrei, 2006; Veazey et al., 2000). However, while HIV can utilize both CCR5 and CXCR4, most SIVs are limited to only CCR5 as a coreceptor. There are some exceptions to this, as several SIV isolates were reported to also use CXCR4, including SIVmnd-1, SIVsab and several SIVsmm strains (Owen et al., 2000; Pandrea et al., 2005; Schols and De Clercq, 1998). Very recently, SIVsmm and SIVagm strains were reported to utilize CXCR6 as a coreceptor (Elliott et al., 2015; Riddick et al., 2016). It has also been reported that CCR6 and CRK-L3, a natural isoform of CCR6, can act as a coreceptor for both primary HIV and SIVsmm isolates (Islam et al., 2013, 2014). This suggests that some SIVs may have an expanded cell tropism besides CD4+ CCR5+ T-cells or an expanded range of potential target cells (Elliott et al., 2015; Riddick et al., 2016).
One of the major exceptions to the usage of CCR5+ as a coreceptor by SIVs is SIVrcm, which uses CCR2b as a coreceptor instead of CCR5. This appears to be an evolutionary feature born from necessity, as the majority of red-capped mangabeys (RCMs), the natural hosts of SIVrcm, lack CCR5 entirely. This is because approximately 83% of RCMs have a Δ24 deletion that silences transcription of their CCR5 gene, making them resistant to viral entry using CCR5 as a coreceptor. This is very similar to the situation in humans, where a small number of people exhibit a Δ32 mutation of their CCR5 gene that renders them resistant to HIV infection (Samson et al., 1996). Consequently, SIVrcm has adapted to use CCR2 or CCR4 to ensure its continued dissemination throughout the RCM population or persistence upon cross-species transmission (Chen et al., 1998b; Gautam et al., 2009). The usage of CXCR4, CXCR6 and CCR6 coreceptors by some SIVagm and SIVsmm lineages suggests that there might have been similar adaption by these viruses to overcome the naturally low availability of CD4+ CCR5+ target cells in natural hosts (see 5.4).
5.3. Natural hosts maintain peripheral T-cell populations and T-cell function
Despite the high levels of ongoing viral replication during both the acute and chronic infection, another defining feature of SIV-infection of natural hosts is the preservation of peripheral CD4+ T-cell populations during chronic infection (Apetrei et al., 2007; Broussard et al., 2001; Diop et al., 2000b; Gordon et al., 2007; Kornfeld et al., 2005; Ma et al., 2014; Onanga et al., 2002, 2006; Pandrea et al., 2003, 2005, 2006a, 2012a; Silvestri et al., 2003). This is in contrast to pathogenic HIV/SIV infections, where chronic peripheral CD4+ T-cell depletion is associated with disease progression and the development of AIDS (Rodríguez B et al., 2006). However, CD4+ T-cells are depleted during the acute infection in both SIV-infection of natural hosts and HIV/SIVmac infection, with partial restoration of CD4+ T-cell populations by chronic infection (see 5.5) (Li et al., 2005; Mattapallil et al., 2005; Mehandru et al., 2004, 2007; Pandrea and Apetrei, 2010; VandeWoude and Apetrei, 2006; Veazey et al., 1998).
The fact that natural hosts can undergo even a partial depletion of CD4+ T-cells and then maintain high levels of viral replication calls into question how they are able to maintain T-cell function. One possible explanation was derived from studies in SMs, where CD4 + T-cell depletion can occur down to AIDS-associated levels without any clinical consequences (Apetrei et al., 2007; Gordon et al., 2007; Milush et al., 2007, 2011; Sumpter et al., 2007). This preservation of immune function was reported to be due to populations of CD4neg CD8neg T-cells that perform helper cell-like functions following SIV-induced CD4+ depletion (Milush et al., 2011). These double negative T-cells arise through down-regulation of CD4 expression by memory T-cells as they enter the memory pool. The double negative T-cells were shown to be able to carry out a variety of CD4+ T-cell associated functions (production of IL-2 and IL-17, FoxP3 and CD40 ligand expression, MHCII restriction) and are resistant to SIV infection (Beaumier et al., 2009; Vinton et al., 2011). In HIV patients, CD4neg CD8neg cells can also arise from down-regulation, but remain susceptible to infection (Kaiser et al., 2007).
5.4. Natural hosts of SIVs exhibit reduced expression of CCR5 and lower total numbers of CD4+ CCR5+ T-cells at mucosal sites
Another defining characteristic of natural hosts of SIV is the low expression levels of the viral coreceptor CCR5 on CD4+ T-cells, particularly on those residing in the gut. In comparison, nonnatural hosts have high numbers of CD4+ CCR5+ T-cells in the gut and other mucosal sites (Pandrea et al., 2007a, 2008b) (Figure 1). This down-regulation of CCR5 expression specifically occurs for CD4+ T-cells, as CCR5 expression on CD8+ T-cells remains in the same range as in pathogenic hosts (Pandrea et al., 2007a). This low levels of expression of CCR5 by the memory CD4+ T- cells occurs even in the absence of SIV-infection in the natural hosts of SIVs (Pandrea et al., 2007a).
In addition to low expression levels of CCR5, some natural hosts also exhibit an general reduction in the overall CD4+ T-cell counts. While this decrease applies to all tissue compartments, it is even more pronounced at mucosal sites, such as the gut (Pandrea et al., 2006a, 2007a, 2008b, 2012a). This trend only holds true for some species of African guenons (tribe Cercopithecini), i.e., AGMs, patas and sun-tailed monkeys (Pandrea et al., 2006a). SMs and mandrills (tribe Papionini) have been reported to have higher CD4+ T-cell counts, similar to those seen in RMs (Pandrea and Apetrei, 2010).
The observation that natural hosts can have high levels of viral replication, but low levels of CD4+ CCR5+ target cells available to support that replication is one of the major paradoxes of SIV infection in natural hosts. One possible explanation for this is that the level of CCR5 expression by CD4+ T-cells does increase in response to infection in natural hosts, but the effect is masked by simultaneous SIV-induced depletion (Pandrea and Apetrei, 2010). Another potential explanation is that there are other target cells in natural hosts apart from CD4+ CCR5+ T-cells, such as macrophages (Pandrea and Apetrei, 2010). This is somewhat supported by the observation that SIVsab and other SIVs are dual-tropic for CCR5 and CXCR4 (see 5.2). The occurance of giant cell disease in the rare cases of AIDS in natural hosts also supports the infection of monocytes/macrophages during natural infection (Pandrea et al., 2009). However, during acute infection, CD4+ T-cells are most depleted in the gut, where they more frequently express CCR5. The depletion is more limited in blood and LNs, where a higher fraction of CD4+ T-cells express CXCR4. This holds true for infections by both natural (AGMs) and nonnatural (RMs and PTMs) hosts, as well as for HIV infections (Kornfeld et al., 2005; Pandrea and Apetrei, 2010; Pandrea et al., 2007b), strongly suggesting that even in cases where SIVs can employ other coreceptors for infection, CD4+ T lymphocytes will be preferentially targeted.
Apart from their obvious depletion, CD4+ T-cells have been implicated as the major cell type supporting SIV replication by in situ hybridization studies showing that SIV RNA colocalizes mostly with CD3+ cells and infrequently with macrophages (Gordon et al., 2008; Pandrea et al., 2008c; Perelson, 2002). Furthermore, when natural hosts experimentally receive ART, the viral decay suggests that the cells predominantly supporting viral replication are short lived, implicating lymphocytes as the principal target cell (Gordon et al., 2008; Pandrea et al., 2008c; Perelson, 2002). Additionally, in a study where CD4+ T-cells were experimentally depleted in naturally SIVsmm-infected SMs using humanized anti-CD4 mAb, VLs decreased sharply in blood, rectal biopsies and bronchoalveolar lavages, and returned to baseline levels after the CD4+ populations were replenished (Klatt et al., 2008). Conversely, the CD4+ depletion had no effect on macrophages, T regulatory cell or CD8+ T-cell populations, indicating that CD4+ T-cells were the primary cell type supporting viral replication (Klatt et al., 2008). Yet, when CD4+ T cells are depleted or are resistant to the virus (such as cells defective for CCR5 expression, like in RCMs), SIVs will infect macrophages in both progressive and nonprogressive hosts (Avalos et al., 2016; Bernard-Stoecklin et al., 2013; Li et al., 2015; Mir et al., 2015). This is best demonstrated by the fact that both progressive and natural hosts that develop AIDS exhibit giant cell disease, a condition characterized by heavy infiltration of tissues by SIV-infected macrophages (Li et al., 1991; Ling et al., 2004; Pandrea et al., 2009). Macrophage tropism of SIVs is not surprising, since lentiviruses first emerged as macrophage-tropic viruses, as is still the case for lentiviruses infecting other mammal species (Maedi-Visna, equine infectious anemia virus or caprine arthritic encephalitis virus) (VandeWoude and Apetrei, 2006).
5.5. Following depletion, peripheral but not the mucosal CD4+ T-cell populations are restored to near preinfection levels in natural hosts
Both natural and nonnatural hosts undergo a similar massive depletion of mucosal CD4+ T-cells during the acute stage of infection. The primary site of CD4+ T-cell depletion is the gut, specifically the GALT and depletion mainly occurs during the acute stage of infection (Brenchley et al., 2004; Gordon et al., 2007; Li et al., 2005; Mattapallil et al., 2005; Mehandru et al., 2004, 2007; Pandrea et al., 2007b; Smit-McBride et al., 1998; Veazey et al., 1998). Natural hosts experience this depletion in spite of the reduced level of CCR5 expression or lower total number of CD4+ cells (see 5.4) (Pandrea et al., 2007a, 2008b).
What sets natural and nonnatural hosts apart are the immunological events following depletion of mucosal CD4+ T-cells (Table 2). In untreated HIV patients and pathogenic SIV infections, the peripheral CD4+ T-cell populations will eventually decline without intervention, leading to the development of AIDS. The scenario in natural hosts is quite different, with recovery of the peripheral CD4+ T-cells population to nearly baseline levels by the chronic infection. Additionally, the GALT-associated CD4+ T-cells partially recover, despite the robust chronic viral replication, though GALT CD4+ T-cells populations never recover fully to preinfection levels (Chahroudi et al., 2012; Haase, 2010; Pandrea and Apetrei, 2010; Sodora et al., 2009). Most of this recovery following acute infection consists of restoration of central memory and naïve T-cells, while effector memory T-cells remain depleted (Pandrea et al., 2007b). This partial recovery is possible in natural hosts because of the control of immune activation and apoptosis (see 5.6), which prevents excessive depletion of the overall CD4+ T-cells population, with the loss due to viral replication being compensated by a normal production of cells (Pandrea and Apetrei, 2010).
Table 2.
Pathological and Immunological Features of Lentiviral Infections in Natural versus Pathogenic Hosts
| Feature | Natural Hosts | Asian NHPs | Humans |
|---|---|---|---|
| Progression to AIDS | No | Yes | Yes |
| Hypercoagulability | No | Yes | Yes |
| Comorbidities | Rare | Yes | Yes |
| Virus cytopathy | Yes | Yes | Yes |
| Acute damage to gut epithelium | Yes | Yes | Yes |
| Microbial translocation | No | Yes | Yes |
| Acute immune activation | Yes | Yes | Yes |
| Acute anti-inflammatory milieu | Yes | No | No |
| Interferon response | Acute only | Yes | Yes |
| T-cell proliferation | Acute only | Yes | Yes |
| Chronic immune activation | No | Yes | Yes |
| Chronic inflammation | No | Yes | Yes |
| Peripheral CD4+ T-cell depletion | Acute only | +++ | +++ |
| Mucosal CD4+ T-cell depletion | ++ | +++ | +++ |
| CD4negCD8neg resistance to infection | Yes | No | No |
| CD4+ Th17-cell depletion | No | Yes | Yes |
| Tscm depletion | No | Yes | Yes |
| CD8+ T-cell response | Yes | Yes | Yes |
| CD20+ B-cell response | Yes | Yes | Yes |
| Treg homeostasis | Yes | No | No |
| γδ T-cell anergy | No | Yes | Yes |
After their restoration, CD4+ central memory cells (TCM) are lost in SIVsmm-infected SMs at rates 20 times slower than those of SIV-infected RMs (McGary et al., 2014). This translates to a half-life of total TCM populations <16 months for RMs, but >17 years for SMs, which is in agreement with the rare cases of AIDS in natural hosts in very old animals. Also, SM CD4+ TCM proliferation levels are lower than in RMs and the RM TCM proliferation correlates with viral replication (McGary et al., 2014). Yet, a recent study of mandrills in Gabon found a significant decrease in memory T-cells populations that was correlated with the duration of infection (Greenwood et al., 2014). However, other studies of the same mandrill cohort did not find similar features (Apetrei et al., 2011).
Besides central memory T-cells, other cell types are also preserved in natural hosts and show divergent dynamics between SIV-infected RMs and SMs. Notably, memory CD4+ T-cells with stem cell-like properties (TSCM) undergo depletion and exhibit increased rates of proliferation and high levels of virus infection in RMs. Conversely, SMs CD4+ TSCM cell populations remained stable, showed no marked increase in proliferation, and had very low rates of direct infection. As TSCM are known to be able to differentiate into central, effector and naïve T-cells, their homeostasis could be crucial to restoration of T-cell populations following depletion in natural hosts (Cartwright et al., 2014; Chahroudi et al., 2015).
5.6. Natural hosts control chronic immune activation, proliferation, and interferon production
One of the most striking features of the SIV infection in natural hosts is their ability to control their levels of chronic immune cell activation. This is reflected by the expression of HLA-DR and CD69 (both markers of immune cell activation) on T-cells, predominately CD8+ T-cells, in natural hosts, which increase slightly during acute infection and returns to baseline levels by chronic infection. In pathogenic infections, the expression levels of these activation markers by T-cells rise dramatically during the acute infection and are never resolved during the chronic infection, being correlated with disease progression (Diop et al., 2000b; Gordon et al., 2007; Kornfeld et al., 2005; Milush et al., 2007; Onanga et al., 2006; Pandrea et al., 2003, 2005, 2006a, 2008c, 2007b; Ploquin et al., 2006; Silvestri et al., 2003; Souquière et al., 2009). Also, expression of PD-1, a cell surface protein that acts as a negative regulator of the immune activation, was found to be rapidly induced in the lymphatic tissue in SMs in response to SIV-infection. This was associated with resolution of acute immune activation and the preservation of CD4+ populations and lymphatic tissue structure (Estes et al., 2008). Resolution of this aberrant, persistent immune activation during the transition from acute-to-chronic infection is therefore thought to be the major mechanism by which natural hosts avoid progression to AIDS (Bosinger et al., 2009, 2011; Estes et al., 2008; Harris et al., 2010; Jacquelin et al., 2009; Pandrea et al., 2007b). However, it should be noted that suppression of the control of immune activation at the transition from acute-to-chronic infection and maintenance of high levels of immune activation throughout the first six months of infection did not result in major alterations of the later stages of chronic infection (Pandrea and Apetrei, unpublished).
Similar to immune cell activation, T-cell proliferation increases only during the acute infection in natural hosts (Gordon et al., 2007; Milush et al., 2007; Muthukumar et al., 2005; Pandrea et al., 2007b; Silvestri et al., 2005). By the chronic phase of infection, proliferation levels return to near their preinfection state (Chakrabarti et al., 2000; Kaur et al., 2008; Muthukumar et al., 2005). This can be observed as a transient increase in expression of the proliferation marker Ki-67 during the acute infection and early chronic infection in AGMs, with return to baseline by late chronic infection (Li et al., 2005; Pandrea et al., 2007b). Conversely, in pathogenic infections, T-cell proliferation continues to increase to the point of excessive cell turnover, likely in an attempt to recover from the depletion of CD4+ T-cells (Grossman et al., 2006; Picker et al., 2004).
Another point of divergence between natural and nonnatural hosts during SIV infection is seen in the production of interferons. A strong and sustained Th1-mediated type I and type II interferon response was observed in the LNs of pigtailed macaques (PTMs) infected with SIVsab, which is pathogenic in PTMs. In contrast, only a transient type I interferon response occurred in SIVsab-infected AGMs, which was resolved after peak viral replication (Lederer et al., 2009). Furthermore, experimental administration of high concentrations of the type I IFN-α to acutely infected AGMs had no impact on immune activation (Jacquelin et al., 2014). Taken together, natural hosts seem to possess the ability to maintain homeostasis of their immune cell populations and resolve immune activation, proliferation and IFN production after the acute SIV infection.
5.7. CD8+ T-cells do not contribute significantly to the prevention of disease progression
The current data indicates that the general CD8+ T-cell populations and their SIV-specific responses are comparable in both infected and uninfected AGMs and RMs (Reina et al., 2009; Schmitz et al., 2009; Zahn et al., 2008, 2010). However, the role that CD8+ T-cells play in different natural host species becomes unclear when looking at the dynamics of viral replication following experimental depletion of CD8+ T-cells. In chronically SIVmac-infected RMs, experimental CD8+ cell depletion results in a 1–3 log increase in plasma viremia (Jin et al., 1999; Schmitz et al., 1999). In contrast, CD8+ depletion in chronically SIVsmm-infected SMs seem to have very little impact on viral replication, though one study did show an increase in VLs following CD8+ depletion (Barry et al., 2007; Wang et al., 2006). Similarly, CD8+ T-cell depletion during acute infection did not increase the peak VLs in AGMs, but it did lead to a plateau of viral replication following peak infection until the CD8+ populations were restored. These findings, along with those of other studies on cellular immune responses in natural hosts, indicate that CD8+ T-cells do play a role in controlling viral replication to some extent (Kaur et al., 2001; Wang et al., 2006; Zahn et al., 2008). However, none of the in vivo depletion experiments had any effect on the actual state of disease progression (Barry et al., 2007; Gaufin et al., 2010). This is in contrast to CD8+ depletion in RMs, which results in increased CD4+ T-cell proliferation and rapid disease progression (Okoye et al., 2009). Consequently, it remains unclear what impact, if any, CD8+ T-cells play in preventing disease progression in natural hosts.
5.8. Natural hosts maintain function of immune cell populations
Apart from the changes in CD4+ and CD8+ T-cell populations, natural hosts also maintain normal populations and functions of a variety of other immune cell subsets, such as NK cells, Th17 T-cells, regulatory T-cells (Tregs), γδ T-cells and mDCs. In contrast, disturbances in these cell populations have been described during pathogenic HIV and SIV infections (Barratt-Boyes and Wijewardana, 2011; Brenchley et al., 2006a, 2008; Favre et al., 2009; Grossman et al., 2006; Wijewardana et al., 2013; Xu et al., 2014). Preservation of these secondary immune cell populations could impact protection from disease progression in natural hosts in a number of ways. For instance, there is evidence to support that maintenance of the Th-17/Treg ratio may be crucial to preventing disease progression and maintaining the integrity of the mucosal barrier during acute infection (Brenchley et al., 2008; Favre et al., 2009). Moreover, preserving Treg homeostasis might confer protection through production of anti-inflammatory cytokines, such as IL-10, or by prevention of excess CD4+ T-cell proliferation following the initial CD4+ depletion in the gut (Pandrea and Apetrei, 2010). Expression of the Treg marker FoxP3 was reported to increase along with anti-inflammatory cytokine production, suggesting that Tregs might play an important role in establishing the anti-inflammatory response (Kornfeld et al., 2005). Tregs are rapidly depleted in the intestines of neonatal RMs infected with SIVmac, supporting the role of the loss of Tregs in pathogenic disease progression (Wang et al., 2015).
In addition to maintaining the homeostasis of Tregs, preventing dysfunction of γδ T-cells may play a crucial role in averting disease progression, as apart from cytokine production, γδ T-cells also perform a variety of other functions, including regulation of tissue healing (Kosub et al., 2008; Vantourout and Hayday, 2013). In HIV infection, it has been shown that γδ T-cells become anergic and can no longer migrate in response to pro-inflammatory cytokines (Martini et al., 2002; Poggi et al., 2004). In contrast, γδ T-cells remain fully functional in SMs (Brenchley et al., 2006a; Milush et al., 2007). It was also shown that γδ T-cells in SMs retain their ability to produce the Th1 cytokines TNF-α and INF-γ postinfection, while this capacity was reduced in γδ T-cells from HIV-infected subjects, along with the overall numbers of γδ T-cells (Kosub et al., 2008). Given the importance of controlling inflammation during primary infection (see 5.10 and 5.11), preservation of regulatory cell populations like Tregs and γδ T-cells likely plays an important role in the prevention of disease progression in natural hosts.
5.9. The humoral response plays little to no role in controlling viral replication following mucosal transmission
The humoral response in SIV-infected natural hosts has been shown to be similar to or lower than what has been reported in pathogenic infections (Gaufin et al., 2009; Gicheru et al., 1999; Hirsch, 2004; Souquière et al., 2009). However, a paucity of SIV Gag-specific antibodies is a shared feature of SIV-infected natural hosts. Oddly, the T-cell response to Gag in natural hosts is comparable to those seen in pathogenic infections, meaning that the absence of anti-Gag antibodies is not due to a lack of Gag-specific T-cells (Reina et al., 2009). Furthermore, some SIVs, like several SIVsmm strains, are actually resistant to antibody neutralization, making it unclear what effect antibodies might have on SIVs (Gautam et al., 2007). Further complicating the situation, experimental depletion of CD20+ B-cells had no effect on either viral replication or disease progression in AGMs, even after the depletion was maintained in our studies for over 6 months (Gaufin et al., 2009; Schmitz et al., 2009). Similarly, in a pair of studies that sought to suppress the adaptive immune response through simultaneous depletion of CD8+ and CD20+ cells during primary SIV infection, the depletion caused no alteration in the state of disease progression in AGMs. In contrast, SIVagm-infected PTMs rapidly progressed to AIDS when both CD8+ and CD20+ populations were depleted (Schmitz et al., 2009; Zahn et al., 2010). In summation, these results strongly suggest that the role of the adaptive immune response in natural hosts is minimal at best and likely has little to do with preventing disease progression.
5.10. Damage to the gut epithelium occurs in both natural and nonnatural hosts, but is quickly resolved in natural hosts
Perhaps one of the most important differences between SIV infection in natural and nonnatural SIV hosts and the one that might play the biggest role in avoiding persistent immune activation is the lack of chronic damage to the gut epithelium in natural hosts. In nonnatural, pathogenic hosts (i.e., humans and RMs), this damage is a consequence of early viral replication, which causes disruption of the gut epithelium and dysregulation of epithelial cell homeostasis (Mohan et al., 2013). The result is damage to the gut epithelium, which allows leakage of microbes and microbial products from the gut lumen into the surrounding tissue, a process known as microbial translocation (MT). Once outside the lumen, microbial antigens bind to Toll-like receptors, stimulating chronic aberrant immune activation. This, in turn, not only creates more available target cells for the virus, but also functionally exhausts the immune system and its capacity for regeneration (Brenchley and Douek, 2012; Brenchley et al., 2006b, 2006b; Gordon et al., 2007; Pandrea et al., 2007b).
It is important to note that natural hosts do sustain some damage to their gut epithelium during the early stages of SIV infection. However, this damage is transient and is rapidly reversed, with no leakage from the lumen of the gut. This is reflected by the absence of bacterial lipopolysaccharide (LPS) in the plasma during both the acute and chronic stages of SIV infection in AGMs (Pandrea et al., 2007b). The importance of avoiding MT was highlighted by two studies where experimentally administration of LPS to AGMs induced general immune activation followed by CD4+ T-cell depletion and an increase in VLs (though there was ultimately no impact on disease progression) (Pandrea et al., 2008d, 2012b). In another study, we administered dextran sulphate, a drug that is directly toxic to intestinal epithelial cells and is known to impair the integrity of the mucosal barrier, to AGMs. This resulted in increases in plasma SIV RNA, sCD14 (see below), CRP and LPS levels, along with loss of mucosal CD4+ T-cells (Hao et al., 2015). Conversely, when acutely SIV-infected PTMs were treated with sevelamer, an LPS chelator, in the gut, the treatment resulted in a dramatic reduction in both inflammation and immune activation, directly demonstrating the significance of MT on SIV pathogenesis (Kristoff et al., 2014).
As the bulk of data regarding MT in natural hosts was derived from studies of animals in captivity, a recent study sought to examine whether or not natural hosts were able to avoid MT in the wild. Because direct measurement of mucosal integrity in wild natural hosts would have been virtually impossible, the sCD14 protein was used as a surrogate marker for MT. CD14 is a coreceptor for LPS expressed by peripheral blood monocytes and tissue macrophages. After LPS stimulation, CD14+ monocytes/macrophages secrete sCD14 and shed surface CD14, which then bind to LPS (Brenchley and Douek, 2012). We did not find any difference in plasma sCD14 levels between wild SIV-positive and SIV-negative vervets and sabaeus (Ma et al., 2013, 2014). These results are in agreement with studies of experimentally infected AGMs, where sCD14 levels remained stable following infection (Pandrea et al., 2006b, 2012a, 2012a, 2007b). A separate study of semi-wild mandrills in Gabon confirmed these results by showing that plasma levels of sCD14 remained unchanged following SIVmnd1-infection (Greenwood et al., 2014).
5.11. Resolution of damage to the gut and prevention of MT primarily hinges on control of apoptosis and establishment of a rapid anti-inflammatory response
The precise means by which natural hosts avoid chronic damage to their gut and the associated MT is still somewhat uncertain. CD4+ T-cell apoptosis levels in natural hosts do not increase significantly during acute or chronic infection (Cumont et al., 2008; Estaquier et al., 1994; Hurtrel et al., 2005; Meythaler et al., 2009; Pandrea et al., 2007b; Silvestri et al., 2003). However, transient CD8+ apoptosis occurs in both natural host and pathogenic hosts (Meythaler et al., 2009). Lack of increased apoptosis of both gut lymphocytes and epithelial cells may contribute to the preservation of the integrity of the gut epithelium (Pandrea and Apetrei, 2010).
Another important mechanism reported to contribute to the preservation of the integrity of the gut epithelium is the establishment of an anti-inflammatory milieu early during infection that represses the effects of pro-inflammatory cytokines. Both natural and nonnatural hosts exhibit increased expression levels of pro-inflammatory cytokines (IFN-γ,TNF-α, IL-1β, IL-12) following infection, albeit at a somewhat lower level in natural hosts. These cytokines are produced by both immune and epithelial cells as part of the innate immune response to viral replication following transmission (Diop et al., 2008; Hirao et al., 2014; Lu et al., 2014; Mandl et al., 2008; Pandrea and Apetrei, 2010). However, this increase is only transient in natural hosts; following acute infection, expression of pro-inflammatory cytokines return to preinfection levels. In HIV-infected patients and SIVmac-infected RMs, pro-inflammatory cytokine production persists into chronic infection, stimulating chronic inflammation and, in turn, immune activation (Kornfeld et al., 2005; Milush et al., 2007; Ploquin et al., 2006). Resolution of this initial pro-inflammatory response to the virus seems to depend on establishment of the anti-inflammatory milieu early during infection of the natural host. This is reflected in a rapid increase in expression of the anti-inflammatory cytokines TGF-β and IL-10 following SIVagm infection of AGMs (Kornfeld et al., 2005). Furthermore, a recent study in RMs established a correlation with internalization of the IL-10 receptor (i.e., lowered responsiveness to IL-10) by mucosal lymphocytes in the gut epithelium with increased levels of apoptosis (Pan et al., 2014).
6. Conclusions
The study of SIV infection in African NHPs that are natural hosts of SIVs has already dramatically contributed to the new paradigm of the pathogenesis of HIV infection; namely, that control of chronic immune activation and inflammation could significantly improve the prognostic, survival and quality of life of chronically HIV-infected patients. But there are many other lessons that can be learned from the study of natural SIV infection in natural hosts, notably with regards to control of virus transmission. In this environment of high rates of SIV transmission despite target cell restriction at the mucosal sites of entry, control of virus transmission to the offspring is remarkable and needs to be investigated to design and implement new strategies for preventing MTIT in humans.
Perhaps the most important lesson that the study of SIV infection in natural hosts should teach us is that it might be more productive to shift our focus from preventing infection to preventing the deleterious consequences of infection. Millions of year of coevolution drove virus-host coadaptation in natural hosts in this direction. Instead of attempting to overcome transmission (which is a Catch-22, where any increase in immune cell effectors results in increases of target cells and consequently fuels transmission), natural hosts developed a tempered ignorance of the virus in which multiple pathways and mechanisms lead to a nonprogressive, albeit very active, “Well-Tempered SIV infection”. Similar to its musical counterpart, “The Well-Tempered Clavier”, SIV infection in the natural hosts is a masterpiece of evolution that is perfectly tuned, and designed to last forever.
Highlights.
Over 40 species of African nonhuman primates carry species-specific SIVs
SIV prevalence in the wild is very high in most of the natural host species
SIV horizontal transmission is efficient in spite of low levels of mucosal target cells
Maternal-to-infant transmission is rare in spite of massive offspring exposure to SIV
Natural hosts of SIVs generally do not progress to AIDS
Control of immune activation is the key factor behind lack of disease progression
Acknowledgments
We thank Beatrice Hahn, Vanessa Hirsch, Ania Jasinska, Mickaela Muller, Martine Peeters, Jason Brenchley, Daniel Douek, Jake Estes, Nelson Freimer, Ruy Ribeiro, David Robertson, Guido Silvestri, Francois Simon and Don Sodora for two decades of helpful discussion. We would like to thank the many groups of scientists that had seminal contributions to this field. We would also like to thank the plethora of people in our laboratories that have generated invaluable data regarding the pathogenesis of SIV infection in natural hosts and whose results were always in contradiction to all the initially proposed hypotheses. This work was supported by funds from grants: RO1 AI064066 (IP) RO1 AI065325 (CA), RO1 AI1193461 (CA) and P01AI088564 (CA/IP) from the National Institute of Allergy and Infectious Diseases (NIAID); RO1 HL117715 (IP) and RO1 HL123096 (IP) from the National Heart, Lung and Blood Institute (NHLBI); R56 DE023508 (CA) from the National Institute of Dental and Craniofacial Research (NIDCR) and RO1 RR025781 (CA and IP) from the National Center for Research Resources (NCRR). KDR was supported by T32 AI065380 (Pitt AIDS Research Training, PART). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AGMs
African green monkeys
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- GALT
gut associated lymphatic tissue
- HIV
human immunodeficiency virus
- LPS
lipopolysaccharide
- MT
microbial translocation
- MTIT
mother-to-infant transmission
- NHPs
nonhuman primates
- PTMs
pig-tailed macaques
- RCM
red-capped mangabeys
- RMs
rhesus macaques
- SIV
simian immunodeficiency virus
- SMs
sooty mangabeys
- TCM
central memory T cells
- Tregs
regulatory T cells
- TSCM
memory T-cells with stem cell-like properties
- TLRs
Toll-like receptors
- VL
viral loads
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghokeng AF, Ayouba A, Mpoudi-Ngole E, Loul S, Liegeois F, Delaporte E, Peeters M. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provide insights into risks for potential new cross-species transmissions. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2010;10:386–396. doi: 10.1016/j.meegid.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrovandi GM, Kuhn L. What babies and breasts can teach us about natural protection from HIV infection. J Infect Dis. 2010;202:S366–S370. doi: 10.1086/655972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan JS, Short M, Taylor ME, Su S, Hirsch VM, Johnson PR, Shaw GM, Hahn BH. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J Virol. 1991;65:2816–2828. doi: 10.1128/jvi.65.6.2816-2828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedee AM, Lacour N, Ratterree M. Mother-to-infant transmission of SIV via breast-feeding in rhesus macaques. J Med Primatol. 2003;32:187–193. doi: 10.1034/j.1600-0684.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Amedee AM, Rychert J, Lacour N, Fresh L, Ratterree M. Viral and immunological factors associated with breast milk transmission of SIV in rhesus macaques. Retrovirology. 2004;1:17. doi: 10.1186/1742-4690-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos JD, Wilks AB, Fouda GG, Smith SD, Colvin L, Mahlokozera T, Ho C, Beck K, Overman RG, DeMarco CT, et al. Lack of B Cell Dysfunction Is Associated with Functional, gp120-Dominant Antibody Responses in Breast Milk of Simian Immunodeficiency Virus-Infected African Green Monkeys. J Virol. 2013;87:11121–11134. doi: 10.1128/JVI.01887-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari AA, Silvestri G. Natural hosts of SIV: implication in AIDS. Amsterdam; Boston: Elsevier: Academic Press; 2014. [Google Scholar]
- Apetrei C, Gormus B, Pandrea I, Metzger M, ten Haaft P, Martin LN, Bohm R, Alvarez X, Koopman G, Murphey-Corb M, et al. Direct Inoculation of Simian Immunodeficiency Virus from Sooty Mangabeys in Black Mangabeys (Lophocebus aterrimus): First Evidence of AIDS in a Heterologous African Species and Different Pathologic Outcomes of Experimental Infection. J Virol. 2004;78:11506–11518. doi: 10.1128/JVI.78.21.11506-11518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Falkenstein S, Bohm R, Koehler J, Traina-Dorge V, et al. Molecular Epidemiology of Simian Immunodeficiency Virus SIVsm in U.S. Primate Centers Unravels the Origin of SIVmac and SIVstm. J Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Gautam R, Sumpter B, Carter AC, Gaufin T, Staprans SI, Else J, Barnes M, Cao R, Garg S, et al. Virus Subtype-Specific Features of Natural Simian Immunodeficiency Virus SIVsmm Infection in Sooty Mangabeys. J Virol. 2007;81:7913–7923. doi: 10.1128/JVI.00281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Gaufin T, Gautam R, Vinton C, Hirsch V, Lewis M, Brenchley J, Pandrea I. Pattern of SIVagm Infection in Patas Monkeys Suggests that Host Adaptation to SIV Infection May Result in Resistance to Infection and Virus Extinction. J Infect Dis. 2010;202:S371–S376. doi: 10.1086/655970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Sumpter B, Souquiere S, Chahroudi A, Makuwa M, Reed P, Ribeiro RM, Pandrea I, Roques P, Silvestri G. Immunovirological Analyses of Chronically Simian Immunodeficiency Virus SIVmnd-1- and SIVmnd-2-Infected Mandrills (Mandrillus sphinx) J Virol. 2011;85:13077–13087. doi: 10.1128/JVI.05693-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos CR, Price SL, Forsyth ER, Pin JN, Shirk EN, Bullock BT, Queen SE, Li M, Gellerup D, O’Connor SL, et al. Quantitation of Productively Infected Monocytes and Macrophages of SIV-Infected Macaques. J Virol. 2016 doi: 10.1128/JVI.00290-16. JVI.00290–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayouba A, Akoua-Koffi C, Calvignac-Spencer S, Esteban A, Locatelli S, Li H, Li Y, Hahn BH, Delaporte E, Leendertz FH, et al. Evidence for continuing cross-species transmission of SIVsmm to humans: characterization of a new HIV-2 lineage in rural Côte d’Ivoire. AIDS Lond Engl. 2013;27 doi: 10.1097/01.aids.0000432443.22684.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggaley RF, White RG, Boily MC. Systematic review of orogenital HIV-1 transmission probabilities. Int J Epidemiol. 2008;37:1255–1265. doi: 10.1093/ije/dyn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes E, Gao F, Bibollet-Ruche F, Courgnaud V, Peeters M, Marx PA, Hahn BH, Sharp PM. Hybrid Origin of SIV in Chimpanzees. Science. 2003;300:1713–1713. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes SM, Wijewardana V. A divergent myeloid dendritic cell response at virus set-point predicts disease outcome in SIV-infected rhesus macaques. J Med Primatol. 2011;40:206–213. doi: 10.1111/j.1600-0684.2011.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AP, Silvestri G, Safrit JT, Sumpter B, Kozyr N, McClure HM, Staprans SI, Feinberg MB. Depletion of CD8+ Cells in Sooty Mangabey Monkeys Naturally Infected with Simian Immunodeficiency Virus Reveals Limited Role for Immune Control of Virus Replication in a Natural Host Species. J Immunol. 2007;178:8002–8012. doi: 10.4049/jimmunol.178.12.8002. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, Cosma A, Dereuddre-Bosquet N, Le Grand R. Semen CD4+ T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques. PLoS Pathog. 2013;9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Ruche F, Galat-Luong A, Cuny G, Sarni-Manchado P, Galat G, Durand JP, Pourrut X, Veas F. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J Gen Virol. 1996;77:773–781. doi: 10.1099/0022-1317-77-4-773. [DOI] [PubMed] [Google Scholar]
- Bibollet-Ruche F, Bailes E, Gao F, Pourrut X, Barlow KL, Clewley JP, Mwenda JM, Langat DK, Chege GK, McClure HM, et al. New Simian Immunodeficiency Virus Infecting De Brazza’s Monkeys (Cercopithecus neglectus): Evidence for a Cercopithecus Monkey Virus Clade. J Virol. 2004;78:7748–7762. doi: 10.1128/JVI.78.14.7748-7762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in SIV infection. Curr Opin HIV AIDS. 2011;6:411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial Translocation Across the GI Tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T Cell Depletion during all Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006a;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006b;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian Immunodeficiency Virus Replicates to High Levels in Naturally Infected African Green Monkeys without Inducing Immunologic or Neurologic Disease. J Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott STC, Collman RG, Bosinger SE, Paiardini M, Vanderford TH, et al. Divergent CD4+ T Memory Stem Cell Dynamics in Pathogenic and Nonpathogenic Simian Immunodeficiency Virus Infections. J Immunol. 2014;192:4666–4673. doi: 10.4049/jimmunol.1303193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Meeker T, Lawson B, Ratcliffe S, Else J, Silvestri G. Mother-to-Infant Transmission of Simian Immunodeficiency Virus Is Rare in Sooty Mangabeys and Is Associated with Low Viremia. J Virol. 2011;85:5757–5763. doi: 10.1128/JVI.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV Hosts: Showing AIDS the Door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Cartwright E, Lee ST, Mavigner M, Carnathan DG, Lawson B, Carnathan PM, Hashempoor T, Murphy MK, Meeker T, et al. Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques. PLOS Pathog. 2014;10:e1003958. doi: 10.1371/journal.ppat.1003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Silvestri G, Lichterfeld M. T memory stem cells and HIV: a long-term relationship. Curr HIV/AIDS Rep. 2015;12:33–40. doi: 10.1007/s11904-014-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, Skulsky E, Ho DD, Cheng-Mayer C, Marx PA. Normal T-Cell Turnover in Sooty Mangabeys Harboring Active Simian Immunodeficiency Virus Infection. J Virol. 2000;74:1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Telfier P, Gettie A, Reed P, Zhang L, Ho DD, Marx PA. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhou P, Ho DD, Landau NR, Marx PA. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gettie A, Ho DD, Marx PA. Primary SIVsm Isolates Use the CCR5 Coreceptor from Sooty Mangabeys Naturally Infected in West Africa: A Comparison of Coreceptor Usage of Primary SIVsm, HIV-2, and SIVmac. Virology. 1998a;246:113–124. doi: 10.1006/viro.1998.9174. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, Lu CY, Aguilar RF, Ho DD, Marx PA. Natural Infection of a Homozygous Δ24 CCR5 Red-capped Mangabey with an R2b-Tropic Simian Immunodeficiency Virus. J Exp Med. 1998b;188:2057–2065. doi: 10.1084/jem.188.11.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenine AL, Ferrantelli F, Hofmann-Lehmann R, Vangel MG, McClure HM, Ruprecht RM. Older Rhesus Macaque Infants Are More Susceptible to Oral Infection with Simian-Human Immunodeficiency Virus 89.6P than Neonates. J Virol. 2005;79:1333–1336. doi: 10.1128/JVI.79.2.1333-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenine AL, Siddappa NB, Kramer VG, Sciaranghella G, Rasmussen RA, Lee SJ, Santosuosso M, Poznansky MC, Velu V, Amara RR, et al. Relative Transmissibility of an R5 Clade C Simian- Human Immunodeficiency Virus Across Different Mucosae in Macaques Parallels the Relative Risks of Sexual HIV-1 Transmission in Humans via Different Routes. J Infect Dis. 2010;201:1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton AA, Emerman M. Convergence and Divergence in the Evolution of the APOBEC3G-Vif Interaction Reveal Ancient Origins of Simian Immunodeficiency Viruses. PLOS Pathog. 2013;9:e1003135. doi: 10.1371/journal.ppat.1003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Feistner A, Evans S, Tsujimoto H, Hayami M. A lack of evidence of sexual transmission of a simian immunodeficiency agent in a semifree-ranging group of mandrills. AIDS Lond Engl. 1989;3:764. [PubMed] [Google Scholar]
- Corbet S, Müller-Trutwin MC, Versmisse P, Delarue S, Ayouba A, Lewis J, Brunak S, Martin P, Brun-Vezinet F, Simon F, et al. env Sequences of Simian Immunodeficiency Viruses from Chimpanzees in Cameroon Are Strongly Related to Those of Human Immunodeficiency Virus Group N from the Same Geographic Area. J Virol. 2000;74:529–534. doi: 10.1128/jvi.74.1.529-534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin DT, Woloszynek S, Morra WA, Honarvar S, Linder JM, Gonder MK, O’Connor MP, Hearn GW. Long-Term Urban Market Dynamics Reveal Increased Bushmeat Carcass Volume despite Economic Growth and Proactive Environmental Legislation on Bioko Island, Equatorial Guinea. PLOS ONE. 2015;10:e0134464. doi: 10.1371/journal.pone.0134464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Le Grand R, Muller-Trutwin M, et al. Early Divergence in Lymphoid Tissue Apoptosis between Pathogenic and Nonpathogenic Simian Immunodeficiency Virus Infections of Nonhuman Primates. J Virol. 2008;82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, Sehgal PK, Schmidt DK, Silva DP, Solomon KR, Hodi FS, Ringler DJ, Hunt RD, King NW. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988;41:601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- D’arc M, Ayouba A, Esteban A, Learn GH, Boué V, Liegeois F, Etienne L, Tagg N, Leendertz FH, Boesch C, et al. Origin of the HIV-1 group O epidemic in western lowland gorillas. Proc Natl Acad Sci. 2015;112:E1343–E1352. doi: 10.1073/pnas.1502022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham W. History of green monkeys in West Indies: migration from Africa. J BHMS. 1981;36:210–229. [Google Scholar]
- Diop OM, Gueye A, Dias-Tavares M, Kornfeld C, Faye A, Ave P, Huerre M, Corbet S, Barre-Sinoussi F, Müller-Trutwin MC. High Levels of Viral Replication during Primary Simian Immunodeficiency Virus SIVagm Infection Are Rapidly and Strongly Controlled in African Green Monkeys. J Virol. 2000a;74:7538–7547. doi: 10.1128/jvi.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop OM, Gueye A, Dias-Tavares M, Kornfeld C, Faye A, Ave P, Huerre M, Corbet S, Barre-Sinoussi F, Müller-Trutwin MC. High Levels of Viral Replication during Primary Simian Immunodeficiency Virus SIVagm Infection Are Rapidly and Strongly Controlled in African Green Monkeys. J Virol. 2000b;74:7538–7547. doi: 10.1128/jvi.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop OM, Ploquin MJY, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, et al. Plasmacytoid Dendritic Cell Dynamics and Alpha Interferon Production during Simian Immunodeficiency Virus Infection with a Nonpathogenic Outcome. J Virol. 2008;82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott STC, Wetzel KS, Francella N, Bryan S, Romero DC, Riddick NE, Shaheen F, Vanderford T, Derdeyn CA, Silvestri G, et al. Dualtropic CXCR6/CCR5 Simian Immunodeficiency Virus (SIV) Infection of Sooty Mangabey Primary Lymphocytes: Distinct Coreceptor Use in Natural versus Pathogenic Hosts of SIV. J Virol. 2015;89:9252–9261. doi: 10.1128/JVI.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, Peeters M, Bedjabaga L, Honoré C, Bussi P, Dixson A, Delaporte E. Prevalence and transmission of simian immunodeficiency virus and simian T-cell leukemia virus in a semi-free-range breeding colony of mandrills in Gabon. AIDS Lond Engl. 1991;5:1385–1386. doi: 10.1097/00002030-199111000-00018. [DOI] [PubMed] [Google Scholar]
- Estaquier J, Idziorek T, de Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci U S A. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early Resolution of Acute Immune Activation and Induction of PD-1 in SIV-Infected Sooty Mangabeys Distinguishes Nonpathogenic from Pathogenic Infection in Rhesus Macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne L, Nerrienet E, LeBreton M, Bibila GT, Foupouapouognigni Y, Rousset D, Nana A, Djoko CF, Tamoufe U, Aghokeng AF, et al. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodytes chimpanzee with AIDS related symptoms. Retrovirology. 2011;8:4. doi: 10.1186/1742-4690-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne L, Locatelli S, Ayouba A, Esteban A, Butel C, Liegeois F, Aghokeng A, Delaporte E, Ngole EM, Peeters M. Noninvasive Follow-Up of Simian Immunodeficiency Virus Infection in Wild-Living Nonhabituated Western Lowland Gorillas in Cameroon. J Virol. 2012;86:9760–9772. doi: 10.1128/JVI.01186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria NR, Rambaut A, Suchard MA, Baele G, Bedford T, Ward MJ, Tatem AJ, Sousa JD, Arinaminpathy N, Pépin J, et al. The early spread and epidemic ignition of HIV-1 in human populations. Science. 2014;346:56–61. doi: 10.1126/science.1256739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, et al. Critical Loss of the Balance between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Fischer W, Apetrei C, Santiago ML, Li Y, Gautam R, Pandrea I, Shaw GM, Hahn BH, Letvin NL, Nabel GJ, et al. Distinct Evolutionary Pressures Underlie Diversity in Simian Immunodeficiency Virus and Human Immunodeficiency Virus Lineages. J Virol. 2012;86:13217–13231. doi: 10.1128/JVI.01862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchet D, Verrier D, Ngoubangoye B, Souquière S, Makuwa M, Kazanji M, Gonzalez JP, Pontier D. Natural simian immunodeficiency virus transmission in mandrills: a family affair? Proc R Soc Lond B Biol Sci. 2012;279:3426–3435. doi: 10.1098/rspb.2012.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda GG, Yates NL, Pollara J, Shen X, Overman GR, Mahlokozera T, Wilks AB, Kang HH, Salazar-Gonzalez JF, Salazar MG, et al. HIV-Specific Functional Antibody Responses in Breast Milk Mirror Those in Plasma and Are Primarily Mediated by IgG Antibodies. J Virol. 2011;85:9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, Gordon TP, Anderson DC, McClure HM. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS Lond Engl. 1990;4:619–625. doi: 10.1097/00002030-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Gaufin T, Pattison M, Gautam R, Stoulig C, Dufour J, MacFarland J, Mandell D, Tatum C, Marx MH, Ribeiro RM, et al. Effect of B-Cell Depletion on Viral Replication and Clinical Outcome of Simian Immunodeficiency Virus Infection in a Natural Host. J Virol. 2009;83:10347–10357. doi: 10.1128/JVI.00880-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaufin T, Ribeiro RM, Gautam R, Dufour J, Mandell D, Apetrei C, Pandrea I. Experimental depletion of CD8+ cells in acutely SIVagm-Infected African Green Monkeys results in increased viral replication. Retrovirology. 2010;7:42. doi: 10.1186/1742-4690-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Carter AC, Katz N, Butler IF, Barnes M, Hasegawa A, Ratterree M, Silvestri G, Marx PA, Hirsch VM, et al. In vitro Characterization of Primary SIVsmm Isolates Belonging to Different Lineages. In Vitro growth on Rhesus Macaque Cells is Not Predictive for In Vivo Replication in Rhesus Macaques. Virology. 2007;362:257–270. doi: 10.1016/j.virol.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Gaufin T, Butler I, Gautam A, Barnes M, Mandell D, Pattison M, Tatum C, Macfarland J, Monjure C, et al. Simian Immunodeficiency Virus SIVrcm, a Unique CCR2-Tropic Virus, Selectively Depletes Memory CD4+ T Cells in Pigtailed Macaques through Expanded Coreceptor Usage In Vivo. J Virol. 2009;83:7894–7908. doi: 10.1128/JVI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni LD, Chen HL, Hodara VL, Chu L, Parodi LM, Smith LM, Sexton V, Cappelli D, Sodora DL. Impact of Mucosal Inflammation on Oral Simian Immunodeficiency Virus Transmission. J Virol. 2013;87:1750–1758. doi: 10.1128/JVI.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicheru MM, Otsyula M, Spearman P, Graham BS, Miller CJ, Robinson HL, Haigwood NL, Montefiori DC. Neutralizing antibody responses in Africa green monkeys naturally infected with simian immunodeficiency virus (SIVagm) J Med Primatol. 1999;28:97–104. doi: 10.1111/j.1600-0684.1999.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Gifford RJ, Katzourakis A, Tristem M, Pybus OG, Winters M, Shafer RW. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc Natl Acad Sci. 2008;105:20362–20367. doi: 10.1073/pnas.0807873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanadurai CW, Pandrea I, Parrish NF, Kraus MH, Learn GH, Salazar MG, Sauermann U, Topfer K, Gautam R, Munch J, et al. Genetic Identity and Biological Phenotype of a Transmitted/Founder Virus Representative of Nonpathogenic Simian Immunodeficiency Virus Infection in African Green Monkeys. J Virol. 2010;84:12245–12254. doi: 10.1128/JVI.01603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Ourmanov I, Brown CR, Beer BE, Elkins WR, Plishka R, Buckler-White A, Hirsch VM. Wide Range of Viral Load in Healthy African Green Monkeys Naturally Infected with Simian Immunodeficiency Virus. J Virol. 2000;74:11744–11753. doi: 10.1128/jvi.74.24.11744-11753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Brown CR, Ourmanov I, Pandrea I, Buckler-White A, Erb C, Nandi JS, Foster GJ, Autissier P, Schmitz JE, et al. Comparison of Simian Immunodeficiency Virus SIVagmVer Replication and CD4+ T-Cell Dynamics in Vervet and Sabaeus African Green Monkeys. J Virol. 2006;80:4868–4877. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, et al. Severe Depletion of Mucosal CD4+ T Cells in AIDS-Free Simian Immunodeficiency Virus-Infected Sooty Mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SN, Dunham RM, Engram JC, Estes J, Wang Z, Klatt NR, Paiardini M, Pandrea IV, Apetrei C, Sodora DL, et al. Short-Lived Infected Cells Support Virus Replication in Sooty Mangabeys Naturally Infected with Simian Immunodeficiency Virus: Implications for AIDS Pathogenesis. J Virol. 2008;82:3725–3735. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood EJD, Schmidt F, Liégeois F, Kondova I, Herbert A, Ngoubangoye B, Rouet F, Heeney JL. Loss of memory CD4+ T-cells in semi-wild mandrills (Mandrillus sphinx) naturally infected with species-specific simian immunodeficiency virus SIVmnd-1. J Gen Virol. 2014;95:201–212. doi: 10.1099/vir.0.059808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- Gueye A, Diop Om, Ploquin Mjy, Kornfeld C, Faye A, Cumont MC, Hurtrel B, Barré-Sinoussi F, Müller-Trutwin Mc. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J Med Primatol. 2004;33:83–97. doi: 10.1111/j.1600-0684.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Haase AT. Early Events in Sexual Transmission of HIV and SIV and Opportunities for Interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, Trubey CM, Smedley J, Klatt NR, Giavedoni LD, et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun. 2015;6:8020. doi: 10.1038/ncomms9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, et al. Downregulation of Robust Acute Type I Interferon Responses Distinguishes Nonpathogenic Simian Immunodeficiency Virus (SIV) Infection of Natural Hosts from Pathogenic SIV Infection of Rhesus Macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry RM, Wells MA, Phelan MA, Schneider AL, Epstein JS, Quinnan GV. Antibodies to simian immunodeficiency virus in African green monkeys in Africa in 1957–62. Lancet Lond Engl. 1986;2:455. doi: 10.1016/s0140-6736(86)92156-2. [DOI] [PubMed] [Google Scholar]
- Heuverswyn FV, Li Y, Bailes E, Neel C, Lafay B, Keele BF, Shaw KS, Takehisa J, Kraus MH, Loul S, et al. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology. 2007;368:155–171. doi: 10.1016/j.virol.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Hirao LA, Grishina I, Bourry O, Hu WK, Somrit M, Sankaran-Walters S, Gaulke CA, Fenton AN, Li JA, Crawford RW, et al. Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 2004;6:40–53. [PubMed] [Google Scholar]
- Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Ho C, Wu S, Amos JD, Colvin L, Smith SD, Wilks AB, DeMarco CT, Brinkley C, Denny TN, Schmitz JE, et al. Transient Compartmentalization of Simian Immunodeficiency Virus Variants in the Breast Milk of African Green Monkeys. J Virol. 2013;87:11292–11299. doi: 10.1128/JVI.01643-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B, Petit F, Arnoult D, Müller-Trutwin M, Silvestri G, Estaquier J. Apoptosis in SIV infection. Cell Death Differ. 2005;12:979–990. doi: 10.1038/sj.cdd.4401600. [DOI] [PubMed] [Google Scholar]
- Islam S, Shimizu N, Hoque SA, Jinno-Oue A, Tanaka A, Hoshino H. CCR6 Functions as a New Coreceptor for Limited Primary Human and Simian Immunodeficiency Viruses. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0073116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Kanbe K, Shimizu N, Ohtsuki T, Jinno-Oue A, Tanaka A, Hoshino H. CKR-L3, a deletion version CCR6-isoform shows coreceptor-activity for limited human and simian immunodeficiency viruses. BMC Infect Dis. 2014;14:354. doi: 10.1186/1471-2334-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat A-S, Kunkel D, Petitjean G, Dillies M-A, Roques P, Butor C, Silvestri G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009 doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Petitjean G, Kunkel D, Liovat AS, Jochems SP, Rogers KA, Ploquin MJ, Madec Y, Barré-Sinoussi F, Dereuddre-Bosquet N, et al. Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection. PLOS Pathog. 2014;10:e1004241. doi: 10.1371/journal.ppat.1004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MJ, Hui H, Robertson DL, Müller MC, Barré-Sinoussi F, Hirsch VM, Allan JS, Shaw GM, Sharp PM, Hahn BH. Mosaic genome structure of simian immunodeficiency virus from west African green monkeys. EMBO J. 1994a;13:2935–2947. doi: 10.1002/j.1460-2075.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MJ, Rogers J, Phillips-Conroy JE, Allan JS, Desrosiers RC, Shaw GM, Sharp PM, Hahn BH. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J Virol. 1994b;68:8454–8460. doi: 10.1128/jvi.68.12.8454-8460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. Dramatic Rise in Plasma Viremia after CD8+ T Cell Depletion in Simian Immunodeficiency Virus–infected Macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Phillips-Conroy JE, Turner TR, Broussard S, Allan JS. SIVagm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops) J Med Primatol. 1996;25:78–83. doi: 10.1111/j.1600-0684.1996.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Kaiser P, Joos B, Niederöst B, Weber R, Günthard HF, Fischer M Study, the S.H.C. Productive Human Immunodeficiency Virus Type 1 Infection in Peripheral Blood Predominantly Takes Place in CD4/CD8 Double-Negative T Lymphocytes. J Virol. 2007;81:9693–9706. doi: 10.1128/JVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Alexander L, Staprans SI, Denekamp L, Hale CL, McClure HM, Feinberg MB, Desrosiers RC, Johnson RP. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur J Immunol. 2001;31:3207–3217. doi: 10.1002/1521-4141(200111)31:11<3207::aid-immu3207>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kaur A, Mascio MD, Barabasz A, Rosenzweig M, McClure HM, Perelson AS, Ribeiro RM, Johnson RP. Dynamics of T- and B-Lymphocyte Turnover in a Natural Host of Simian Immunodeficiency Virus. J Virol. 2008;82:1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, et al. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009a;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009b;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Takeuchi JS, Ren F, Matsuda K, Sato K, Kimura Y, Misawa N, Yoshikawa R, Nakano Y, Yamada E, et al. Characterization of redcapped mangabey tetherin: implication for the co-evolution of primates and their lentiviruses. Sci Rep. 2014;4 doi: 10.1038/srep05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld C, Ploquin MJY, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosub DA, Lehrman G, Milush JM, Zhou D, Chacko E, Leone A, Gordon S, Silvestri G, Else JG, Keiser P, et al. Gamma/Delta T-Cell Functional Responses Differ after Pathogenic Human Immunodeficiency Virus and Nonpathogenic Simian Immunodeficiency Virus Infections. J Virol. 2008;82:1155–1165. doi: 10.1128/JVI.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyos RD, Gordon SN, Staprans SI, Silvestri G, Regoes RR. Similar Impact of CD8+ T Cell Responses on Early Virus Dynamics during SIV Infections of Rhesus Macaques and Sooty Mangabeys. PLOS Comput Biol. 2010;6:e1000901. doi: 10.1371/journal.pcbi.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, Stock JL, He T, Mobley AD, Ross S, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest. 2014;124:2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. Transcriptional Profiling in Pathogenic and Non-Pathogenic SIV Infections Reveals Significant Distinctions in Kinetics and Tissue Compartmentalization. PLOS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leendertz SAJ, Junglen S, Hedemann C, Goffe A, Calvignac S, Boesch C, Leendertz FH. High Prevalence, Coinfection Rate, and Genetic Diversity of Retroviruses in Wild Red Colobus Monkeys (Piliocolobus badius badius) in Taï National Park, Côte d’Ivoire. J Virol. 2010;84:7427–7436. doi: 10.1128/JVI.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leendertz SAJ, Locatelli S, Boesch C, Kücherer C, Formenty P, Liegeois F, Ayouba A, Peeters M, Leendertz FH. No evidence for transmission of SIVwrc from western red colobus monkeys (piliocolobus badius badius) to wild west african chimpanzees (pan troglodytes verus) despite high exposure through hunting. BMC Microbiol. 2011;11:24. doi: 10.1186/1471-2180-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Li SL, Kaaya EE, Feichtinger H, Putkonen P, Parravicini C, Böttiger D, Biberfeld G, Biberfeld P. Monocyte/macrophage giant cell disease in SIV-infected cynomolgus monkeys. Res Virol. 1991;142:173–182. doi: 10.1016/0923-2516(91)90054-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Ndjango JB, Learn GH, Ramirez MA, Keele BF, Bibollet-Ruche F, Liu W, Easlick JL, Decker JM, Rudicell RS, et al. Eastern Chimpanzees, but Not Bonobos, Represent a Simian Immunodeficiency Virus Reservoir. J Virol. 2012;86:10776–10791. doi: 10.1128/JVI.01498-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang G, Duan L, Lu W, Katze MG, Lewis MG, Haase AT, Li Q. SIV Infection of Lung Macrophages. PloS One. 2015;10:e0125500. doi: 10.1371/journal.pone.0125500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois F, Schmidt F, Boué V, Butel C, Mouacha F, Ngari P, Ondo BM, Leroy E, Heeney JL, Delaporte E, et al. Full-Length Genome Analyses of Two New Simian Immunodeficiency Virus (SIV) Strains from Mustached Monkeys (C. Cephus) in Gabon Illustrate a Complex Evolutionary History among the SIVmus/mon/gsn Lineage. Viruses. 2014;6:2880–2898. doi: 10.3390/v6072880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Apetrei C, Pandrea I, Veazey RS, Lackner AA, Gormus B, Marx PA. Classic AIDS in a Sooty Mangabey after an 18-Year Natural Infection. J Virol. 2004;78:8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli S, Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS Lond Engl. 2012;26:659–673. doi: 10.1097/QAD.0b013e328350fb68. [DOI] [PubMed] [Google Scholar]
- Locatelli S, McKean KA, Clee PRS, Gonder MK. The Evolution of Resistance to Simian Immunodeficiency Virus (SIV): A Review. Int J Primatol. 2014;35:349–375. [Google Scholar]
- Locher CP, Witt SA, Herndier BG, Tenner-Racz K, Racz P, Levy JA. Baboons as an animal model for human immunodeficiency virus pathogenesis and vaccine development. Immunol Rev. 2001;183:127–140. doi: 10.1034/j.1600-065x.2001.1830111.x. [DOI] [PubMed] [Google Scholar]
- Locher CP, Witt SA, Herndier BG, Abbey NW, Tenner-Racz K, Racz P, Kiviat NB, Murthy KK, Brasky K, Leland M, et al. Increased Virus Replication and Virulence after Serial Passage of Human Immunodeficiency Virus Type 2 in Baboons. J Virol. 2003;77:77–83. doi: 10.1128/JVI.77.1.77-83.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstine LJ, Pedersen NC, Higgins J, Pallis KC, Uyeda A, Marx P, Lerche NW, Munn RJ, Gardner MB. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys) Int J Cancer. 1986;38:563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Lowenstine LJ, Lerche NW, Yee JL, Uyeda A, Jennings MB, Munn RJ, McClure HM, Anderson DC, Fultz PN, Gardner MB. Evidence for a lentiviral etiology in an epizootic of immune deficiency and lymphoma in stump-tailed macaques (Macaca arctoides) J Med Primatol. 1992;21:1–14. [PubMed] [Google Scholar]
- Lu W, Ma F, Churbanov A, Wan Y, Li Y, Kang G, Yuan Z, Wang D, Zhang C, Xu J, et al. Virus-Host Mucosal Interactions During Early SIV Rectal Transmission. Virology. 2014;464–465:406–414. doi: 10.1016/j.virol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Sosa NM, et al. SIVagm Infection in Wild African Green Monkeys from South Africa: Epidemiology, Natural History, and Evolutionary Considerations. PLOS Pathog. 2013;9:e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He T, Raehtz K, Schmitt CA, Jung Y, Cramer JD, et al. Factors Associated with Siman Immunodeficiency Virus Transmission in a Natural African Nonhuman Primate Host in the Wild. J Virol. 2014;88:5687–5705. doi: 10.1128/JVI.03606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZM, Dutra J, Fritts L, Miller CJ. Lymphatic Dissemination of Simian Immunodeficiency Virus after Penile Inoculation. J Virol. 2016;90:4093–4104. doi: 10.1128/JVI.02947-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLOS Pathog. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- Martini F, Poccia F, Goletti D, Carrara S, Vincenti D, D’Offizi G, Agrati C, Ippolito G, Colizzi V, Pucillo LP, et al. Acute Human Immunodeficiency Virus Replication Causes a Rapid and Persistent Impairment of Vγ9Vδ2 T Cells in Chronically Infected Patients Undergoing Structured Treatment Interruption. J Infect Dis. 2002;186:847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- McGary CS, Cervasi B, Chahroudi A, Micci L, Taaffe J, Meeker T, Silvestri G, Davenport MP, Paiardini M. Increased Stability and Limited Proliferation of CD4+ Central Memory T Cells Differentiate Nonprogressive Simian Immunodeficiency Virus (SIV) Infection of Sooty Mangabeys from Progressive SIV Infection of Rhesus Macaques. J Virol. 2014;88:4533–4542. doi: 10.1128/JVI.03515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 Infection Is Associated with Preferential Depletion of CD4+ T Lymphocytes from Effector Sites in the Gastrointestinal Tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, et al. Mechanisms of Gastrointestinal CD4+ T-Cell Depletion during Acute and Early Human Immunodeficiency Virus Type 1 Infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, Ling B, Marx PA, O’Neil S, Kaur A. Differential CD4+ T-Lymphocyte Apoptosis and Bystander T-Cell Activation in Rhesus Macaques and Sooty Mangabeys during Acute Simian Immunodeficiency Virus Infection. J Virol. 2009;83:572–583. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milush JM, Kosub D, Marthas M, Schmidt K, Scott F, Wozniakowski A, Brown C, Westmoreland S, Sodora DL. Rapid dissemination of SIV following oral inoculation. AIDS Lond Engl. 2004;18:2371–2380. [PubMed] [Google Scholar]
- Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar Alagar, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, et al. Virally Induced CD4+ T Cell Depletion Is Not Sufficient to Induce AIDS in a Natural Host. J Immunol. 2007;179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, Reeves JD, Anton E, O’Neill E, Butler E, et al. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest. 2011;121:1102–1110. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir KD, Bosinger SE, Gasper M, Ho O, Else JG, Brenchley JM, Kelvin DJ, Silvestri G, Hu S, Sodora DL. Simian Immunodeficiency Virus-Induced Alterations in Monocyte Production of Tumor Necrosis Factor Alpha Contribute to Reduced Immune Activation in Sooty Mangabeys. J Virol. 2012;86:7605–7615. doi: 10.1128/JVI.06813-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir KD, Mavigner M, Wang C, Paiardini M, Sodora DL, Chahroudi AM, Bosinger SE, Silvestri G. Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors. J Virol. 2015;89:10136–10144. doi: 10.1128/JVI.00710-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Kaushal D, Aye PP, Alvarez X, Veazey RS, Lackner AA. Focused Examination of the Intestinal Epithelium Reveals Transcriptional Signatures Consistent with Disturbances in Enterocyte Maturation and Differentiation during the Course of SIV Infection. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 Coreceptors—Central to Understanding the Transmission and Pathogenesis of Human Immunodeficiency Virus Type 1 Infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Muthukumar A, Zhou D, Paiardini M, Barry AP, Cole KS, McClure HM, Staprans SI, Silvestri G, Sodora DL. Timely triggering of homeostatic mechanisms involved in the regulation of T-cell levels in SIVsm-infected sooty mangabeys. Blood. 2005;106:3839–3845. doi: 10.1182/blood-2005-01-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel C, Etienne L, Li Y, Takehisa J, Rudicell RS, Bass IN, Moudindo J, Mebenga A, Esteban A, Heuverswyn FV, et al. Molecular Epidemiology of Simian Immunodeficiency Virus Infection in Wild-Living Gorillas. J Virol. 2010;84:1464–1476. doi: 10.1128/JVI.02129-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerrienet E, Amouretti X, Müller-Trutwin MC, Poaty-Mavoungou V, Bedjebaga I, Nguyen HT, Dubreuil G, Corbet S, Wickings EJ, Barre-Sinoussi F, et al. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res Hum Retroviruses. 1998;14:785–796. doi: 10.1089/aid.1998.14.785. [DOI] [PubMed] [Google Scholar]
- Ogino T, Nishimura J, Barman S, Kayama H, Uematsu S, Okuzaki D, Osawa H, Haraguchi N, Uemura M, Hata T, et al. Increased Th17-Inducing Activity of CD14+ CD163low Myeloid Cells in Intestinal Lamina Propria of Patients With Crohn’s Disease. Gastroenterology. 2013;145:1380–1391. e1. doi: 10.1053/j.gastro.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Masuda T, Tsujimoto H, Ishikawa KI, Kodama T, Morikawa S, Nakai M, Honjo S, Hayami M. Isolation of simian immunodeficiency virus from african green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int J Cancer. 1988;41:115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, et al. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onanga R, Kornfeld C, Pandrea I, Estaquier J, Souquière S, Rouquet P, Mavoungou VP, Bourry O, M’Boup S, Barré-Sinoussi F, et al. High Levels of Viral Replication Contrast with Only Transient Changes in CD4+ and CD8+ Cell Numbers during the Early Phase of Experimental Infection with Simian Immunodeficiency Virus SIVmnd-1 in Mandrillus sphinx. J Virol. 2002;76:10256–10263. doi: 10.1128/JVI.76.20.10256-10263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onanga R, Souquière S, Makuwa M, Mouinga-Ondeme A, Simon F, Apetrei C, Roques P. Primary Simian Immunodeficiency Virus SIVmnd-2 Infection in Mandrills (Mandrillus sphinx) J Virol. 2006;80:3301–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsyula MG, Gettie A, Suleman M, Tarara R, Mohamed I, Marx P. Apparent lack of vertical transmission of simian immunodeficiency virus (SIV) in naturally infected African green monkeys, Cercopithecus aethiops. Ann Trop Med Parasitol. 1995;89:573–576. doi: 10.1080/00034983.1995.11812990. [DOI] [PubMed] [Google Scholar]
- Owen SM, Masciotra S, Novembre F, Yee J, Switzer WM, Ostyula M, Lal RB. Simian Immunodeficiency Viruses of Diverse Origin Can Use CXCR4 as a Coreceptor for Entry into Human Cells. J Virol. 2000;74:5702–5708. doi: 10.1128/jvi.74.12.5702-5708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Kenway-Lynch CS, Lala W, Veazey RS, Lackner AA, Das A, Pahar B. Lack of Interleukin-10-Mediated Anti-Inflammatory Signals and Upregulated Interferon Gamma Production Are Linked to Increased Intestinal Epithelial Cell Apoptosis in Pathogenic Simian Immunodeficiency Virus Infection. J Virol. 2014;88:13015–13028. doi: 10.1128/JVI.01757-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C. Where the Wild Things Are: Pathogenesis of SIV Infection in African Nonhuman Primate Hosts. Curr HIV/AIDS Rep. 2010;7:28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Rouquet P, Bourry O, Ngari P, Wickings EJ, Roques P, Apetrei C. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS Lond Engl. 2001;15:2461–2462. doi: 10.1097/00002030-200112070-00019. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, Telfer PT, Abernethy K, White LT, Ngari P, et al. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317:119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Kornfeld C, Ploquin MJY, Apetrei C, Faye A, Rouquet P, Roques P, Simon F, Barre-Sinoussi F, Muller-Trutwin MC, et al. Impact of Viral Factors on Very Early In Vivo Replication Profiles in Simian Immunodeficiency Virus SIVagm-Infected African Green Monkeys. J Virol. 2005;79:6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, et al. Simian Immunodeficiency Virus SIVagm. sab Infection of Caribbean African Green Monkeys: a New Model for the Study of SIV Pathogenesis in Natural Hosts. J Virol. 2006a;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch V, Apetrei C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol. 2006b;35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007a;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the Wild: Simian Immunodeficiency Virus (SIV) Infection in Natural Hosts. Trends Immunol. 2008a;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, Rouquet P, Silvestri G, Simon F, Roques P, et al. Paucity of CD4+ CCR5+ T Cells May Prevent Transmission of Simian Immunodeficiency Virus in Natural Nonhuman Primate Hosts by Breast-Feeding. J Virol. 2008b;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Ribeiro RM, Gautam R, Gaufin T, Pattison M, Barnes M, Monjure C, Stoulig C, Dufour J, Cyprian W, et al. Simian Immunodeficiency Virus SIVagm Dynamics in African Green Monkeys. J Virol. 2008c;82:3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Gaufin T, Brenchley JM, Gautam R, Monjure C, Gautam A, Coleman C, Lackner AA, Ribeiro RM, Douek DC, et al. Cutting Edge: Experimentally Induced Immune Activation in Natural Hosts of Simian Immunodeficiency Virus Induces Significant Increases in Viral Replication and CD4+ T Cell Depletion. J Immunol. 2008d;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Silvestri G, Apetrei C. AIDS in african nonhuman primate hosts of SIVs: a new paradigm of SIV infection. Curr HIV Res. 2009;7:57–72. doi: 10.2174/157016209787048456. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, et al. Mucosal Simian Immunodeficiency Virus Transmission in African Green Monkeys: Susceptibility to Infection Is Proportional to Target Cell Availability at Mucosal Sites. J Virol. 2012a;86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, Xu C, Haret-Richter GS, Trichel A, Apetrei C, et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012b;120:1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007b;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MAM, Kessing B, Pontius J, Roelke M, Rumpler Y, et al. A Molecular Phylogeny of Living Primates. PLOS Genet. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol. 2002;2:28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- Permar SR, Kang HH, Wilks AB, Mach LV, Carville A, Mansfield KG, Learn GH, Hahn BH, Letvin NL. Local replication of simian immunodeficiency virus in the breast milk compartment of chronically-infected, lactating rhesus monkeys. Retrovirology. 2010a;7:7. doi: 10.1186/1742-4690-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permar SR, Wilks AB, Ehlinger EP, Kang HH, Mahlokozera T, Coffey RT, Carville A, Letvin NL, Seaman MS. Limited Contribution of Mucosal IgA to Simian Immunodeficiency Virus (SIV)-Specific Neutralizing Antibody Response and Virus Envelope Evolution in Breast Milk of SIV-Infected, Lactating Rhesus Monkeys. J Virol. 2010b;84:8209–8218. doi: 10.1128/JVI.00656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe Van de Perre PAR. HIV-1 Reservoirs in Breast Milk and Challenges to Elimination of Breast-Feeding Transmission of HIV-1. Sci Transl Med. 2012;4:143sr3. doi: 10.1126/scitranslmed.3003327. [DOI] [PubMed] [Google Scholar]
- Phillips-Conroy JE, Jolly CJ, Petros B, Allan JS, Desrosiers RC. Sexual transmission of SIVagm in wild grivet monkeys. J Med Primatol. 1994;23:1–7. doi: 10.1111/j.1600-0684.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Wang C, et al. Insufficient Production and Tissue Delivery of CD4+Memory T Cells in Rapidly Progressive Simian Immunodeficiency Virus Infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-Free Virus in Breast Milk of HIV-1-Seropositive Women. J Acquir Immune Defic Syndr. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- Plantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemée V, Damond F, Robertson DL, Simon F. A new human immunodeficiency virus derived from gorillas. Nat Med. 2009;15:871–872. doi: 10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- Ploquin MJY, Desoutter JF, Santos PR, Pandrea I, Diop OM, Hosmalin A, Butor C, Barre-Sinoussi F, Müller-Trutwin MC. Distinct expression profiles of TGF-β1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology. 2006;3:37. doi: 10.1186/1742-4690-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Carosio R, Fenoglio D, Brenci S, Murdaca G, Setti M, Indiveri F, Scabini S, Ferrero E, Zocchi MR. Migration of Vδ1 and Vδ2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1–infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205–2213. doi: 10.1182/blood-2003-08-2928. [DOI] [PubMed] [Google Scholar]
- Prince AM, Brotman B, Lee DH, Andrus L, Valinsky J, Marx P. Lack of Evidence for HIV Type 1-Related SIVcpz Infection in Captive and Wild Chimpanzees (Pan troglodytes verus) in West Africa. AIDS Res Hum Retroviruses. 2002;18:657–660. doi: 10.1089/088922202760019356. [DOI] [PubMed] [Google Scholar]
- Reina JML, Favre D, Kasakow Z, Mayau V, Nugeyre MT, Ka T, Faye A, Miller CJ, Scott-Algara D, McCune JM, et al. Gag p27-Specific B- and T-Cell Responses in Simian Immunodeficiency Virus SIVagm-Infected African Green Monkeys. J Virol. 2009;83:2770–2777. doi: 10.1128/JVI.01841-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg EJ, Engelbrecht S, Mwenda J, Laten JD, Robson BA, Stander T, Chege GK. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J Gen Virol. 1998;79:1809–1814. doi: 10.1099/0022-1317-79-7-1809. [DOI] [PubMed] [Google Scholar]
- Rey-Cuillé MA, Berthier JL, Bomsel-Demontoy MC, Chaduc Y, Montagnier L, Hovanessian AG, Chakrabarti LA. Simian Immunodeficiency Virus Replicates to High Levels in Sooty Mangabeys without Inducing Disease. J Virol. 1998;72:3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NE, Wu F, Matsuda K, Whitted S, Ourmanov I, Goldstein S, Goeken RM, Plishka RJ, Buckler-White A, Brenchley JM, et al. Simian Immunodeficiency Virus SIVagm Efficiently Utilizes Non-CCR5 Entry Pathways in African Green Monkey Lymphocytes: Potential Role for GPR15 and CXCR6 as Viral Coreceptors. J Virol. 2016;90:2316–2331. doi: 10.1128/JVI.02529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez B, Sethi AK, Cheruvu VK, et al. PRedictive value of plasma hiv rna level on rate of cd4 t-cell decline in untreated hiv infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- Rudicell RS, Jones JH, Wroblewski EE, Learn GH, Li Y, Robertson JD, Greengrass E, Grossmann F, Kamenya S, Pintea L, et al. Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics. PLOS Pathog. 2010;6:e1001116. doi: 10.1371/journal.ppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudicell RS, Piel AK, Stewart F, Moore DL, Learn GH, Li Y, Takehisa J, Pintea L, Shaw GM, Moore J, et al. High Prevalence of Simian Immunodeficiency Virus Infection in a Community of Savanna Chimpanzees. J Virol. 2011;85:9918–9928. doi: 10.1128/JVI.05475-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht RM, Baba TW, Liska V, Ray NB, Martin LN, Murphey-Corb M, Rizvi TA, Bernacky BJ, Bernacky ME, McClure HM, et al. Oral Transmission of Primate Lentiviruses. J Infect Dis. 1999;179:S408–S412. doi: 10.1086/314794. [DOI] [PubMed] [Google Scholar]
- Rychert J, Amedee AM. The antibody response to SIV in lactating rhesus macaques. J Acquir Immune Defic Syndr 1999. 2005;38:135–141. doi: 10.1097/01.qai.0000148947.03416.b5. [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Learn GH, Fouda GG, Kang HH, Mahlokozera T, Wilks AB, Lovingood RV, Stacey A, Kalilani L, et al. Origin and Evolution of HIV-1 in Breast Milk Determined by Single-Genome Amplification and Sequencing. J Virol. 2011;85:2751–2763. doi: 10.1128/JVI.02316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapouméroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, Fruteau C, Noë R, Peeters M, Brookfield JFY, et al. Simian Immunodeficiency Virus Infection in Free-Ranging Sooty Mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d’Ivoire: Implications for the Origin of Epidemic Human Immunodeficiency Virus Type 2. J Virol. 2005;79:12515–12527. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of Viremia in Simian Immunodeficiency Virus Infection by CD8+ Lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Zahn RC, Brown CR, Rett MD, Li M, Tang H, Pryputniewicz S, Byrum RA, Kaur A, Montefiori DC, et al. Inhibition of Adaptive Immune Responses Leads to a Fatal Clinical Outcome in SIV-Infected Pigtailed Macaques but Not Vervet African Green Monkeys. PLOS Pathog. 2009;5:e1000691. doi: 10.1371/journal.ppat.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Ma ZM, Hagan EA, Wilks AB, Furr KL, Linde CH, Zahn RC, Brenchley JM, Miller CJ, Permar SR. Memory CD4+ T Lymphocytes in the Gastrointestinal Tract Are a Major Source of Cell-Associated Simian Immunodeficiency Virus in Chronic Nonpathogenic Infection of African Green Monkeys. J Virol. 2012;86:11380–11385. doi: 10.1128/JVI.01556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols D, De Clercq E. The simian immunodeficiency virus mnd(GB-1) strain uses CXCR4, not CCR5, as coreceptor for entry in human cells. J Gen Virol. 1998;79:2203–2205. doi: 10.1099/0022-1317-79-9-2203. [DOI] [PubMed] [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS Pandemic. Cold Spring Harb Perspect Med. 2011;1:a006841–a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV Infection of Sooty Mangabeys Is Characterized by Limited Bystander Immunopathology Despite Chronic High-Level Viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent Host Responses during Primary Simian Immunodeficiency Virus SIVsm Infection of Natural Sooty Mangabey and Nonnatural Rhesus Macaque Hosts. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T Lymphocytes Retain High Potential for Cytokine Responses but Have Severe CD4+ T-Cell Depletion at All Stages of Simian Immunodeficiency Virus Infection Compared to Peripheral Lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquière S, Bibollet-Ruche F, Robertson DL, Makuwa M, Apetrei C, Onanga R, Kornfeld C, Plantier JC, Gao F, Abernethy K, et al. Wild Mandrillus sphinx Are Carriers of Two Types of Lentivirus. J Virol. 2001;75:7086–7096. doi: 10.1128/JVI.75.15.7086-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquière S, Onanga R, Makuwa M, Pandrea I, Ngari P, Rouquet P, Bourry O, Kazanji M, Apetrei C, Simon F, et al. Simian immunodeficiency virus types 1 and 2 (SIV mnd 1 and 2) have different pathogenic potentials in rhesus macaques upon experimental cross-species transmission. J Gen Virol. 2009;90:488–499. doi: 10.1099/vir.0.005181-0. [DOI] [PubMed] [Google Scholar]
- Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ. Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract. PLOS Pathog. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, et al. Correlates of Preserved CD4+ T Cell Homeostasis during Natural, Nonpathogenic Simian Immunodeficiency Virus Infection of Sooty Mangabeys: Implications for AIDS Pathogenesis. J Immunol. 2007;178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- Takehisa J, Kraus MH, Ayouba A, Bailes E, Heuverswyn FV, Decker JM, Li Y, Rudicell RS, Learn GH, Neel C, et al. Origin and Biology of Simian Immunodeficiency Virus in Wild-Living Western Gorillas. J Virol. 2009;83:1635–1648. doi: 10.1128/JVI.02311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura T, Hayami M. Phylogenetic analysis of SIV derived from mandrill and drill. Front Biosci J Virtual Libr. 2004;9:513–520. doi: 10.2741/1242. [DOI] [PubMed] [Google Scholar]
- Tomonaga K, Katahira J, Fukasawa M, Hassan MA, Kawamura M, Akari H, Miura T, Goto T, Nakai M, Suleman M, et al. Isolation and characterization of simian immunodeficiency virus from African white-crowned mangabey monkeys (Cercocebus torquatus lunulatus) Arch Virol. 1993;129:77–92. doi: 10.1007/BF01316886. [DOI] [PubMed] [Google Scholar]
- Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, Villinger F, Bosinger SE, Silvestri G. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood. 2012;119:5750–5757. doi: 10.1182/blood-2012-02-411496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S, Apetrei C. Going Wild: Lessons from Naturally Occurring T-Lymphotropic Lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164–164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, Woodward R, Silvestri G, Pandrea I, Apetrei C, et al. CD4-Like Immunological Function by CD4− T Cells in Multiple Natural Hosts of Simian Immunodeficiency Virus. J Virol. 2011;85:8702–8708. doi: 10.1128/JVI.00332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu H, Shen C, Alvarez X, Liu D, Pahar B, Ratterree MS, Doyle-Meyers LA, Lackner AA, Veazey RS. Profound loss of intestinal Tregs in acutely SIV-infected neonatal macaques. J Leukoc Biol. 2015;97:391–400. doi: 10.1189/jlb.4A0514-266RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Metcalf B, Ribeiro RM, McClure H, Kaur A. Th-1-Type Cytotoxic CD8+ T-Lymphocyte Responses to Simian Immunodeficiency Virus (SIV) Are a Consistent Feature of Natural SIV Infection in Sooty Mangabeys. J Virol. 2006;80:2771–2783. doi: 10.1128/JVI.80.6.2771-2783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijewardana V, Kristoff J, Xu C, Ma D, Haret-Richter G, Stock JL, Policicchio BB, Mobley AD, Nusbaum R, Aamer H, et al. Kinetics of Myeloid Dendritic Cell Trafficking and Activation: Impact on Progressive, Nonprogressive and Controlled SIV Infections. PLoS Pathog. 2013;9:e1003600. doi: 10.1371/journal.ppat.1003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks AB, Perry JR, Ehlinger EP, Zahn RC, White R, Gauduin MC, Carville A, Seaman MS, Schmitz JE, Permar SR. High Cell-Free Virus Load and Robust Autologous Humoral Immune Responses in Breast Milk of Simian Immunodeficiency Virus-Infected African Green Monkeys. J Virol. 2011;85:9517–9526. doi: 10.1128/JVI.00796-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LF, Chahroudi A, Chen H-L, Jaspan HB, Sodora DL. The Oral Mucosa Immune Environment and Oral Transmission of HIV/SIV. Immunol Rev. 2013;254 doi: 10.1111/imr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey M, Telfer P, Souquière S, Hunter M, Coleman CA, Metzger MJ, Reed P, Makuwa M, Hearn G, Honarvar S, et al. Island Biogeography Reveals the Deep History of SIV. Science. 2010;329:1487–1487. doi: 10.1126/science.1193550. [DOI] [PubMed] [Google Scholar]
- Xing J, Wang H, Zhang Y, Ray DA, Tosi AJ, Disotell TR, Batzer MA. A mobile element-based evolutionary history of guenons (tribe Cercopithecini) BMC Biol. 2007;5:5. doi: 10.1186/1741-7007-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Veazey RS. Th17 Cells Coordinate with Th22 Cells in Maintaining Homeostasis of Intestinal Tissues and both are Depleted in SIV-Infected Macaques. J AIDS Clin Res. 2014;5 doi: 10.4172/2155-6113.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn RC, Rett MD, Korioth-Schmitz B, Sun Y, Buzby AP, Goldstein S, Brown CR, Byrum RA, Freeman GJ, Letvin NL, et al. Simian Immunodeficiency Virus (SIV)-Specific CD8+ T-Cell Responses in Vervet African Green Monkeys Chronically Infected with SIVagm. J Virol. 2008;82:11577–11588. doi: 10.1128/JVI.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn RC, Rett MD, Li M, Tang H, Korioth-Schmitz B, Balachandran H, White R, Pryputniewicz S, Letvin NL, Kaur A, et al. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood. 2010;115:3070–3078. doi: 10.1182/blood-2009-10-245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lou B, Lal RB, Gettie A, Marx PA, Moore JP. Use of Inhibitors To Evaluate Coreceptor Usage by Simian and Simian/Human Immunodeficiency Viruses and Human Immunodeficiency Virus Type 2 in Primary Cells. J Virol. 2000;74:6893–6910. doi: 10.1128/jvi.74.15.6893-6910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S, Fa JE, Wohlfart C, Streit B, Jacob S, Wegmann M. Mapping Bushmeat Hunting Pressure in Central Africa. Biotropica. 2016;48:405–412. [Google Scholar]