Occupational therapy intervention that includes aerobic and strengthening exercises may help improve independence in ADLs and improve physical performance in people with AD.
MeSH TERMS: activities of daily living, Alzheimer disease, exercise therapy, occupational therapy, treatment outcome
Abstract
OBJECTIVE. Alzheimer’s disease (AD) results in a loss of independence in activities of daily living (ADLs), which in turn affects the quality of life of affected people and places a burden on caretakers. Limited research has examined the influence of physical training (aerobic, balance, and strength training) on ADL performance of people with AD.
METHOD. Six randomized controlled trials (total of 446 participants) fit the inclusion criteria. For each study, we calculated effect sizes for primary and secondary outcomes.
RESULTS. Average effect size (95% confidence interval) for exercise on the primary outcome (ADL performance) was 0.80 (p < .001). Exercise had a moderate impact on the secondary outcome of physical function (effect size = 0.53, p = .004).
CONCLUSION. Occupational therapy intervention that includes aerobic and strengthening exercises may help improve independence in ADLs and improve physical performance in people with AD. Additional research is needed to identify specific components of intervention and optimal dosage to develop clinical guidelines.
Alzheimer’s disease (AD) is a progressive neurological disorder characterized by loss in cognitive function, abnormal behavior, and decreased ability to perform basic activities of daily living (ADLs; Mayeux, 2010). Ultimately, these symptoms affect quality of life of both people with AD and their caregivers. AD is the most common cause of dementia, accounting for 60% to 80% of all cases (Jellinger & Attems, 2007; Schneider, Arvanitakis, Bang, & Bennett, 2007). An estimated 5.4 million Americans, nearly two-thirds of whom are women, have been diagnosed with AD (Hebert, Scherr, Bienias, Bennett, & Evans, 2003; Plassman et al., 2007; Seshadri et al., 1997). It is predicted that by 2030, the number of Americans age 65 yr and older with AD will increase to 7.7 million (Hebert et al., 2003).
AD results in tremendous societal and economic strain. Caring for people with AD often leads to depression, emotional anxiety, and a host of other negative consequences for caregivers (Monin & Schulz, 2009; Schulz et al., 2003; Yaffe et al., 2002). Caring for people with AD and other forms of dementia cost the U.S. economy $183 billion in 2011, an amount that is expected to increase to $1.1 trillion by 2050 (Alzheimer’s Association, 2010). Because no known cure exists for AD, treatment options are limited to lifestyle interventions, including diet and exercise. The objective of treatment is to slow disease progression while improving quality of life (Mayeux, 2010).
Interventions aimed at maintaining independence in ADLs in people with AD typically use occupations such as functional ADL training, instrumental ADL (IADL) training, and leisure and social activity training (Letts et al., 2011). Although physical training is a routine part of occupational therapy practice, its effect has been examined primarily on physical outcomes such as fall prevention (Jensen & Padilla, 2011). The effect of physical training on ADL performance has not been systematically examined. Because physical training (including neuromuscular reeducation, gait training, and therapeutic exercises) is a reimbursable service in occupational therapy (American Occupational Therapy Association, n.d.), it is important to examine the evidence supporting such interventions.
Objectives
The purpose of this systematic review was to examine the effectiveness of physical training (aerobic, balance, and strength training) on ADL performance in people with AD. We also examined the effect of exercise on two secondary outcomes: (1) physical performance and (2) cognition and mood.
Method
Studies Included
Recent recommendations for systematic reviews in AD call for inclusion of randomized controlled trials (RCTs) with large sample sizes, computation of effect size, and inclusion of studies conducted in diverse practice settings (Arbesman & Lieberman, 2011). We therefore included RCTs with a sample size greater than 15 that tested ambulatory older adults (men and women age 65 yr or older) diagnosed with AD, living either in long-term care facilities or in the community. Participants in the studies included people in the early and advanced stages of AD. We excluded studies that tested people diagnosed with dementia associated with mild cognitive impairment or Parkinson’s disease.
Types of Intervention
Interventions that included aerobic, strength, and balance training or any combination of the three were examined. Comparison groups included other forms of intervention or routine medical care. We also included studies that compared one active treatment with another.
Outcome Measures
Primary Outcomes.
Outcome measures used to measure the primary outcome, ADL performance, were the Katz Index of Independence in ADLs (Rolland et al., 2007; Santana-Sosa, Barriopedro, López-Mojares, Pérez, & Lucia, 2008), the Barthel Index of ADLs (Santana-Sosa et al., 2008; Venturelli, Scarsini, & Schena, 2011; Vreugdenhil, Cannell, Davies, & Razay, 2012), the Physical Functioning subscale of the SF–36 (Teri et al. 2003), and the Acute Care Index of Function (ACIF; Roach, Tappen, Kirk-Sanchez, Williams, & Loewenstein, 2011).
Secondary Outcomes.
Secondary outcome measures included measures of physical function and of cognition and mood. Physical performance was measured using the 6-min walk test (6–MWT; Roach et al., 2011; Venturelli et al., 2011), the Tinetti Performance Oriented Mobility Assessment (Santana-Sosa et al., 2008), the Senior Fitness Test (Santana-Sosa et al., 2008) and Functional Reach Test, Timed Up and Go test, sit to stand (Vreugdenhil et al., 2012), 6-m walking speed, standing balance, the one-leg balance test (Rolland et al., 2007), and the SF–36 Physical Functioning subscale (Vreugdenhil et al., 2012). Cognition and mood were measured using the Alzheimer’s Disease Assessment Scale (ADAS–Cog; Vreugdenhil et al., 2012), the Mini-Mental State Examination (MMSE; Venturelli et al., 2011; Vreugdenhil et al., 2012), the Cornell Scale for Depression in Dementia, the Hamilton Rating Scale for Depression (Teri et al. 2003), and the Montgomery–Asberg Depression Rating Scale (Roach et al., 2011).
Search Methods
We searched the following electronic databases: PubMed, the Cochrane Library, MEDLINE, CINAHL, Google Scholar, and ISI Web of Knowledge. We used the following MeSH headings: “(activities of daily living OR ADL) AND (occupational therapy OR physical therapy OR rehabilitation) AND (exercise OR aerobic exercise OR balance training OR functional activities OR walking OR gait) AND Alzheimer’s disease AND (randomized controlled trial OR randomized clinical trial).” The MEDLINE search terms were adjusted for use in the Cochrane Library and ISI Web of Knowledge searches. We also searched reference lists of relevant articles and of two books (Brawley, 2006; Kandel, Schwartz, & Jessell, 2000). Our search also found one systematic review (Coelho, Santos-Galduroz, Gobbi, & Stella, 2009).
Analysis
We selected studies through a two-step process. First, we read the abstracts of articles that conformed to the inclusion criteria. If a study met the inclusion criteria, one author reviewed it and discussed the findings with the other authors. A study was included in the review when all authors were in agreement. Any disagreement was resolved through discussion. Each article was assessed using the GATE–Lite (Jackson et al., 2006) and CanChild forms (Lammi & Law, 2003) while applying a predetermined data abstraction procedure. Questions about articles were discussed among the authors to resolve any lack of clarity.
We calculated effect sizes to compare studies that used different interventions and outcomes. Effect size was calculated by finding the difference between means of the posttreatment outcome measures and the baseline values divided by the pooled standard deviation of the primary outcome measure. We entered the effect size into RevMan5 software (Cochrane Collaboration, 2011), which mapped it on a forest plot with a fixed-effects model and calculated the I 2, q, and z for an overall effect. We repeated the calculations for the secondary outcomes.
Results
The initial search located 2,875 references. Two additional citations were references from textbooks. After duplicates were removed, 1,573 articles remained. The authors filtered out irrelevant studies and identified and inspected 91 studies. All authors agreed that 16 articles fit the inclusion criteria. Of these, 10 were excluded because they did not include ADL performance as the primary outcome or published only the study protocol.
Six studies (total N = 446) published between 2003 and 2012 met the inclusion criteria. Sample sizes ranged from 16 to 153 participants. All studies included in this review were Level 1b or 2b RCTs according to the Oxford Centre for Evidence Based Medicine (2013) levels of evidence system.
All studies included people with AD who completed an exercise program consisting of aerobic, strength, or balance training or any combination of the three. The length of the exercise programs varied from 12 weeks to 12 months. In 3 studies, the exercise programs were performed in the participants’ nursing home (Roach et al., 2011; Rolland et al., 2007; Santana-Sosa et al., 2008), whereas in the other 3 studies the exercise programs were performed in participants’ homes in the community (Teri et al., 2003; Venturelli et al., 2011; Vreugdenhil et al., 2012).
Interventions consisted of walking programs, functional activity performance, stretching, strength and resistance training, balance exercises, and aerobic and endurance training, as well as conversation programs. All studies included a routine medical care comparison group. ADL performance was the common primary outcome of all 6 studies. Two studies (Rolland et al., 2007; Santana-Sosa et al., 2008) used the Katz Index as the measure of ADL performance. Three studies (Santana-Sosa et al., 2008; Venturelli et al., 2011; Vreugdenhil et al., 2012) used the Barthel Index to measure ADL.
Risk of Bias
Because of the nature of the interventions, it was difficult to blind participants to their allocation to the control or intervention group. All 6 studies reported blinding of testers and were given a low risk of bias.
We identified other potential sources of bias in 4 of the 6 studies (Rolland et al., 2007; Santana-Sosa et al., 2008; Venturelli et al., 2011; Vreugdenhil et al., 2012). Rolland et al. (2007) did not address cross-contamination that may have occurred because participants in both the control and intervention groups lived in the same facility. Moreover, the study had a small sample size from a very specific population. Similarities between groups were not provided. In addition, the time allowed for familiarization with each exercise was not specified. In the Venturelli et al. (2011) study, caregivers recorded the results from each exercise session, which may have skewed the results for the intervention group. Vreugdenhil et al. (2012) were unclear about whether exercise or social interaction was responsible for the change in outcome; they provided evidence to support both. In addition, lack of participant blinding may have led to an expectation of improvement among the intervention group. Two studies had no other potential biases (Roach et al., 2011; Teri et al., 2003); therefore, we judged these to have a low risk of bias.
Effects of Intervention on Activities of Daily Living
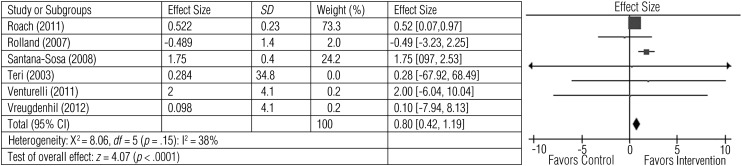
Six studies involving 446 participants tested the effect of exercise on ADL performance (Roach et al., 2011; Rolland et al., 2007; Santana-Sosa et al., 2008; Teri et al., 2003; Venturelli et al., 2011; Vreugdenhil et al., 2012). Figure 1 illustrates that exercise had a large and significant effect on ADL performance (z = 4.07, p < .0001; average effect size = 0.80).
Figure 1.
Forest plot of effect sizes for the comparison of exercise versus control groups on the primary outcome of activity of daily living performance.
Note. CI = confidence interval; SD = standard deviation.
Two studies reported significant improvements in the Katz Index scores after 12 mo (Rolland et al., 2007) or 12 wk (Santana-Sosa et al., 2008) of intervention. Three studies (Santana-Sosa et al., 2008; Venturelli et al., 2011; Vreugdenhil et al., 2012), with a total of 77 participants, measured ADL with the Barthel Index. Santana-Sosa et al. (2008) reported a post hoc analysis on 16 participants. After intervention, the exercise group had significantly improved scores (p < .05), whereas the control group did not (p > .05). Similarly, Venturelli et al. (2011) found that a walking exercise group (n = 11) produced significant (p < .05) improvement in Barthel Index scores after 6 mo of intervention, whereas the control group (n = 10) maintained baseline scores. Vreugdenhil et al. (2012) reported a significant increase (p = .047) in Barthel Index scores after 4 mo of exercise intervention; control participants’ ADL scores declined from baseline.
One study (N = 21) assessed IADLs (Vreugdenhil et al., 2012). ADL scores for the intervention group increased significantly (p = .007), whereas those for the control group declined from baseline.
Teri et al. (2003) reported ADL scores of 153 participants using the Physical Functioning subscale of the SF–36. At 3 mo, the exercise group demonstrated improved scores (p < .001), whereas scores of the control group declined. At 24-mo follow-up, the difference in scores between the exercise intervention and control groups was maintained (p = .003).
Roach et al. (2011) tested 82 participants and measured ADLs using subscales of the ACIF. The 6–MWT served as a proxy for the mobility component of the scale. The transfer component of the ACIF scale increased significantly (p = .04) among the exercise intervention group, whereas ACIF scores decreased for the conversation and walking groups. The bed mobility component of the ACIF and the 6–MWT proxy did not change significantly from baseline to postintervention among the three groups.
Effects of Intervention on Physical Function Measures
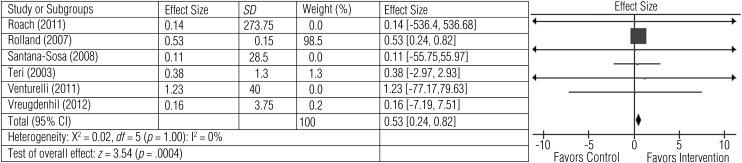
All 6 studies measured physical function. Exercise improved physical function assessed on measures such as the 6–MWT (Roach et al., 2011; Venturelli et al., 2011), 6-m walking speed (Rolland et al., 2007), one-leg balance test (Rolland et al., 2007), Timed Up and Go test (Vreugdenhil et al., 2012), functional reach (Vreugdenhil et al., 2012), sit to stand (Vreugdenhil et al., 2012), Senior Fitness Test (Santana-Sosa et al., 2008), Tinetti Performance Oriented Mobility Assessment (Santana-Sosa et al., 2008), and SF–36 Physical Functioning subscale (Teri et al., 2003). Figure 2 shows the effect size for comparison of exercise with control interventions. The average effect size (0.53), although moderate, was statistically significant (z = 3.54, p < .0004).
Figure 2.
Forest plot of effect sizes for the comparison of exercise versus control groups on the secondary outcome of physical function.
Note. CI = confidence interval; SD = standard deviation.
Effects of Intervention on Cognition and Mood
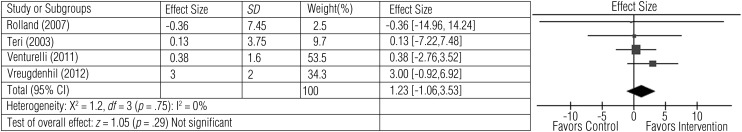
Four studies involving 346 participants measured cognition and mood (Rolland et al., 2007; Teri et al., 2003; Venturelli et al., 2011; Vreugdenhil et al., 2012). Exercise was related to improvement in overall cognitive function as seen by an improvement in scores on the MMSE (Venturelli et al., 2011; Vreugdenhil et al., 2012) and the ADAS–Cog (Vreugdenhil et al., 2012). Two studies reported the beneficial effects of exercise on mood as measured by improvement in scores on the Cornell Scale for Depression in Dementia (Teri et al., 2003), the Hamilton Rating Scale for Depression (Teri et al., 2003), and the Montgomery–Asberg Depression Rating Scale (Rolland et al., 2007). The average effect size (1.23), although high, was not statistically significant (z = 1.05, p = .29), likely because of high variation in the range of effect sizes across studies (Figure 3).
Figure 3.
Forest plot of effect sizes for the comparison of exercise versus control groups on the secondary outcome of cognition and mood.
Note. CI = confidence interval; SD = standard deviation.
Discussion
This systematic review examined the effects of exercise on ADL performance in people with AD. Only 6 RCTs satisfied the inclusion criteria, and the studies varied with regard to the number of participants, types of intervention (including frequency, intensity, duration, and typology), reported outcomes, intervention settings, and statistical methodology. Because the studies did not present data for individual participants, our analysis was limited to calculation of effect sizes to compare across studies.
Regarding length of intervention, longer duration of intervention was not associated with better outcomes. Rather, larger effect sizes were seen in studies with shorter and midrange intervention duration. These findings may help program planners identify optimal exercise dosage for people with AD. The shortest intervention lasted 12 wk (Santana-Sosa et al., 2008), and the longest lasted 12 mo (Rolland et al., 2007). Successful exercise programs were carried out with relatively good adherence in all settings, including long-term care facilities and community- and home-based locations. The reasons for good study adherence were twofold: (1) Participants carried out interventions with the assistance of either a specialist (e.g., occupational therapy practitioner) or a caregiver and (2) interventions occurred in the setting in which the participant lived. Of interest, adherence was a significant predictor of change in ADL score (Rolland et al., 2007).
Most participants tolerated exercise well. Several possible adverse outcomes included syncope, malaise, falls, fractures, hospitalization, and death (Rolland et al., 2007; Teri et al., 2003). Only 1 study directly reported adverse events (five falls) in the exercise group (Rolland et al., 2007). Given the advanced age of participants and the physiological response to exercise in aging, these events should be anticipated and extra safety precautions taken.
All 6 studies provided evidence that exercise (with aerobic, strength, balance, and coordination components) either decreased decline in performance of ADLs or increased ability to perform ADLs. These positive effects were apparent with programs ranging in length from 12 wk (Santana-Sosa et al., 2008; Teri et al., 2003) and intermediate length of 16 wk (Roach et al., 2011; Vreugdenhil et al., 2012) to 6 mo (Venturelli et al., 2011) and 12 mo (Rolland et al., 2007). Furthermore, the positive effects of a 3-mo intervention lasted 24 mo (Teri et al., 2003). Although no clear relationship was found between length of intervention and effect size, the longest study intervention (Rolland et al., 2007) did not produce a large effect size, so longer interventions should be prescribed with caution; longer interventions may increase the burden on both clients and caregivers. No adverse effects of exercise on ADL performance were noted.
The study with the largest effect size implemented a walking and aerobic program of only 30 min four times a week (Venturelli et al., 2011). However, Roach et al. (2011) showed that a walking program produced a decline in ADL performance and that an intervention focused on strength, balance, and endurance resulted in improvements. Therefore, it is not clear which form of exercise is best for reducing decline in ADL performance in people with AD.
Exercise training also improved physical performance in people with AD as measured by a variety of outcome measures, including the 6-MWT, Tinetti Performance Oriented Mobility Assessment, Senior Fitness Test, and Timed Get Up and Go test. Although increased fitness levels appear to be correlated with ability to perform ADLs, firm conclusions cannot be drawn because statistical analyses were not possible in this review.
Four studies addressed the effect of exercise on cognitive function and mood (Rolland et al., 2007; Teri et al., 2003; Venturelli et al., 2011; Vreugdenhil et al., 2012). In 3 studies, a trend toward improved cognition (or decrease in decline) and depression was shown in the exercise groups (Teri et al., 2003; Venturelli et al., 2011; Vreugdenhil et al., 2012). One study reported no differences in cognition and depression between the exercise and control groups (Rolland et al., 2007). Correlations between improved cognition, mood, and exercise were not the focus of this analysis but rather were used as secondary outcomes, and conclusions were not statistically analyzed.
Currently, no standard of care exists for people with AD to improve overall function. This review provides insight into the potential use of exercise interventions to improve ADL performance and quality of life in people living with AD. Finding the right balance between the appropriate type and duration of exercise can help in the development of clinical guidelines. Most of the studies we reviewed dealt with people living in a nursing home or in the community. The use of a caregiver when implementing the exercise intervention also varied. Guidelines that address these issues can help improve the management of people with AD in nursing home facilities, improve their overall function, and decrease caregiver burden.
Implications for Occupational Therapy Practice and Research
The findings of this review have the following implications for occupational therapy practice:
Physical training, which is often included in occupational therapy practice, can help improve ADL performance in people with Alzheimer’s disease. Occupational therapy practitioners may be involved in planning and delivering physical training, identifying and correcting compensatory mechanisms, and providing support to minimize adverse events such as falls. The studies reviewed highlight the clinical feasibility of an exercise program for people with AD.
Intervention should include components of aerobic, strength, balance, and coordination training.
Physical training was equally effective in long-term care facilities and in home-based settings.
Occupational therapy practitioners may enlist the help of caregivers or trained assistants (e.g., aides) in improving adherence to physical training programs. However, interventions should be structured so as not to increase caregiver burden.
Although physical training is not routinely recommended for people with AD, this review provides evidence to support inclusion of aerobic exercise and strength, balance, and coordination training in occupational therapy practice. Physical training is a reimbursable service and may be included in occupational therapy practice.
In addition, the findings of this review have the following implications for occupational therapy research:
Well-designed randomized controlled trials with large sample sizes are needed to assess the effects of exercise on ADL performance in people with AD.
Additional studies are needed to clarify the specific components of physical training that are most effective and clinically feasible.
Research is needed to examine optimal length and intensity of intervention to develop clinical guidelines for exercise in this population.
Acknowledgments
Ashwini K. Rao has received research support from the National Institute of Child Health and Human Development (1K01HD060912, Principal Investigator) and the National Institute of Neurological Disorders and Stroke (5R01NS042859-06A2, Coinvestigator).
References
- Alzheimer’s Association. Changing the trajectory of Alzheimer’s disease: A national imperative. 2010. Retrieved from www.alz.org/documents_custom/trajectory.pdf.
- American Occupational Therapy Association. AOTA guide to Medicare local coverage determinations. n.d.. Retrieved from http://www.aota.org/∼/media/Corporate/Files/Advocacy/Reimb/Pay/Medicare/LCDs/Resources/LCD%20Advocacy%20Packet1.ashx.
- Arbesman M., Lieberman D. Methodology for the systematic reviews on occupational therapy for adults with Alzheimer’s disease and related dementias. American Journal of Occupational Therapy. 2011;65:490–496. doi: 10.5014/ajot.2011.002576. http://dx.doi.org/10.5014/ajot.2011.002576 . [DOI] [PubMed] [Google Scholar]
- Brawley E. C. Design innovations for aging and Alzheimer’s: Creating caring environments. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Cochrane Collaboration. Review Manager. RevMan, Version 5.2.3. 2011. Retrieved from http://ims.cochrane.org/revman/download.
- Coelho F. G., Santos-Galduroz R. F., Gobbi S., Stella F. [Systematized physical activity and cognitive performance in elderly with Alzheimer’s dementia: A systematic review] Revista Brasileira de Psiquiatria (Sao Paulo, Brazil) 2009;31:163–170. doi: 10.1590/s1516-44462009000200014. http://dx.doi.org/10.1590/S1516-44462009000200014 . [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. http://dx.doi.org/10.1001/archneur.60.8.1119 . [DOI] [PubMed] [Google Scholar]
- Jackson R., Ameratunga S., Broad J., Connor J., Lethaby A., Robb G., …, Heneghan C. The GATE frame: Critical appraisal with pictures. Evidence-Based Medicine. 2006;11:35–38. doi: 10.1136/ebm.11.2.35. http://dx.doi.org/10.1136/ebm.11.2.35 . [DOI] [PubMed] [Google Scholar]
- Jellinger K. A., Attems J. Neuropathological evaluation of mixed dementia. Journal of the Neurological Sciences. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. http://dx.doi.org/10.1016/j.jns.2007.01.045 . [DOI] [PubMed] [Google Scholar]
- Jensen L. E., Padilla R. Effectiveness of interventions to prevent falls in people with Alzheimer’s disease and related dementias. American Journal of Occupational Therapy. 2011;65:532–540. doi: 10.5014/ajot.2011.002626. http://dx.doi.org/10.5014/ajot.2011.002626 . [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H., Jessell T. M. Principles of neural science. 4th ed. New York: McGraw-Hill; 2000. [Google Scholar]
- Lammi B. M., Law M. The effects of family-centred functional therapy on the occupational performance of children with cerebral palsy. Revue Canadienne D’Ergothérapie. 2003;70:285–297. doi: 10.1177/000841740307000505. http://dx.doi.org/10.1177/000841740307000505 . [DOI] [PubMed] [Google Scholar]
- Letts L., Edwards M., Berenyi J., Moros K., O’Neill C., O’Toole C., McGrath C. Using occupations to improve quality of life, health and wellness, and client and caregiver satisfaction for people with Alzheimer’s disease and related dementias. American Journal of Occupational Therapy. 2011;65:497–504. doi: 10.5014/ajot.2011.002584. http://dx.doi.org/10.5014/ajot.2011.002584 . [DOI] [PubMed] [Google Scholar]
- Mayeux R. Clinical practice: Early Alzheimer’s disease. New England Journal of Medicine. 2010;362:2194–2201. doi: 10.1056/NEJMcp0910236. http://dx.doi.org/10.1056/NEJMcp0910236 . [DOI] [PubMed] [Google Scholar]
- Monin J. K., Schulz R. Interpersonal effects of suffering in older adult caregiving relationships. Psychology and Aging. 2009;24:681–695. doi: 10.1037/a0016355. http://dx.doi.org/10.1037/a0016355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford Centre for Evidence Based Medicine Levels of Evidence Working Group. OCEBM levels of evidence system. 2013. Retrieved from http://www.cebm.net/index.aspx?o=5653.
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B., …, Wallace R. B. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. http://dx.doi.org/10.1159/000109998 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach K. E., Tappen R. M., Kirk-Sanchez N., Williams C. L., Loewenstein D. A randomized controlled trial of an activity specific exercise program for individuals with Alzheimer disease in long-term care settings. Journal of Geriatric Physical Therapy. 2011;34:50–56. doi: 10.1519/JPT.0b013e31820aab9c. http://dx.doi.org/10.1519/JPT.0b013e31820aab9c . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y., Pillard F., Klapouszczak A., Reynish E., Thomas D., Andrieu S., …, Vellas B. Exercise program for nursing home residents with Alzheimer’s disease: A 1-year randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. http://dx.doi.org/10.1111/j.1532-5415.2007.01035.x . [DOI] [PubMed] [Google Scholar]
- Santana-Sosa E., Barriopedro M. I., López-Mojares L. M., Pérez M., Lucia A. Exercise training is beneficial for Alzheimer’s patients. International Journal of Sports Medicine. 2008;29:845–850. doi: 10.1055/s-2008-1038432. http://dx.doi.org/10.1055/s-2008-1038432 . [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Arvanitakis Z., Bang W., Bennett D. A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. http://dx.doi.org/10.1212/01.wnl.0000271090.28148.24 . [DOI] [PubMed] [Google Scholar]
- Schulz R., Mendelsohn A. B., Haley W. E., Mahoney D., Allen R. S., Zhang S., …, Belle S. H., Resources for Enhancing Alzheimer’s Caregiver Health Investigators. End-of-life care and the effects of bereavement on family caregivers of persons with dementia. New England Journal of Medicine. 2003;349:1936–1942. doi: 10.1056/NEJMsa035373. http://dx.doi.org/10.1056/NEJMsa035373 . [DOI] [PubMed] [Google Scholar]
- Seshadri S., Wolf P. A., Beiser A., Au R., McNulty K., White R., D’Agostino R. B. Lifetime risk of dementia and Alzheimer’s disease: The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. http://dx.doi.org/10.1212/WNL.49.6.1498 . [DOI] [PubMed] [Google Scholar]
- Teri L., Gibbons L. E., McCurry S. M., Logsdon R. G., Buchner D. M., Barlow W. E., …, Larson E. B. Exercise plus behavioral management in patients with Alzheimer disease: A randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. http://dx.doi.org/10.1001/jama.290.15.2015 . [DOI] [PubMed] [Google Scholar]
- Venturelli M., Scarsini R., Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. American Journal of Alzheimer’s Disease and Other Dementias. 2011;26:381–388. doi: 10.1177/1533317511418956. http://dx.doi.org/10.1177/1533317511418956 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil A., Cannell J., Davies A., Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scandinavian Journal of Caring Sciences. 2012;26:12–19. doi: 10.1111/j.1471-6712.2011.00895.x. http://dx.doi.org/10.1111/j.1471-6712.2011.00895.x . [DOI] [PubMed] [Google Scholar]
- Yaffe K., Fox P., Newcomer R., Sands L., Lindquist K., Dane K., Covinsky K. E. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. http://dx.doi.org/10.1001/jama.287.16.2090 . [DOI] [PubMed] [Google Scholar]