Abstract
The lesion-deficit model dominates neuropsychology. This is unsurprising given powerful demonstrations that focal brain lesions can affect specific aspects of cognition. Nowhere is this more evident than in patients with bilateral hippocampal damage. In the last sixty years the amnesia and other impairments exhibited by these patients have helped to delineate the functions of the hippocampus and shape the field of memory. We do not question the value of this approach. However, less prominent are the cognitive processes that remain intact following hippocampal lesions. Here, we collate the piecemeal reports of preservation of function following focal bilateral hippocampal damage, highlighting a wealth of information often veiled by the field’s focus on deficits. We consider how a systematic understanding of what is preserved as well as what is lost could add an important layer of precision to models of memory and the hippocampus.
Keywords: Hippocampus, memory, navigation, deficits, neuropsychology, scene construction
Overview
Memory is fundamental to everyday cognition. Consequently, a key goal of cognitive psychology and neuropsychology is to understand how memories are formed, represented and recollected. Studies of patients with damage to a particular brain region, the hippocampus, have been pivotal in illuminating the organisation of the memory system by showing, for example, that memory is not a unitary phenomenon. The importance of the hippocampus for memory was first formally demonstrated nearly 60 years ago by patient HM (Scoville & Milner 1957). Removal of HM’s medial temporal lobes (MTL; which includes the hippocampi) for the relief of intractable seizures left him with profound amnesia, unable to recall any new personal experiences (episodic/autobiographical memories) while pre-surgical autobiographical memories were also compromised to a degree. Nevertheless, HM’s cognition did not collapse; he retained an above-average IQ, apparently intact perceptual and language capabilities, and aspects of his memory - working memory and procedural learning - were also preserved. Furthermore, while elements of his recognition memory were impaired, his familiarity was not, and while his autobiographical memory was affected, his semantic memory for the same time periods was intact (Augustinack et al 2014).
Scoville and Milner (1957) appreciated that HM’s cognitive and memory profile had two equally important components – what was impaired and what was preserved - and that only by considering both could the structure of memory and its functional anatomy be properly understood. In the decades since the case of HM was first reported there has been a wealth of studies investigating patients with damage to the MTL including those with focal lesions to the hippocampi (reviewed in Spiers et al 2001, Winocur & Moscovitch 2011). However, this work has predominantly focussed on patients’ deficits. Indeed, even HM’s purportedly preserved abilities have been questioned in recent years, with visual perception (Lee et al 2012) and working memory (Ranganath & Blumenfeld 2005) reported to be impaired in patients with focal bilateral hippocampal damage. In other work, the remit of the hippocampus has been extended beyond autobiographical memory, to include spatial navigation (Maguire et al 1998, Maguire et al 2006, O'Keefe & Dostrovsky 1971, O'Keefe & Nadel 1978) and imagining the future (Hassabis et al 2007a), with deficits in these domains also apparent following hippocampal lesions.
Consequent upon the dominance of the lesion-deficit model in neuropsychology, consideration of preserved functions has been eclipsed by the field’s emphasis on unearthing impairments. We believe this narrow focus could impede our ability to achieve a full understanding of hippocampal functionality and the organisation of memory and related cognition. It is not that the field is devoid of evidence relating to the intact abilities of patients with hippocampal damage, but rather that what is there is piecemeal and has not been considered in its totality. Our main aim in this article is to redress the balance by collating some of the evidence that exists in the literature concerning preservation of function in the context of bilateral hippocampal damage. We then consider how a systematic understanding of what is preserved as well as what is lost could be used to inform a theoretically-enriched understanding of memory and its neural substrates.
We focus primarily on patients with putative focal bilateral hippocampal damage. Where relevant, mention is made of animal work, patients with less focal hippocampal lesions and functional MRI (fMRI) studies.
Why is preservation problematic?
Before examining the pertinent empirical data, it is interesting to first consider why preservation has failed to achieve parity with reports of deficits in patients with bilateral hippocampal damage. As noted, neuropsychological research is traditionally based on the lesion-deficit model. The logic here is if a patient cannot do X, then the execution of X must depend upon the lesioned area. Investigations are therefore aimed at highlighting cognitive impairments following brain damage by finding statistically significant performance differences between patients and matched healthy participants, usually leading to conclusions about the necessity of a brain region for a specific task or function. Here preservation runs into its first problem. The usual analysis employed within psychology is that of null hypothesis significance testing. This test asks, at the simplest level, whether two means are different to each other. Finding a significant result (often with a threshold of p<0.05 – a deficit in function) lends itself to a simple conclusion – that the means of patients and control subjects are different. However, a non-significant or “null” result (typically p>0.05 – preservation of function) is not typically regarded as the reverse conclusion. Null results can occur for multiple reasons, for example, type II errors (accepting a false null hypothesis), low study power (the probability of correctly rejecting the null hypothesis when it is false) and poor experimental design.
Problems with preservation do not end there as further issues arise in relation to interpreting the results. If, following hippocampal damage, some aspects of cognition are preserved, the simplest assumption is that the hippocampus is not required for those tasks to be performed. However, there are other potential explanations for preservation. First, brain structure and function are not fixed. Environmental stimulation or memory encoding can alter the structure of the brain, for example when trainee taxi drivers learn the layout of ~25,000 streets around London (UK) this is associated, within subjects, with increased grey matter volume in the posterior hippocampus (Woollett & Maguire 2011). Second, brain regions are not always selective in their responsivity – in the congenitally blind, visual areas have been found to activate during fMRI studies of braille reading (i.e. tactile inputs) and verbal memory (Amedi et al 2003). As such, it is possible that following damage to the hippocampus other brain regions may be able to compensate to some degree. This may be particularly relevant in developmental amnesia (Vargha-Khadem et al 1997), where early life insult to the hippocampi may lead to a reorganisation of function within the brain.
It is also uncertain to what extent remnant tissue contributes to cognitive tasks. It is typically assumed in neuropsychology that damage to brain structures of the extent usually observed in patients with hippocampal damage could in effect equate to a near complete loss of functionality. That is, as stated by Gold & Squire (2005): “These observations suggest that a reduction in hippocampal volume of approximately 40%, as estimated from MRI scans, likely indicates the nearly complete loss of hippocampal neurons. The tissue collapses with the result that the hippocampus is markedly reduced in volume, but the tissue does not disappear entirely. Thus, a loss of approximately 40% of hippocampal volume as measured from MRI scans should not be taken to mean that 60% of the hippocampus remains functional” (p. 84-85). However, several fMRI studies conducted with patients have shown that even where hippocampal tissue volume is reduced by 50%, it nevertheless activates during successful performance on tasks that are thought to be hippocampal dependent (e.g. Maguire et al 2001, Maguire et al 2010a, Mullally et al 2012a). Preservation may thus, in some cases, be supported by remnant portions of the hippocampus that are still functional.
People also differ in the strategies they employ to perform a task. These different cognitive styles and strategies can influence the brain networks engaged (Sanfratello et al 2014) and this could be expressed in different patterns of preservation following brain damage. Further, the brain contains degeneracy, that is many-to-one structure-function relationships (Price & Friston 2002). As such, and related to the use of different cognitive strategies and plasticity, preservation of function following lesions does not necessarily mean that the damaged region is never involved in a particular cognitive process.
So there is much to muddy the waters in preservation and it is perhaps not surprising, therefore, that journal editors and individual scientists have long been wary of null results (Ferguson & Heene 2012, Rosenthal 1979) and concomitant conclusions regarding preservation of function. It should be noted that preserved performance on one task observed in the context of impaired performance on a second task within the same experimental design can temper some of the above concerns, although these single dissociations are not without their own interpretive issues (Dunn & Kirsner 2003).
Deficits are also difficult
Studies investigating deficits are not, however, immune from problems either. They are also susceptible to, in this case, type I errors (incorrect rejection of the null hypothesis) and poor experimental design. One of the most contentious issues is in relation to study power and sample size. It is often the case in neuropsychology that single patients are studied and compared to a small group of control subjects. This substantially raises the likelihood of type I errors, and also makes it difficult to gauge the generalizability of findings. Discussion of these important issues are beyond the scope of this article, but see Rosenbaum et al (2014) for recent consideration of these matters.
Beyond statistical issues, interpretation of deficits is not straightforward. In patients with bilateral hippocampal damage, the accusation is sometimes levelled that the lesions are not truly focal to the hippocampus and that damage to other areas might have contributed to the impairment, thus making conclusions specific to the hippocampus impossible (e.g. Kim et al 2015, Squire et al 2010). It has been further suggested that pathology arising from limbic encephalitis (LE) is invariably more diffuse compared with aetiologies like anoxia (Kim et al 2015, Squire et al 2010). However, post-mortem studies of certain types of LE document highly selective hippocampal damage (Dunstan & Winer 2006, Khan et al 2009, Park et al 2007). On the other hand, patients who have been described as having selective hippocampal-damaged from non-LE pathologies can have wider brain damage (e.g. Kim et al 2015: patient DA, heroin overdose, bilateral globus pallidus lesions; patient KE, toxic shock syndrome, basal ganglia lesions). We therefore believe that arguments about the selectivity of lesions are specious because many pathological processes produce widespread brain damage, but only those rare patients with apparently selective hippocampal lesions are typically included in studies where the prime or sole interest is in the hippocampus (e.g. Mullally et al 2012b).
Even where high-resolution MRI scanning of patients’ brains has been undertaken and subsequent painstaking measurement of hippocampus and other regions confirm the circumscribed nature of the lesions, covert pathology that is undetectable using current technologies might be present. Indeed, even when a more remote brain area has not itself been directly damaged, diaschisis may have occurred – this is is a sudden loss or change of function in a portion of the brain connected to a distant damaged brain area (e.g. Campo et al 2012). For example, studies in rats using immediate-early gene imaging as a marker of neuronal activity found that lesions in the anterior thalamic nuclei and hippocampus both produce marked retrosplenial cortex (RSC) dysfunction (Albasser et al 2007, Jenkins et al 2004). This suggests that the functional impact of hippocampal lesions could be exacerbated by distal dysfunctions in RSC. These lesions had little or no effect on RSC cell numbers (Jenkins et al 2004, Poirier & Aggleton 2009), so that seemingly intact cytoarchitecture (that might appear normal on an MRI scan) was combined with a functional abnormality. In humans also, focal brain lesions have been found to cause disruption to network organisation across the brain during fMRI scanning (Gratton et al 2012, Hayes et al 2012).
The lesion-deficit model encourages conclusions about the necessity of a brain area for a specific function. But no brain region is an island, and so at best this is an over-simplification. Thus, even when strenuous efforts are made to confirm the circumscribed nature of hippocampal lesions in the context of a deficit (including subsequently at post-mortem, e.g. Zola-Morgan et al 1986), this must always be caveated by the possibility that the function in question may not be solely the domain of the hippocampus.
Why bother testing patients?
Given the difficulties outlined above, one might well question the usefulness of testing patients at all. We firmly believe in the value of neuropsychological research; it has been transformative for the field of memory and continues to hold its own even in these days of functional neuroimaging (Rorden & Karnath 2004). In fact, our aim in highlighting the issues faced by patient studies is to make the point that examining or theorising about preservation is no more flawed or inappropriate than postulating about deficits. Importantly, neuropsychology is constantly striving to improve its methods (see Rosenbaum et al 2014 for more on this). For instance, complementing neuropsychological studies with fMRI in healthy control subjects helps to establish convergent evidence (e.g. Hassabis et al 2007a, Hassabis et al 2007b). Similarly, conducting fMRI scanning on the patients themselves (during tasks they are able to perform) is becoming more common and this can provide clues about the potential functionality of remnant tissue (Maguire et al 2010b, Mullally et al 2012a). Moreover, detailed statistical and methodological reporting can overcome some of the concerns associated with null results (see sidebar ‘Interpretation of null results’). The interpretation of deficits arising after bilateral hippocampal lesions has also benefitted from meta-analyses and in depth reviews (Kessels et al 2001, Spiers et al 2001, Squire 1992, Winocur & Moscovitch 2011), which have included some consideration of preservation, specifically in the domain of learning (Cohen 1984, Schacter & Graf 1986). As far as we are aware, however, there have been no reviews of preservation of function across multiple domains.
Sidebars. Interpretation of null results.
Why bother testing patients?
While it is not statistically possible to accept the null hypothesis, meaningful conclusions can still be made from null findings. Interpretation does, however, require further reporting of the data, including effects sizes and confidence intervals as well as the usual p-values (e.g. Aberson 2002). While this is recommended in the most recent edition (6th) of the American Psychological Association publication manual (American Psychological Association 2010), it is not yet common practice. Effect sizes provide a standardised measure of the extent of the difference between two means (e.g. Cohen’s d) or the proportion of variance explained (e.g. eta-squared or r2). If effect sizes are very small, then differences between groups are likely to be non-significant even with greater experimental power. Confidence intervals around means and effect sizes measure the deviation around these variables – small confidence intervals that largely overlap suggest high similarity between groups, large confidence intervals or smaller regions of overlap suggest possible differences given greater sensitivity and power. Thus, more detailed description of the results, including where possible the data for each patient and every control subject, can increase the interpretability of the statistics leading to a null result.
Therefore, after a brief overview of hippocampal anatomy and extant theoretical frameworks, in the subsequent sections we will consider a range of cognitive functions with which the hippocampus has been associated, starting with two of the ‘classics’, navigation and autobiographical memory. Naturally we will refer to deficits, but our prime focus will be on highlighting those functions that seem to be completely intact as well as instances where pockets of preservation have been observed within the context of an overarching impairment. While not exhaustive, this survey reveals some unexpected abilities, new angles on extant beliefs, and surprising gaps in our knowledge.
Hippocampal anatomy
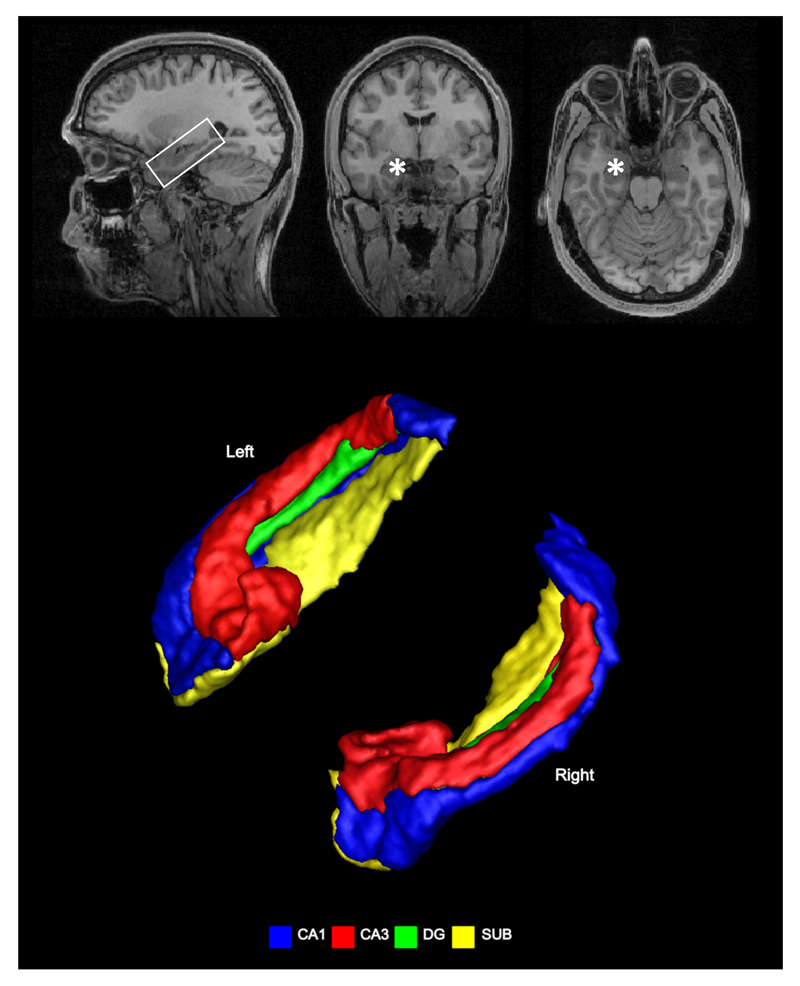
The hippocampus is a brain structure thought to be common to all mammals (West 1990), and is located in the MTL of each hemisphere (Figure 1). The hippocampal formation consists of two laminae rolled up inside each other. One is termed the ‘hippocampus proper’ or ‘cornu ammonis’. It is subdivided according to differences in cellular structure into ‘subfields’ named CA1, CA2, CA3 and CA4. The other lamina is the dentate gyrus (DG). The entorhinal cortex mediates connections to and from the hippocampus, while there are also direct connections between hippocampus and subcortical regions via the fornix. In rodents, the hippocampus runs along a dorsal-ventral axis, corresponding to a posterior-anterior axis in humans. Different parts of this axis have distinct connections to other regions of the brain, suggesting potential functional differentiation between dorsal and ventral hippocampus – an idea that receives support from a range of empirical data (e.g. Fanselow & Dong 2010, Maguire et al 2000, Moser & Moser 1998, Poppenk et al 2013, Strange et al 2014).
Figure 1.
The anatomy of the human hippocampus. Upper panels show the structural MRI scan of a healthy individual in sagittal view (within the white box), coronal and axial views – where the (left) hippocampus is indicated with a white asterisk and the right hippocampus is free to view. Lower panels show 3-D rendering of two hippocampi with the subfields colour-coded.
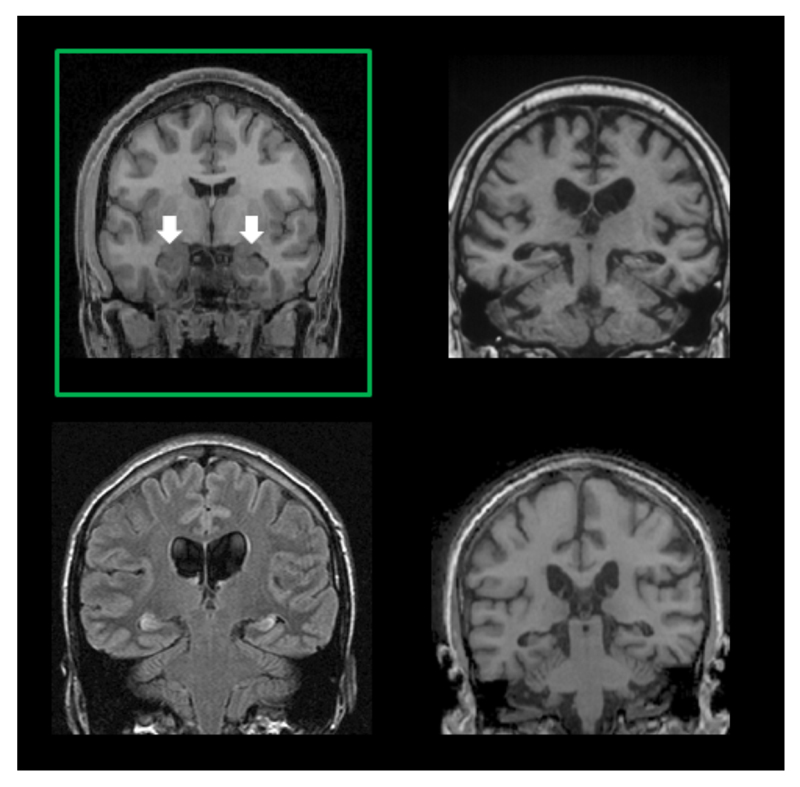
The hippocampus in humans is susceptible to a range of common pathologies including Alzheimer’s disease, epilepsy, limbic encephalitis and stroke. The typical aetiologies that gave rise to the focal bilateral hippocampal damage (as far as can be determined with current techniques) in the patients we will consider here, are anoxia, ischemia and some forms of limbic encephalitis (Figure 2).
Figure 2.
Examples of bilateral hippocampal damage in three patients. Within the upper left green box is a coronal section from a healthy brain with the two hippocampi indicated by white arrows. The three other panels show coronal sections from patients with damage to the two hippocampi.
Theories of hippocampal function
An in-depth description of theories of hippocampal function is beyond the scope of this piece and they are amply covered elsewhere (Eichenbaum & Cohen 2014, Konkel & Cohen 2009, Maguire & Mullally 2013, O'Keefe & Nadel 1978, Squire & Zola-Morgan 1991). Here we will briefly mention the main theories and their germane points, reserving further elaboration for the relevant sections below.
O'Keefe and Dostrovsky (1971) discovered that cells in the hippocampus encode the location of a rat in its environment. Each ‘place cell’ fires when the rat enters the cell’s preferred area (its place field) irrespective of where the rat is looking. These findings were formalised into the cognitive map theory (O'Keefe & Nadel 1978) which proposes that the hippocampus in rats and other mammals, including humans, provides a world-centred or allocentric spatial framework, as opposed to a framework where space is egocentric and represented relative to the observer him/herself. Allocentric spatial representations facilitate flexible navigation strategies, as well potentially providing a spatial scaffold upon which episodic/autobiographical memories can be built.
An alternative view of the relationship between spatial and episodic memory is offered by the relational theory, which argues that the primary function of the hippocampus is not spatial, but should instead be thought of as the representation of associations between disparate elements (Cohen & Eichenbaum 1993, Eichenbaum 2004). Specifically, this theory posits the existence of three elemental cognitive processes that are all mediated by the hippocampus - associative representation, sequential organisation, and relational networking. According to this view, these fundamental properties can fully account for the spatial processing found within the hippocampus, and are flexible enough to explain possible non-spatial hippocampal processes. Relational theory has parallels with other models, including the constructive episodic simulation hypothesis (Schacter & Addis 2007, Schacter et al 2012), which suggests that the hippocampus and connected brain regions flexibly recombine elements of existing episodic memories to create new (e.g. future) scenarios, and another theory that emphasises the hippocampal role in binding objects and contexts (Ranganath 2010).
By contrast, Hassabis and Maguire (2007, 2009, see also Maguire & Mullally 2013) have proposed that a primary function of the hippocampus is to support a process called ‘scene construction’. This is defined as the process of mentally generating and maintaining a complex and coherent scene - a scene is a spatially coherent representation of the world, small or large-scale, within which we can potentially operate, e.g. a scene of your local park or of your desk top. This necessitates the retrieval and integration of the relevant components of the scene from modality-specific cortex, which are then bound into a spatially coherent scene representation. Notably, this concept is flexible enough to account for both newly imagined scenes and retrieved episodic memories, as this core process is held to be involved in both. The authors also argue that scene construction may also be critical for other functions such as spatial navigation and planning for the future. This, and the constructive episodic simulation hypothesis (Schacter & Addis 2007, Schacter et al 2012), are consistent with a large body of evidence suggesting that episodic memory is not simply a perfect record of past events, but instead should be considered more of a reconstructive process (Bartlett 1932, Conway & Pleydell-Pearce 2000, Schacter et al 1998). Scene construction theory differs from the cognitive map theory in placing the creation and representation of scenes at the centre of hippocampal processing, although space is important to both views.
As well as theoretical positions focusing on the nature of the information being processed by the hippocampus, some theories have considered the timescale of hippocampal involvement in memory. There is general agreement that episodic/autobiographical memories depend on the hippocampus during initial encoding (Scoville & Milner 1957). However, its role in supporting such memories when they are more remote is contentious. The standard model of consolidation argues that memories (semantic and autobiographical) become less dependent on the hippocampus, eventually eschewing the need for its involvement altogether during retrieval (Marr 1971, Squire 1992, Squire & Wixted 2011, Teyler & DiScenna 1985) Alternative theories, in particular the multiple trace theory (and also the scene construction theory) propose instead that the hippocampus is necessary for retrieving vivid, contextually rich and detailed autobiographical memories in perpetuity (Nadel & Moscovitch 1997, Winocur & Moscovitch 2011), while semantic information is consolidated over time such that hippocampal involvement is no longer necessary for retrieval.
The array of views outlined above demonstrates that, despite knowing a great deal about the hippocampus, there is still not wide agreement on the information it represents, the processes it performs and the timescale of its involvement. By collating here instances of preserved function in the context of hippocampal damage we hope to contribute to a more fully rounded view of the hippocampus, but also to examine whether consideration of preservation can help to reconcile, or adjudicate between, these theoretical views of hippocampal function.
Navigation
Animal work points to a key role for the hippocampus in allocentric spatial navigation (O'Keefe & Dostrovsky 1971, O'Keefe & Nadel 1978). Studies in humans also support the idea of a spatial function for the hippocampus. For instance, increased grey matter volume in the posterior hippocampus is observed in individuals who have to learn very large and complex spatial layouts (Maguire et al 2000, Woollett & Maguire 2011). During fMRI scanning the hippocampus is engaged when subjects are mentally or virtually navigating (Spiers & Maguire 2006), while intracranial recording from electrodes implanted in the hippocampi of patients being considered for epilepsy surgery show navigation-related responses (Ekstrom et al 2003).
Neuropsychological studies also appear to confirm the necessity of the hippocampus for spatial memory and navigation. Patients with MTL lesions have difficulty learning visual (Milner 1965), tactile (Corkin 1965) and in situ mazes (Astur et al 2002). The latter has also been found following focal hippocampi damage (Goodrich-Hunsaker et al 2010). As in the rat literature, the spatial impairments are typically interpreted as being allocentric in nature (Holdstock et al 2000).
While patient studies have also confirmed that hippocampal damage prevents the spatial learning of new environments (Maguire et al 2006, Rosenbaum et al 2000, Teng & Squire 1999), evidence exists which suggests the hippocampus is not essential for the recall of remotely learned spatial memories. Patient EP (who had extensive damage to his medial and anterior temporal lobes), while being unable to learn new environments, had preserved ability to navigate in an environment he had learned many years prior to his illness (Teng & Squire 1999). He was able to describe routes between his home and local places, between different local locations, between locations when some streets were blocked, and was able to determine the direction (by pointing) to particular landmarks when in a specific location. Another patient, KC (who also had widespread brain damage including the hippocampi) also demonstrated a similar pattern of results (Rosenbaum et al 2000). These two cases speak against the idea of the hippocampus being necessary for allocentric spatial tasks (as proposed by the cognitive mapping theory) and, moreover, imply that remote spatial memories are not hippocampal-dependent (discordant with the multiple trace and scene construction theories).
However, it has been argued that the environments which EP and KC recalled with such accuracy had highly regular, predictable, grid-pattern layouts that may have been over-learned and therefore did not require true allocentric spatial processing (Spiers & Maguire 2007). This is interesting in itself, the fact that some spatial layouts could lend themselves, over time and with extensive experience, to becoming more akin to semantic information and so not require the hippocampus for retrieval. The data from EP and KC therefore highlight that, just as with memory in general, spatial memory may also fractionate along hippocampal-dependent (allocentric) and non-hippocampal-dependent (semantic) lines. But what aspect of navigation is actually impaired in the context of focal hippocampal damage, and is it correct to let the cognitive mapping theory off the hook, so to speak, by interpreting EP and KC’s preservation akin to preserved semantic knowledge?
Another patient has extended our understanding further. TT was a licensed London taxi driver of long-standing who suffered primary damage to his hippocampi (Maguire et al 2006). TT, as with other patients with hippocampal damage, could not learn new environments. But how was his knowledge of and navigation in London, a city noted for its chaotic, unpredictable and complex layout? Compared to matched London taxi driver controls, TT could recognise and describe in detail London landmarks, he had accurate representations of their spatial relationships and the absolute distances between them. Furthermore, he could place landmarks on a map of London, and could point to the location of landmarks with high accuracy. TT could also navigate through (virtual reality) London using main artery or ‘A’ roads. Thus, with more focal hippocampal damage and a more complex environment than EP and KC, patient TT too showed remarkably persevered allocentric spatial ability. It is therefore incorrect to say, as is often the case in the literature, that the hippocampus is essential for navigation in its entirety. These three cases show that characterising hippocampal function in terms of allocentricity alone may not be adequate. Moreover, these findings also suggest that representations of basic relationships between landmarks, and binding or combining of information can occur without the hippocampus at least for material learned prior to the lesions being sustained (Eichenbaum & Cohen 2014, Konkel & Cohen 2009).
Interestingly, TT became lost when navigation depended on the complex network of London’s smaller roads (Maguire et al 2006). This is unlikely to be explained by a lack of detailed knowledge of London, which TT undoubtedly possessed. Instead, TT may not have been able to visualise in advance where he needed to turn off the A roads onto the smaller roads. Indeed, TT was also significantly impaired at imagining scenes (Hassabis et al 2007a). The data from TT also show that the hippocampus remains necessary for spatial navigation even in environments learned long ago, but in a specific way that might involve visualising scenes of key points in the environment.
Autobiographical memory, semantic memory and time
While navigation is a function shared across species, research into the human hippocampi has also focussed on memory processes that are not easily accessible in non-humans. Considering two forms of memory in particular, episodic or autobiographical memory concerns memories of our personal past experiences, and semantic memory refers to general knowledge (see Tulving 1972, Tulving 2002). Patients with focal bilateral hippocampal damage are consistently reported as being unable to form lasting memories of new autobiographical events, and this is widely accepted by all theoretical models of hippocampal function. There is less agreement about whether patients can acquire new semantic information (Mishkin et al 1998, Squire & Zola 1998, Tulving & Markowitsch 1998). Impairments in both recall and recognition for news events, famous faces and whether famous individuals were living or dead have been found in patients with selective hippocampal damage (e.g. Reed & Squire 1998). By contrast, patient YR could recognise famous people and events that came to prominence after her hippocampal damage and could categorise famous people regarding the nature of their fame. She was impaired, however, at categorising events and dating names and events (Holdstock et al 2002). The status of semantic learning post-hippocampal lesion is therefore not clear.
By contrast, semantic memory for pre-lesion information is preserved (e.g. Andelman et al 2010, Lee et al 2005a, Winocur & Moscovitch 2011) and again most theoretical positions accept that semantic information can be retrieved without the hippocampus. However, the question of whether the hippocampus is required for recalling remote autobiographical memories is hotly debated. Two patterns emerge from the literature. Some patients suffer complete loss of autobiographical memories across all time points – recent and remote (e.g. Cipolotti et al 2001, Maguire et al 2006, Viskontas et al 2000). There are others for whom there is a temporal gradient, typically with recent memories lost and then preserved autobiographical memories that are more remote (e.g. Squire & Zola 1998). How remote the memories need to be before they are preserved is not clear-cut, with some patients reported to have intact autobiographical memories stretching back decades (Bayley et al 2003, Kapur & Brooks 1999, Kirwan et al 2008). In reviewing the neuropsychological literature Winocur and Moscovitch (2011) estimate that there is equal support across cases reported in the literature for hippocampal damage to be associated with complete loss of autobiographical memories on the one hand and preservation of more remote memories on the other.
Several possible reasons for these differing patterns have been proposed. For example, it is likely that many of our autobiographical memories become less detailed and more like semantic memories over time (so-called sematicization). This could explain the apparent preservation of remote autobiographical memory in some patients (Winocur & Moscovitch 2011). It could also be the case that those patients with more extensive loss of remote autobiographical memories had more widespread and covert damage (e.g. Reed & Squire 1998). However, neither of these explanations is adequate to explain away all of the contrary evidence. Autobiographical memory and consolidation are of central importance to memory neuroscience because they speak fundamentally to the type of information the hippocampus represents and, by inference, the timescale of its involvement and the processes involved. Yet the neuropsychology seems deadlocked.
FMRI studies of healthy participants have more often supported the view that the hippocampus is necessary for retrieving vivid autobiographical memories in perpetuity (Gilboa et al 2004, Maguire et al 2001, Maguire & Frith 2003, Ryan et al 2001). Of course, fMRI shows brain areas that are involved in, but not whether they are necessary for, a task. Recently, Bonnici et al. (2012) used a different type of method to analyse fMRI data acquired during autobiographical memory recall. Multi-voxel pattern analysis (MVPA) can be used to establish whether information about a memory is represented in the hippocampus from the patterns of fMRI activity across voxels (Chadwick et al 2012). Bonnici et al. (2012) found that information about both recent (2 week old) and remote (10 year old) autobiographical memories (matched across factors such as vividness and detail) was represented in the anterior hippocampus (MVPA classification accuracies were significantly above chance for the two types of memories and were not significantly different from each other). On the other hand, in the posterior hippocampus (and ventromedial prefrontal cortex), classification accuracies were significantly higher for remote memories than recent memories. Further examination revealed that the posterior hippocampal findings were specific to subregions CA3 and DG (Bonnici et al 2013).
These results suggest that some kind of change has indeed taken place between recent and remote autobiographical memories and this is reflected not only in a change in hippocampal-cortical involvement, but within the hippocampus itself – which is not predicted by any theory. The anterior hippocampus may perform a function that is common to both recent and remote memories (perhaps scene construction - Zeidman et al 2014), while remote memories require more of whatever process is going on in the posterior hippocampus – perhaps the reinstatement of the spatial context (Woollett & Maguire 2011). More generally, these data indicate that the mixed pattern of preserved or impaired remote autobiographical memory observed within the neuropsychological literature may depend on the location and extent of the damage within the hippocampus itself.
The precise role of the hippocampus in autobiographical memory is crucial to resolve, and going forward we believe that using fMRI with hippocampal-damaged patients could reveal more about the functionality of remaining hippocampal tissue and its location, as can techniques such as MVPA. What is urgently needed are longitudinal fMRI studies that follow autobiographical memory representations over long time periods (i.e. years) to examine if and how memory traces change over time and concomitant neural responses. These are very challenging fMRI studies to conduct, however, and there are none that we are aware of in the literature to date.
Implicit in autobiographical memory is the notion of time – memories can be recent or from far back in time, and we can also project ourselves forward in time, so called ‘mental time travel’ (Tulving 1972, Tulving 2002). Neurons that appear to respond to time have been found in the rodent hippocampus (MacDonald et al 2011), although an fMRI study in humans found that frontal and parietal cortices, but not the hippocampus, supported mental time travel (Nyberg et al 2010). Time, however, can be construed in different ways and in-depth discussion of time is beyond the scope of this article (see Eichenbaum 2014, Hassabis & Maguire 2007 for reviews). However, it is interesting to consider some instances of preserved time-related processing.
We know, for example, that patients with bilateral hippocampal damage can do basic tasks like arranging pictures into a sequence to make a logical story, as well as being able recombine elements in narratives to ensure a logical story unfolds (Mullally & Maguire 2014). While patients have lost the ability to recall their past (and imagine the future – see Imagination section), they still understand the concept of time (Craver et al 2014a). For example, patient KC, who has widespread damage that includes the hippocampi bilaterally, performed comparably to control participants in being willing to trade a smaller sooner reward for a larger delayed reward (temporal discounting; Kwan et al 2012). If his concept of time was impaired, we would expect him to always choose the reward in the present regardless of the value of a future reward. Furthermore, KC made decisions which would affect future rewards in the same way as control participants demonstrating that while he could not imagine future experiences, he could, on some level, still travel in time (Craver et al 2014b).
Emotion and theory of mind
Even though patients with bilateral hippocampal damage cannot form new autobiographical memories, they can still be affected emotionally by events over time. Fear (Bechara et al 1995) and eyeblink (Gabrieli et al 1995) conditioning are unaffected following bilateral hippocampal lesions while explicit memory for the conditioning was lost. Additionally, after viewing emotional film clips (either happy or sad), patients’ mood remained inducted even though they could not remember details of the film (Feinstein et al 2010). Further, patients could produce as many emotional memories from before their lesion (the majority of which are positive) as controls, which, when rated on emotional intensity by independent raters, was equal to controls (Buchanan et al 2005). On the other hand, when rated for emotional intensity by the patients themselves, memories were rated as less intense.
Yet, while patients with hippocampal damage seem to have retained emotional responses (i.e. the emotion generated when an event is happening), their anxiety response is impaired. For example, in response to a standardised stress test (public speaking) patients showed increased heart rate and affective responses as did control participants, but no cortisol response – cortisol has been associated with anxiety (Buchanan et al 2009). Additionally, in a virtual foraging environment patients were not affected by changes in threat level, spending less time in the “safe place” and behaving less cautiously over time compared to controls despite explicit knowledge of the threat level (Bach et al 2014). Thus, patients show reduced approach-avoidance behaviour when there is a potential threat (i.e. anxiety, not fear). This differentiation seen in patients accords with animal literature suggesting a distinction between the brain circuitry involved in fear, which is thought to be processed by the amygdala, and anxiety, thought to be mediated by the hippocampus (Gray & McNaughton 2003, McHugh et al 2004). Another study suggests that even though patients can experience emotions, their empathy – the ability to share and understand the feelings of others - is reduced. Questionnaire measures (completed by patients and family members) suggested lower trait empathy, and patients were unaffected by empathy inductions via auditory recording and written notes (Beadle et al 2013).
The ability to empathise would seem to be related to theory of mind. This allows an individual to infer the mental states of others. Theory of mind has been reported as intact following hippocampal damage. Patient KC (and a second similar patient ML), who had widespread damage that included the hippocampi bilaterally, performed at the same level as controls on a range of theory of mind tasks (Rosenbaum et al 2007). This finding is interesting to consider in the light of the report of reduced empathy following hippocampal lesions (Beadle et al 2013). Being able to logically understand what someone else knows including their emotional state (as in theory of mind) may be different to fully experiencing someone else’s emotional state (as in empathy). Support for this difference comes from counterfactual thinking, where patients with focal bilateral hippocampal pathology showed intact high-level causal inference which allowed them to logically infer the thoughts and emotional state of a protagonist in an emotional event, without needing to simulate or experience the event or emotions (Mullally & Maguire 2014). In a way, this distinction could be considered similar to that between semantic and episodic memory. It is also interesting to note that when healthy participants were presented with a situation depicting another individual in difficulty, imagining themselves helping the person (episodic simulation) or recalling an event from their past where they helped another (episodic memory) led to increased prosocial intentions (Gaesser & Schacter 2014). That is, engaging elements of episodic processing to help fully experience the event rather than just observing it boosted participants’ empathy, thus suggesting a link between episodic memory and empathy.
Overall, therefore, the ability to feel ‘in the moment’ emotions and basic emotional processing, as well as being able to logically infer in factual terms the thoughts and feelings of others, appears preserved in patients with bilateral hippocampal damage. What they cannot seem to do is imagine another person’s situation in order to fully experience that person’s emotions – it may therefore be, not a deficit in emotion per se, but an impairment of constructing another’s situation.
Recognition memory
The recall or re-experiencing of autobiographical memories is often contrasted with another type of memory. Recognition memory is the ability to recognise previously encountered events, objects, or people. It is typically subdivided into two component processes: recollection and familiarity, often referred to as “remembering” and “knowing”, respectively. Recollection is the retrieval of contextual details associated with the previously experienced event. By contrast, familiarity is the feeling that the event was previously experienced, but without recollection of the associated details or context (see Gardiner & Parkin 1990, Tulving & Thomson 1973, Yonelinas 2002 for reviews).
The role of the hippocampus in recognition memory is hotly debated. Some researchers suggest that all recognition memory (with the exception of faces, see below) requires the hippocampus (e.g. Smith et al 2014a). Others believe that recollection is dependent on the hippocampus but familiarity is not (Brown & Aggleton 2001, Eichenbaum et al 2007). Another view is that hippocampal involvement is stimulus dependent, being required for recognition of across-domain pairs of items (e.g. a picture and a sound) but not single items or within-domain (e.g. picture-picture) pairs (e.g. Mayes et al 2007).
This is an entrenched debate and, as with autobiographical memory above, there is neuropsychological evidence from patients with focal hippocampal damage to support each perspective. We cannot do this substantial literature justice in a short space here, and others have written eloquently and at length about it elsewhere (Brown & Aggleton 2001, Eichenbaum et al 2007, Yonelinas 2002). Therefore we limit ourselves here to making just a few observations.
One consistent result is that of preserved face recognition (Aggleton & Shaw 1996, Bird & Burgess 2008, Mayes et al 2004, Smith et al 2014a), although this may only be at short delays (Smith et al 2014a). In an exceptionally thorough examination of recognition memory across different types of stimuli (including words, faces, buildings, objects), patient YR’s forced choice, Yes/No, intra-item associations and associations between items of the same category was preserved (Mayes et al 2004). YR was only impaired on recognition tests for associations between items of different kinds (e.g. words and faces), a finding that has been replicated (e.g. Holdstock et al 2005, Konkel et al 2008).
The consistent finding of preserved face recognition may seem at odds with YR’s impaired recognition of associations across domains. However, a face in contrast to other complex stimuli, is thought to be processed as a whole entity and not as multiple component parts (e.g. Tsao & Livingstone 2008). An interesting contrast to faces is that of scenes. Scene stimuli are complex stimuli in that they are made up of multiple features. Unlike faces however, scenes are thought to be processed by combining each individual feature. Notably, despite preserved ability to recognise faces, patients with focal hippocampal damage are typically impaired at recognising scenes (Taylor et al 2007).
Thus, preserved recognition memory following hippocampal damage may occur for two reasons: first, if a familiarity processes can be used and not a recollective one (Eichenbaum et al 2007), or second, provided the internal representation of a spatially coherent scene/context is not required (Lee et al 2012, Maguire & Mullally 2013, Zeidman et al 2014).
Working memory
When performing a number of the above tasks, working memory may be engaged – this is the transient holding online of information, for example, maintaining stimuli in mind to decide upon whether they are old or new at short delays. Working memory has traditionally been regarded as immune from hippocampal damage. Indeed standard tests of working memory (e.g. digit span) are preserved following such damage (e.g. Andelman et al 2010, Goodrich-Hunsaker & Hopkins 2009, Hopkins et al 2004, Victor & Agamanolis 1990, Warren et al 2012).
Experimental tests of working memory also indicate preservation. Hippocampal-damaged patients’ eye movements had similar patterns to that of control participants when shown a manipulated scene soon after the original, suggesting spared working memory (Ryan et al 2000, Ryan & Cohen 2004). Further, during the spatial exploration of masked scenes (the scene could only be seen through a moveable window), hippocampal damaged patients were able to successfully relocate to their original start location from the goal object within each trial (Yee et al 2014). Additionally, working memory has been shown to be preserved in patients for single objects or single locations (Olson et al 2006a, Olson et al 2006b).
However, more recent investigations suggest that working memory might not be completely hippocampal independent (e.g. Ranganath & Blumenfeld 2005, Yonelinas 2013). While working memory is preserved for single items, more complex associations requiring combining elements together led to impaired working memory, for object-location (Olson et al 2006a, Olson et al 2006b), face-scene and object-scene relations (Hannula et al 2006, Hannula et al 2015) and topographical stimuli (Hartley et al 2007). Moreover, MEG work suggests increased hippocampal theta synchronicity with occipital and temporal regions during working memory maintenance of scenes (Cashdollar et al 2009).
Thus, working memory seems to be preserved following hippocampal damage for single items and locations. However, when using more complex stimuli, typically involving scenes, impairments are reported.
Verbal memory
While debates rage about hippocampal contributions to some of the memory types outlined above, there is one form of memory that is invariably compromised by bilateral hippocampal lesions and that is verbal memory. Patients cannot recall lists of single words (Buchanan et al 2005), word-pair associates (Cipolotti et al 2006) and verbal narratives (Barense et al 2007). Why might this be the case, given that single words do not require any associative binding and none of this verbal material appears to involve allocentric processing, object-context binding or the internal construction of scenes?
Standardised verbal memory tests (e.g. Warrington Word Recognition Memory Test, Wechsler Memory Scale word pair associates and logical memory subtests, Rey Auditory Verbal Learning Test) all use concrete words which represent specific imaginable items (Paivio 1969). One speculation offered by Maguire and Mullally (2013) is that people may automatically use imagery, such as scenes, during encoding and retrieval of concrete verbal material. For instance, we might visualise the scene within which a story is unfolding, or place the items described in word pairs in a simple scene together. Despite the rise and fall of imagery-based memory theories across the decades (Paivio 1969), there is evidence to suggest that visual imagery not only boosts pair-associate recall in healthy participants, but also enabled patients with left temporal lobectomies to partially compensate for their verbal memory deficits (Jones 1974). If verbal memory tasks routinely benefit from the use of imagery-based mnemonic strategies, and if hippocampal amnesic patients have difficulty imagining scenes (Hassabis et al 2007a), they would be disadvantaged on such tasks. This, then, gives rise to the clear prediction that the patients should be less impaired when learning and recalling abstract words.
Abstract words typically represent ideas and concepts and, as such, they are much less imageable. From the literature it is surprisingly difficult to ascertain whether memory for abstract words is preserved following focal bilateral hippocampal damage, as most tests and studies have used concrete, imageable words. If we instead consider patients who had unilateral temporal lobectomies for the relief of intractable epilepsy, then abstract words have been examined. Right temporal lobectomy patients were found to have impaired memory for concrete word pairs but preserved memory for abstract word pairs compared to controls (Jones-Gotman & Milner 1978, see also Jones-Gotman 1979). Moreover, while greater hippocampal lesion extent was associated with a bigger drop in performance on concrete word pairs, lesion size had no effect on abstract word pair performance.
Further work suggests that imageability may be key to understanding this pattern of preservation and impairment (Jones-Gotman 1979). Patients and control participants were presented with a list of mixed concrete and abstract words. For some words, they were asked to visualise the word, whereas for others they were asked to pronounce the word. After a delay they had to recall the word list - right temporal lobectomy patients had comparable performance to control participants for both concrete and abstract words when the words were previously pronounced, but inferior performance on both abstract and concrete words when the words were previously visualised. Greater extent of hippocampal lesion was associated with impaired performance on visualised concrete words, but had no relationship with the other conditions. Imageability of words seems, therefore, to be important to understanding the relationship between words and the hippocampus. In further support of this, Gold et al (2006) and Kirwan et al (2010) found that patients with bilateral hippocampal damage were impaired at both word recognition and recall with mixed concrete and abstract words. However, for the abstract words they explicitly required the patients to learn by imagining an indoor or outdoor scene, which likely explains their impairment, given that hippocampal damaged patients are unable to imagine scenes (Andelman et al 2010, Hassabis et al 2007a, Mullally et al 2012a, Race et al 2011, Rosenbaum et al 2009).
It could be that abstract words simply require greater effort and memory search to develop a representational image (Kieras 1978), but thereafter are processed like concrete words. If this is the case, then there should be significant overlap in the brain networks supporting the processing of concrete and abstract words in neuroimaging studies of healthy participants. However, extant data show differences in the brain networks for processing abstract and concrete words (e.g. Binder et al 2005, Wang et al 2013).
The majority of hippocampal theories have a visuospatial bias (Bird et al 2012, Maguire & Mullally 2013, Moscovitch et al 2006, O'Keefe & Nadel 1978, Ranganath 2010, Schacter & Addis 2009). Accounting for verbal memory deficits is therefore challenging. If, however, there is a distinction between abstract and concrete memoranda, and processing of the former is preserved following bilateral hippocampal lesions, this would have important implications for understanding and conceptualising hippocampal processing. It is therefore surprising that abstract verbal material has featured so little across the decades of research involving patients with bilateral hippocampal damage. This gap in our knowledge clearly needs to be addressed.
Learning
As observed previously, acquisition of new episodic information, such as autobiographical events, is compromised in the context of bilateral hippocampal damage, while there are mixed reports concerning the preservation of semantic learning. There is a group of patients who sustained their bilateral hippocampal damage very early in life who display instances of preserved learning and other interesting features (see sidebar on ‘Developmental amnesia’). However, our main focus here is in asking whether patients whose bilateral hippocampal damage occurred in adulthood can learn and retain any kind of new information. There is an extensive literature on preserved priming and implicit learning in amnesia that we cannot cover here, and so we refer the reader to a recent review (Reber 2013). We therefore limit this section to reflections on other aspects of preserved learning that have not received such extensive coverage.
Developmental amnesia (DA).
Learning
DA occurs following a hypoxic/ischemic incident perinatally or in early childhood, resulting in bilateral hippocampal pathology (Gadian et al 2000, Vargha-Khadem et al 1997, Vargha-Khadem et al 2003). A distinguishing feature of DA compared to hippocampal damage sustained in adulthood is that the content of semantic memory and world knowledge, which is rich and age appropriate, has been learned after the onset of hippocampal pathology. This contrasts with their autobiographical memory which is impaired. This may indicate that semantic learning is hippocampal independent, although reorganisation of the developing brain in the presence of hippocampal damage could be a contributing factor. Also in contrast to adult patients (e.g. Hassabis et al 2007a), individuals with DA appear to have preserved ability to imagine fictitious and future scenes (Cooper et al 2011, Hurley et al 2011, Maguire et al 2010b). However, this seems to rely on their intact semantic and world knowledge - they describe it as an effortful process and one where they are unable to actually visualise the scenes in their mind’s eye. Moreover, unlike control participants, in well-characterised DA patient Jon, his remnant hippocampal tissue was not significantly activated while he constructed scenes during fMRI (Mullally et al 2014).
Patient HM displayed some implicit learning, for example, his motor skills improved on a tapping task and two tracking tasks (where a drum rotated and he was required to keep contact with a specified track with just one hand or with both hands simultaneously) even though he had no memory of previously performing the task (Corkin 1968). Implicit learning has also been shown for digit, spatial location, word and pseudo word sequences (e.g. Gagnon et al 2004), and for procedural learning including geometric figure tracing, weaving and pouring liquid into multiple containers from a height (Cavaco et al 2004). Long lasting priming (up to 7 days) involving verbal material (e.g. word stem completion and word pairs) and object naming has also been reported in patients (Schacter et al 1993, Tulving & Schacter 1990). By contrast, mixed results have been reported for visuospatial search where participants had to locate a rotated “T” within a display of rotated “L” distractors. Originally, results suggested that patients showed no priming (Chun & Phelps 1999), while a later study suggested otherwise (Manns & Squire 2001). Thus, priming over a more complex scene display may be reduced compared to single items.
Some elements of probabilistic learning are also preserved in patients with bilateral hippocampal damage. This concerns tasks where the associations between stimuli and responses are probabilistic, and so information from a single trial is not reliable and therefore not as relevant as information accrued across many trials. During initial learning trials, learning rates have been reported as equal between patients and controls (Knowlton et al 1996, Reber et al 1996). However, after continuous training (e.g. more than 50 learning trials) controls began to outperform patients (Knowlton et al 1994), possibly due to controls beginning to use more complex strategies to learn outcomes (Meeter et al 2006). However, while initial learning could take place, when outcome probabilities were changed, patients did not change their responses, suggesting an impairment in flexibly using the acquired knowledge (Shohamy et al 2008).
By contrast, in another study probabilistic learning in patients was found to be impaired across the board (Hopkins et al 2004). Here, while patients’ scores remained at around the same level as previously reported, control participants’ scores increased to much higher levels. Further, using a similar paradigm but deterministic learning and configural (i.e. combined) elements, patients were impaired compared to control participants (Kumaran et al 2007). Two patients in this latter study showed better learning than the other patients, suggesting some ability to combine information, but on debriefing they indicated that while they had formed associations between outcomes and individual combined patterns, they could not relate the patterns to each other. Thus, it seems that even when some basic elements of associative learning are retained, the ability to integrate and use this information may be lost in the context of hippocampal damage.
Another type of learning, collaborative learning or learning within a “common ground”, has been found to be intact following bilateral hippocampal lesions (Duff et al 2006). Over time, patients needed to use fewer words to generate labels for abstract objects when describing them to a known partner. Further, this label knowledge was retained at 6 months. However, the patients could not remember the objects themselves – while the shortened labels created in common ground were retained, the objects needed to be present for the patients to describe them (Rubin et al 2011).
In another examination of associative learning, patients with damage to the hippocampus and wider MTL learned (and retained at 1 week) arbitrary associations via “fast mapping”, despite impairment on a matched standard association task (Merhav et al 2014, Sharon et al 2011). Fast mapping is the process by which children rapidly acquire new words (Carey & Bartlett 1978) and involves actively discovering associations instead of deliberate learning. However, two other studies failed to find preserved learning following fast mapping (Smith et al 2014b, Warren & Duff 2014). The reasons for this disparity are not clear, and more work on fast-mapping is required to better understand the parameters within which such learning might be possible.
In summary, patients with bilateral hippocampal damage are able to form arbitrary associations, particularly when learning is implicit. Yet, patients typically do not remember how or where the information was obtained, nor can they flexibly use the acquired information. The knowledge therefore seems to lack a backdrop or a context, a time or place – a theme running through several hippocampal theories (Buzsaki & Moser 2013, Eichenbaum & Cohen 2014, Maguire & Mullally 2013).
Visual perception
The hippocampus receives a large number of inputs from multiple sensory modalities and in particular from vision (Felleman & Van Essen 1991). It may be that preserved or impaired cognitive functions could in fact arise from a basic processing level, namely that of visual perception. Traditionally, visual perception has been reported as preserved following hippocampal damage (Scoville & Milner 1957, see also Lee et al 2005a, Spiers et al 2001). Moreover, a series of studies suggests that focal bilateral hippocampal damage predominantly leaves visual discrimination abilities intact for material such as faces, single objects, abstract art, and colours. There is one exception; patients could not discriminate between scenes (Graham et al 2006, Lee et al 2005a, Lee et al 2005b).
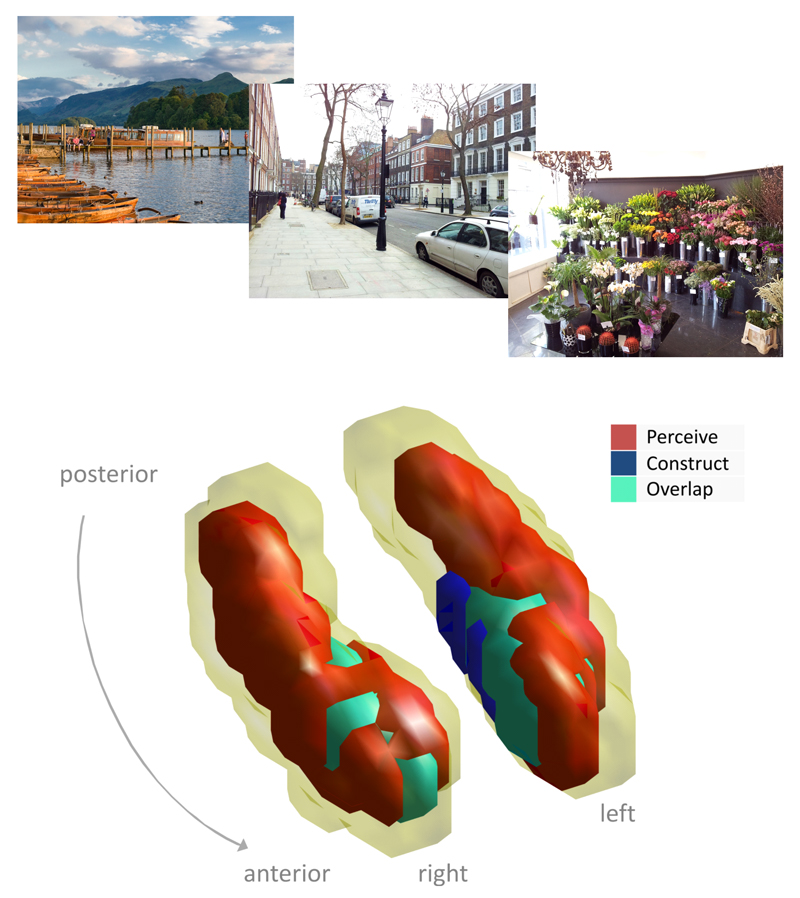
FMRI studies of healthy participants have shown hippocampal engagement during the perception and discrimination of scenes (Barense et al 2010, Lee et al 2008, Mundy et al 2012). Zeidman et al (2014) recently investigated the hippocampal response to visually perceiving scenes, constructing scenes in the imagination and maintaining scenes in working memory. They found extensive hippocampal activation for perceiving scenes, and a circumscribed area of anterior medial hippocampus common to scene perception and scene construction (Figure 3). There was significantly less hippocampal activity for maintaining scenes in working memory. Further evidence from patients and fMRI in healthy participants suggests that the hippocampus is engaged in perception when discriminating “strength based perception” (the global entity) but not “state-based perception” (local visual features; Aly et al 2013), highlighting region CA1 in particular (Elfman et al 2014).
Figure 3.
Scene processing and the hippocampus. Upper panels are examples of scenes used in the scene viewing condition of Zeidman et al’s (2014) fMRI study. Lower panels show a schematic of the two hippocampi from that study indicating activity associated with viewing scenes (perception), constructing scenes in the mind’s eye and an area in anterior medial hippocampus of maximal overlap in the activity associated with the two conditions.
However, patients have also been reported to have preserved visual perception and stimulus discrimination regardless of the stimuli used (Kim et al 2011, Shrager et al 2006). These authors suggest that impairments in visual perception observed elsewhere are in fact due to discrimination ability not improving over time as in control participants, overloading of working memory or long term memory encoding deficits (Kim et al 2011, Knutson et al 2012). Zeidman et al (2014) recently described how differences across the patient scene perception literature could be reconciled. They propose that whether scene perception is preserved or impaired in patients with hippocampal lesions may depend on whether a task requires an internal model of a spatially coherent scene to be constructed. We elaborate further on this idea below.
Imagination
When, in 1992, HM was asked what he believed he would do tomorrow he replied “whatever is beneficial” and appeared to have “no database to consult when asked what he would do the next day, week, or in years to come” (S. Corkin, personal communication; cited in de Vito & Della Sala 2011). Similar anecdotal evidence of problems imagining the future was reported in patient KC (Rosenbaum et al 2005, Tulving 1985; see also patient DB in Klein et al 2002). Hassabis et al (2007a) formally tested a group of patients with more focal bilateral hippocampal pathology and found they were unable to imagine personal future scenarios and fictitious scenes. They reported that their attempted scenes were spatially fragmented. Providing the scene elements to the patients did not improve their performance. Subsequently, this scene construction deficit was replicated across different laboratories and in different sets of hippocampal-damaged patients (Andelman et al 2010, Mullally et al 2012b, Race et al 2011, Rosenbaum et al 2009), except in one study where scene construction ability was reported as preserved (Squire et al 2010). It is notable that the patients in this latter study did not exhibit pervasive autobiographical memory loss (see also Kirwan et al 2008). As such, this finding in fact provides further support for the scene construction theory which posits that if patients have intact autobiographical memory then they should also have preserved scene construction ability, because the former depends on the latter (Maguire & Mullally 2013; see also Maguire & Hassabis 2011 and Mullally et al 2012a for more on this). FMRI studies of healthy participants have confirmed hippocampal engagement during scene construction/simulation tasks (Addis et al 2007, Hassabis et al 2007b). Interestingly Hassabis et al (2007a) found that one patient with bilateral hippocampal damage could construct scenes, and during fMRI this was associated with significant activation of the remnant tissue of his right hippocampus (Mullally et al 2012a).
While the inability to imagine fictitious or future scenes is striking, it is equally informative to consider related preservations. Patients were able to imagine single isolated objects, and could list relevant associated items; they simply could not visualise them in a coherent scene (Hassabis et al 2007a, Mullally et al 2012a). Patients tested by Mullally et al (2012b) and Race et al (2013) could richly describe pictures of scenes that were put in front of them, which in the latter study included forming detailed narrative descriptions of scene images, suggesting basic scene perception was intact. This seems at odds with the findings in the previous section of impaired scene perception.
Further clues about the hippocampal role in scene processing come from the study of boundary extension (BE; Intraub & Richardson 1989). BE is a where people erroneously remember seeing more of a scene than was present in the sensory input, and occurs because when we view a scene, we implicitly extrapolate beyond the borders to form an extended representation of that scene. In the absence of the original visual input, this extended scene is remembered instead of the original input, causing a memory error. BE is a robust and consistent effect and, of note, only occurs in relation to scenes and not single isolated objects (Gottesman & Intraub 2002), a dissociation that mirrors the imagination dichotomy observed in hippocampal-damaged patients (Hassabis et al 2007a).
Mullally et al (2012b) found that patients with focal bilateral hippocampal damage had significantly attenuated BE. They did not extrapolate as much as controls beyond the view in scenes they were shown, and this paradoxically led to significantly better memory for the scenes compared to the control participants (see Kapur 2011 for other examples of paradoxical facilitation following brain lesions). BE depends on the ability to imagine beyond the view in a scene, and having lost this ability, the patients were then less susceptible to the BE effect. An fMRI study of healthy participants confirmed the engagement of the hippocampus during BE (Chadwick et al 2013). Mullally et al (2012b) also showed patients a picture of a scene and asked them to imagine what might be beyond the view. Although they could generate as many details as control participants appropriate to the context and could associate them with each other and the context, they made significantly fewer spatial references, and were unable to visualise the extended scenes in their mind’s eye.
Kim et al (2015), testing most of the same patients that were examined by Squire et al (2010), have recently reported that their patients showed normal BE and thus disputed the idea that the hippocampus is required for scene construction. They also questioned the degree of hippocampal volume loss in Mullally et al’s (2012b) patients. In fact, Kim et al (2015) made a factual error on this latter point. They incorrectly claimed that two of Mullally et al’s (2012b) patients had hippocampal volume loss greater than 70%. As stated by Mullally et al (2012b), the volumes were reduced to (not by) 68.7%-78.33% of normal, rendering redundant their arguments about this point.
Concerning their BE findings, it is not surprising that Kim et al (2015) found normal BE in their patients given that BE depends upon scene construction ability which was shown to be intact in these patients (Squire et al 2010). Moreover, Kim et al (2015) changed critical elements in how BE was tested which may have fostered null results. Critically, BE weakens as stimulus view widens (Hubbard et al 2010). To enhance sensitivity to group differences, in Mullally et al (2012b) very tight close-ups were selected for the BE drawing task (objects filled 43.4% of the space). By contrast, Kim et al (2015) used more wide-angled photographs (objects filled 30.2% of the space), thus reducing the ability to distinguish group differences. In addition, Kim et al (2015) more than doubled the number of trials typical for this method (Mullally et al 2012b, see others in Hubbard et al 2010). This raises concerns, given that such trial limitations were used to minimize inter-trial effects on memory. Kim et al’s (2015) experiment 2b is especially surprising, because participants were explicitly discouraged from selecting the correct (‘the same’) response, thus biasing the experiment away from finding attenuated BE and consequently, once again, restricting the opportunity for detecting group differences. We therefore believe that the patients tested in Squire et al (2010) and Kim et al (2015), without pervasive autobiographical memory deficits, and features of the testing in both studies may go some way towards accounting for the anomalies between their results and others in the literature.
One other apparent preservation is relevant to consider here. We often engage in counterfactual (CF) thinking, which involves reflecting on ‘what might have been’. Creating alternative versions of reality seems to have parallels with recollecting the past and imagining the future (for more on CF thinking see Schacter et al 2015). Given that these are impaired in patients with hippocampal damage, we might predict that CF thinking would be compromised following hippocampal damage. Testing non-personal CF thinking, Mullally and Maguire (2014) found that patients could deconstruct reality, add in and recombine elements, change relations between temporal sequences of events, enabling them to determine plausible alternatives of complex episodes. However, a difference between the patients and control participants emerged in the patients’ subtle avoidance of CF simulations that required the construction of an internal spatial representation. These findings suggest that mental simulation in the form of non-episodic CF thinking, does not seem to depend upon the hippocampus unless there is the added requirement for construction of a coherent spatial scene within which to play out scenarios.
In summary, it may be that healthy individuals are never passively perceiving scenes because the boundary extension effect, underpinned by scene construction, always occurs and engages the hippocampus. Thus, without a model of a scene being constructed in the hippocampus, the scene currently in view can only be comprehended in isolation and cannot be extended beyond its borders or in one’s imagination. It is for this reason Zeidman et al (2014) proposed that scene perception tasks that require the generation of an internal model of a scene (as is typically required in scene discrimination tasks) are dependent upon the hippocampus. Maguire and Mullally (2013) go further and suggest that any task or any aspect of cognition that requires an internal model of a scene will be adversely affected by bilateral hippocampal lesions.
Theoretical implications and conclusions
Here we surveyed the literature across numerous cognitive tasks to collate instances of preserved, and even facilitated, performance in patients with focal bilateral hippocampal damage. What is striking in the first instance is the nature of the preservations. For example, we are accustomed to reading in the literature that navigation is impaired in patients with focal bilateral hippocampal damage, but this kind of sweeping statement belies the facts. In terms of environments learned pre-lesion, only one specific aspect of navigation seems to be impaired, while performance on the majority of tasks assessing even high level allocentric spatial memory and knowledge are all intact. In other instances, a lack of empirical studies precludes a proper evaluation of hippocampal involvement. Verbal memory is a case in point, held to be a paradigmatic example of impairment following hippocampal lesions, and yet there is a dearth of studies examining patients’ ability to learn abstract verbal memoranda. Given such gaps in our knowledge and accepting that theories are formulated on more than neuropsychological evidence alone, how do the theoretical accounts we outlined earlier hold up when the preservations described here are considered?
Rather than focusing on specific instances of preservation and their implications for each theory, it is perhaps more useful to ask whether any clear or unifying themes emerge from the data as a whole. By concentrating on commonalities across different aspects of memory and cognition we can to some degree guard against the interpretational issues summarised at the outset.
We believe that the patterns of preservation noted here help isolate a core problem that patients with focal bilateral hippocampal damage face. They cannot construct a spatially coherent model of the world. In other words, they are unable to construct internal representations of scenes. They seem unable to visualise in advance when to make turnings onto smaller roads during navigation, they cannot reconstruct scenes of past events or imagine scenes in the future, they are impaired at constructing another person’s situation to experience that person’s emotions, and they have deficits in recognition memory and working memory specifically for scenes. Their learning is devoid of a context, their perception specifically of scenes is compromised when internal models of scenes need to be generated. They show attenuated boundary extension, leaving them access to only what is in front of their eyes.
Considering current theories, we believe the scene construction theory can best account for the patterns of impairment and preservation across these functional domains (Hassabis & Maguire 2007, 2009, Maguire & Mullally 2013). A purely allocentric account (O'Keefe & Nadel 1978), or a purely associative/relational model (Konkel et al 2008) are not completely satisfactory given that patients appear to retain aspects of these abilities in some shape or form. By viewing the core function of the hippocampus as constructing spatially coherent scenes, this helps to explain the hippocampus’ role in a diversity of cognitive functions that extend beyond memory. As noted by Maguire & Mullally (2013), the hippocampus is not solely responsible for all of these functions, but rather it contributes a key ingredient - scene construction. This is why considering preservations is vital for aiding interpretation; the many aspects of navigation and memory that are preserved following hippocampal damage make sense because they do not require the internal construction of scenes. We note that at this time the scene construction theory has not been tested directly in relation to all the preservations and deficits that follow hippocampal damage. This in particular applies to verbal memory, although the majority of hippocampal theories have a similar visuospatial bias (Bird et al 2012, Maguire & Mullally 2013, Moscovitch et al 2006, O'Keefe & Nadel 1978, Ranganath 2010, Schacter & Addis 2009), and accounting for verbal memory deficits is a universal challenge. We also appreciate that others have different views and have noted evidence purported to speak against the scene construction theory that the reader may wish to take into account (e.g. Kim et al 2015, Squire et al 2010).
Finally, we acknowledge that we are stakeholders in the scene construction theory, and that others with divergent views may come to different conclusions after reflecting on the patterns of preservation we have collated here. We welcome debates that consider all of the evidence. Overall, our hope is that people take preservations into account to a greater extent in their empirical studies and their theoretical models of the hippocampus and memory, that they begin to make principled predictions about preservations as well as deficits, and that the findings surveyed here stimulate new questions about the old issues of what does the hippocampus do and how does memory work.
Summary points.
Neuropsychological studies are dominated by the lesion-deficit model while preservation of function following brain damage receives less attention.
Considering preservation of function following focal bilateral hippocampal damage could help to inform, refine or refute extant models of the hippocampus and memory.
We collated evidence across a range of functional domains concerning preservations following focal bilateral hippocampal lesions.
While not exhaustive, this survey revealed some unexpected abilities, new angles on extant beliefs, and surprising gaps in our knowledge.
Overall, when considered in their totality, the data appear to suggest that patients cannot construct spatially coherent models, or scenes, of the world, and this may explain their pattern of performance across disparate aspects of cognition.
We believe that the scene construction theory may be best able to account for the preservations and deficits that arise from focal bilateral hippocampal damage.
Future issues.
Presentation of neuropsychological data would be improved by routine reporting of effect sizes, confidence intervals and by showing all data from each participant. In this way preservations in particular can be interpreted more accurately.
Scanning patients using fMRI could provide insights into the functionality of remnant hippocampal tissue and aid in interpreting preservations.
Researchers should be confident to make principled predictions about preservations as well as deficits when assessing patients with focal bilateral hippocampal damage.
Putting a spotlight on preservations has revealed gaps in our knowledge, for example, concerning verbal memory, that need to be pursued.
If we understand more about preservation following hippocampal damage, we may be better placed to approach rehabilitation in a more efficacious way in the future.
We believe that the scene construction theory is currently in the best position to account for the patterns of preservation and impairments observed following focal bilateral hippocampal damage. But how is scene construction realised by the hippocampus and what are the mechanisms involved?
Acknowledgements
Support for the authors came from a Wellcome Trust Principal Research Fellowship to E.A.M. The authors thank Narinder Kapur for helpful discussions, and Sinéad Mullally and Helene Intraub for their contributions to the discussion of boundary extension.
Acronyms and Definitions List
- MTL
Medial Temporal Lobes
- Allocentric space
A world-centred spatial framework of the environment
- Egocentric space
Space is represented relative to the observer him/her self
- Scene
A spatially coherent representation of the world, small or large-scale, within which we can potentially operate
- Autobiographical memory
Memories of our personal past experiences
- Semantic memory
General and world knowledge
- Recognition memory
The ability to recognise previously encountered events, objects, or people
- Recollection
The retrieval of contextual details associated with a previously experienced event
- Familiarity
The feeling that an event was previously experienced, but without recollection of the associated details or context
- Implicit memory
When previous experience enhances performance, but without explicit awareness of that previous experience
- Fast mapping
The process by which children rapidly acquire new words, whereby new associations are discovered and not deliberately learnt
- Working memory
The transient holding online of information
- Empathy
The ability to share and understand the feelings of others
- Theory of mind
The ability to infer the mental states of others
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- Aberson C. Interpreting null results: Improving presentation and conclusions with confidence intervals. Journal of Articles in Support of the Null Hypothesis. 2002;1:36–42. [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Shaw C. Amnesia and recognition memory: A re-analysis of psychometric data. Neuropsychologia. 1996;34:51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Warburton EC, Aggleton JP. Hippocampal lesions halve immediate–early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. Eur J Neurosci. 2007;26:1254–66. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- Aly M, Ranganath C, Yonelinas Andrew P. Detecting changes in scenes: The hippocampus is critical for strength-based perception. Neuron. 2013;78:1127–37. doi: 10.1016/j.neuron.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–66. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Publication Manual of the American Psychological Association. Washington D.C: American Psychological Association; 2010. [Google Scholar]
- Andelman F, Hoofien D, Goldberg I, Aizenstein O, Neufeld MY. Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase. 2010;16:426–35. doi: 10.1080/13554791003623318. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, van der Kouwe AJW, Salat DH, Benner T, Stevens AA, et al. H.M.'s contributions to neuroscience: A review and autopsy studies. Hippocampus. 2014 doi: 10.1002/hipo.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Guitart-Masip M, Packard PA, Miró J, Falip M, et al. Human hippocampus arbitrates approach-avoidance conflict. Curr Biol. 2014;24:541–47. doi: 10.1016/j.cub.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–74. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Lee ACH, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2010;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett FC. Remembering: A study in experimental and social psychology. Cambridge, England: Cambridge University Press; 1932. [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38:135–44. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Beadle JN, Tranel D, Cohen NJ, Duff MC. Empathy in hippocampal amnesia. Front Psychol. 2013;4:69. doi: 10.3389/fpsyg.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–18. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Binder J, Westbury C, McKiernan K, Possing E, Medler D. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 2005;17:905–17. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus supports recognition memory for familiar words but not unfamiliar faces. Curr Biol. 2008;18:1932–36. doi: 10.1016/j.cub.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Bird CM, Bisby JA, Burgess N. The hippocampus and spatial constraints on mental imagery. Front Hum Neurosci. 2012;6:142. doi: 10.3389/fnhum.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, Maguire EA. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J Neurosci. 2012;32:16982–91. doi: 10.1523/JNEUROSCI.2475-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Maguire EA. Representations of recent and remote autobiographical memories in hippocampal subfields. Hippocampus. 2013;23:849–54. doi: 10.1002/hipo.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. J Neurosci. 2005;25:3151–60. doi: 10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav. 2009;56:44–50. doi: 10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–38. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo P, Garrido MI, Moran RJ, Maestú F, García-Morales I, et al. Remote effects of hippocampal sclerosis on effective connectivity during working memory encoding: A case of connectional diaschisis? Cereb Cortex. 2012;22:1225–36. doi: 10.1093/cercor/bhr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Bartlett E. Acquiring a single new word. Pap R Child. 1978;15:17–29. [Google Scholar]
- Cashdollar N, Malecki U, Rugg-Gunn FJ, Duncan JS, Lavie N, Duzel E. Hippocampus-dependent and -independent theta-networks of active maintenance. Proc Natl Acad Sci. 2009;106:20493–98. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Allen JS, Castro-Caldas A, Damasio H. The scope of preserved procedural memory in amnesia. Brain. 2004;127:1853–67. doi: 10.1093/brain/awh208. [DOI] [PubMed] [Google Scholar]
- Chadwick MJ, Bonnici HM, Maguire EA. Decoding information in the human hippocampus: A user's guide. Neuropsychologia. 2012;50:3107–21. doi: 10.1016/j.neuropsychologia.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Mullally SL, Maguire EA. The hippocampus extrapolates beyond the view in scenes: An fMRI study of boundary extension. Cortex. 2013;49:2067–79. doi: 10.1016/j.cortex.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–47. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, et al. Long-term retrograde amnesia… the crucial role of the hippocampus. Neuropsychologia. 2001;39:151–72. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: A case study. Neuropsychologia. 2006;44:489–506. doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cohen NJ. Preserved learning capacity in amnesia: Evidence for multiple memory systems. In: Squire LR, Butters N, editors. Neuropsychology of Memory. New York: Guilford Press; 1984. pp. 83–103. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–88. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Vargha-Khadem F, Gadian DG, Maguire EA. The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia. 2011;49:1843–50. doi: 10.1016/j.neuropsychologia.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Tactually-guided maze learning in man: Effects of unilateral cortical excisions and bilateral hippocampal lesions. Neuropsychologia. 1965;3:339–51. [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–65. [Google Scholar]
- Craver CF, Kwan D, Steindam C, Rosenbaum RS. Individuals with episodic amnesia are not stuck in time. Neuropsychologia. 2014a;57:191–95. doi: 10.1016/j.neuropsychologia.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Craver CF, Cova F, Green L, Myerson J, Rosenbaum RS, et al. An Allais paradox without mental time travel. Hippocampus. 2014b;24:1375–80. doi: 10.1002/hipo.22318. [DOI] [PubMed] [Google Scholar]
- de Vito S, Della Sala S. Predicting the future. Cortex. 2011;47:1018–22. doi: 10.1016/j.cortex.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nat Neurosci. 2006;9:140–46. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Kirsner K. What Can we Infer from Double Dissociations? Cortex. 2003;39:1–7. doi: 10.1016/s0010-9452(08)70070-4. [DOI] [PubMed] [Google Scholar]
- Dunstan EJ, Winer JB. Autoimmune limbic encephalitis causing fits, rapidly progressive confusion and hyponatraemia. Age Ageing. 2006;35:536–37. doi: 10.1093/ageing/afl045. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–70. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci. 2014;15:732–44. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–88. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Elfman KW, Aly M, Yonelinas AP. Neurocomputational account of memory and perception: Thresholded and graded signals in the hippocampus. Hippocampus. 2014;24:1672–86. doi: 10.1002/hipo.22345. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Duff MC, Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proc Natl Acad Sci. 2010 doi: 10.1073/pnas.0914054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ, Heene M. A vast graveyard of undead theories: Publication bias and psychological science’s aversion to the null. Perspect Psychol Sci. 2012;7:555–61. doi: 10.1177/1745691612459059. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JF. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci. 1995;109:819–27. doi: 10.1037//0735-7044.109.5.819. [DOI] [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic–ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Schacter DL. Episodic simulation and episodic memory can increase intentions to help others. Proc Natl Acad Sci. 2014;111:4415–20. doi: 10.1073/pnas.1402461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon S, Foster J, Turcotte J, Jongenelis S. Involvement of the hippocampus in implicit learning of supra-span sequences: The case of SJ. Cogn Neuropsychol. 2004;21:867–82. doi: 10.1080/02643290342000609. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Parkin A. Attention and recollective experience in recognition memory. Mem Cognit. 1990;18:579–83. doi: 10.3758/bf03197100. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–25. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, et al. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. Proc Natl Acad Sci. 2006;103:9351–56. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hopkins RO. Word memory test performance in amnesic patients with hippocampal damage. Neuropsychology. 2009;23:529–34. doi: 10.1037/a0015444. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20:481–91. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Gottesman CV, Intraub H. Surface construal and the mental representation of scenes. J Exp Psychol Hum Percept Perform. 2002;28:589–99. doi: 10.1037//0096-1523.28.3.589. [DOI] [PubMed] [Google Scholar]
- Graham KS, Scahill VL, Hornberger M, Barense MD, Lee ACH, et al. Abnormal categorization and perceptual learning in patients with hippocampal damage. J Neurosci. 2006;26:7547–54. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Pérez F, D'Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci. 2012;24:1275–85. doi: 10.1162/jocn_a_00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, McNaughton N. The neuropsychology of anxiety. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–59. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Allen JS, Kirchhoff BA, Nickel AE, Cohen NJ. Memory for items and relationships among items embedded in realistic scenes: Disproportionate relational memory impairments in amnesia. Neuropsychology. 2015;29:126–38. doi: 10.1037/neu0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci. 2007a;104:1726–31. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007b;27:14365–74. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc Lond, Ser B: Biol Sci. 2009;364:1263–71. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, Verfaellie M. Default network connectivity in medial temporal lobe amnesia. J Neurosci. 2012;32:14622–29. doi: 10.1523/JNEUROSCI.0700-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Aggleton JP, Roberts N. A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia. 2000;38:410–25. doi: 10.1016/s0028-3932(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Isaac CL, Gong Q, Roberts N. Differential involvement of the hippocampus and temporal lobe cortices in rapid and slow learning of new semantic information. Neuropsychologia. 2002;40:748–68. doi: 10.1016/s0028-3932(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–15. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Myers CE, Shohamy D, Grossman S, Gluck M. Impaired probabilistic category learning in hypoxic subjects with hippocampal damage. Neuropsychologia. 2004;42:524–35. doi: 10.1016/j.neuropsychologia.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hubbard TL, Hutchison JL, Courtney JR. Boundary extension: Findings and theories. Q J Exp Psychol. 2010;63:1467–94. doi: 10.1080/17470210903511236. [DOI] [PubMed] [Google Scholar]
- Hurley NC, Maguire EA, Vargha-Khadem F. Patient HC with developmental amnesia can construct future scenarios. Neuropsychologia. 2011;49:3620–28. doi: 10.1016/j.neuropsychologia.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intraub H, Richardson M. Wide-angle memories of close-up scenes. J Exp Psychol Learn Mem Cogn. 1989;15:179–87. doi: 10.1037//0278-7393.15.2.179. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Vann SD, Amin E, Aggleton JP. Anterior thalamic lesions stop immediate early gene activation in selective laminae of the retrosplenial cortex: evidence of covert pathology in rats? Eur J Neurosci. 2004;19:3291–304. doi: 10.1111/j.0953-816X.2004.03421.x. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M, Milner B. Right temporal-lobe contribution to image-mediated verbal learning. Neuropsychologia. 1978;16:61–71. doi: 10.1016/0028-3932(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M. Incidental learning of image-mediated or pronounced words after right temporal lobectomy. Cortex. 1979;15:187–97. doi: 10.1016/s0010-9452(79)80024-6. [DOI] [PubMed] [Google Scholar]
- Jones MK. Imagery as a mnemonic aid after left temporal lobectomy: Contrast between material-specific and generalized memory disorders. Neuropsychologia. 1974;12:21–30. doi: 10.1016/0028-3932(74)90023-2. [DOI] [PubMed] [Google Scholar]
- Kapur N, Brooks DJ. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. 1999;9:247–54. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kapur N. The paradoxical brain. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- Kessels RPC, de Haan EHF, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res Rev. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Khan NL, Jeffree MA, Good C, Macleod W, Al-Sarraj S. Histopathology of VGKC antibody-associated limbic encephalitis. Neurology. 2009;72:1703–05. doi: 10.1212/WNL.0b013e3181a55eb3. [DOI] [PubMed] [Google Scholar]
- Kieras D. Beyond pictures and words: Alternative information-processing models for imagery effect in verbal memory. Psychol Bull. 1978;85:532–54. [Google Scholar]
- Kim S, Jeneson A, van der Horst AS, Frascino JC, Hopkins RO, Squire LR. Memory, visual discrimination performance, and the human hippocampus. J Neurosci. 2011;31:2624–29. doi: 10.1523/JNEUROSCI.5954-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dede AJO, Hopkins RO, Squire LR. Memory, scene construction, and the human hippocampus. Proc Natl Acad Sci. 2015;112:4767–72. doi: 10.1073/pnas.1503863112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Bayley PJ, Galván VV, Squire LR. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proc Natl Acad Sci. 2008;105:2676–80. doi: 10.1073/pnas.0712155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. A demonstration that the hippocampus supports both recollection and familiarity. Proc Natl Acad Sci. 2010;107:344–48. doi: 10.1073/pnas.0912543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Soc Cognition. 2002;20:353–79. [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Memory. 1994;1:106–20. [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knutson AR, Hopkins RO, Squire LR. Visual discrimination performance, memory, and medial temporal lobe function. Proc Natl Acad Sci. 2012;109:13106–11. doi: 10.1073/pnas.1208876109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel D, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: Representations and methods. Front Neurosci. 2009;3:166–74. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, Spiers HJ, Vann SD, Vargha-Khadem F, Maguire EA. Impaired spatial and non-spatial configural learning in patients with hippocampal pathology. Neuropsychologia. 2007;45:2699–711. doi: 10.1016/j.neuropsychologia.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Boyer P, Rosenbaum RS. Future decision-making without episodic mental time travel. Hippocampus. 2012;22:1215–19. doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, et al. Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 2005a;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005b;15:782–97. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 2008;18:683–96. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Yeung L-K, Barense MD. The hippocampus and visual perception. Front Hum Neurosci. 2012;6:91. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald Christopher J, Lepage Kyle Q, Eden Uri T, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–49. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–24. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci. 2000;97:4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–70. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–07. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Kumaran D, Hassabis D, Kopelman MD. Autobiographical memory in semantic dementia: A longitudinal fMRI study. Neuropsychologia. 2010a;48:123–36. doi: 10.1016/j.neuropsychologia.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Hassabis D. Imagining fictitious and future experiences: Evidence from developmental amnesia. Neuropsychologia. 2010b;48:3187–92. doi: 10.1016/j.neuropsychologia.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Hassabis D. Role of the hippocampus in imagination and future thinking. Proc Natl Acad Sci. 2011;108:E39. doi: 10.1073/pnas.1018876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. The hippocampus: A manifesto for change. J Exp Psychol Gen. 2013;142:1180–89. doi: 10.1037/a0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Perceptual learning, awareness, and the hippocampus. Hippocampus. 2001;11:776–82. doi: 10.1002/hipo.1093. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond Ser B: Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–35. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–84. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RMJ, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Meeter M, Myers CE, Shohamy D, Hopkins RO, Gluck MA. Strategies in probabilistic categorization: Results from a new way of analyzing performance. Learn Memory. 2006;13:230–39. doi: 10.1101/lm.43006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhav M, Karni A, Gilboa A. Neocortical catastrophic interference in healthy and amnesic adults: A paradoxical matter of time. Hippocampus. 2014;24:1653–62. doi: 10.1002/hipo.22353. [DOI] [PubMed] [Google Scholar]
- Milner B. Visually-guided maze learning in man: Effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia. 1965;3:317–38. [Google Scholar]
- Mishkin M, Vargha-Khadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8:212–16. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–90. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moser M-B, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Hassabis D, Maguire EA. Scene construction in amnesia: an FMRI study. J Neurosci. 2012a;32:5646–53. doi: 10.1523/JNEUROSCI.5522-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Intraub H, Maguire EA. Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr Biol. 2012b;22:261–68. doi: 10.1016/j.cub.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. Counterfactual thinking in patients with amnesia. Hippocampus. 2014;24:1261–66. doi: 10.1002/hipo.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Vargha-Khadem F, Maguire EA. Scene construction in developmental amnesia: An fMRI study. Neuropsychologia. 2014;52:1–10. doi: 10.1016/j.neuropsychologia.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy ME, Downing PE, Graham KS. Extrastriate cortex and medial temporal lobe regions respond differentially to visual feature overlap within preferred stimulus category. Neuropsychologia. 2012;50:3053–61. doi: 10.1016/j.neuropsychologia.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–27. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Kim ASN, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proc Natl Acad Sci. 2010;107:22356–59. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–75. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006a;26:4596–601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006b;18:1087–97. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Paivio A. Mental imagery in associative learning and memory. Psychol Rev. 1969;76:241–63. [Google Scholar]
- Park DC, Murman DL, Perry KD, Bruch LA. An autopsy case of limbic encephalitis with voltage-gated potassium channel antibodies. Eur J Neurol. 2007;14:e5–e6. doi: 10.1111/j.1468-1331.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- Poirier GL, Aggleton JP. Post-surgical interval and lesion location within the limbic thalamus determine extent of retrosplenial cortex immediate-early gene hypoactivity. Neuroscience. 2009;160:452–69. doi: 10.1016/j.neuroscience.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–40. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–21. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–69. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus. 2013;23:268–77. doi: 10.1002/hipo.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–80. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–90. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Knowlton BJ, Squire LR. Dissociable properties of memory systems: Differences in the flexibility of declarative and nondeclarative knowledge. Behav Neurosci. 1996;110:861–71. doi: 10.1037//0735-7044.110.5.861. [DOI] [PubMed] [Google Scholar]
- Reber PJ. The neural basis of implicit learning and memory: A review of neuropsychological and neuroimaging research. Neuropsychologia. 2013;51:2026–42. doi: 10.1016/j.neuropsychologia.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. J Neurosci. 1998;18:3943–54. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:812–19. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Priselac S, Köhler S, Black SE, Gao F, et al. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat Neurosci. 2000;3:1044–48. doi: 10.1038/79867. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Köhler S, Schacter DL, Moscovitch M, Westmacott R, et al. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47:2181–87. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Moscovitch M. Case studies continue to illuminate the cognitive neuroscience of memory. Ann N Y Acad Sci. 2014;1316:105–33. doi: 10.1111/nyas.12467. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–41. [Google Scholar]
- Rubin RD, Brown-Schmidt S, Duff MC, Tranel D, Cohen NJ. How do I remember that I know you know that I know? Psychol Sci. 2011;22:1574–82. doi: 10.1177/0956797611418245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–61. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, et al. Hippocampal complex and retrieval of recent and very remote autobiographical memories: Evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–14. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Sanfratello L, Caprihan A, Stephen JM, Knoefel JE, Adair JC, et al. Same task, different strategies: How brain networks can be influenced by memory strategy. Hum Brain Mapp. 2014;35:5127–40. doi: 10.1002/hbm.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Graf P. Preserved learning in amnesic patients: Perspectives from research on direct priming. J Clin Exp Neuropsychol. 1986;8:727–43. doi: 10.1080/01688638608405192. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Chiu CY, Ochsner KN. Implicit memory: a selective review. Annu Rev Neurosci. 1993;16:159–82. doi: 10.1146/annurev.ne.16.030193.001111. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. Constructive memory: The ghosts of past and future. Nature. 2007;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci. 2009;364:1245–53. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–94. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: Intersections between memory and decisions. Neurobiol Learn Mem. 2015;117:14–21. doi: 10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon T, Moscovitch M, Gilboa A. Rapid neocortical acquisition of long-term arbitrary associations independent of the hippocampus. Proc Natl Acad Sci. 2011;108:1146–51. doi: 10.1073/pnas.1005238108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Hopkins RO, Sage J, Gluck MA. Distinct hippocampal and basal ganglia contributions to probabilistic learning and reversal. J Cogn Neurosci. 2008;21:1820–32. doi: 10.1162/jocn.2009.21138. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26:2235–40. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Jeneson A, Frascino JC, Kirwan CB, Hopkins RO, Squire LR. When recognition memory is independent of hippocampal function. Proc Natl Acad Sci. 2014a;111:9935–40. doi: 10.1073/pnas.1409878111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Urgolites ZJ, Hopkins RO, Squire LR. Comparison of explicit and incidental learning strategies in memory-impaired patients. Proc Natl Acad Sci. 2014b;111:475–79. doi: 10.1073/pnas.1322263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–82. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–40. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. The neuroscience of remote spatial memory: A tale of two cities. Neuroscience. 2007;149:7–27. doi: 10.1016/j.neuroscience.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Squire L, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–86. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SGR, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci. 2010;107:19044–48. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–88. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Taylor KJ, Henson RNA, Graham KS. Recognition memory for faces and scenes in amnesia: Dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2428–38. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–77. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The role of hippocampus in memory: A hypothesis. Neurosci Biobehav Rev. 1985;9:377–89. doi: 10.1016/0149-7634(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–37. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, editor. Organization of memory. New York: Academic; 1972. [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352–73. [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Tulving E, Schacter D. Priming and human memory systems. Science. 1990;247:301–06. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Salmond CH, Watkins KE, Friston KJ, Gadian DG, Mishkin M. Developmental amnesia: Effect of age at injury. Proc Natl Acad Sci. 2003;100:10055–60. doi: 10.1073/pnas.1233756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Agamanolis D. Amnesia due to lesions confined to the hippocampus: A clinical-pathologic study. J Cogn Neurosci. 1990;2:246–57. doi: 10.1162/jocn.1990.2.3.246. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J Neurosci. 2000;20:5853–57. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Baucom LB, Shinkareva SV. Decoding abstract and concrete concept representations based on single-trial fMRI data. Hum Brain Mapp. 2013;34:1133–47. doi: 10.1002/hbm.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Magnotta V, Capizzano AA, Cassell MD, Tranel D. Long-term neuropsychological, neuroanatomical, and life outcome in hippocampal amnesia. Clin Neuropsychol. 2012;26:335–69. doi: 10.1080/13854046.2012.655781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC. Not so fast: Hippocampal amnesia slows word learning despite successful fast mapping. Hippocampus. 2014;24:920–33. doi: 10.1002/hipo.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological studies of the hippocampus: a comparison of the hippocampal subdivisions of diverse species including hedgehogs, laboratory rodents, wild mice and men. Prog Brain Res. 1990;83:13–36. doi: 10.1016/s0079-6123(08)61238-8. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17:766–80. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring “the Knowledge” of London's layout drives structural brain changes. Curr Biol. 2011;21:2109–14. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee LTS, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24:154–64. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Mullally SL, Maguire EA. Constructing, perceiving, and maintaining scenes: Hippocampal activity and connectivity. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–67. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]