Abstract
Background
The positive relationship between lean mass (LM) and bone health is well known, but a positive association between insulin and LM has also been described. Insulin has some anabolic properties on bone through the stimulation of osteoblast differentiation, yet the role of LM as a confounder or mediator in this relationship remains uncertain.
Objective
To examine whether the association between insulin levels and bone health is mediated by LM.
Methods
A cross-sectional study was conducted at the Castilla La Mancha University (Spain) involving 466 young adults (113 young men; 19.5±2.3 years). LM and total-body bone mineral content (BMC) were measured by dual energy x-ray absorptiometry, and insulin was measured in fasting serum samples.
Results
Young adults with high total LM had higher values of total-body BMC than their peers after controlling for age and sex, this relationship persisted after adjusting for insulin levels (p<0.001). In mediation analyses, insulin levels were positively associated with total-body BMC (b = 0.05; p<0.001) and total LM acted as an intermediate variable, attenuating the association between insulin levels and total-body BMC (b = -31.98; p>0.05) as indicated by Sobel test values for indirect effect (z = 4.43; p<0.001).
Conclusions
LM plays an important role in the relationship between insulin levels and bone health, in such a way that while increases in LM have a positive influence on bone health, they are also negatively associated with insulin levels.
Introduction
Osteoporotic fractures are a major cause of morbidity and mortality in developed countries [1]. These fractures are clinical consequences of osteoporosis, a systemic skeletal disease that contributes to bone fragility [2]. Evidence consistently supports that peak bone mass, defined as the amount of bone acquired at the end of skeletal development that usually occurs between the second and third decades of life [3,4], is an important determinant of lifelong skeletal health and a key determinant of future fracture risk during adulthood [5,6].
Bone mass variability is determined by several factors, including genetics, mechanical and endocrine factors [7–9]. Lean mass (LM) is considered the best predictor of bone mineral content (BMC) in adolescents and young adults [10,11], though its relationship with bone health is complex due to the multiple associations in which this body composition component is involved. On the other hand, LM is an excellent indicator of bone mechanical stimulation and its changes are highly correlated with bone health [12–14]. A linear relationship between LM and BMC during growth has been reported, forming bone and muscle a functional unit [15,16]. Moreover, LM is positively associated with body weight and fat mass, and both influence bone turnover [13,17]. Lastly, LM is associated with insulin levels, because insulin can stimulate amino acid transport and protein synthesis [18,19] by inhibiting proteolysis [20] in skeletal muscle. These physiological effects have been used for therapeutic purposes and to increase muscle mass in individuals involved in sport activities [21].
Insulin has an anabolic effect on bone [22,23], through the stimulation of osteoblast differentiation, which enhances production of osteocalcin [24]. Even though the role of LM as a confounder or mediator in the relationship between insulin and bone remains uncertain, it seems plausible that the metabolic effect of insulin resistance on muscle mass could influence bone health, since a positive association between lean mass and bone outcomes is well known.
Although the relationship between insulin and LM has been repeatedly described, no studies have jointly examined the association of these predictors with bone outcomes. Furthermore, most published studies have been conducted using statistical multivariate procedures (ANCOVA, multiple linear regression or logistic regression) in order to control for potential confounders, but these statistical procedures are unable to distinguish between confounding and mediating variables.
The present study aimed to determine whether the relationship between insulin and bone health is mediated by LM in young adults.
Subjects and methods
Study design and participants
This was a cross-sectional ancillary study of a previously conducted population-based study [25,26] aimed at assessing changes in lifestyle and cardiovascular risk that occur during an individual’s time at university. A study, which included all first-year university students of the 2009–2010 academic year from the Castilla-La Mancha University in Cuenca Campus, Spain, were performed. A total of 770 students were invited and 683 (88.7%) agreed to participate. In this report, we use data from a subsample of 466 university students in which BMC (by dual energy x-ray absorptiometry [DXA]) was measured. The young adults included in the data analysis for this study did not differ in age, sex or parental socioeconomic status from the whole sample of young adults participating in the trial.
The study protocol was approved by the Clinical Research Ethics Committee of the Hospital Virgen de la Luz in Cuenca, once participants were informed verbally and in writing, they were asked to sign a consent form as a condition to participate in the study. Because there were no participants aged less than 18 years and that is the legal age in Spain, written informed consent was individually obtained from each participant. Documents with the signed consent were recorded. The Ethics Committee approved the study protocol including permissions and informed consent documents.
Study variables
Anthropometry
Weight was measured twice with the subject barefoot and wearing light clothing using a Seca-770 scale. Height was measured twice with the subject barefoot and upright, with the sagittal midline at the midline of the stadiometer, using a Seca-222 wall-mounted stadiometer. Body mass index was calculated as weight in kilograms divided by the square of height in meters (kg/m2) using the means of the weight and height measurements.
Body composition
The young adults were scanned in the supine position using DXA (Lunar iDXA, GE Medical Systems Lunar, Madison, WI 53718, USA). The analyses were performed using enCoreTM 2008 software version 12.30.008. DXA equipment accuracy was checked daily before each scanning session using the GE Lunar calibration phantom, as recommended by the manufacturer. All scans were performed at high resolution by the same trained researcher. Bone mineral density (BMD) (g/cm2), fat mass (g), and LM (g) were obtained for each individual from total analysis of the whole body scan. BMC (g) and LM were calculated as follows: BMC = [BMD x area] and LM = [total mass—(fat mass + BMC)]. For all analyses, total LM was categorized as follows: low (1st quartile), medium (2nd and 3rd quartiles) and high (4th quartile).
Serum biochemistries
Insulin and glucose were measured in serum blood samples and they were collected via a cubital vein puncture under standard conditions [27] between 8:15 and 9:00 AM, after at least 12 hours of fasting. The samples were processed in a COBAS C711 system from Roche Diagnostics, blood glucose concentration was determined by the hexokinase method and blood insulin concentration was determined by the one-step chemiluminescent microparticle immunoassay and processing on a platform composed of two ARCHITECT i2000SR systems from Abbott Laboratories. The variation coefficient of fasting insulin ranged from 2.47 to 3.34%, at the lower and higher levels, respectively. For all analyses, insulin levels were categorized as follows: low (1st quartile), medium (2nd and 3rd quartiles) and high (4th quartile).
Statistical analysis
Both statistical (Kolmogorov–Smirnov test) and graphical methods (normal probability plots) were used to examine fitting to a normal distribution for each continuous variable. Insulin levels were not normally distributed and were log transformed.
ANCOVA models were used to test mean differences in total-body BMC by insulin level categories. Age and sex were covariates in model 1, and age, sex and total LM were covariates in model 2. Similarly, when we used total LM categories as fixed factors, we used as covariates age and sex in model 1, and age, sex and insulin levels in model 2.
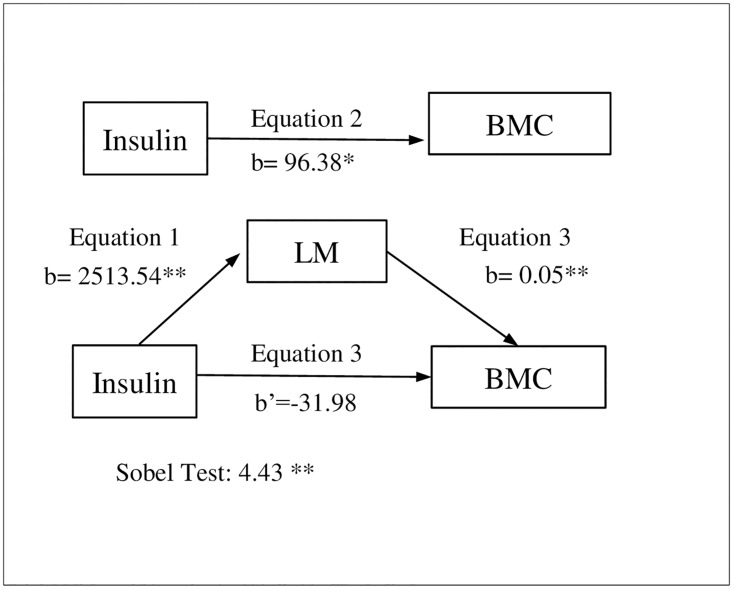
Linear regression analyses were conducted to test the potential mediating effect of total LM in the association between insulin levels and total-body BMC, following the criteria outlined by Baron and Kenny [28] namely: 1) the independent variable must be significantly related to the mediator, 2) the independent variable must be significantly related to the dependent variable, 3) the mediator must be significantly related to the dependent variable, and 4) the association between the independent and dependent variables must be attenuated when the mediator is included in the regression model. In addition, we tested mediation effect using the steps outlined by Sobel [29]: 1) we estimated the attenuation or indirect effect (i.e. the effect of the independent variable on the mediator from the first regression model multiplied by the effect of the mediator on the dependent variable obtained from the third regression model), and 2) we divided the indirect effect by its standard error and performed a z-test under the null hypothesis that the indirect effect is equal to zero. The regression model was adjusted for age and sex (Fig 1).
Fig 1. Simple mediation models of the relationship between insulin and total-body Bone Mineral Content (BMC) using total Lean Mass (LM) as a mediator, and controlling for age and sex.
*p<0.050; **p<0.001.
Simple mediation models were estimated using the PROCESS macro for SPSS. This macro uses bootstrapping methods as recommended by Preacher and Hayes [30] for testing mediation hypotheses (we used a resample procedure of 10,000 bootstrap samples). In the mediation analysis with the three variables, BMC, insulin and LM were used in their original quantitative scale. The study data are shown in S1 File. Statistical analyses were performed with SPSS-IBM (Software, v.19.0 SPSS Inc., Chicago, IL, USA) and the level of significance was set at α = 0.05.
Results
Descriptive characteristics (mean ± standard deviation [SD]) of the study sample are shown in Table 1. All variables differed significantly by sex except insulin levels, fasting glucose and age. Table 2 shows the mean-adjusted differences in total-body BMC by insulin levels and total LM categories, after controlling for potential confounders. Young adults with high insulin levels showed higher total-body BMC than their peers, though the differences did not reach statistical significance after controlling for age and sex (model 1), and additionally for total LM (model 2). Moreover, young adults with high total LM had significantly higher total-body BMC than those with low total LM after controlling for age and sex (model 1), and also when insulin levels were controlled for (model 2).
Table 1. Descriptive characteristics of the study sample (mean ± SD).
| All (466) | Boys (113) | Girls (353) | p | |
|---|---|---|---|---|
| Age (years) | 19.5±2.3 | 19.8±2.3 | 19.4±2.2 | 0.094 |
| Body mass (kg) | 62.1±12.1 | 73.3±11.8 | 58.6±9.8 | <0.001 |
| Height (cm) | 166.0±8.4 | 175.9±7.5 | 162.8±5.6 | <0.001 |
| BMI (Kg/m2) | 22.5± 3.5 | 23.6±3.3 | 22.1±3.5 | 0.001 |
| Total LM (g) | 41516.7±8898.5 | 54518.8±6742.3 | 37354.5±4300.5 | <0.001 |
| Insulin levels (IU/mL | 8.2±3.5 | 7.7±2.9 | 8.3±3.7 | 0.100 |
| Fasting glucose (mmol/l) | 86.7±15.4 | 88.4±0.6 | 86.1±0.9 | 0.189 |
| Total body BMC (g) | 2446.6±453.5 | 3010.2±0.5 | 2266.2±267.2 | <0.001 |
BMI body mass index; LM lean mass; BMC bone mineral content.
Table 2. ANCOVA models comparing means of total-body Bone Mineral Content (BMC) by insulin levels and total Lean Mass (LM) categories in young adults.
| BMC (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Insulin levels | Total lean mass | |||||||
| Low | Medium | High | p | Low | Medium | High | p | |
| n = 113 | n = 224 | n = 109 | n = 116 | n = 234 | n = 116 | |||
| Model 1 | 2590.94±35.17 | 2636.88±25.23 | 2697.05±41.90 | 0.158 | 2046.82±25.15 | 2421.63±40.14 | 2912.25±37.45 a, b | <0.001 |
| Model 2 | 2417.16±22.65 | 2405.54±17.81 | 2394.47±28.22 | 0.801 | 2042.22±25.95 | 2426.10±40.30 | 2912.08±38.24 a, b | <0.001 |
Covariates for insulin levels: Model 1 (age and sex); Model 2 (Model 1+ total lean mass).
Covariates for total lean mass: Model 1 (age and sex); Model 2 (Model 1+ insulin levels).
Superscript letters indicate statistical significance (p≤0.050) for post-hoc hypothesis test determinates by using the Bonferroni correction for multiple comparisons:
a High>Medium>Low;
b High>Low
Simple mediation analysis
We tested the mediator role of total LM (Fig 1) in the relationship between insulin levels and total-body BMC. The relationship between insulin levels and total LM was positive (b = 2513.54; p<0.001) in the first regression equation, and between insulin levels and total-body BMC (b = 96.38; p<0.050) in the second regression equation. In the third regression equation, the relationship between total LM and total-body BMC was positive (b = 0.05; p<0.001), though between insulin levels and total-body BMC it was attenuated when the mediator was included in the regression model (b = -31.98; p>0.050). Thus, total LM acted as a mediator of the relationship between insulin levels and total-body BMC, as shown by the Sobel test for indirect effect (z = 4.43; p<0.001). The percentage of total effect mediated by total LM was 26.8%.
Discussion
To the best of our knowledge, this is the first study in young adults analysing whether total-body BMC levels are related to insulin levels regardless of total LM or, conversely, whether the latter acts as mediator in the association between insulin levels and total-body BMC. The main findings of this study are: (1) young adults with high total LM have more total-body BMC than those with lower total LM after controlling for relevant confounders, including insulin levels; and (2) total LM is a total mediator in the relationship between insulin levels and total-body BMC.
Insulin-like growth factor and insulin play an important role in muscle development [31]. The anabolic actions of insulin are of interest to people, such as athletes and body builders who want to increase their muscle mass, and to those concerned with preventing sarcopenia. It is well known that insulin inhibits protein catabolism, and increases the synthesis of glycogen and proteins in muscle, promoting the entry of glycogen and amino acids into muscle cells [32,33]. In addition, physiological hyperinsulinemia has been shown to enhance the activity of amino acid transport and protein synthesis in muscle mass [18]. Accordingly, our study shows a positive relationship between insulin and lean mass.
The mechanostat theory describes muscle as a mediator that transfers the ground reaction forces and the forces generated during muscle contractions to bone [34]. Literature consistently considers LM as the best predictor of bone health in adolescents and young adults [10,35]. Similar to previous results, our findings show that total-body BMC levels are positively associated with LM in young adults.
The role of insulin-like growth factor in the regulation, development and homeostasis maintenance of bone is well known [36,37], as well as the fact that its homologue, insulin, has some anabolic properties for bone. Insulin may work by stimulating osteoblast differentiation, which in turn would enhance the production of osteocalcin [24,38]. Moreover, insulin might exert an effect on bone cells through direct binding to the insulin receptor, which has been detected in primary human osteoblasts differentiated from bone marrow-derived mesenchymal stem cells [39]. Our data in is line with previous reports that show high insulin levels were related with higher total-body BMC, though the differences did not reach statistical significance after controlling for confounders such as LM, which might explain this relationship.
There is consistent evidence regarding the bivariate association of LM with both insulin [8,24,38,40] and bone [11,41,42]. Likewise, the relationship between insulin and bone in humans has been established [22,43]. In addition, a recent study has showed that lean body mass is an important intermediary factor in the insulin-like growth factor 1 and bone relationship in premenarcheal girls [44]. However, it has not been fully illustrated whether LM acts as a confounder or as a mediator in the association between insulin levels and total-body BMC in young adults. Our study confirms the independent relationship between LM and insulin levels with total-body BMC, and it clarifies the mediating role of LM in the relationship between insulin levels and total-body BMC.
The current study has several limitations that should be acknowledged. First, the cross-sectional design does not allow us to make cause–effect inferences and no study design has the statistical power or is as free of bias as prospective intervention studies. However, in terms of feasibility, such a study design would require a large sample size or a long follow-up period, apart from the ethical considerations for these kinds of studies. This is probably why most mediation analysis are cross-sectional [45–47]. Second, our results are based on analyses of insulin levels measured through fasting insulin rather than an index of insulin resistance such as the homeostasis model assessment of insulin (HOMA-IR). However, insulin levels are well correlated with HOMA-IR (r = 0.85; p<0.001) [48]; thus, in the current population, HOMA-IR offers no advantages in evaluating insulin resistance. Finally, the relationships analysed were likely influenced by more than one mediator variable; future studies using structural equation procedures might be useful to more specifically clarify the potential mediator role of each factor.
Conclusions
Our data are relevant from a clinical perspective as they disclose that the association between insulin and bone mass in young adults seems to be mediated by LM. Thus, LM may influence not only glucose metabolism but also bone health.
Supporting information
(XLSX)
Acknowledgments
We thank all participants of the study. This study was funded mainly by the Foundation for Health Research of Castilla-La Mancha (FISCAM) (AN/2008/31). Additional funding was obtained from the Research Network in Preventive and Health Promotion Activities (Red de Investigación en Actividades Preventivas y de Promoción de Salud) (RD06/0018/0038) and from the Ministry of Science and Technology (RYC-2010-05957)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded mainly by Foundation for Health Research of Castilla-La Mancha (FISCAM) (AN/2008/31). Additional funding was obtained from Research Network in Preventive and Health Promotion Activities (Red de Investigación en Actividades Preventivas y de Promoción de Salud) (RD06/0018/0038) and from Ministry of Science and Technology (RYC-2010-05957). CAB is supported by a grant from the Spanish Ministry of Education, Culture and Sport (FPU13/03137). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lofgren B, Stenevi-Lundgren S, Dencker M, Karlsson MK (2010) The mode of school transportation in pre-pubertal children does not influence the accrual of bone mineral or the gain in bone size—two year prospective data from the paediatric osteoporosis preventive (POP) study. BMC Musculoskelet Disord 11: 25 10.1186/1471-2474-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16 Suppl 2: S3–7. [DOI] [PubMed] [Google Scholar]
- 3.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26: 1729–1739. 10.1002/jbmr.412 [DOI] [PubMed] [Google Scholar]
- 4.Henry YM, Fatayerji D, Eastell R (2004) Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int 15: 263–273. 10.1007/s00198-003-1542-9 [DOI] [PubMed] [Google Scholar]
- 5.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA (2010) Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46: 294–305. 10.1016/j.bone.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Zemel B (2013) Bone mineral accretion and its relationship to growth, sexual maturation and body composition during childhood and adolescence. World Rev Nutr Diet 106: 39–45. [DOI] [PubMed] [Google Scholar]
- 7.Tobias JH, Steer CD, Mattocks CG, Riddoch C, Ness AR (2007) Habitual levels of physical activity influence bone mass in 11-year-old children from the United Kingdom: findings from a large population-based cohort. J Bone Miner Res 22: 101–109. 10.1359/jbmr.060913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulzele K, Clemens TL (2012) Novel functions for insulin in bone. Bone 50: 452–456. 10.1016/j.bone.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 9.Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C (1996) Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol 270: E320–327. [DOI] [PubMed] [Google Scholar]
- 10.El Hage RP, Courteix D, Benhamou CL, Jacob C, Jaffre C (2009) Relative importance of lean and fat mass on bone mineral density in a group of adolescent girls and boys. Eur J Appl Physiol 105: 759–764. 10.1007/s00421-008-0959-4 [DOI] [PubMed] [Google Scholar]
- 11.Zhu K, Briffa K, Smith A, Mountain J, Briggs AM, Lye S, et al. (2014) Gender differences in the relationships between lean body mass, fat mass and peak bone mass in young adults. Osteoporos Int 25: 1563–1570. 10.1007/s00198-014-2665-x [DOI] [PubMed] [Google Scholar]
- 12.Vicente-Rodriguez G, Ara I, Perez-Gomez J, Dorado C, Calbet JA (2005) Muscular development and physical activity as major determinants of femoral bone mass acquisition during growth. Br J Sports Med 39: 611–616. 10.1136/bjsm.2004.014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gracia-Marco L, Ortega FB, Jimenez-Pavon D, Rodriguez G, Castillo MJ, Vicente-Rodriguez G, et al. (2012) Adiposity and bone health in Spanish adolescents. The HELENA study. Osteoporos Int 23: 937–947. 10.1007/s00198-011-1649-3 [DOI] [PubMed] [Google Scholar]
- 14.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard M B (2011) Mechanical loads and cortical bone geometry in healthy children and young adults. Bone 48: 1103–1108. 10.1016/j.bone.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost HM (2003) Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275: 1081–1101. 10.1002/ar.a.10119 [DOI] [PubMed] [Google Scholar]
- 16.Schoenau E, Saggese G, Peter F, Baroncelli GI, Shaw NJ, Crabtree N J, et al. (2004) From bone biology to bone analysis. Horm Res 61: 257–269. 10.1159/000076635 [DOI] [PubMed] [Google Scholar]
- 17.Reid IR (2008) Relationships between fat and bone. Osteoporos Int 19: 595–606. 10.1007/s00198-007-0492-z [DOI] [PubMed] [Google Scholar]
- 18.Bonadonna RC, Saccomani MP, Cobelli C, DeFronzo RA (1993) Effect of insulin on system A amino acid transport in human skeletal muscle. J Clin Invest 91: 514–521. 10.1172/JCI116230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolster DR, Jefferson LS, Kimball SR (2004) Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc 63: 351–356. 10.1079/PNS2004355 [DOI] [PubMed] [Google Scholar]
- 20.Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ (1995) Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest 96: 1722–1729. 10.1172/JCI118217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham MR, Evans P, Davies B, Baker JS (2008) AAS, growth hormone, and insulin abuse: psychological and neuroendocrine effects. Ther Clin Risk Manag 4: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawlor DA, Sattar N, Sayers A, Tobias JH (2012) The association of fasting insulin, glucose, and lipids with bone mass in adolescents: findings from a cross-sectional study. J Clin Endocrinol Metab 97: 2068–2076. 10.1210/jc.2011-2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H (2000) Correlations between insulin sensitivity and bone mineral density in non-diabetic men. Diabet Med 17: 124–129. [DOI] [PubMed] [Google Scholar]
- 24.Klein GL (2014) Insulin and bone: Recent developments. World J Diabetes 5: 14–16. 10.4239/wjd.v5.i1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Palencia NM, Solera-Martinez M, Gracia-Marco L, Silva P, Martinez-Vizcaino V, Canete-Garcia-Prieto J, et al. (2015) Levels and Patterns of Objectively Assessed Physical Activity and Compliance with Different Public Health Guidelines in University Students. PLoS One 10: e0141977 10.1371/journal.pone.0141977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diez-Fernandez A, Sanchez-Lopez M, Nieto JA, Gonzalez-Garcia A, Miota-Ibarra J, Ortiz-Galeano I, et al. (2017) Relationship between cardiorespiratory fitness and blood pressure in young adults: a mediation analysis of body composition. Hypertens Res. [DOI] [PubMed] [Google Scholar]
- 27.(1989) Comisión de lípidos y lipoproteínas de la Sociedad Española de Química Clínica. Protocolo para la obtención de especímenes en las determinaciones de lípidos y lipoproteínas. Quim Clin 8: 349–351. [Google Scholar]
- 28.Baron RM, Kenny DA (1986) The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 29.Sobel M. (1982) Asymptotic confidence intervals for indirect effects in structural equation models In: Leinhardt S, editor. Sociological methodology. Washington DC: American Sociological Association; 290–312. [Google Scholar]
- 30.Preacher KJ, Hayes AF (2008) Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40: 879–891. [DOI] [PubMed] [Google Scholar]
- 31.Velloso CP (2008) Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568. 10.1038/bjp.2008.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biolo G, Declan Fleming RY, Wolfe RR (1995) Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819. 10.1172/JCI117731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonksen PH (2001) Insulin, growth hormone and sport. J Endocrinol 170: 13–25. [DOI] [PubMed] [Google Scholar]
- 34.Frost HM (2000) Muscle, bone, and the Utah paradigm: a 1999 overview. Med Sci Sports Exerc 32: 911–917. [DOI] [PubMed] [Google Scholar]
- 35.Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB (2005) The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone 37: 474–481. 10.1016/j.bone.2005.04.038 [DOI] [PubMed] [Google Scholar]
- 36.Soot T, Jurimae T, Jurimae J (2006) Relationships between bone mineral density, insulin-like growth factor-1 and sex hormones in young females with different physical activity. J Sports Med Phys Fitness 46: 293–297. [PubMed] [Google Scholar]
- 37.Breen ME, Laing EM, Hall DB, Hausman DB, Taylor RG, Isales CM, et al. (2011) 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab 96: E89–98. 10.1210/jc.2010-0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142: 296–308. 10.1016/j.cell.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avnet S, Perut F, Salerno M, Sciacca L, Baldini N (2012) Insulin receptor isoforms are differently expressed during human osteoblastogenesis. Differentiation 83: 242–248. 10.1016/j.diff.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 40.Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner W S, et al. (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho-Pham LT, Nguyen UD, Nguyen TV (2014) Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab 99: 30–38. 10.1210/jc.2013-3190 [DOI] [PubMed] [Google Scholar]
- 42.Torres-Costoso A, Gracia-Marco L, Sanchez-Lopez M, Garcia-Prieto JC, Garcia-Hermoso A, Diez-Fernandez A,et al. (2015) Lean mass as a total mediator of the influence of muscular fitness on bone health in schoolchildren: a mediation analysis. J Sports Sci 33: 817–830. 10.1080/02640414.2014.964750 [DOI] [PubMed] [Google Scholar]
- 43.Riddle RC, Clemens TL (2014) Insulin, osteoblasts, and energy metabolism: why bone counts calories. J Clin Invest 124: 1465–1467. 10.1172/JCI75554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kindler JM, Pollock NK, Laing EM, Jenkins NT, Oshri A, et al. (2016) Insulin Resistance Negatively Influences the Muscle-Dependent IGF-1-Bone Mass Relationship in Premenarcheal Girls. J Clin Endocrinol Metab 101: 199–205. 10.1210/jc.2015-3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres-Costoso A, Gracia-Marco L, Sanchez-Lopez M, Notario-Pacheco B, Arias-Palencia N, Martinez-Vizcaino V (2015) Physical activity and bone health in schoolchildren: the mediating role of fitness and body fat. PLoS One 10: e0123797 10.1371/journal.pone.0123797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Díez-Fernández A, Sanchez-Lopez M, Mora-Rodríguez R, Notario-Pacheco B, Torrijos-Niño C, Martínez-Vizcaíno V (2013) Obesity as a mediator of the influence of cardiorespiratory fitness on cardiometabolic risk: a mediation analysis. Diabetes Care 37(3): 855–862. 10.2337/dc13-0416 [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Hermoso A, Martinez-Vizcaino V, Sanchez-Lopez M, Recio-Rodriguez JI, Gomez-Marcos MA, Garcia-Ortiz L (2015) Moderate-to-vigorous physical activity as a mediator between sedentary behavior and cardiometabolic risk in Spanish healthy adults: a mediation analysis. Int J Behav Nutr Phys Act 12: 78 10.1186/s12966-015-0244-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere M B, et al. (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.