Abstract
OBJECTIVE
Aortic stiffness is an important predictor of future morbidity and mortality. Diabetes is associated with increased aortic stiffness, but the importance of nondiabetic glucometabolic status for accelerated aortic stiffening is unclear. We tested the hypothesis that adverse glucometabolic status is associated with accelerated aortic stiffening in individuals without diabetes, independently of known risk factors for arterial stiffening.
RESEARCH DESIGN AND METHODS
Glucometabolic status and other cardiovascular risk factors were assessed at baseline in 2008–09, and carotid femoral pulse wave velocity (cfPWV) at baseline and follow-up in 2012–13, in 4,386 participants without diabetes of the Whitehall II Study.
RESULTS
The mean age of the cohort at cfPWV baseline was 60 years, and 74% were male. cfPWV increased from (mean ± SE) 8.30 ± 0.03 to 8.98 ± 0.04 m/s during 4 years of follow-up. At baseline, cfPWV was associated with fasting and 2-h postload glucose, HbA1c, and HOMA-insulin resistance (HOMA-IR). HbA1c and HOMA-IR were associated with progression of cfPWV after adjusting for physiological confounders and cardiovascular risk factors. A 1 SD higher HbA1c and HOMA-IR were associated with greater increases in cfPWV (0.11 m/s per 5 years [95% CI 0.04, 0.18], P = 0.003 and 0.09 m/s per 5 years [0.01, 0.17], P = 0.03, respectively). Additional adjustment for BMI weakened the association with HOMA-IR but not with HbA1c.
CONCLUSIONS
HbA1c is independently associated with accelerated progression of aortic stiffness in individuals without diabetes. These findings suggest that long-term glucometabolic status, even in individuals without diabetes, could be an important target for preventative strategies against vascular aging.
Introduction
Aortic stiffness provides important prognostic information on overall cardiovascular risk and mortality (1). In the most recent meta-analysis, carotid femoral pulse wave velocity (cfPWV), the current gold standard measure of aortic stiffness, was an independent predictor of future cardiovascular events and improved risk classification beyond that provided by traditional risk factors (2). This has added to the view that aortic stiffness is a measure of vascular health or vascular age (3,4) and has created considerable interest in aortic stiffness as a novel therapeutic target.
The precise mechanisms underlying aortic stiffening remain poorly understood. The heterogeneity in the rate of stiffening suggests it is not an inevitable consequence of the aging process (5). A number of risk factors for accelerated aortic stiffening have been described, including elevated blood pressure, renal disease, systemic inflammation, and adiposity (6–10). Diabetes has been linked with increased vascular stiffness in several case-control studies (11–13), and HbA1c level is associated with an accelerated age-related increase in cfPWV in individuals with type 2 diabetes (14). Cross-sectional studies in individuals without diabetes suggest an association between aortic stiffness, glycemia, and indices of insulin resistance (11,15,16). However, a prospective analysis using Whitehall II data are equivocal, finding associations only in men (17). The evidence from longitudinal studies is also conflicting. Only one of seven previous longitudinal studies identified plasma glucose as an independent predictor of the rate of progression of cfPWV after adjusting for confounders, but only in women (18). The remaining longitudinal studies either report no independent association (19–22) or did not examine glucose as a risk factor for stiffening (23,24). None of the studies provide any information concerning HbA1c and aortic stiffening.
We hypothesized that impaired glycemic control is associated with accelerated aortic stiffening in individuals without diabetes and that this would be independent of known risk factors for arterial stiffening. Our aim was to test this hypothesis in the Whitehall II longitudinal study. This cohort study provides data on progression of cfPWV during a 4-year interval, together with repeated assessments of glycemia, insulin resistance, anthropometric parameters, and cardiovascular risk factors. We excluded individuals with clinical or biochemical evidence of diabetes to reduce the confounding effects of treatment and diabetic complications, such as renal disease, which may themselves alter stiffness.
Research Design and Methods
Participants
All participants were drawn from the Whitehall II cohort, a longitudinal observational study of 10,308 civil servants recruited between 1985 and 1988 when aged 35–55 years (25). Participants have been followed up every 4–5 years with detailed clinical examinations and self-administered questionnaires in 1991–94, 1997–99, 2003–04, 2008–09, and 2012–13. Research Ethics Committee approval was obtained for each examination phase, and participants gave written informed consent.
The cfPWV was assessed in 4,347 of 6,225 participants seen at the screening clinic in 2008–09 and in 4,485 of 5,660 seen in 2012–13. The present analysis was based on participants without diabetes. Diabetes was defined by self-report/doctor diagnosis, the use of antidiabetic medication, fasting glucose ≥7.0, a 2-h glucose ≥11.1 mmol/L during an oral glucose tolerance test, or HbA1c ≥6.5% (48 mmol/mol). Excluded from the analysis were 617 participants who met these criteria at baseline and 582 at follow-up, together with a further 95 participants at baseline and 301 at follow-up who had missing data on covariates. This left 4,386 participants without diabetes who underwent cfPWV assessment during the 2008–09 (n = 3,685) and/or 2012–13 (n = 3,602) examinations who make up the analytic sample. The cfPWF was assessed in 2,901 participants at both time points. The same measurement protocol was used at each examination.
Measurements
PVW
Aortic stiffness was assessed by cfPWV, the current noninvasive gold standard (26). Higher values of cfPWV indicate a faster speed of wave travel between the arterial sites and, hence, a stiffer aorta. Measurements were made after a 15-min supine rest, in duplicate, using the SphygmoCor system (AtCor Medical, Sydney, NSW, Australia), as previously described (27). Briefly, brachial blood pressure was measured and then cfPWV assessed between the carotid and femoral sites. Path length was determined with a tape measure by subtracting the carotid-sternal notch distance from the femoral-sternal notch distance. If the difference between repeated measurements was >0.5 m/s, a third measurement was taken, and the average of the measurements was used in the analysis. Heart rate was derived from the SphygmoCor software, and blood pressure was measured using a validated oscillometric device immediately before cfPWV. Mean arterial pressure (MAP) was calculated as diastolic pressure plus one-third of the pulse pressure.
Vascular Disease, Diabetes, and Antihypertensive Medication
Prevalent vascular disease status (myocardial infarction [MI] and/or stroke) at the 2008–09 assessment was determined using self-report doctor diagnosis and hospitalization with verification from medical records where available. Prevalent diabetes was determined by oral glucose tolerance test, self-report, doctor diagnosis, and/or medication (28).
Anthropometry and Other Covariates
Anthropometric measures and cardiovascular risk factors were measured in 2003–04 and 2008–09 to provide mean exposure in the 5 years before the baseline cfPWV assessment in 2008–09. Weight, height, and waist and hip circumferences were measured using standard protocols (29). Serum, fluoride plasma, and blood drawn into EDTA tubes was collected after an overnight fast or ≥5 h after a fat-free breakfast for participants presenting in the afternoon. Serum total cholesterol, HDL cholesterol, triglycerides, and plasma glucose were measured.
Glucometabolic Measures
Samples were handled according to standard protocols. Venous blood samples were taken in fasted individuals (≥8 h fasting or ≥5 h for afternoon visits), before a standard 2-h oral glucose tolerance test was administered in all participants (29). Glucose samples were drawn into fluoride Monovette tubes and insulin samples into native tubes that were centrifuged on site within 1 h. Plasma or serum was immediately moved into microtubes and stored at –70°C. Blood glucose was measured using the glucose oxidase method (30) on a YSI model 2300 STAT PLUS analyzer (2003–04 and 2007–09; mean coefficient of variation of 1.4–3.1%) (YSI Corporation, Yellow Springs, OH), and serum insulin with a DAKO insulin ELISA kit (DakoCytomation Ltd., Ely, U.K.) (31) (2003–04, mean coefficient of variation 4.2–9.3%, 2007–09). HOMA insulin sensitivity and HOMA β-cell function were calculated from model-derived estimates (rather than linear approximations) with the HOMA2 version 2.2 calculator (32). HbA1c was measured in whole blood, drawn into EDTA Monovette tubes, using the validated (33) Tosoh G8 high performance liquid chromatography platform (Tosoh Bioscience, Tessenderlo, Belgium).
The exposures and covariates used in the analyses were the mean values of risk factors assessed in 2003–04 and 2008–09 because, compared with a single measurement in 2008–09, these provide more reliable estimates of exposure in the 5 years before the first cfPWV measurement.
Statistical Analysis
Distributions of glucometabolic indices among people without diabetes were categorized in sex-specific quintiles and also expressed in standardized units. Linear mixed models were used to estimate the relation of glucometabolic indices with cfPWV in 2008–09 and change in cfPWV between 2008–09 and 2012–13. These models used all available cfPWV data, including cases where only one cfPWV measurement was available, which reduced selection bias and allowed better estimates of the associations of potential confounding factors. The models also accounted for correlation between repeated measures within individuals. We fitted the models with a random intercept and slope to account for individual differences in cfPWV at baseline and rate of change during follow-up. From these models, the effect of each glucometabolic index on cfPWV at baseline (2008–09) is estimated by the coefficient for the main effect of the glucometabolic index, and the effect on progression of cfPWV between 2008–09 and 2012–13 is estimated by the interaction of the main effect with time. The longitudinal effects of the glucometabolic indices are expressed as 5-year changes in cfPWV to allow direct comparisons with previous studies (18,20,24). All estimates were initially adjusted for age, sex, ethnic group, heart rate, and MAP at the time of cfPWV measurement. Baseline cfPWV and progression of cfPWV per 5 years were estimated from these models by quintile of each glucometabolic index distribution and per 1 SD increment in each index. This allowed us to examine associations with cfPWV across the distribution of each glucometabolic index and whether the coefficients increased linearly across quintiles. Tests of heterogeneity were conducted using likelihood ratio tests that compared the fit of the models with and without the quintiles of each glucometabolic measure. Two further models cumulatively adjusted for 1) systolic blood pressure, antihypertensive medication, lipid-lowering medication, smoking status, prevalent MI or stroke, and mean triglyceride and HDL-cholesterol between 2003–04 and 2008–09 and 2) mean BMI between 2003–04 and 2008–09 and estimated glomerular filtration rate in 2008–09. Sensitivity analysis was conducted to compare characteristics of participants with and without measurements of cfPWV to exclude the possibility of selection bias. Further sensitivity analyses used the glucometabolic measures from baseline (2008–09) or prebaseline (2003–04) rather than averaging the glycemic measures across the two phases.
Results
The cohort at the time of the baseline cfPWV assessment was a mean age of 60 years, 74% male, and predominantly of white ethnic origin. Fewer than 5% had chronic disease, and ∼30% were taking antihypertensive medication. A comparison of participants with and without cfPWV assessments across the entire cohort revealed that those individuals who did not have cfPWV measured were more likely to be female and have generally poorer health, in terms of chronic disease and taking antihypertensive or lipid-lowering medication (Supplementary Table 1). Detailed participant characteristics at the time of the two cfPWV examinations are provided in Table 1. Measures of exposure that were averaged across the prebaseline (2003–04) and baseline (2008–09) cfPWV visits are presented separately for each examination phase in Supplementary Table 2.
Table 1.
Characteristics of participants without diabetes at baseline
| Characteristic | Baseline (2008–09) (N = 3,685) |
Follow-up (2012–13) (N = 3,602) |
||
|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | |
| Age, years | 60.1 (5.7) | 65.0 (5.6) | ||
| Female | 25.6 | 25.7 | ||
| Ethnic group | ||||
| White | 94.0 | 94.6 | ||
| South Asian | 3.2 | 2.8 | ||
| Black | 2.1 | 1.9 | ||
| Other | 0.7 | 0.7 | ||
| Diabetes | 0.0 | 1.1 | ||
| MI or stroke | 4.9 | 4.5 | ||
| Antihypertensive medication | 29.1 | 36.0 | ||
| Lipid-lowering medication | 26.7 | 36.6 | ||
| Former smoker | 45.2 | 48.8 | ||
| Current smoker | 5.2 | 3.1 | ||
| BMI, kg/m2 | 26.0 (3.7) | 26.0 (3.9) | ||
| Systolic blood pressure, mmHg | 124.2 (15.3) | 126.7 (15.9) | ||
| Diastolic blood pressure, mmHg | 70.8 (10.0) | 70.8 (9.8) | ||
| Triglyceride, mmol/L | 1.16 (0.65) | 1.13 (0.57) | ||
| HDL cholesterol, mmol/L | 1.63 (0.45) | 1.68 (0.47) | ||
| LDL cholesterol, mmol/L | 3.14 (0.94) | 2.98 (0.94) | ||
| Fasting glucose, mmol/L† | 5.05* (0.10) | 5.16* (0.10) | ||
| 2-h glucose, mmol/L† | 6.23* (0.26) | Not done | ||
| HbA1c, %† | 5.58* (0.07) | 5.64* (0.06) | ||
| HbA1c, mmol/mol† | 37.4* (0.07) | 38.4* (0.06) | ||
| HOMA-IR† | 1.39* (0.67) | 1.68* (0.66) | ||
| Heart rate, bpm | 65.8 (11.3) | 68.1 (11.6) | ||
| cfPWV, m/s | 8.30 (1.93) | 8.98 (2.39) | ||
| MAP, mmHg | 89.4 (10.5) | 93.8 (10.9) | ||
Characteristics of the participants included in the present analyses at the baseline (2008–09) and follow-up (2012–13) visits.
*Geometric mean and SD of logged values.
†For glucometabolic indices, the baseline values represent the average of values at the 2003–04 and 2008–09 assessments. The mean for the 4,386 participants included in the analyses is provided in Supplementary Table 2, together with the separate means for 2003–04 and 2008–09.
There was no evidence that the associations of the glucometabolic indices with baseline cfPWV and progression of cfPWV differed between men and women (Supplementary Table 3). Cross-sectional associations between cfPWV and glucometabolic indices are shown by quintile in Table 2, adjusted for age, sex, ethnicity, MAP, and heart rate. Significant positive associations were found with fasting glucose, 2-h glucose, HbA1c, and HOMA-insulin resistance (HOMA-IR). cfPWV increased by between 0.19 and 0.40 m/s when moving from the lowest to highest quintile of each determinant. Additional adjustment for other potential confounders, including drug therapy and cardiovascular risk factors, only modestly attenuated these associations (Supplementary Table 4). When glucometabolic measures were treated as continuous variables, glucose and HOMA-IR were strongly associated and HbA1c was weakly associated with baseline cfPWV (Table 3). These associations were weakened with additional adjustment for potential confounders.
Table 2.
The association of glucometabolic indices at baseline with cfPWV measured at baseline and progression of cfPWV during the follow-up period
| Glucometabolic measurea |
cfPWV at baseline |
Change in cfPWV (per 5 years) |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Persons observed (n) | Meanb | Differencec (95% CI) | P value | Meanb | Differencec (95% CI) | P value | |
| Fasting glucose, mmol/L | 7,287 | |||||||
| Q1-lowest quintile | 4.65 | 1,512 | 8.30 | Ref | — | 0.58 | Ref | — |
| Q2 | 4.93 | 1,251 | 8.33 | 0.04 (−0.13, 0.21) | 0.64 | 0.60 | 0.02 (−0.21, 0.25) | 0.89 |
| Q3 | 5.10 | 1,521 | 8.39 | 0.11 (−0.05, 0.27) | 0.18 | 0.59 | 0.01 (−0.20, 0.23) | 0.91 |
| Q4 | 5.35 | 1,603 | 8.50 | 0.22 (0.06, 0.37) | 0.007 | 0.57 | 0.00 (−0.21, 0.22) | 0.99 |
| Q5-highest quintile | 5.70 | 1,400 | 8.52 | 0.27 (0.11, 0.43) | 0.001 | 0.59 | 0.02 (−0.20, 0.24) | 0.87 |
| Heterogeneity (P value) | 0.005 | 1.0 | ||||||
| Per 1 SD higher fasting glucose | 0.08 (0.03, 0.13) | 0.004 | 0.01 (−0.07, 0.08) | 0.89 | ||||
| 2-h glucose, mmol/L | 7,055 | |||||||
| Q1-lowest quintile | 4.65 | 1,468 | 8.15 | Ref | — | 0.53 | Ref | — |
| Q2 | 5.55 | 1,488 | 8.26 | 0.11 (−0.05, 0.27) | 0.18 | 0.53 | 0.00 (−0.22, 0.21) | 0.97 |
| Q3 | 6.20 | 1,379 | 8.32 | 0.17 (0.00, 0.33) | 0.05 | 0.52 | −0.01 (−0.24, 0.21) | 0.90 |
| Q4 | 6.90 | 1,409 | 8.25 | 0.10 (−0.06, 0.26) | 0.22 | 0.56 | 0.03 (−0.19, 0.26) | 0.76 |
| Q5-highest quintile | 8.25 | 1,311 | 8.46 | 0.32 (0.15, 0.48) | <0.001 | 0.73 | 0.20 (−0.03, 0.43) | 0.09 |
| Heterogeneity (P value) | 0.006 | 0.36 | ||||||
| Per 1 SD higher 2-h glucose | 0.11 (0.06, 0.16) | <0.001 | 0.07 (−0.01, 0.14) | 0.07 | ||||
| HbA1c, % | 7,283 | |||||||
| Q1-lowest quintile | 5.0 | 1,472 | 8.35 | Ref | — | 0.44 | Ref | — |
| Q2 | 5.2 | 1,197 | 8.26 | −0.08 (−0.25, 0.09) | 0.33 | 0.58 | 0.14 (−0.09, 0.37) | 0.24 |
| Q3 | 5.4 | 1,930 | 8.39 | 0.04 (−0.11, 0.19) | 0.60 | 0.49 | 0.05 (−0.15, 0.26) | 0.62 |
| Q4 | 5.6 | 1,418 | 8.56 | 0.21 (0.04, 0.37) | 0.01 | 0.55 | 0.11 (−0.11, 0.33) | 0.34 |
| Q5-highest quintile | 5.8 | 1,266 | 8.54 | 0.19 (0.02, 0.36) | 0.03 | 0.83 | 0.39 (0.15, 0.62) | 0.001 |
| Heterogeneity (P value) | 0.003 | 0.01 | ||||||
| Per 1 SD higher HbA1c | 0.05 (0.00, 0.11) | 0.05 | 0.12 (0.04, 0.19) | 0.002 | ||||
| HOMA-IR | 7,189 | |||||||
| Q1-lowest quintile | 0.73 | 1,583 | 8.21 | Ref | — | 0.41 | Ref | — |
| Q2 | 1.12 | 1,480 | 8.24 | 0.03 (−0.12, 0.19) | 0.67 | 0.47 | 0.06 (−0.16, 0.27) | 0.61 |
| Q3 | 1.53 | 1,474 | 8.46 | 0.26 (0.10, 0.41) | 0.002 | 0.60 | 0.19 (−0.03, 0.40) | 0.09 |
| Q4 | 2.12 | 1,415 | 8.55 | 0.35 (0.19, 0.51) | <0.001 | 0.62 | 0.20 (−0.02, 0.42) | 0.07 |
| Q5-highest quintile | 3.52 | 1,237 | 8.61 | 0.40 (0.23, 0.57) | <0.001 | 0.80 | 0.39 (0.15, 0.62) | 0.001 |
| Heterogeneity (P value) | <0.001 | 0.01 | ||||||
| Per 1 SD higher HOMA-IR | 0.15 (0.10, 0.21) | <0.001 | 0.11 (0.04, 0.19) | 0.004 | ||||
aValues are the averages of measurements made in 2003–04 and 2008–09.
bMeans are adjusted for age, sex, ethnic group, heart rate, and MAP at the time of the cfPWV measurement and are shown adjusted to white men without diabetes at age 65 with a MAP of 90 mmHg.
cDifferences are adjusted for age, sex, ethnic group, and MAP at the time of the cfPWV measurement.
Table 3.
The associations of glucometabolic indices with cfPWV and progression of cfPWV after adjustment for confounding factors and other cardiovascular risk factors
| Glucometabolic measurea | Model adjustments | cfPWV at baseline | Change in cfPWV (per 5 years) | ||
|---|---|---|---|---|---|
| Differenceb (95% CI) | P value | Increaseb (95% CI) | P value | ||
| Fasting glucose | Model 1c | 0.08 (0.03, 0.13) | 0.004 | 0.01 (−0.07, 0.08) | 0.89 |
| Model 2d | 0.05 (−0.01, 0.10) | 0.08 | −0.01 (−0.08, 0.07) | 0.87 | |
| Model 3e | 0.05 (−0.01, 0.10) | 0.09 | −0.03 (−0.10, 0.04) | 0.44 | |
| 2-h glucose | Model 1c | 0.11 (0.06, 0.16) | <0.001 | 0.07 (−0.01, 0.14) | 0.07 |
| Model 2d | 0.06 (0.01, 0.12) | 0.03 | 0.06 (−0.02, 0.13) | 0.12 | |
| Model 3e | 0.06 (0.00, 0.11) | 0.04 | 0.04 (−0.03, 0.12) | 0.24 | |
| HbA1c | Model 1c | 0.05 (0.00, 0.11) | 0.05 | 0.12 (0.04, 0.19) | 0.002 |
| Model 2d | 0.04 (−0.02, 0.09) | 0.19 | 0.11 (0.04, 0.18) | 0.003 | |
| Model 3e | 0.03 (−0.02, 0.09) | 0.22 | 0.10 (0.03, 0.17) | 0.008 | |
| HOMA-IR | Model 1c | 0.15 (0.10, 0.21) | <0.001 | 0.11 (0.04, 0.19) | 0.004 |
| Model 2d | 0.07 (0.01, 0.13) | 0.03 | 0.09 (0.01, 0.17) | 0.03 | |
| Model 3e | 0.07 (0.00, 0.13) | 0.05 | 0.02 (−0.07, 0.11) | 0.63 | |
aValues are the averages of measurements made in 2003–04 and 2008–09.
bDifferences and increases in PVW are per 1 SD higher value for each glucometabolic measure.
cModel 1 is adjusted for age, sex, ethnic group, heart rate, and MAP at the time of the cfPWV measurement.
dModel 2 is adjusted as for model 1 plus systolic blood pressure, antihypertensive medication, lipid-lowering medication, prevalent MI or stroke, smoking status, and mean triglyceride and HDL-cholesterol between 2003–04 and 2008–09.
eModel 3 is adjusted as for model 2 plus mean BMI between 2003–04 and 2008–09.
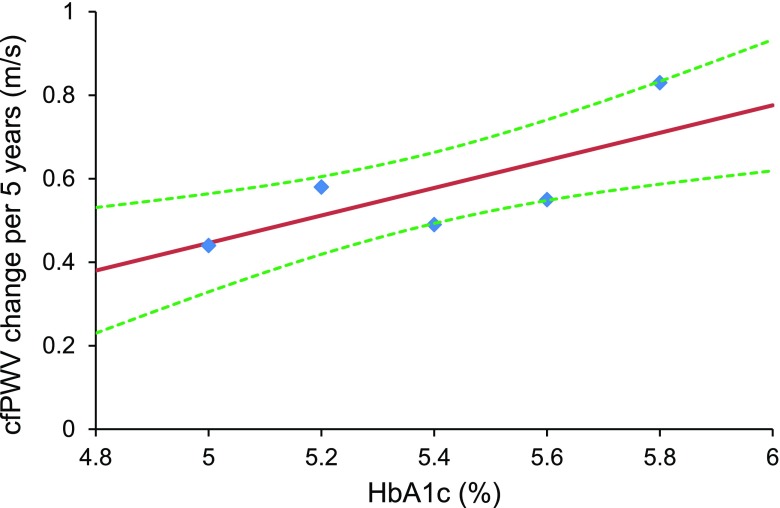
After adjustment for age, sex, ethnicity, MAP, and heart rate, only HbA1c and HOMA-IR were significantly associated with progression of cfPWV. There was a 0.39 m/s greater increase in cfPWV over 5 years in individuals in the top quintile of either parameter compared with those in the lowest quintile (Table 2 and Fig. 1). Adjustment for treatment and other risk factors (Supplementary Table 5) did not significantly attenuate these associations. However, after the addition of BMI to the models, there was no longer an association with HOMA-IR. Analyses using the continuous variables revealed similar findings: a 1 SD higher HbA1c or HOMA-IR at baseline was associated with a ∼0.12 m/s greater increase in cfPWV over 5 years (Table 3). In the fully adjusted model, only HbA1c was associated with progression in cfPWV.
Figure 1.
The association between HbA1c and change in cfPWV. Plotted points show the cfPWV change per 5 years for each quintile of HbA1c, plotted at the median of each quintile. The solid line shows the linear association, and the dashed lines show the 95% CI. Values shown are adjusted to white men without diabetes at age 65 with a MAP of 90 mmHg. Test for quadratic (nonlinear) effect gave a P value = 0.48.
Repeating these analyses using glucometabolic measures from baseline (2008–09) or prebaseline (2003–04), rather than averaging across the two phases, did not meaningfully alter the results (Supplementary Tables 6 and 7, respectively). Similarly, excluding the 1.1% of individuals who developed diabetes during follow-up had no influence on the results (data not shown). To exclude the possibility of a selection bias influencing our results because cfPWV was not measured in all participants, a separate analysis using the entire cohort explored the relationship between HbA1c and blood pressure, which is closely related to cfPWV. At baseline and follow-up, HbA1c was ∼3% higher in hypertensive individuals than nonhypertensive individuals, in those with and without cfPWV assessment, after adjusting for confounding factors (Supplementary Table 8). This supports our observed association between HbA1c and cfPWV and argues against the possibility that selection bias might be influencing our findings.
Conclusions
Our main findings are that glucose, HbA1c, and insulin resistance are all cross-sectionally associated with aortic stiffness and that longitudinal analysis shows HbA1c and HOMA-IR are associated with the progression of aortic stiffness during a 4-year period. These associations were independent of other cardiovascular risk factors. The association between HbA1c and aortic stiffening was also independent of BMI. These observations suggest that factors underlying glucometabolic status may affect aortic stiffening even within the normoglycemic range, which may have important implications for developing antistiffening strategies and exploiting novel therapeutic targets.
Aortic stiffness, and cfPWV in particular, is an important risk factor for future cardiovascular disease independently of other cardiovascular risk factors (2). However, the biological processes underlying aortic stiffening are incompletely understood. Cross-sectional analyses have provided inconsistent results (6) and are limited in their ability to attribute causality. Existing longitudinal data highlight the importance of age and blood pressure, but the importance of other potential risk factors, including indices of glucose homeostasis, is unclear because of lack of replication studies, variable lengths of follow-up, poor availability of data on individual risk factors, and variation in adjustment for confounders. Whitehall II is a large cohort with prospective data on cfPWV and other risk factors, including a variety of glucometabolic indices, making it well suited to examine the relationship between glycemia and aortic stiffening in individuals without diabetes.
As expected, we found significant cross-sectional relationships between indices of glucose homeostasis and insulin resistance with cfPWV. Importantly, this remained after adjustment for physiological confounders of stiffness such as MAP and heart rate (34). Similar results have been reported previously using a variety of study designs, indices of stiffness, and varying levels of adjustment for physiological confounders (11,15,16,35,36). In the current study, adjustment for other potential risk factors for arterial stiffening or cardiovascular disease only modestly reduced the strength of association with glucose measures, but the associations with HOMA-IR and HbA1c were more markedly attenuated, with an approximate halving of the β-values. This contrasts with cross-sectional findings from a cohort of 263 African Americans (16), in whom HbA1c but not fasting or 2-h glucose levels remained independently associated with cfPWV. This disparity possibly reflects ethnic differences in the association between HbA1c and arterial stiffness or may reflect a lack of appropriate statistical power in the African American study. In addition, we a priori excluded individuals with diabetes, which may have removed any association with HbA1c in our data. Alternatively, residual confounding may explain our observed associations with plasma glucose.
In contrast to the cross-sectional observations, fasting and 2-h glucose were not associated with progression of aortic stiffening in the current study, which is consistent with previous observations (19–22). However, HbA1c and HOMA-IR were associated with accelerated progression of cfPWV independently of potential confounders. Previously, one small study reported no association between cfPWV progression and HOMA (19), but HbA1c has been associated with progressive carotid artery stiffening in the Atherosclerosis Risk in Communities (ARIC) Study (37), although, unfortunately, cfPWV was not assessed. In the current study, cfPWV increased by ∼0.7 m/s, which is consistent with previous longitudinal studies, which have reported increases of between 0.2 and 0.6 m/s per 5 years in participants aged ∼60 years (18,20,24). Moreover, modest differences of 0.07% in HbA1c or 0.67 units in HOMA-IR were associated with an ∼0.1 m/s greater increase in cfPWV over 5 years, equating to ∼12–14% of the overall change in stiffness. Although the rate of stiffening is strongly dependent on age, our data suggest that even in individuals without diabetes, modest differences in glucometabolic status have a meaningful effect on arterial stiffening, consistent with our hypothesis of accelerated vascular aging.
A strong mechanistic relationship exists between measures of adiposity, insulin resistance, and glycemia. We and others have previously reported that measures of adiposity, including BMI, are associated with accelerated aortic stiffening independent of other risk factors (10,18,20,22); therefore, we additionally adjusted for BMI. After this, HbA1c but not HOMA-IR remained predictive of aortic stiffening. These findings suggest that adiposity and insulin resistance share common pathways leading to aortic stiffening but that these pathways may be independent of glycemia, although this hypothesis requires further examination. A number of mechanisms may be responsible, including visceral and perivascular fat accumulation and the vascular effects of insulin. Indeed, fasting insulin concentrations are positively associated with cfPWV in the general population (38). In addition, abdominal and vascular adiposity alter adipokine levels, increase circulating proinflammatory stimuli, and may directly inflame the vasculature (39–41). Low adiponectin levels have previously been associated with obesity and increased cfPWV progression (19), and in the Whitehall II cohort, we have previously shown that a panel of inflammatory markers was associated with increased cfPWV 16 years later (17).
Systemic inflammation is also associated with cfPWV (7,42). Interestingly, the peroxisome proliferator–activated receptor γ agonist, pioglitazone, improves inflammation and glycemic control in obese patients with diabetes (43). Pioglitazone was also effective in preventing strokes (44) and in reducing the progression to diabetes and major cardiovascular events (45) in a high-risk population without diabetes. Peroxisome proliferator–activated receptor γ agonists may thus represent one potential therapeutic strategy to retard aortic stiffening in individuals without diabetes. In addition, formation of advanced glycation end products, which accumulate in tissues over time and with increased plasma glucose levels (46), correlate with aortic stiffness in individuals without diabetes (47,48). Experimental cross-link breakers reduce cfPWV and pulse pressure in animals (49) and humans (50), and our longitudinal finding for HbA1c suggests that advanced glycation end products may represent another novel antistiffening target, even in individuals without diabetes. However, both of these hypotheses need to be tested in well-designed intervention studies.
The current study has a number of limitations. We were restricted to 4-year follow-up data and cannot exclude the possibility that differences in relative strength of the glucometabolic risk factor effects may be observed with longer follow-up, or indeed, in younger adults. However, our data are consistent with observations made in other cohorts with similar lengths of follow-up. Moreover, we used the gold standard method of cfPWV to assess aortic stiffness and an identical protocol at both examinations. However, use of a 5-h fast for afternoon examinations may have meant that those participants were not truly fasted. We are unable to comment on the effect of diabetes per se because we a priori excluded individuals with diabetes. This allowed us to minimize potential confounding influences, such as therapy. However, we were able to demonstrate meaningful differences in progression of aortic stiffness, even within what is considered a normal range of HbA1c. Given that glucometabolic indices determine the development of diabetes up to 15 years in advance (51) and cfPWV predicts future cardiovascular risk (2), we believe that our observations are clinically important and suggest that further mechanistic and intervention studies of arterial stiffening should examine factors related to longer-term glucometabolic status. These could involve lifestyle approaches and/or trials of glucose-lowering therapies in individuals without diabetes, which could ultimately influence public health strategies.
In summary, a higher HbA1c and HOMA-IR are associated with increased aortic stiffening in individuals without diabetes. This was independent of potential confounders or other cardiovascular risk factors and, in the case of HbA1c, also independent of BMI. In contrast, point estimates of glucose, either fasting or 2 h after a standard glucose tolerance test, were not associated with progression of aortic stiffness. Our data suggest that higher average glucose levels may be causally related to accelerated vascular aging through long-term mechanisms rather than short-term dynamic changes in the arterial wall. Improving glucometabolic status may thus represent a strategy to improve vascular health.
Supplementary Material
Article Information
Acknowledgments. The authors thank all of the participating civil service departments and their welfare personnel and establishment officers, the Occupational Health and Safety Agency, the Council of Civil Service Unions, all of the participating civil servants in the Whitehall II Study, and all members of the Whitehall II Study team. The Whitehall II Study team is composed of research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants, and data entry staff, who make the study possible.
Funding. This work was supported by the British Heart Foundation (RG/13/2/30098), the British Medical Research Council (K013351), the British Department of Health, the British Stroke Association (TSA 2008/05), the National Heart, Lung, and Blood Institute (R01-HL-036310), and the National Institute on Aging (R01-AG-013196 and R01-AG-034454). C.M.M. and I.B.W. receive support from the National Institute for Health Research Cambridge Biomedical Research Centre and the British Heart Foundation Centre of Excellence. I.B.W. is a British Heart Foundation Senior Fellow. N.B.J. and D.R.W. are supported by the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. M.K. is supported by the Medical Research Council, the Economic and Social Research Council, and NordForsk, the Nordic Programme on Health and Welfare. M.J.S. is partially supported by the British Heart Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.M. drafted the manuscript. I.B.W. conceived the idea for the manuscript and reviewed and edited the manuscript. N.B.J. and A.S.-M. reviewed and edited the manuscript. D.R.W., M.K., and A.G.T. contributed to the discussion and reviewed and edited the manuscript. E.J.B. contributed to the analysis and discussion and reviewed and edited the manuscript. M.J.S. performed statistical analysis and reviewed and edited the manuscript. M.J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1773/-/DC1.
References
- 1.Townsend RR, Wilkinson IB, Schiffrin EL, et al.; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015;66:698–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, et al. . Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson PM, Khalili P, Franklin SS. Blood pressure and pulse wave velocity as metrics for evaluating pathologic ageing of the cardiovascular system. Blood Press 2014;23:17–30 [DOI] [PubMed] [Google Scholar]
- 4.Brunner EJ, Shipley MJ, Witte DR, et al. . Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension 2011;57:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson IB, McEniery CM. Arteriosclerosis: inevitable or self-inflicted? Hypertension 2012;60:3–5 [DOI] [PubMed] [Google Scholar]
- 6.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009;54:1328–1336 [DOI] [PubMed] [Google Scholar]
- 7.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol 2004;24:969–974. [DOI] [PubMed]
- 8.Tomlinson L, Ben-Shlomo Y, Caplin B, et al. . Renal disease is associated with accelerated vascular ageing: initial results of the UK Research Alliance into Kidney Disease and Arterial Stiffness (Ureka) Collaboration. J Hypertens 2010;28:E417 [Google Scholar]
- 9.McEniery CM, Yasmin, Maki-Petaja KM, et al.; Anglo-Cardiff Collaboration Trial Investigators . The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III). Hypertension 2010;56:591–597 [DOI] [PubMed] [Google Scholar]
- 10.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, et al. . Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension 2015;66:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schram MT, Kostense PJ, Van Dijk RA, et al. . Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002;20:1743–1751 [DOI] [PubMed] [Google Scholar]
- 12.Wahlqvist ML, Lo CS, Myers KA, Simpson RW, Simpson JM. Putative determinants of arterial wall compliance in NIDDM. Diabetes Care 1988;11:787–790 [DOI] [PubMed] [Google Scholar]
- 13.Yapei Y, Xiaoyan R, Sha Z, et al. . Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: an up-to-date meta-analysis. Med Sci Monit 2015;21:2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira MT, Leite NC, Cardoso CR, Salles GF. Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care 2015;38:897–904 [DOI] [PubMed] [Google Scholar]
- 15.Henry RM, Kostense PJ, Spijkerman AM, et al.; Hoorn Study . Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 2003;107:2089–2095 [DOI] [PubMed] [Google Scholar]
- 16.Stakos DA, Schuster DP, Sparks EA, et al. . Association between glycosylated hemoglobin, left ventricular mass and aortic function in nondiabetic individuals with insulin resistance. Eur J Endocrinol 2007;157:63–68 [DOI] [PubMed] [Google Scholar]
- 17.Johansen NB, Vistisen D, Brunner EJ, et al. . Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS One 2012;7:e37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlGhatrif M, Strait JB, Morrell CH, et al. . Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 2013;62:934–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Khoudary SR, Barinas-Mitchell E, White J, et al.; ERA JUMP Study Group . Adiponectin, systolic blood pressure, and alcohol consumption are associated with more aortic stiffness progression among apparently healthy men. Atherosclerosis 2012;225:475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wildman RP, Farhat GN, Patel AS, et al. . Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005;45:187–192 [DOI] [PubMed] [Google Scholar]
- 21.Benetos A, Adamopoulos C, Bureau JM, et al. . Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–1207 [DOI] [PubMed] [Google Scholar]
- 22.Birru MS, Matthews KA, Thurston RC, et al.; SWAN Heart Study . African-American ethnicity and cardiovascular risk factors are related to aortic pulse-wave velocity progression. Am J Hypertens 2011;24:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scuteri A, Morrell CH, Orrù M, et al. . Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014;64:1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaess BM, Rong J, Larson MG, et al. . Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012;308:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 26.Laurent S, Cockcroft JR, van Bortel LM, et al. . Abridged version of the expert consensus document. Artery Res 2007;1:2–12 [Google Scholar]
- 27.Wilkinson IB, Fuchs SA, Jansen IM, et al. . Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998;16:2079–2084 [DOI] [PubMed] [Google Scholar]
- 28.Brunner EJ, Shipley MJ, Marmot MG, Kivimaki M, Witte DR. Do the Joint British Society (JBS2) guidelines on prevention of cardiovascular disease with respect to plasma glucose improve risk stratification in the general population? Prospective cohort study. Diabet Med 2010;27:550–555 [DOI] [PubMed] [Google Scholar]
- 29.Brunner EJ, Marmot MG, Nanchahal K, et al. . Social inequality in coronary risk: central obesity and the metabolic syndrome. evidence from the Whitehall II study. Diabetologia 1997;40:1341–1349 [DOI] [PubMed] [Google Scholar]
- 30.Cooper GR. Methods for determining the amount of glucose in blood. CRC Crit Rev Clin Lab Sci 1973;4:101–145 [DOI] [PubMed] [Google Scholar]
- 31.Andersen L, Dinesen B, Jørgensen PN, Poulsen F, Røder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem 1993;39:578–582 [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 33.Chapelle JP, Teixeira J, Maisin D, et al. . Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med 2010;48:365–371 [DOI] [PubMed] [Google Scholar]
- 34.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators . Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Huang Y, Li X, et al. . Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine 2009;36:262–267 [DOI] [PubMed] [Google Scholar]
- 36.Lukich E, Matas Z, Boaz M, Shargorodsky M. Increasing derangement of glucose homeostasis is associated with increased arterial stiffness in patients with diabetes, impaired fasting glucose and normal controls. Diabetes Metab Res Rev 2010;26:365–370 [DOI] [PubMed] [Google Scholar]
- 37.Rubin J, Nambi V, Chambless LE, et al. . Hyperglycemia and arterial stiffness: the Atherosclerosis Risk in the Communities study. Atherosclerosis 2012;225:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Relation between insulin and aortic stiffness: a population-based study. J Hum Hypertens 2004;18:1–7 [DOI] [PubMed] [Google Scholar]
- 39.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2009;29:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henrichot E, Juge-Aubry CE, Pernin A, et al. . Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005;25:2594–2599 [DOI] [PubMed] [Google Scholar]
- 41.Gao H, Fall T, van Dam RM, et al. . Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 2013;62:1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mäki-Petäjä KM, Hall FC, Booth AD, et al. . Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006;114:1185–1192 [DOI] [PubMed] [Google Scholar]
- 43.Tripathy D, Daniele G, Fiorentino TV, et al. . Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study. Diabetologia 2013;56:2153–2163 [DOI] [PubMed] [Google Scholar]
- 44.Kernan WN, Viscoli CM, Furie KL, et al.; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inzucchi SE, Viscoli CM, Young LH, et al.; IRIS Trial Investigators . Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care 2016;39:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 1995;46:223–234 [DOI] [PubMed] [Google Scholar]
- 47.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens 2009;22:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semba RD, Sun K, Schwartz AV, et al.; Health ABC Study . Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens 2015;33:797–803; discussion 803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaitkevicius PV, Lane M, Spurgeon H, et al. . A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A 2001;98:1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kass DA, Shapiro EP, Kawaguchi M, et al. . Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2001;104:1464–1470 [DOI] [PubMed] [Google Scholar]
- 51.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.