Abstract
OBJECTIVE
High triglyceride (TG) levels and low HDL cholesterol (HDL-C) levels are risk factors for cardiovascular disease. It is unclear whether this relationship depends on glycemic dysregulation, sex, or LDL cholesterol (LDL-C) level.
RESEARCH DESIGN AND METHODS
We studied 3,216 participants (40% men, 41% with diabetes) who were free of cardiovascular disease at baseline in a community-based, prospective cohort of American Indians (median follow-up 17.7 years). Cox models estimated hazard ratios (HRs) and 95% CIs for incident ischemic stroke and coronary heart disease (CHD) in relation to combined TG and HDL-C status, where a fasting TG level ≥150 mg/dL was “high” and a fasting HDL-C level <40 mg/dL for men (<50 mg/dL for women) was “low.” Models included age, sex, BMI, smoking, diabetes, fasting LDL-C level, antihypertensive medications, physical activity, estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio.
RESULTS
Participants with high TG and low HDL levels had a 1.32-fold greater HR (95% CI 1.06–1.64) for CHD than those with normal TG and normal HDL levels. It was observed in participants with diabetes, but not in those without diabetes, that high TG plus low HDL levels were associated with a 1.54-fold greater HR (95% CI 1.15–2.06) for CHD (P value for interaction = 0.003) and a 2.13-fold greater HR (95% CI 1.06–4.29) for stroke (P value for interaction = 0.060). High TG and low HDL level was associated with CHD risk in participants with an LDL-C level of ≥130 mg/dL, but this was not observed in those participants with lower LDL-C levels. Sex did not appear to modify these associations.
CONCLUSIONS
Adults with both high TG and low HDL-C, particularly those with diabetes, have increased risks of incident CHD and stroke. In particular, those with an LDL-C level ≥130 mg/dL may have an increased risk of incident stroke.
Introduction
High levels of triglyceride (TG) and low levels of HDL cholesterol (HDL-C) are considered to be risk factors for coronary heart disease (CHD) (1) and ischemic stroke (2,3). Lowering the LDL cholesterol (LDL-C) level with statin therapy is the primary target in reducing atherosclerotic cardiovascular disease (ASCVD) (4,5). Yet, high TG and low HDL-C levels remain prevalent in some statin-treated adults (6). Prospective studies (7–10) have suggested that a high TG level in combination with a low HDL-C level, also called “atherogenic dyslipidemia,” may be a clinical marker to identify adults who are at risk for ASCVD. This may be particularly true of individuals with obesity and metabolic disease, as insulin resistance (IR) directly contributes to such dyslipidemic abnormalities (11–14). Moreover, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, participants with diabetes who were receiving treatment with a statin for LDL-C control and had both high TG and low HDL-C experienced cardiovascular protection with fenofibrate, which lowers TG and raises HDL-C levels, among other favorable lipid-modifying effects (15).
In view of the links between metabolic disease and atherogenic dyslipidemia, and amid the ongoing obesity and diabetes epidemics affecting contemporary societies (16,17), understanding the role of abnormal levels of TG and HDL-C as cardiovascular risk factors in different dysmetabolic states is of considerable clinical and public health importance. It remains unclear whether the high TG and low HDL-C levels used to define atherogenic dyslipidemia are associated with different risks of incident CHD or ischemic stroke in distinct populations defined by diabetes status, sex, or LDL-C level (7–10). Evidence analyzing electronic medical records has suggested that there are increased CHD risks related to high TG and low HDL-C levels in individuals with diabetes, but important patient characteristics were not well controlled (18). We hypothesized that high fasting plasma TG level in combination with low HDL-C level is a risk factor for CHD and ischemic stroke among American Indians, who have a high prevalence of obesity and diabetes, and that the magnitude of these relationships depends on diabetes status, sex, and LDL-C level.
Research Design and Methods
Study Population
The Strong Heart Study (SHS) is an ongoing, community-based, prospective cohort study of American Indians from South Dakota, North Dakota, Oklahoma, and Arizona (19). A total of 4,549 participants (age range 45–74 years) were enrolled at the baseline examination between 1989 and 1991. Participants returned for follow-up visits in 1993–1995 and 1998–1999. Morbidity and mortality have been updated and recorded annually. Detailed information about study design and data collection in the SHS has been reported previously (20,21) and is accessible at the website (http://strongheart.ouhsc.edu/). The current analytic sample consisted of 3,216 eligible individuals at SHS baseline, after excluding 331 participants who had a history of cardiovascular disease (CVD) at baseline, 68 people who had missing baseline measurements of TG or HDL-C, and 934 participants from one community who withdrew consent for the study. Informed consent was obtained from each participant. The participating tribes and the institutional review boards of the participating institutions and the Indian Health Service approved the SHS.
Baseline Data Collection
At SHS baseline, each participant underwent an in-person interview, physical examination, and structured questionnaire data collection. All participants also provided blood and urine specimens after a 12-h fast. The following markers were measured in these specimens using standard procedures: plasma lipid panel (TG, total cholesterol, LDL-C, and HDL-C) (22) and lipoproteins (23), insulin (24), glucose (22), creatinine (25), fibrinogen (25), urinary albumin, and creatinine (26). Participants with no known diabetes also underwent a 2-h oral glucose tolerance test (consisting of a blood draw, to measure glucose, 2 h after a 75-g oral glucose load) (20). In all participants, blood pressure and anthropometric measurements (weight, height, and waist circumference) were performed according to standardized procedures by trained and certified study staff (20). BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Demographic information, lifestyle habits (alcohol use, tobacco smoking, physical activity), medical history, medication use, family history of illnesses, and physical activity were collected using structured questionnaires (20). Diabetes status was defined according to 1998 World Health Organization criteria: taking any diabetes medication, fasting glucose concentration ≥126 mg/dL, or 2-h blood glucose concentration ≥200 mg/dL after a 75-g oral glucose tolerance test. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or taking antihypertensive drugs. LDL-C levels were estimated by the Friedewald formula in participants with a TG level <400 mg/dL. Microalbuminuria and macroalbuminuria were defined as urinary albumin-to-creatinine ratio (UACR) >30 but <300 mg/g, and ≥300 mg/g, respectively. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated equation for Modification of Diet in Renal Disease (eGFR-MDRD), as follows: 186.3 × (serum creatinine [Scr])−1.154 × (age)−0.203 × (0.742 in females) (27). Separately, eGFR was also calculated based on the Chronic Kidney Disease Epidemiology Collaboration equation (eGFR-CKD-EPI), as follows: 141 × (minimum of 1 or standardized Scr [mg/dL]/κ)α × (maximum of 1 or standardized Scr [mg/dL]/κ)−1.209 × 0.993age × (1.018 if female), where κ is 0.7 in females and 0.9 in males, and α is −0.329 in females and −0.411 in males (28,29). Physical activity was captured in a questionnaire regarding activities related to leisure time and occupation in the past year (30). The questionnaire for physical activity has been applied and assessed in the Pima Indian Study of Arizona using Caltrac activity monitors (Accusplit Corporation, Livermore, CA). Correlation between past-week self-reported physical activity and activity monitor counts was high (ρ = 0.8). Test-retest correlations for past-year occupational and leisure time activities ranged from 0.63 to 0.92 (30,31). Total physical activity was the sum of the MET hours per week (MET-h/week) for leisure time and occupational activities.
Ascertainment of Incident CHD and Stroke
The current study included incident fatal or nonfatal events of ischemic stroke and CHD from the SHS baseline to 31 December 2007. These events were identified by annual morbidity and mortality surveillance, including a review of tribal and Indian Health Service hospital records and death certificates or by direct contact with participants and/or their families at each follow-up visit, with subsequent adjudication by study physicians using standard criteria as described previously (20,32). CHD included fatal myocardial infarction, sudden death due to CHD, other fatal CHD event, or nonfatal definite CHD, as defined previously (32). Stroke included ischemic fatal and nonfatal strokes, as described previously (33).
Statistical Analyses
Fasting TG levels were categorized into “normal TG” (<150 mg/dL) or “high TG” (≥150 mg/dL), and fasting HDL-C levels were categorized into “normal HDL” (≥40 mg/dL for men and ≥50 mg/dL for women) or “low HDL” (<40 mg/dL for men and <50 mg/dL for women), per the Adult Treatment Panel III (34) and the 2013 American College of Cardiology/American Heart Association guidelines (35). Using these cut points, we also defined TG-HDL status into the following four categories based on both TG and HDL-C levels: 1) “normal TG-normal HDL” (the referent category, defined as having normal TG and normal HDL-C levels); 2) “normal TG-low HDL”; 3) “high TG-normal HDL”; and 4) “high TG-low HDL.” A priori, we hypothesized the following: 1) that TG-HDL status confers different risks for incident CHD and ischemic stroke; and 2) that these relations depend on sex, diabetes status, and LDL-C level (7–10).
Distributions for continuous variables were examined to identify outliers and violations of normality. ANOVA was used to compare the mean values of continuous variables with normal distributions. For variables with skewed distributions, we instead conducted the nonparametric median score test and presented the median and interquartile range (first and third quartiles). χ2 tests were used to compare categorical variables. The time-to-event distribution (for incident ischemic stroke and CHD, separately) was assessed using the Kaplan-Meier method and compared according to the TG-HDL status, using the log-rank test. Incidence rates of stroke and CHD per 1,000 person-years were calculated by dividing the cumulative number of stroke or CHD cases by all at-risk person-years during follow-up. The person-year was estimated from the date of the baseline examination (1989–1991) to the first date of incident stroke or CHD diagnosis, date of death, or 31 December 2007, whichever occurred first. If a participant experienced an incident stroke and an incident CHD event, the event that occurred first was included for analysis. The 95% CIs for the crude incidence rates were estimated by generalized linear model, assuming a Poisson distribution for the probability distribution of outcome of count data (number of participants with incident CHD or stroke) during follow-up and an α-value of 0.05.
Cox proportional hazards models were conducted to estimate hazard ratios (HRs) and 95% CIs relating each of the following: TG level alone, HDL-C level alone, and TG-HDL status, to incident CHD and separately to incident stroke. Initially, the multivariable models were stratified on the location of the participating SHS study sites and included baseline age and sex (basic model). The full multivariable models additionally controlled for risk factors for CHD and stroke, including smoking, BMI, estimated LDL-C level, use of antihypertensive medications (yes/no), diabetes (yes/no), categorical physical activity (MET-h/week in quartile), eGFR-MDRD, and UACR. We stratified the Cox model on the location of the participating SHS study sites, which allowed different baseline hazards at each site and estimated the coefficients for TG-HDL status in relation to CHD or ischemic stroke by using the product of the partial likelihood functions for each individual stratum. Models excluded 246 participants (8%) who had missing information on one or more of the covariates, including 88 people who had undetermined LDL-C levels due to a TG concentration of ≥400 mg/dL. Full models did not include lipid-lowering medication use or the HOMA-IR because <3% of participants used lipid-lowering medication at baseline (27) and HOMA-IR was not applicable for participants with diabetes. The final models did not include alcohol use because it was not observed to be associated with either CHD or ischemic stroke in the SHS population (27,36).
We also explored associations between TG-HDL status and risks of nonfatal and, separately, fatal CHD. However, these analyses were limited because of the smaller subgroup sample sizes. We did not explore analogous relationships regarding incident nonfatal and fatal stroke because of having few fatal incident stroke events (n = 11).
To test whether sex (male/female), diabetes (yes/no), or LDL-C status (high/not high) modified relationships between TG-HDL status and the risks of incident CHD or ischemic stroke, 1) TG-HDL status by sex, 2) TG-HDL status by diabetes status, and 3) TG-HDL status by LDL-C status were each separately added to the full model. LDL-C level was defined to be high if it was ≥130 mg/dL. We considered a P value for interaction of <0.05 to be statistically significant for effect modification. All statistical analyses were performed using the SAS statistical software package version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics by TG and HDL-C Status
Of the 3,216 participants at baseline (mean age 56 years), 37% had normal TG and normal HDL, 30% had normal TG and low HDL, and 24% had high TG and low HDL, whereas only 9% had high TG and normal HDL (Table 1). About 50% were 45–55 years old, and the age distribution was not observed to vary by TG-HDL status. Physical activity (in MET-h/week) appeared similar across the four groups of TG-HDL status in men and women. Other baseline characteristics differed. About 65% of participants with low HDL-C levels, regardless of TG status, were female, whereas high TG-normal HDL or normal TG-normal HDL was as likely in men as women. Overall, 41% of participants (481 men and 853 women) had diabetes. Having diabetes, higher fasting plasma glucose levels, and higher fasting insulin levels varied by TG-HDL status (P < 0.001 for each). Sixty percent of participants with high TG and low HDL levels and only 30% of those with normal TG and normal HDL levels had diabetes. About 40% of those with high TG and normal HDL levels or normal TG and low HDL levels had diabetes. Higher LDL-C level, hypertension, microalbuminuria, and macroalbuminuria were more common in participants with high TG, regardless of HDL-C. Larger waist circumference and higher BMI were more prevalent in participants with low HDL-C, regardless of TG level.
Table 1.
Baseline characteristics of 3,216 subjects without known CHD and ischemic stroke at SHS baseline, by TG and HDL-C status
| Total population (n = 3,216) | TG and HDL-C status* (n = 3,216) |
|||||
|---|---|---|---|---|---|---|
| High TG, low HDL-C | High TG, normal HDL-C | Normal TG, low HDL-C | Normal TG, normal HDL-C | P value | ||
| Sample size, n (%) | 775 (24) | 288 (9) | 975 (30) | 1,178 (37) | ||
| TG (mg/dL), median (IQR), | 118 (82, 170) | 215 (175, 284) | 187 (163, 221) | 103 (80, 124) | 85 (64, 113) | <0.001a |
| HDL-C (mg/dL), median (IQR) | ||||||
| Men | 41 (34, 49) | 32 (28, 35) | 45 (42, 49) | 35 (32, 37) | 49 (43, 57) | <0.001a |
| Women | 46 (40, 56) | 39 (34, 43) | 58 (52, 63) | 42 (38, 46) | 58 (53, 67) | <0.001a |
| Age (years), mean (SD) | 56 (8) | 56 (8) | 56 (8) | 56 (8) | 56 (8) | 0.264b |
| 45–55, n (%) | 1,617 (50) | 394 (50) | 150 (52) | 500 (51) | 573 (49) | 0.881b |
| 56–65, n (%) | 1,037 (32) | 248 (32) | 92 (32) | 307 (31) | 390 (33) | |
| ≥66, n (%) | 562 (18) | 133 (17) | 46 (16) | 168 (17) | 215 (18) | |
| Female, n (%) | 1,916 (60) | 495 (64) | 147 (51) | 662 (68) | 612 (52) | <0.001b |
| Diabetes, n (%) | 1,334 (41) | 457 (60) | 116 (41) | 422 (44) | 339 (30) | <0.001b |
| Fasting plasma glucose (mg/dL), median (IQR) | 110 (98, 152) | 128 (106, 232) | 113 (100, 169) | 111 (99, 155) | 104 (95, 121) | <0.001a |
| Fasting insulin (μU/mL), median (IQR) | 15 (9, 24) | 20 (12, 30) | 16 (9, 24) | 16 (11, 26) | 11 (6, 18) | <0.001a |
| BMI (kg/m2), mean (SD) | 30 (6) | 31 (5) | 30 (5) | 32 (6) | 29 (6) | <0.001b |
| Waist circumference (cm), mean (SD) | ||||||
| Men | 102 (13) | 106 (11) | 103 (11) | 105 (14) | 99 (13) | <0.001b |
| Women | 105 (15) | 107 (12) | 103 (13) | 107 (15) | 101 (16) | <0.001b |
| Total physical activity (MET-h/week), median (IQR)** | ||||||
| Men | 78 (20, 159) | 83 (19, 173) | 106 (39,187) | 61 (13, 153) | 77 (22, 149) | 0.060a |
| Women | 45 (10, 105) | 48 (8, 102) | 59 (13, 106) | 37 (10, 106) | 46 (11, 106) | 0.073a |
| Smoking, n (%) | 0.037b | |||||
| Current | 1,239 (39) | 327 (42) | 96 (33) | 355 (36) | 461 (39) | 0.021b |
| Past | 1,040 (32) | 249 (32) | 101 (35) | 309 (32) | 381 (32) | 0.760b |
| Never | 935 (29) | 199 (26) | 91 (32) | 311 (32) | 334 (28) | |
| LDL-C (mg/dL), median (IQR) | 119 (98, 141) | 123 (102, 146) | 122 (99, 148) | 118 (97, 138) | 117 (96, 138) | <0.001a |
| Hypertension, n (%)*** | 1143 (36) | 315 (41) | 133 (46) | 320 (33) | 375 (32) | <0.001b |
| Antihypertensive medication, n (%) | 562 (17) | 177 (17) | 62 (17) | 162 (13) | 161 (11) | <0.001a |
| Blood pressure (mmHg), median (IQR) | ||||||
| SBP | 124 (113, 136) | 125 (115, 137) | 129 (118, 141) | 122 (111, 134) | 124 (113, 135) | <0.001a |
| DBP | 76 (70, 83) | 77 (70, 83) | 79 (72, 86) | 75 (69, 82) | 76 (69, 82) | <0.001a |
| Microalbuminuria, n (%)# | 507 (16) | 158 (21) | 54 (19) | 153 (16) | 142 (12) | <0.001b |
| Macroalbuminuria, n (%)# | 237 (7) | 97 (13) | 36 (13) | 48 (5) | 56 (5) | <0.001b |
| eGFR-MDRD (mL/min), mean (SD) | 82 (23) | 83 (33) | 82 (22) | 80 (18) | 82 (18) | 0.277b |
DBP, diastolic blood pressure; IQR, interquartile range; SBP, systolic blood pressure.
*High TG means TG levels ≥150 mg/dL; low HDL-C means HDL-C levels <40 mg/dL (men) or 50 mg/dL (women).
**Total physical activity included leisure time and occupational physical activities over the past year and was presented in MET-h/week to capture all energy expenditure in all activities (30).
***Hypertension: SBP ≥140 mmHg, DBP ≥90 mmHg, or receiving antihypertensive medication.
#Microalbuminuria and macroalbuminuria: UACR ≥30 mg/g but <300 mg/g and creatinine ratio ≥300 mg/g, respectively.
aP values: χ2 test (categorical variables) or ANOVA test (for continuous variables with normal distribution).
bP value for nonparametric median scores test.
Incidence Rates of Ischemic Stroke and CHD
During a median follow-up time of 17.7 years (interquartile range 15.2, 18.6 years), 789 participants (394 men, 385 women) experienced incident CHD (202 were fatal), and 158 participants (67 men, 89 women) experienced incident ischemic stroke (11 were fatal), with a total of 42,427 at-risk person-years for CHD and 42,456 at-risk person-years for ischemic stroke. Overall, the incidence rates of CHD and ischemic stroke were 18.6 (95% CI 17.6–19.3) and 3.7 (95% CI 3.1–4.3) per 1,000 person-years, respectively. Among participants with incident CHD, 59% (200 men, 266 women) had diabetes at baseline, and among those with incident stroke, 55% (29 men, 59 women) had diabetes. Women without diabetes had the lowest incidence rates of CHD and stroke (7.6 and 1.9 per 1,000 person-years, respectively). Men with diabetes had the highest incidence of CHD (39.1 per 1,000 person-years), and men and women with diabetes had similar rates of stroke.
TG-HDL Status and Risks of Incident CHD and Ischemic Stroke
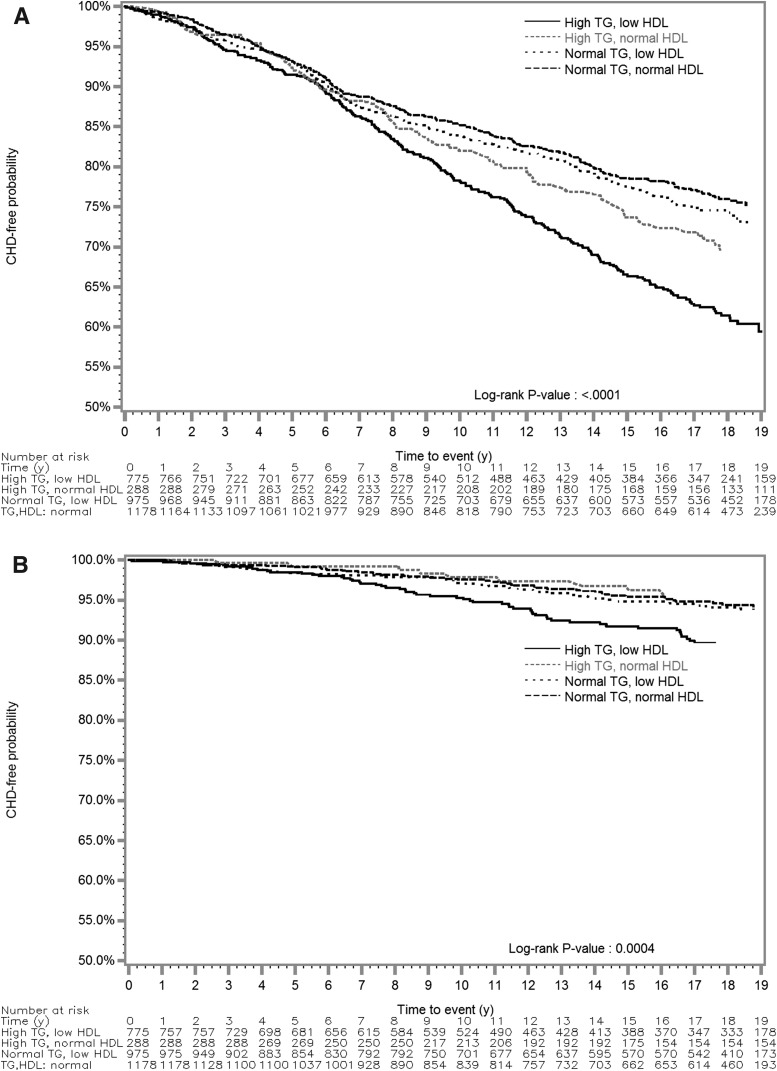
By TG-HDL status, participants with high TG and low HDL levels were least likely to be free of CHD or stroke during follow-up (Fig. 1). High TG and low HDL-C levels were each associated with incident CHD, but with not incident ischemic stroke, after adjustment for age, sex, BMI, smoking, LDL-C level, diabetes, use of antihypertensive medications, physical activity, UACR, and eGFR-MDRD (Table 2). Participants with high TG levels had a 19% greater hazard of CHD (adjusted HR 1.19; 95% CI 1.00–1.42) than those with normal TG levels. Participants with low HDL-C levels had a nearly significant 17% greater hazard of CHD (adjusted HR 1.17; 95% CI 0.99–1.35) than those with normal HDL-C levels. High TG level alone was associated with an adjusted HR estimate of 1.28 for stroke, compared with normal TG level, and a low HDL-C level was associated with an adjusted HR of 1.35 for stroke, compared with normal HDL-C, but their CIs also included unity. High TG and low HDL levels were associated with a 32% greater adjusted hazard of CHD (adjusted HR 1.32; 95% CI 1.06–1.64) compared with normal TG and normal HDL levels. Participants with high TG and low HDL levels had an adjusted HR estimate of 1.46 for incident ischemic stroke, but the CI included unity (95% CI 0.92–2.33).
Figure 1.
Kaplan-Meier time-to-event curves and log-rank tests for CHD (A) and ischemic stroke (B) by TG and HDL-C status during a median follow-up time of 17.7 years in the SHS. A fasting TG level of ≥150 mg/dL was “high.” An HDL-C level of <40 mg/dL (men) or <50 mg/dL (women) was “low.”
Table 2.
HR estimates of CHD and ischemic stroke associated with TG and HDL-C status in the SHS
| CHD |
Ischemic stroke |
|||||||
|---|---|---|---|---|---|---|---|---|
| Noncase | Case | Basic model1 | Full model2 | Noncase | Case | Basic model1 | Full model2 | |
| TG3 | ||||||||
| Normal TG | 1,695 (79) | 459 (21) | Reference | Reference | 2,063 (96) | 91 (4) | Reference | Reference |
| High TG | 734 (69) | 330 (31) | 1.57 (1.36–1.81) | 1.19 (1.00–1.42) | 997 (94) | 67 (6) | 1.64 (1.19–2.25) | 1.28 (0.84–1.94) |
| HDL-C3 | ||||||||
| Normal HDL-C | 1,154 (79) | 313 (21) | Reference | Reference | 1,410 (96) | 57 (4) | Reference | Reference |
| Low HDL-C | 1,275 (73) | 476 (27) | 1.44 (1.24–1.67) | 1.17 (0.99–1.39) | 1,649 (94) | 102 (6) | 1.69 (1.22–2.34) | 1.35 (0.89–2.06) |
| TG-HDL status | ||||||||
| Normal TG-HDL | 940 (80) | 238 (20) | Reference | Reference | 1,131 (96) | 47 (4) | Reference | Reference |
| Normal TG, low HDL | 754 (77) | 221 (23) | 1.27 (1.05–1.53) | 1.08 (0.87–1.33) | 931 (95) | 44 (5) | 1.26 (0.83–1.91) | 0.99 (0.62–1.58) |
| High TG, normal HDL | 213 (74) | 75 (26) | 1.39 (1.06–1.81) | 1.04 (0.76–1.41) | 279 (97) | 9 (3) | 0.85 (0.42–1.74) | 0.57 (0.24–1.38) |
| High TG, low HDL | 520 (67) | 255 (33) | 1.89 (1.58–2.27) | 1.32 (1.06–1.64) | 717 (93) | 58 (7) | 2.20 (1.50–3.25) | 1.46 (0.92–2.33) |
Data are reported as n (%) or HR (95% CI).
1Basic models stratified on SHS centers and included age and sex.
2Full models additionally included BMI, smoking (current, past, ever), estimated LDL-C level, diabetes (yes/no), antihypertensive medications (yes/no), categorical physical activity (MET-h/week in quartile), UACR (continuous), and eGFR-MDRD (continuous).
3High TG: fasting TG ≥150 mg/dL; low HDL-C: HDL-C <40 mg/dL (men) or <50 mg/dL (women).
Among 789 incident CHD cases, 587 were nonfatal. The HR estimates for incident nonfatal CHD were comparable to those for overall incident CHD. For example, participants with high TG and low HDL levels had a 47% increased adjusted hazard (adjusted HR 1.47; 95% CI 1.14–1.89). For the smaller subgroup with incident fatal CHD, the HR estimates appeared lower than those for nonfatal CHD; for example, participants with high TG and low HDL levels had a 10% increased adjusted hazard (adjusted HR 1.10; 95% CI 0.72–1.68). However, the 95% CIs were wide, contained unity, and overlapped with their counterparts for nonfatal CHD.
Diabetes status and possibly LDL-C level, but not sex, were observed to modify the relationships between TG-HDL status and the risks of incident CHD and incident stroke. In participants with diabetes, high TG alone was associated with an increased CHD risk (HR 1.47; 95% CI 1.08–2.00, per SD change [207 mg/dL] in TG), and increased HDL-C level alone was associated with reduced stroke risk (HR 0.72; 95% CI 0.53–0.97, per SD increase [12 mg/dL] in HDL-C) (Table 3). The relationship of TG-HDL status to the risks of incident CHD and stroke depended on diabetes status (P value for interaction = 0.003 and 0.060, respectively) (Table 3). High TG-low HDL was associated with a 1.54-fold greater hazard of incident CHD (HR 1.54; 95% CI 1.15–2.06) and a 2.13-fold greater hazard of incident stroke (HR 2.13; 95% CI 1.06–4.29) in participants with diabetes, but associations were not observed in participants without diabetes. Additional adjustment for A1C levels in the multivariable models did not substantially alter the hazard estimates for CHD risk (HR 1.41; 95% CI 1.04–1.90) but attenuated the hazards for stroke (HR 1.78; 95% CI 0.88–3.63). In participants without diabetes, the estimated associations between TG-HDL status and incident CHD and stroke did not substantially change after additional adjustment for HOMA-IR.
Table 3.
TG-HDL status in relation with CHD or ischemic stroke in participants without and with diabetes
| No diabetes |
Diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Noncase, n (%) | Case, n (%) | Full model1 |
Noncase, n (%) | Case, n (%) | Full model1 |
|||
| HR | (95% CI) | HR | (95% CI) | |||||
| CHD (P interaction = 0.003) | ||||||||
| TG (per SD increase)2 | 1.07 | (0.92–1.24) | 1.47 | (1.08–2.00) | ||||
| HDL-C (per SD increase)3 | 0.87 | (0.75–1.01) | 0.95 | (0.83–1.08) | ||||
| TG-HDL status | ||||||||
| Normal TG-HDL | 681 (85) | 122 (15) | 1 (reference) | 233 (69) | 103 (30) | 1 (reference) | ||
| Normal TG, low HDL | 435 (81) | 103 (19) | 1.26 | (0.94–1.69) | 306 (73) | 111 (27) | 0.92 | (0.67–1.26) |
| High TG, normal HDL | 138 (82) | 30 (18) | 1.14 | (0.74–1.76) | 71 (62) | 43 (38) | 0.99 | (0.64–1.54) |
| High TG, low HDL | 251 (83) | 50 (17) | 0.88 | (0.60–1.29) | 258 (57) | 192 (43) | 1.54 | (1.15–2.06) |
| Ischemic stroke (P interaction = 0.060) | ||||||||
| TG (per SD increase)2 | 1.15 | (0.82–1.62) | 1.43 | (0.74–2.73) | ||||
| HDL-C (per SD increase)3 | 0.93 | (0.69–1.26) | 0.72 | (0.53–0.97) | ||||
| TG-HDL status | ||||||||
| Normal TG-HDL | 773 (96) | 34 (4) | 1 (reference) | 326 (96) | 13 (4) | 1 (reference) | ||
| Normal TG, low HDL | 526 (98) | 13 (2) | 0.53 | (0.23–1.19) | 391 (93) | 31 (7) | 1.82 | (0.91–3.66) |
| High TG, normal HDL | 164 (98) | 4 (2) | 0.53 | (0.15–1.81) | 112 (97) | 4 (3) | 0.77 | (0.21–2.83) |
| High TG, low HDL | 287 (94) | 17 (6) | 1.16 | (0.55–2.45) | 417 (91) | 40 (9) | 2.13 | (1.06–4.29) |
High TG, fasting TG ≥150 mg/dL; low HDL-C, HDL-C <40 mg/dL (men) or <50 mg/dL (women).
1Full model stratified on SHS center and included age, sex, BMI, smoking (current, past, ever), estimated LDL-C level, antihypertensive medications (yes/no), categorical physical activity (MET-h/week in quartile), UACR (continuous), and eGFR (continuous).
2Per SD change in TG: 80 mg/dL in participants with no diabetes, 207 mg/dL participants with diabetes.
3Per SD change in HDL-C: 15 mg/dL in participants with no diabetes, 12 mg/dL in participants with diabetes.
The relationship of TG-HDL status and incident CHD risk may have depended on LDL-C level (P value for interaction = 0.064). High TG and low HDL levels were associated with an adjusted HR of 1.42 (95% CI 1.02–1.97), and normal TG and low HDL levels were associated with an HR of 1.48 (95% CI 1.07–2.05) for CHD in participants with LDL-C levels of ≥130 mg/dL, but no associations were observed in those with LDL-C <130 mg/dL. LDL-C level did not appear to modify TG-HDL status in relation to ischemic stroke (P value for interaction = 0.435). Sex was not observed to modify the relationships of TG-HDL status to incident CHD or stroke risks (P value for interaction = 0.827 for CHD, P value for interaction = 0.887 for stroke).
The correlation coefficient for eGFR-MDRD and eGFR-CKD-EPI was 0.898. Using eGFR-CKD-EPI instead of eGFR-MDRD in the full models did not change the HRs or 95% CIs for the overall population or by diabetes status.
Conclusions
As efforts to personalize strategies against CVD persist, this longitudinal prospective study observed that fasting high TG levels combined with low HDL-C levels in the context of diabetes status and LDL-C level might identify subpopulations at increased risk of incident CHD and/or ischemic stroke. Particularly in men and women with diabetes, fasting high TG levels combined with low HDL-C levels was associated with increased risks of incident CHD and ischemic stroke. In particular, adults with fasting LDL-C levels of ≥130 mg/dL who have high TG levels combined with low HDL-C levels may also have an increased CHD risk. However, the degree of IR among adults without diabetes and the degree of glycemic control among adults with diabetes did not appear to modify the relationship of TG-HDL status and incident CHD or ischemic stroke.
Diabetes was present in >40% of our baseline cohort. Previous large prospective studies of high TG-low HDL dyslipidemia and CVD risk included few, if any, participants with diabetes, and some did not differentiate CHD from ischemic stroke (9,10). The current study observed that high TG and low HDL levels are related to a multivariable-adjusted estimated 54% increased risk of incident CHD in adults with diabetes, regardless of A1C level, and this relationship was not observed in adults without diabetes. In an analysis of electronic medical records, a diabetic population with an LDL-C concentration of <100 mg/dL and high TG and low HDL levels had an estimated 35–62% greater risk of incident CHD during the 10-year follow-up compared with those with normal TG and HDL-C levels (18). An isolated low HDL-C or high TG level was each associated with CHD risk in men, but not in women, with diabetes (18). However, lifestyle factors such as physical activity were not well controlled in that study.
The current study observed that high TG and low HDL levels are related to an increased risk, particularly in adults with diabetes or a higher LDL-C level. Reducing the TG level and increasing the HDL-C level by using fenofibrate or niacin failed to demonstrate improved cardiovascular outcomes in patients with diabetes (ACCORD trial) (15) or established CVD (AIM-HIGH trial) (37), who also had LDL-C levels at goal. However, fenofibrate reduced CVD risk in the subpopulation of participants with diabetes who had high TG and low HDL-C levels at baseline in the ACCORD trial (15).
Insulin plays an important role in lipid metabolism, and IR, together with hyperglycemia, may lead to dyslipidemia associated with ASCVD in type 2 diabetes (11–14). Increased levels of TG with decreased levels of HDL-C is common in diabetes and likely due to the overproduction of TG-rich, large VLDL particles and reduced hepatic clearance for TG (12,13). Large VLDL particles promote cholesteryl ester transfer protein activity, attracts cholesteryl ester from LDL-C and HDL-C in exchange for TG, and enhances HDL-C catabolism by hepatic lipase. Subsequently, HDL-C levels decrease, TG content in LDL-C and HDL-C increases, and TG-rich atherogenic small, dense LDL particles are produced in adults with diabetes (14).
This study has several strengths. The SHS provided an excellent large, prospective cohort of both men and women with a high prevalence of diabetes. Diabetes status and also measures of glycemic dysregulation were available. Incident ischemic stroke (and other stoke subtypes) and CHD were adjudicated during a median follow-up time of 17.7 years. The lipid profile and other covariates were obtained at baseline in participants with no history of CVD. The temporality ensures that measurements of lipids and risk factors preceded the CHD and stroke events. There are several limitations. First, these results should be confirmed in other ethnic populations since the analysis is restricted to American Indians, who have the highest prevalence of diabetes among all ethnic groups in the U.S. Also, the present findings are not necessarily generalizable to populations at lower risk based on a lower prevalence of smoking, a higher prevalence of treated hypertension, or lower LDL-C levels. The generalizability of the findings to other populations warrants caution and separate evaluation. However, the diabetes and obesity epidemics have been rising in the U.S. population during the time course of the SHS (38,39). The SHS population provides valuable information for high-risk populations with similar cardiovascular characteristics. No evidence has suggested that the pathogenesis of diabetes or CVD in American Indians is different qualitatively from that in other ethnic groups. Second, because fewer cases of stroke were observed, analyses for stroke risk may be statistically unstable. The sample size also constrained the potential sex-specific analyses by diabetes status (and by LDL-C status). Third, TG levels were obtained during a fasting state. TG levels typically become elevated after a meal, and nonfasting TG has been associated with increased CVD risk (40). Most TG and HDL-C measures are obtained in a fasting state in the clinical setting; thus, interpretation of the study results could be clinically relevant. Fourth, multiple comparisons, although conducted with a priori hypotheses, could have increased the probability of a chance significant finding. Fifth, interpretation of the results of subgroup analyses is limited because of smaller numbers of actual cases in the subgroups.
This prospective cohort study observed that the relationship between TG-HDL status and risk of CVD depends on diabetes status. A high fasting TG level in combination with a low HDL-C level was associated with increased risks of incident CHD and ischemic stroke, particularly in those with diabetes or with an LDL-C level of ≥130 mg/dL, independent of other ASCVD risk factors. Recognition of these patterns may help to enhance lipid-targeted strategies to prevent CHD and ischemic stroke.
Article Information
Acknowledgments. The authors thank all the participants in the SHS.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the Indian Health Service.
Funding. This manuscript was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant R01-HL-107899 (J.S.L.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.S.L. conceptualized and designed the research for and wrote this manuscript and contributed to data analysis. P.-Y.C. conducted the data analysis and contributed to the writing of the manuscript. Y.Z. contributed to the data and reviewed and edited the methods and manuscript. J.R.K., L.G.B., and B.V.H. reviewed and edited the manuscript and contributed to the discussion. J.S.L. and P.-Y.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Di Angelantonio E, Sarwar N, Perry P, et al.; Emerging Risk Factors Collaboration . Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanne D, Koren-Morag N, Graff E, Goldbourt U. Blood lipids and first-ever ischemic stroke/transient ischemic attack in the Bezafibrate Infarction Prevention (BIP) Registry: high triglycerides constitute an independent risk factor. Circulation 2001;104:2892–2897 [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MJ, Xavier D, Liu L, et al.; INTERSTROKE investigators . Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–123 [DOI] [PubMed] [Google Scholar]
- 4.Carey VJ, Bishop L, Laranjo N, Harshfield BJ, Kwiat C, Sacks FM. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am J Cardiol 2010;106:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005;46:1225–1228 [DOI] [PubMed] [Google Scholar]
- 6.Leiter LA, Lundman P, da Silva PM, Drexel H, Junger C, Gitt AK; DYSIS investigators . Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med 2011;28:1343–1351 [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med 2001;161:361–366 [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Maksoud MF, Eckel RH, Hamman RF, Hokanson JE. Risk of coronary heart disease is associated with triglycerides and high-density lipoprotein cholesterol in women and non-high-density lipoprotein cholesterol in men. J Clin Lipidol 2012;6:374–381 [DOI] [PubMed] [Google Scholar]
- 9.Rana JS, Visser ME, Arsenault BJ, et al. Metabolic dyslipidemia and risk of future coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Int J Cardiol 2010;143:399–404 [DOI] [PubMed] [Google Scholar]
- 10.Andersson C, Lyass A, Vasan RS, Massaro JM, D'Agostino RB Sr., Robins SJ. Long-term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham Heart Study. Am Heart J 2014;168:878–883.e1 [DOI] [PubMed] [Google Scholar]
- 11.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2012;32:2104–2112 [DOI] [PubMed] [Google Scholar]
- 12.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 2015;58:886–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soran H, Schofield JD, Adam S, Durrington PN. Diabetic dyslipidaemia. Curr Opin Lipidol 2016;27:313–322 [DOI] [PubMed] [Google Scholar]
- 14.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–1236 [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg HN, Elam MB, Lovato LC, et al.; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 18.Rana JS, Liu JY, Moffet HH, et al. Metabolic dyslipidemia and risk of coronary heart disease in 28,318 adults with diabetes mellitus and low-density lipoprotein cholesterol <100 mg/dl. Am J Cardiol 2015;116:1700–1704 [DOI] [PubMed] [Google Scholar]
- 19.Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation 1999;99:2389–2395 [DOI] [PubMed] [Google Scholar]
- 20.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 1990;132:1141–1155 [DOI] [PubMed] [Google Scholar]
- 21.Howard BV, Welty TK, Fabsitz RR, et al. Risk factors for coronary heart disease in diabetic and nondiabetic Native Americans. The Strong Heart Study. Diabetes 1992;41(Suppl. 2):4–11 [DOI] [PubMed] [Google Scholar]
- 22.Robbins DC, Welty TK, Wang WY, Lee ET, Howard BV. Plasma lipids and lipoprotein concentrations among American Indians: comparison with the US population. Curr Opin Lipidol 1996;7:188–195 [DOI] [PubMed] [Google Scholar]
- 23.Department of Health, Education, and Welfare. Lipid Research Clinics Program: Manual of Laboratory Operations. Washington, DC, U.S. Govt. Printing Office, 1994 (DHEW publ. no. 75-628)
- 24.Morgan CR, Lazarow A. Immunoassay of insulin using a two-antibody system. Proc Soc Exp Biol Med 1962;110:29–32 [DOI] [PubMed] [Google Scholar]
- 25.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol 1957;17:237–246 [in German] [DOI] [PubMed] [Google Scholar]
- 26.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 1960;30:207–212 [PubMed] [Google Scholar]
- 27.Xu J, Lee ET, Peterson LE, et al. Differences in risk factors for coronary heart disease among diabetic and nondiabetic individuals from a population with high rates of diabetes: the Strong Heart Study. J Clin Endocrinol Metab 2012;97:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shara NM, Wang H, Mete M, et al. Estimated GFR and incident cardiovascular disease events in American Indians: the Strong Heart Study. Am J Kidney Dis 2012;60:795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fretts AM, Howard BV, Kriska AM, et al. Physical activity and incident diabetes in American Indians: the Strong Heart Study. Am J Epidemiol 2009;170:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 1990;13:401–411 [DOI] [PubMed] [Google Scholar]
- 32.Lee ET, Cowan LD, Welty TK, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45-74 years, 1984-1988. The Strong Heart Study. Am J Epidemiol 1998;147:995–1008 [DOI] [PubMed] [Google Scholar]
- 33.Karas MG, Devereux RB, Wiebers DO, et al. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the Strong Heart Study. Stroke 2012;43:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 35.Stone NJ, Robinson JG, Lichtenstein AH, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl. 2):S1–S45 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Galloway JM, Welty TK, et al. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation 2008;118:1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boden WE, Probstfield JL, Anderson T, et al.; AIM-HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267 [DOI] [PubMed] [Google Scholar]
- 38.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 39.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195–1200 [DOI] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308 [DOI] [PubMed] [Google Scholar]