Abstract
OBJECTIVE
The pancreas in type 1 diabetes exhibits decreased size (weight/volume) and abnormal exocrine morphology. Serum trypsinogen levels are an established marker of pancreatic exocrine function. As such, we hypothesized that trypsinogen levels may be reduced in patients with pre–type 1 diabetes and type 1 diabetes compared with healthy control subjects.
RESEARCH DESIGN AND METHODS
Serum trypsinogen levels were determined in 100 persons with type 1 diabetes (72 new-onset, 28 established), 99 autoantibody-positive (AAb+) subjects at varying levels of risk for developing this disease, 87 AAb-negative (AAb−) control subjects, 91 AAb− relatives with type 1 diabetes, and 18 patients with type 2 diabetes.
RESULTS
Trypsinogen levels increased significantly with age in control subjects (r = 0.71; P < 0.0001) and were significantly lower in patients with new-onset (mean ± SD 14.5 ± 6.1 ng/mL; P < 0.0001) and established type 1 diabetes (16.7 ± 6.9 ng/mL; P < 0.05) versus AAb− control subjects (25.3 ± 11.2 ng/mL), AAb− relatives (29.3 ± 15.0 ng/mL), AAb+ subjects (26.5 ± 12.1 ng/mL), and patients with type 2 diabetes (31.5 ± 17.3 ng/mL). Multivariate analysis revealed reduced trypsinogen in multiple-AAb+ subjects (P < 0.05) and patients with type 1 diabetes (P < 0.0001) compared with AAb− subjects (control subjects and relatives combined) and single-AAb+ (P < 0.01) subjects when considering age and BMI.
CONCLUSIONS
These findings further support the interplay between pancreatic endocrine and exocrine dysfunction. Longitudinal studies are warranted to validate trypsinogen as a predictive biomarker of type 1 diabetes progression.
Introduction
An exhaustive body of literature supports the notion that type 1 diabetes represents a T-cell–mediated autoimmune disease that involves the specific destruction of insulin-producing pancreatic β-cells (1). Indeed, an individual’s risk for developing this disorder often is determined through testing for type 1 diabetes–associated autoantibodies (AAbs) against β-cell antigens (e.g., GAD antibody [GADA], insulin, IA-2 antigen [IA-2A], zinc transporter 8 antibody [ZnT8A]). Aside from the contribution of genetic factors to type 1 diabetes, the appearance and number of AAbs cumulatively modify the risk for this disease. Individuals without diabetes having no such AAbs are at extremely low risk for type 1 diabetes development, those with one AAb have a low risk, and those with an increasing risk have two or more such biomarkers (2,3). Taken together with other pathogenic investigations of type 1 diabetes, these findings have led to a predominance of research attention toward the endocrine pancreas in general and the pancreatic β-cell in particular.
However, studies of living subjects or tissues obtained from organ donors with type 1 diabetes have revealed that in addition to the autoimmune-mediated β-cell destruction that classically defines the disease, the pancreas in type 1 diabetes is characterized by decreased pancreatic weight and volume, increased immune infiltrates within the exocrine pancreas, and exocrine atrophy (4–7). Some of these observations also have been made in AAb-positive (AAb+) individuals without type 1 diabetes, suggesting that exocrine pancreas abnormalities precede disease onset (8).
With respect to tests diagnostic for exocrine pancreatic function, serum levels of trypsinogen, also known as immunoreactive trypsinogen, provide one well-accepted clinical biomarker (9). Indeed, testing of serum trypsinogen levels is used in a variety of pediatric settings, including newborn screens for cystic fibrosis and meconium ileus and in infants and older children with symptoms that suggest cystic fibrosis (10). In terms of its biology and function, trypsinogen represents the inactive precursor of the digestive enzyme trypsin that becomes active once cleaved by enteropeptidases produced in the intestinal mucosa (11). Pancreatitis has been attributed to aberrant activation of trypsinogen within the acinar cell or pancreatic ducts (12). Hence, in adults, trypsinogen levels often are used as an indicator of pancreatitis, although amylase and lipase are now preferred as pancreatitis biomarkers (13,14). Nonetheless, serum trypsinogen remains a reliable clinical tool in measuring pancreatic exocrine function across all ages.
Decades ago (i.e., in the 1970s), serum levels of trypsinogen were noted as reduced in patients with type 1 diabetes (15–18); however, with the absence of overtly symptomatic exocrine disease in most patients with type 1 diabetes, interest in their use as a diagnostic biomarker failed to develop. Further contributing to this situation was the aforementioned emergence of type 1 diabetes–associated AAbs, factors that have proven to be of immense value to both the diagnosis of type 1 diabetes and their use as a predictive biomarker of disease (19). Our recent findings regarding reduced pancreatic weight and exocrine atrophy at the onset of type 1 diabetes (7) prompted us to address the novel question of whether evidence of altered exocrine function (i.e., reduced serum trypsinogen levels) before hyperglycemic onset would be present. Specifically, we examined this well-validated biomarker in a series of individuals with or at varying levels of risk for type 1 diabetes to quantitate the levels for this marker of exocrine function throughout the natural history of the disease.
Research Design and Methods
Subject Enrollment
Study subjects were recruited from the outpatient clinics at the University of Florida (Gainesville, FL), Nemours Children’s Hospital (Orlando, FL), and Emory University (Atlanta, GA) for studies evaluating the natural history of diabetes. Informed consent was obtained from each subject before study enrollment, and all procedures were conducted in accordance with the Declaration of Helsinki and approved by the institutional review boards at each institution. Random serum samples were collected from nonfasting subjects and stored at −20°C. Samples were selected from 395 of the enrolled subjects, including 87 AAb-negative (AAb−) healthy control subjects (age range 2.6–40.7 years); 91 AAb− first-degree relatives (age range 4.1–46.6 years); 99 AAb+ subjects (age range 2.8–51.3 years); 72 patients with new-onset type 1 diabetes (duration <3 months; mean duration 1.08 months; age range 4.3–31.8 years); 28 subjects with established type 1 diabetes (duration >3 months; mean duration 8.89 years; age range 4.0–45.9 years); and 18 patients with type 2 diabetes (age range 13.8–80.1 years). Additional demographic data are listed in Table 1. AAb+ subjects included 65 with one AAb, 22 with two AAbs, and 12 with three AAbs (i.e., GADA, IA-2A, ZnT8A). Diabetes was diagnosed according to American Diabetes Association criteria (20).
Table 1.
Subject demographics
| Characteristic | AAb− control | AAb− relative | Single AAb+ | Two AAb+ | Three AAb+ | New-onset T1D | Established T1D | T2D |
|---|---|---|---|---|---|---|---|---|
| Total number of subjects | 87 | 91 | 65 | 21 | 13 | 72 | 28 | 18 |
| Sex | ||||||||
| Female | 41 (47.1) | 41 (45.1) | 42* (65.6) | 6 (28.6) | 7 (53.8) | 33 (45.8) | 19 (67.9) | 9 (50.0) |
| Male | 46 (52.9) | 50 (54.9) | 22* (34.4) | 15 (71.4) | 6 (46.2) | 39 (54.2) | 9 (32.1) | 9 (50.0) |
| Ethnicity | ||||||||
| Caucasian | 59 (67.8) | 54 (59.3) | 41 (63.1) | 14 (66.7) | 9 (69.2) | 51 (70.8) | 20 (71.4) | 7 (38.9) |
| Black/African American | 10 (11.5) | 19 (20.9) | 9 (13.8) | 4 (19.0) | 1 (7.7) | 9 (12.5) | 2 (7.1) | 2 (11.1) |
| Hispanic/Latino | 7 (8.0) | 16 (17.6) | 10 (15.4) | 2 (9.5) | 3 (23.1) | 9 (12.5) | 4 (14.3) | 8 (44.4) |
| Other/unknown | 11 (12.6) | 2 (2.2) | 5 (7.7) | 1 (4.8) | 0 (0.0) | 3 (4.2) | 2 (7.1) | 1 (5.6) |
| Age (years) | 21.6 ± 10.3 | 22.4 ± 12.1 | 27.0 ± 13.8 | 22.6 ± 11.4 | 18.8 ± 12.7 | 12.4 ± 4.5 | 20.1 ± 11.0 | 48.3 ± 16.3 |
| Weight (kg)† | 60.9 ± 22.9 | 63.9 ± 29.9 | 70.0 ± 30.1 | 57.1 ± 20.3 | 61.6 ± 24.2 | 49.3 ± 20.9 | 58.7 ± 17.9 | 95.3 ± 24.2 |
| Height (m)† | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.5 | 1.6 ± 0.2 | 1.6 ± 0.1 |
| BMI (kg/m2)† | 22.1 ± 5.0 | 24.6 ± 7.0 | 26.9 ± 8.2 | 20.7 ± 3.8 | 24.4 ± 5.6 | 20.8 ± 6.0 | 23.8 ± 4.0 | 35.9 ± 10.0 |
| Diabetes duration (years) | NA | NA | NA | NA | NA | 0.1 ± 0.1 | 8.9 ± 11.0 | 4.8 ± 5.8 |
Data are n (%) or mean ± SD unless otherwise indicated. Information is presented for AAb− control subjects, AAb− relatives, patients with new-onset T1D, patients with established T1D, AAb+ subjects without diabetes, and patients with T2D. NA, not applicable; T1D, type 1 diabetes; T2D, type 2 diabetes.
*For one single-AAb+ subject, biological sex was not known.
†Provision of height and weight information was voluntary; therefore, these data are not available for all study subjects.
Trypsinogen Measurement
Quantitative determination of serum immunoreactive trypsinogen was measured under blinded conditions in duplicate by radioimmunoassay (DiaSorin, Stillwater, MN) at the ARUP Laboratories (Salt Lake City, UT) as previously described (21). The reported laboratory reference range for serum trypsinogen in healthy individuals is 10.0–57.0 ng/mL (http://ltd.aruplab.com/Tests/Pub/2014025). Of note, this reference range is not notated with respect to subject age.
AAb Measurement
Serum levels of GADA, IA-2A, and ZnT8A were determined with ELISA kits (Kronos Incorporated, Star, ID) according to the manufacturer’s instructions (22).
Statistical Methods
Single-variable statistical analyses were performed with SPSS version 16.0 software (IBM Corporation, Chicago, IL), and results were plotted by using GraphPad Prism version 6.0 software (GraphPad, La Jolla, CA). Data are presented as mean ± SD. One-way ANOVA with post hoc Tukey analysis was used for comparisons across multiple groups. Nonlinear regression with Spearman correlation was performed for trypsinogen level versus age. Statistical tests were two-sided. Multivariate analyses were performed with R-3.3.1 software. Individuals were classified into groups on the basis of detectable serum AAbs or time after diagnosis of type 1 diabetes. By using a forward variable selection method, a multinomial logistic regression model was built to assess whether age, BMI, sex, and serum trypsinogen concentrations (log transformed) were associated with disease status. Because height and weight were self-reported, modeling first used the complete data (nonmissing). Imputation with the median of each group was used to incorporate all data for modeling. P < 0.05 was considered significant.
Results
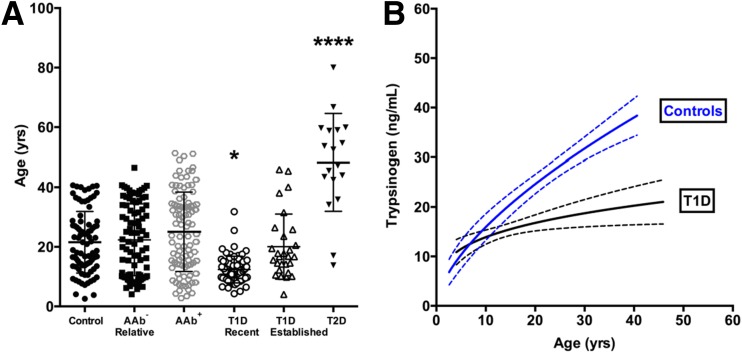
We compared serum levels of trypsinogen, a biomarker of pancreas exocrine capacity, across subjects with or at varying levels of risk for type 1 diabetes as well as for patients with type 2 diabetes and control subjects. Overall, for the majority of subjects, trypsinogen levels fell within the laboratory-reported reference range (10.0–57.0 ng/mL). Of note, the proportion of subjects with trypsinogen levels below the reference range (<10.0 ng/mL) was 25.0% (18 of 72 with new-onset type 1 diabetes), 17.9% (5 of 28 with established type 1 diabetes), 3.0% (3 of 99 AAb+), 2.2% (2 of 91 AAb− relatives), and 5.6% (1 of 18 with type 2 diabetes). All control subjects were within the reference range. With respect to age matching, as expected because of disease presentation, patients with type 2 diabetes were significantly older than all other groups examined (P < 0.0001); similarly, patients with new-onset type 1 diabetes were significantly younger than all other groups (P < 0.05) (Fig. 1A).
Figure 1.
Age-related effects on serum trypsinogen levels in control subjects and patients with type 1 diabetes (T1D). A: Patients with new-onset T1D were significantly younger than AAb− control subjects, AAb− relatives, AAb+ subjects, patients with established T1D, and patients with type 2 diabetes (T2D), whereas patients with T2D were significantly older than all other groups investigated. Data are mean ± SD, one-way ANOVA. *P < 0.05; ****P < 0.0001. B: Nonlinear least squares regression analysis demonstrates increasing serum trypsinogen levels with age for control subjects (P < 0.0001) and patients with T1D (P < 0.05, Spearman correlation). Best-fit curves were significantly different for control subjects vs. patients with T1D (P < 0.0001, extra sum-of-squares F test). Data are plotted as mean (solid lines) with 95% CI (dashed lines).
Among the 99 AAb+ subjects, 65 were single AAb+ (54 GADA, 4 IA-2A, 7 ZnT8A), 21 were double AAb+ (7 GADA and IA-2A, 12 GADA and ZnT8A, 2 IA-2A and ZnT8A), and 13 were triple AAb+. We found that age was associated with serum trypsinogen levels in AAb− control subjects (r = 0.71, P < 0.0001) and patients with type 1 diabetes (r = 0.04, P < 0.05), but for the two groups, the trajectories of trypsinogen over time were significantly different (P < 0.0001) (Fig. 1B). We conducted a multinomial logistic regression analysis controlling for age and BMI. Trypsinogen concentrations were right skewed, so a log transformation was used when modeling the data. Sex was not significantly associated with differences in trypsinogen concentration (data not shown; P > 0.05 for all) and thus was not included in the model. Observed associations between type 1 diabetes and log trypsinogen concentration (relative risk ratio [RRR] 0.10 [95% CI 0.04, 0.24]) are shown in Table 2. Significant associations were also observed for log trypsinogen levels and multiple AAb+ (RRR 0.29 [95% CI 0.09, 0.88]), but not for single AAb+ (RRR 1.98 [95% CI 0.76, 5.14]) (Table 2). These significant associations remained valid when single-AAb+ or multiple-AAb+ subjects were compared as the reference group (Table 2). A significant interaction between log trypsinogen concentration and BMI was not observed (Table 2). However, this finding needs to be confirmed given that self-reporting of height and weight was voluntary, and data were not available for all subjects.
Table 2.
Multivariable multinomial logistic regression model investigating factors associated with AAb status and type 1 diabetes
| Single AAb+ |
Multiple AAb+ |
Type 1 diabetes |
||||
|---|---|---|---|---|---|---|
| Reference group and characteristic | RRR (95% CI) | P value | RRR (95% CI) | P value | RRR (95% CI) | P value |
| AAb− | ||||||
| Log trypsinogen level (ng/mL) | 1.98 (0.76, 5.14) | 0.16 | 0.29 (0.09, 0.88) | <0.05 | 0.10 (0.04, 0.24) | <0.0001 |
| Age (years) | 0.99 (0.94, 1.04) | 0.73 | 1.02 (0.96, 1.08) | 0.56 | 0.92 (0.88, 0.98) | <0.01 |
| BMI (kg/m2) | 1.06 (0.99, 1.14) | 0.08 | 0.98 (0.88, 1.08) | 0.62 | 1.05 (0.98, 1.13) | 0.17 |
| Single AAb+ | ||||||
| Log trypsinogen level (ng/mL) | 0.08 (0.02, 0.40) | <0.01 | 0.02 (0.005, 0.11) | <0.0001 | ||
| Age (years) | 0.99 (0.93, 1.07) | 0.87 | 0.89 (0.83, 0.96) | <0.01 | ||
| BMI (kg/m2) | 0.90 (0.80, 1.02) | 0.11 | 0.97 (0.87, 1.08) | 0.60 | ||
| Multiple AAb+ | ||||||
| Log trypsinogen level (ng/mL) | 0.20 (0.05, 0.79) | <0.05 | ||||
| Age (years) | 0.89 (0.83, 0.97) | <0.01 | ||||
| BMI (kg/m2) | 1.07 (0.96, 1.20) | 0.22 | ||||
Data are presented for single-AAb+ subjects without diabetes, multiple-AAb+ subjects without diabetes, and patients with type 1 diabetes (new-onset and established combined) compared with the reference group. AAb− subjects are control and relatives combined.
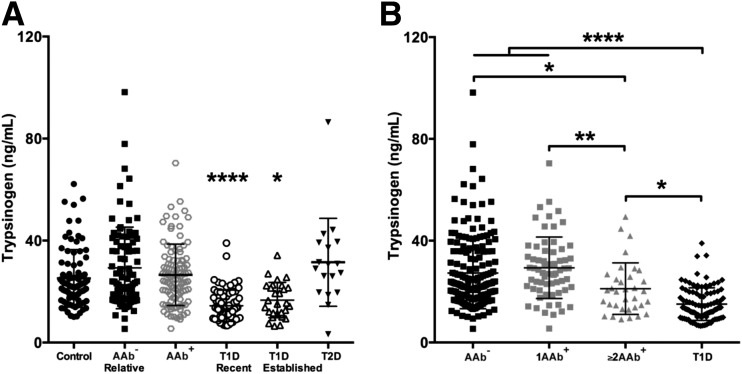
Although not age matched (for obvious reasons) to subjects with type 1 diabetes, we determined trypsinogen levels in patients with type 2 diabetes as an additional control for potential effects of hyperglycemia. Again, serum trypsinogen concentrations were significantly lower in patients with new-onset (mean ± SD 14.5 ± 6.1 ng/mL; P < 0.0001) and established type 1 diabetes (16.7 ± 6.9 ng/mL; P < 0.05) than in healthy control subjects (25.3 ± 11.2 ng/mL), AAb− relatives (29.3 ± 15.0 ng/mL), AAb+ subjects (26.5 ± 12.1 ng/mL), and patients with type 2 diabetes (31.5 ± 17.3 ng/mL) (Fig. 2A).
Figure 2.
Serum trypsinogen concentrations are significantly reduced in patients with type 1 diabetes (T1D) and subjects with multiple T1D-related AAbs. A: Serum trypsinogen is significantly reduced in patients with new-onset and established T1D compared with AAb− control subjects, AAb− relatives, AAb+ (single [1AAb+] vs. multiple [≥2AAb+]) subjects without diabetes, and patients with type 2 diabetes (T2D). B: When AAb+ subjects were stratified by the number of AAbs present, trypsinogen was significantly reduced in T1D (new-onset and established combined) as well as ≥2AAb+ vs. AAb− subjects (control subjects and relatives combined) and 1AAb+ subjects. Trypsinogen levels were lower in patients with T1D vs. ≥2AAb+ subjects. Data are mean ± SD, one-way ANOVA. *P < 0.05; **P < 0.01; ****P < 0.0001.
We also sought to understand trypsinogen levels as a function of degree of β-cell autoimmunity. When AAb+ subjects were stratified according to the number of AAbs present, both multiple-AAb+ subjects (mean ± SD = 21.15 ± 10.1 ng/mL) and patients with type 1 diabetes (15.1 ± 6.4 ng/mL [new-onset and established combined]) had significantly lower trypsinogen levels than single-AAb+ subjects (29.3 ± 12.2 ng/mL; type 1 diabetes vs. single AAb+, P < 0.0001; multiple AAb+ vs. single AAb+, P < 0.01) and AAb− subjects (type 1 diabetes vs. AAb−, P < 0.0001; AAb+ vs. AAb−, P < 0.05) (Fig. 2B). Single-AAb+ subjects and AAb− subjects were comparable (P = 0.62), whereas serum trypsinogen levels were significantly higher in multiple-AAb+ subjects compared with patients with type 1 diabetes (P < 0.05) (Fig. 2B).
Conclusions
Considering the physiological interaction between the exocrine and endocrine pancreas, that primary disorders of the exocrine pancreas also affect endocrine tissues (e.g., cystic fibrosis–related diabetes) and vice versa is not surprising. Accordingly, previous studies have suggested that pancreatic exocrine insufficiency can be observed in patients with type 1 diabetes, including reduced fecal elastase-1 concentrations in both patients with new-onset type 1 diabetes and those with established type 1 diabetes as well as trypsinogen levels in the latter group (4,18,23). In this study, we examined serum trypsinogen concentration in subjects considered to be at various stages of type 1 diabetes progression—from pre–type 1 diabetes (AAb+ subjects) to new-onset and established type 1 diabetes—to explore the potential role of pancreatic exocrine insufficiency over the natural history of the disease (3). Our observation that single-AAb+ subjects are comparable with control subjects suggests that the establishment of autoimmunity may precede exocrine pancreatic dysfunction in pre–type 1 diabetes. However, the significantly lower trypsinogen levels in both multiple-AAb+ subjects and patients with type 1 diabetes compared with control and single-AAb+ subjects suggest that these reductions might precede the symptomatic onset of type 1 diabetes. Our observation that multiple-AAb+ subjects exhibit higher trypsinogen levels than do patients with type 1 diabetes suggests a somewhat intermediate phenotype in the late prediabetes phase. Indeed, to further discern these effects, longitudinal studies that perhaps include the measurement of insulin AAb and islet cell AAb along with the three AAbs tested here are needed. A potential limitation of this study lies in the available sample size. AAb+ individuals without type 1 diabetes are rare among the general population, and blood samples donated by these persons considered to be in the prediabetes phase of the disease are considered precious for studies of type 1 diabetes pathogenesis and biomarker identification. Thus, we included all double- and triple-AAb+ samples available from our serum bank. These samples were collected and stored over the past 7 years from donors throughout the U.S., which speaks further to the paucity of these AAb+ individuals and underscores the importance of investigating this and other potential biomarkers in these rare subjects.
The influence of age on serum trypsinogen levels in healthy individuals conflicts within the literature, with some studies suggesting an increase with age, whereas others did not (24,25). Hence, the influence of age in previous studies may not have been adequately addressed but has been studied here. Beyond the potential influence of age on serum trypsinogen levels, additional factors such as mealtime effects or potential variation in trypsinogen clearance from the bloodstream may be of importance to future investigations of trypsinogen levels.
The loss of exocrine cells in type 1 diabetes has been attributed to multiple factors, including insulin deficiency leading to decreased trophic action on acinar cells; inflammatory or microvascular injury to exocrine tissue; and hyperglycemia, which could inhibit exocrine secretion (26). These factors are believed to also explain the decreased pancreas weight and volume in patients with long-standing type 1 diabetes. Moreover, recent efforts have revealed reduced pancreas weight in patients with new-onset type 1 diabetes as potential evidence of exocrine pancreas damage even before onset of the disease (7), which agrees with the current findings. To this end and consistent with said notion, preliminary studies have suggested that a direct linear relationship exists between pancreatic volume, as determined through MRI, and serum trypsinogen levels (M.C.-T., M.J.H., unpublished observations) in control and AAb+ subjects.
Elevated serum trypsinogen concentrations in patients with type 2 versus type 1 diabetes suggest that this biomarker might prove useful as a clinical test to help with diagnosis, particularly among older patients, yet additional type 2 diabetes samples from a broader age range are needed to demonstrate this definitively. The results of the current study support a view that exocrine secretion of trypsinogen fails to increase proportionally with age before type 1 diabetes onset, as shown in high-risk multiple-AAb+ subjects, and seems unchanged after type 1 diabetes diagnosis. When combined with other emerging evidence in pathological exocrine abnormalities (7,27–29), the current findings support the notion that pancreatic exocrine function may be diminished late in the natural history of pre–type 1 diabetes. The clinical significance of this reduction remains unknown, and given their preliminary nature, these data should be considered to be hypothesis generating. Of course, trypsinogen levels alone are not likely a specific indicator for type 1 diabetes but may be useful in identifying pancreatic damage in combination with classical markers of autoimmunity. Additional investigations, including longitudinal studies, are warranted to understand the natural history of serum trypsinogen in patients with type 1 diabetes and to validate the utility of serum trypsinogen as a biomarker of type 1 diabetes progression.
Article Information
Acknowledgments. The authors thank the members of the University of Florida Health Shands Hospital Core Laboratory for administrative assistance. They also thank Suwa Xu (University of Florida) for assisting with data analysis and Theresa Sumrall, Joshua Peterson, Ana Gabriela Rocha, and Leeana Peters (University of Florida) for technical assistance.
Funding. This project was supported by funding from the National Institutes of Health (AI-42288), National Institute of Diabetes and Digestive and Kidney Diseases (1DP-3DK-101120-01 to M.C.-T., D.S., M.J.H., and M.A.A.), JDRF (Career Development Award 2-2012-280 to T.M.B.), the American Diabetes Association, and the Jeffrey Keene Family Professorship.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.L. researched the data and wrote the manuscript. M.C.-T. conceived of the study, analyzed the data, and reviewed and edited the manuscript. C.H.W. contributed to the discussion and reviewed and edited the manuscript. K.M. researched the data and reviewed and edited the manuscript. A.P. contributed to the discussion and wrote the manuscript. A.R.S., J.S., and F.L. analyzed the data and reviewed and edited the manuscript. T.M.B. and A.M. contributed to the discussion and reviewed and edited the manuscript. D.S. contributed to the discussion, analyzed the data, and reviewed/edited the manuscript. M.J.H. conceived of the study and reviewed and edited the manuscript. M.A.A. conceived of the study and wrote the manuscript. M.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larger E, Philippe MF, Barbot-Trystram L, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med 2012;29:1047–1054 [DOI] [PubMed] [Google Scholar]
- 5.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 9.Moore DJ, Forstner GG, Largman C, Cleghorn GJ, Wong SS, Durie PR. Serum immunoreactive cationic trypsinogen: a useful indicator of severe exocrine dysfunction in the paediatric patient without cystic fibrosis. Gut 1986;27:1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zybert K, Mierzejewska E, Sands D. Clinical status and somatic development of patients with or without meconium ileus diagnosed through neonatal screening for cystic fibrosis. Dev Period Med 2015;19:41–49 [PubMed] [Google Scholar]
- 11.Kitamoto Y, Yuan X, Wu Q, McCourt DW, Sadler JE. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proc Natl Acad Sci U S A 1994;91:7588–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer J, Rau B, Schoenberg MH, Beger HG. Mechanism and role of trypsinogen activation in acute pancreatitis. Hepatogastroenterology 1999;46:2757–2763 [PubMed] [Google Scholar]
- 13.Kemppainen E, Hietaranta A, Puolakkainen P, Hedström J, Haapiainen R, Stenman UH. Time course profile of serum trypsinogen-2 and trypsin-2-alpha1-antitrypsin in patients with acute pancreatitis. Scand J Gastroenterol 2000;35:1216–1220 [DOI] [PubMed] [Google Scholar]
- 14.Basnayake C, Ratnam D. Blood tests for acute pancreatitis. Aust Prescr 2015;38:128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandona P, Elias E, Beckett AG. Serum trypsin concentrations in diabetes mellitus. BMJ 1978;2:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adrian TE, Barnes AJ, Bloom SR. Hypotrypsinaemia in diabetes mellitus. Clin Chim Acta 1979;97:213–216 [DOI] [PubMed] [Google Scholar]
- 17.Dandona P, Freedman DB, Foo Y, et al. Exocrine pancreatic function in diabetes mellitus. J Clin Pathol 1984;37:302–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landin-Olsson M, Borgström A, Blom L, Sundkvist G, Lernmark A; The Swedish Childhood Diabetes Group . Immunoreactive trypsin(ogen) in the sera of children with recent-onset insulin-dependent diabetes and matched controls. Pancreas 1990;5:241–247 [DOI] [PubMed] [Google Scholar]
- 19.Zhao Z, Miao D, Michels A, et al. A multiplex assay combining insulin, GAD, IA-2 and transglutaminase autoantibodies to facilitate screening for pre-type 1 diabetes and celiac disease. J Immunol Methods 2016;430:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 21.Elias E, Redshaw M, Wood T. Diagnostic importance of changes in circulating concentrations of immunoreactive trypsin. Lancet 1977;2:66–68 [DOI] [PubMed] [Google Scholar]
- 22.Wasserfall C, Montgomery E, Yu L, et al. Validation of a rapid type 1 diabetes autoantibody screening assay for community-based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol 2016;185:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalot F, Bonomo K, Perna P, et al. Pancreatic elastase-1 in stools, a marker of exocrine pancreas function, correlates with both residual beta-cell secretion and metabolic control in type 1 diabetic subjects. Diabetes Care 2004;27:2052–2054 [DOI] [PubMed] [Google Scholar]
- 24.Köhn HD, Wider G, Bayer PM, Mostbeck A. Immunoreactive trypsin, alpha-amylase and lipase in serum—is there an age-dependence? Clin Biochem 1982;15:49–51 [DOI] [PubMed] [Google Scholar]
- 25.Itkonen O, Kylänpää L, Zhang WM, Stenman UH. Reference intervals for and validation of recalibrated immunoassays for trypsinogen-1 and trypsinogen-2. Clin Chem 2012;58:1494–1496 [DOI] [PubMed] [Google Scholar]
- 26.Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 1981;22:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) program: goals, operational model and emerging findings. Pediatr Diabetes 2014;15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed]
- 29.Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]