Abstract
OBJECTIVE
The characterization of diverse subtypes of diabetes is a dynamic field of clinical research and an active area of discussion. The objective of this study was to identify new antigenic determinants in the neuroendocrine autoantigen IA-2 (ICA512) and assess whether circulating autoantibodies directed to new IA-2 epitopes identify autoimmune diabetes in young and adult populations with diabetes.
RESEARCH DESIGN AND METHODS
Clinically diagnosed patients with type 2 diabetes (n = 258; diabetes duration: 0.01–31 years) were evaluated using a new biomarker detecting autoantibodies directed to the extracellular domain of the neuroendocrine autoantigen IA-2 (IA-2ec). The proportion of IA-2ec autoantibodies was also evaluated in newly diagnosed patients with type 1 diabetes (n = 150; diabetes duration: 0.04–0.49 years). In addition, IA-2 (intracellular domain), GAD65, and zinc transporter 8 autoantibodies were assayed.
RESULTS
IA-2ec autoantibodies were detected in patients with type 1 diabetes and, surprisingly, in 5% of patients with type 2 diabetes without serologic responses to other IA-2 antigenic epitopes or other islet autoantigens. We also assessed the ability of IA-2ec–derived peptides to elicit CD4+ T-cell responses by stimulating peripheral blood mononuclear cells from patients with type 1 diabetes (n = 18) and HLA-matched healthy subjects (n = 13) with peptides and staining with the peptide/DQ8-specific tetramers, observing disease-associated responses to previously unreported epitopes within IA-2ec.
CONCLUSIONS
We developed a new antibody biomarker identifying novel antigenic determinants within the N terminus of IA-2. IA-2ec autoantibodies can be detected in patients with type 1 diabetes and in a subgroup of adult autoimmune patients with type 2 diabetes phenotype negative for conventional islet autoantibody testing. These observations suggest that islet autoimmunity may be more common in clinically diagnosed type 2 diabetes than previously observed.
Introduction
Autoimmune diabetes is considered to be the end result of an immune-mediated injury of the β-cells within the islets of Langerhans (1,2). Circulating autoantibodies and T-cell responses to islet autoantigens have allowed the development of diagnostic biomarkers that aid in the identification of subjects at risk for type 1 diabetes and of a subset of type 2 diabetes with evidence for islet autoimmunity (3–6). The latter condition is often termed latent autoimmune diabetes in adults (LADA) (7,8).
In autoimmune diabetes, several elements of the secretory pathway of pancreatic β-cells, such as insulin and protein islet tyrosine phosphatase-like protein (IA-2), are targeted by autoantibody and T-cell responses. In type 1 diabetes, the neuroendocrine molecule IA-2 is one of major targets of immune-mediated responses (9–12). IA-2 is a transmembrane glycoprotein of the tyrosine phosphatase-like protein family, which is localized in the insulin-secretory granules of the pancreatic β-cell. This molecule contains three domains: the N-terminal extracellular (or luminal) domain (amino acids 1–556), the transmembrane domain (amino acids 557–600), and the C terminus intracellular (or cytoplasmic) domain (amino acids 601–979) containing a juxtamembrane domain (amino acids 601–686) and an inactive protein tyrosine phosphatase domain (amino acids 687–979). IA-2 is a pseudophosphatase that plays a number of roles within the pancreatic islet such as contributing in β-cell proliferation, aiding in regulating insulin exocytosis, and acting to tether secretory granules to the cytoskeleton. During insulin secretion, the cytoplasmic domain of IA-2/ICA512 is cleaved and traffics to the nucleus, whereby it stimulates the transcription of the insulin gene. Albeit the biological role of IA-2 extracellular domain (IA-2ec) has not been entirely elucidated, stability of pro-ICA512/IA-2 and its targeting to insulin secretory granules require β4-sheet–mediated dimerization of its ectodomain in the endoplasmic reticulum (13).
We previously found indirect evidence for autoantibodies binding to IA-2ec. The presence of these autoantibodies was associated with a high risk of progression of type 1 diabetes (14). We hypothesized that antigenic determinants are present within IA-2ec and identified both autoantibody and T-cell responses specifically directed to the NH2 terminus of IA-2. We provide evidence for humoral responses directed to IA-2ec in patients with type 1 diabetes and, surprisingly, in a subgroup of patients with clinically diagnosed type 2 diabetes. Finally, we demonstrated cell-mediated immunity directed against posttranslationally modified epitopes of the extracellular domain of IA-2 in patients with type 1 diabetes, suggesting that IA-2ec may play a role in the pathogenesis of autoimmune diabetes.
Research Design and Methods
Subjects
The study population consists of 150 patients with type 1 diabetes (74 male and 76 female; mean age 13.40 ± 10.35 years) and 258 patients with type 2 diabetes (135 male and 123 female; mean age 52.67 ± 9.14 years). The sex distribution in the two groups was not statistically significantly different (P = 0.6078). Patients with type 2 diabetes were significantly older than patients with type 1 diabetes (P < 0.0001).
We assayed sera from 150 patients with type 1 diabetes from the Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine; 100 patients with clinically diagnosed type 2 diabetes from the Division of Metabolism, Endocrinology and Diabetes, University of Michigan Health System; and 158 patients with clinically diagnosed type 2 diabetes from the Diabetes Clinic, Azienda Ospedaliera G. Brotzu, Cagliari, Italy. The study was approved by the respective Institutional Review Boards.
The percentage of Caucasians was 87 and 92% in the patients with type 1 and type 2 diabetes, respectively. Diabetes was diagnosed according to standard American Diabetes Association criteria (15). Serum samples from 178 healthy control subjects (72 male and 106 female; mean age 34.62 ± 10.15 years; and 76% Caucasian) were assayed for IA-2ec autoantibodies.
Subjects with type 2 diabetes evaluated in this study were treated with diet, oral hypoglycemic agents, or insulin therapy. All participants were assayed for GAD65, IA-2ic, IA-2ec, and zinc transporter 8 (ZnT8) autoantibodies.
IA-2 Autoantibody Radiobinding Assays
The IA-2ec construct (amino acids 26–577) contains the entire extracellular domain minus the signal peptide (14,16). IA-2ec was in vitro transcribed/translated in the presence of [35S]methionine (PerkinElmer) using the TNT-coupled rabbit reticulocyte system (Promega, Madison, WI) with T7 RNA polymerase. IA-2ec autoantibodies were detected by radiobinding assay using 50% protein A–Sepharose to separate free [35S]methionine from antibody-bound labeled products. The assay was run in triplicate, and the results were expressed as an index calculated as follows: index = (serum sample counts per minute [cpm] − negative control cpm)/(positive control cpm − negative control cpm). We used the protein tyrosine phosphatase IA-2 Q-20 antibody (sc-54749; Santa Cruz Biotechnology, Santa Cruz, CA) as positive control and serum from individuals without diabetes as negative controls. The cutoff point was established as the 99th percentile of values from serum samples obtained from 178 healthy volunteers. The interassay coefficient of variation (CV) was 13.4% (n = 10), and the intra-assay CV was 5.45% (n = 15). The IA-2ec autoantibody assay achieved ratings of 4% sensitivity and 99% specificity at the Islet Autoantibody Standardization Program 2012.
The intracellular domain of IA-2 (IA-2ic) construct (amino acid residues 605–979) was provided by Dr. E. Bonifacio (DFG-Center for Regenerative Therapies Dresden, TU Dresden, Dresden, Germany). The IA-2ic autoantibody radioimmunoassay has a similar assay format as that to detect IA-2ec autoantibodies (14). The interassay CV was 9.9%, and the intra-assay CV was 4.8%. IA-2ic autoantibodies achieved ratings of 72% sensitivity and 99% specificity at the 2007 4th assay proficiency evaluation of the Diabetes Autoantibody Standardization Program.
IA-2ec Antibody Specificity
To demonstrate specificity of IA-2ec autoantibodies, we performed inhibition of autoantibody binding studies. Unlabeled IA-2ec (amino acid residues 26–577) and IA-2ic (amino acid residues 605–979) were expressed in the reticulocyte lysate system with amino acid mixture containing unlabeled methionine (Fig. 3). For competitive binding studies, unlabeled antigens were each incubated separately with test human serum sample for 16 h at 4°C; after centrifugation at 12,000 × g for 20 min at 4°C, the supernatants were then incubated with 20,000 cpm of [35S]methionine-labeled or unlabeled antigen (IA-2ec or IA-2ic) for 16 h at 4°C. Immunoprecipitates were processed, analyzed by scintillation counting, and expressed as an index. All serum samples were assayed in triplicate. The results were presented as the means of three different experiments. Unabsorbed serum was used as a positive control to provide a baseline total cpm for the assay.
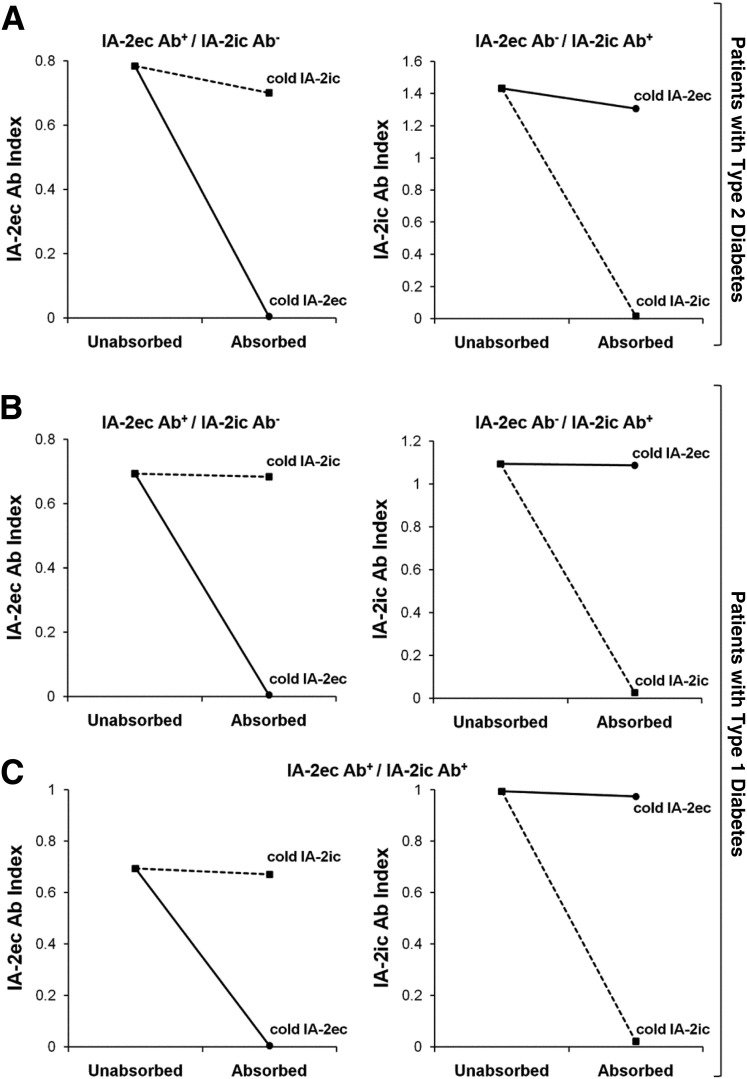
Figure 3.
Specific inhibition of autoantibody binding to IA-2ec (circles) and to IA-2ic (squares) with sera from patients with type 1 and 2 diabetes. A: Binding to IA-2ec of serum of a patient with type 2 diabetes positive for IA-2ec antibodies was not inhibited by preincubation with unlabeled IA-2ic, whereas it was specifically inhibited by preincubation with unlabeled IA-2ec (left panel). Binding to labeled IA-2ic of serum of a patient with type 2 diabetes positive for IA-2ic antibodies was not inhibited by preincubation with unlabeled IA-2ec, but it was specifically inhibited by preincubation with unlabeled IA-2ic (right panel). B and C: Specific inhibition of autoantibody binding to IA-2ec and IA-2ic in patients with type 1 diabetes with different combinations of these autoantibodies. Ab, antibody.
GAD65 and ZnT8 Autoantibody Radiobinding Assay
All serum samples including controls were analyzed in triplicate for autoantibodies targeted against GAD65 and ZnT8 using in vitro transcribed/translated [35S]methionine-labeled recombinant human GAD65 and [35S]methionine-labeled recombinant human ZnT8, as previously reported (17). The GAD65 construct was donated by Dr. Å. Lernmark (Lund University/CRC, Department of Clinical Sciences Diabetes & Celiac Disease Unit, Malmö, Sweden), whereas the ZnT8 construct was donated by Dr. J. Hutton (Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine). The results were expressed as an index as previously reported (17,18). The cutoff points for these autoantibodies were based on the 99th percentiles of values from serum samples obtained from healthy volunteers (17,18).
Insulin Autoantibody Radioimmunoassay
Insulin autoantibody testing was performed at the Barbara Davis Center for Childhood Diabetes according to previously published protocols (19). This assay is currently used for protocols supported by TrialNet and The Environmental Determinants of Diabetes in the Young studies.
Tetramer Staining of In Vitro Expanded CD4+ T Cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll underlay, resuspended in T-cell media (RPMI, 10% human serum, 1% penicillin-streptomycin, and 1% L-glutamine) at 4 × 106 cells/mL, and stimulated with IA-2 peptides (20 µg/mL total) in 48-well plates for 14 days, adding medium and IL-2 as needed starting on day 7. Four peptide sequences that were recently shown to be epitopes were used for stimulation: IA-2 198–216 (SLSYEPALLEPYLFHEFGS), IA-2 467–482 (AAEEYGYIVTDEKPLS), IA-2 523–536 (QNLSLADVTEEAGL), and IA-2 545–562 (TGLEILETGVGEREEAAA). Each of these peptide sequences was modified to contain deamidated glutamate residues (at positions 207, 213, 478, 532, 533, 548, 551, and 556, respectively), as such modifications are required for presentation and recognition. To visualize responses, cells were stained with the corresponding DQ8 tetramers (or a negative control tetramer loaded with a nonstimulatory chromogranin A peptide) for 60 min at 37°C and then with CD4-PerCP (BD Biosciences), CD3-allophycocyanin (eBioscience), and CD25-FITC (BioLegend) for 15 min at 4°C and analyzed on an FACSCalibur (BD Biosciences) and using FlowJo software (Tree Star). As a positive control, PBMCs were stimulated with a well-characterized influenza peptide (matrix protein 97–116 VKLYRKLKREITFHGAKEIS) and stained with the corresponding DRB1*0401 tetramer. Matrix protein 97–116 (MP-97) is a conserved epitope essentially present in any seasonal influenza vaccine. Positive responses were gated based on the negative control tetramer. Each in vitro expansion was standardized as follows: we added IL-2 on day 7 of the culture. Subsequently, the cells were given fresh medium and IL-2 every 2 days, splitting the cells into new wells as needed to keep each well between 50 and 100% confluence.
Statistical Analysis
Data were analyzed using Prism 6 (GraphPad Software, La Jolla, CA) and SPSS 23.0 (SPSS, Chicago, IL). The χ2 and Fisher exact tests were used to compare proportions and evaluate statistically significant associations between two categorical variables. The t test for independent samples was used to compare continuous variables between two groups. Venn diagrams were used to show the autoantibody frequencies. Bonferroni correction was used for multiple comparisons. Differences between T-cell responses to IA-2ec in patients with type 1 diabetes and control subjects were evaluated using an unpaired t test (two-tailed) with Welch’s correction. A P value <0.05 was considered significant.
Results
IA-2ec Autoantibodies Are Present in Patients With Both Type 1 and Type 2 Diabetes
In an effort to identify novel humoral epitopes within the neuroendocrine autoantigen IA-2, we initially performed immunoprecipitation studies using recombinant IA-2ec (amino acid residues 26–577), sera from patients with type 1 diabetes, patients with type 2 diabetes, and healthy volunteers. Immunoprecipitation of the IA-2ec protein can also be visualized by autoradiography following incubation with serum from patients with type 1 and type 2 diabetes with no detectable IA-2ic autoantibodies (Fig. 1A, lanes 1 and 2). In contrast, serum from a healthy control subject did not immunoprecipitate the IA-2ec protein (Fig. 1A, lane 3).
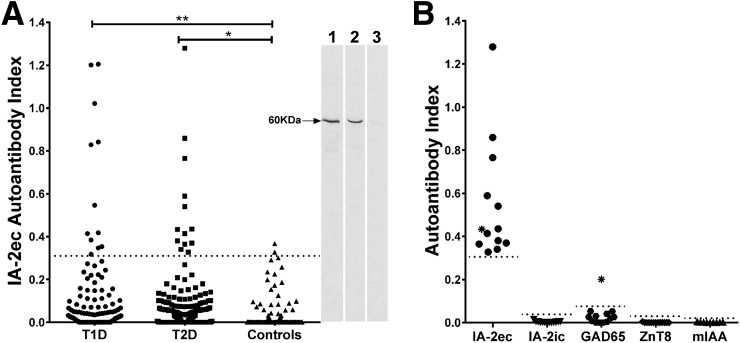
Figure 1.
A: Autoantibodies directed to IA-2ec (amino acids 26–577) can be detected in patients with type 1 and 2 diabetes. Dashed line represents the cutoff point for the assays. Eight percent of patients with type 1 diabetes and 5% of patients with type 2 diabetes exhibited antibody responses to IA-2ec. *P = 0.0371; **P = 0.0023. Two-dimensional SDS-PAGE fractionation of IA-2ec immunoprecipitates followed by autoradiography illustrates that serum from one patient with type 1 diabetes and from another patient with type 2 diabetes (blot, lanes 1 and 2, respectively) strongly reacted with a 60-kDa band (IA-2ec), unlike serum from a healthy volunteer (blot, lane 3). B: The majority of patients with clinically diagnosed type 2 diabetes carrying IA-2ec autoantibodies tested negative for IA-2ic, GAD65, ZnT8, and mIAA. *Positive for both IA-2ec and GAD65 autoantibodies.
We then found that the proportion of autoantibodies against IA-2ec was higher in patients with type 1 as well as type 2 diabetes as compared with that of healthy control subjects (8% [12 out of 150] vs. 1% [2 out of 178], P = 0.0023; 5% [13 out of 258] vs. 1% [2 out of 178], P = 0.0317) (Fig. 1A). Interestingly, the majority of patients with type 2 diabetes who tested positive for IA-2ec autoantibodies were negative for all traditional islet autoantibodies. In the latter group, only one subject tested positive for GAD65 autoantibodies (Fig. 1B). Four of those patients with type 2 diabetes were on insulin therapy.
Surprisingly, 13 patients with type 2 diabetes tested positive for IA-2ec autoantibodies and negative for IA-2ic autoantibodies (Fig. 2B), whereas 1 out of 12 patients with type 1 diabetes tested positive for both IA-2ec and IA-2ic autoantibodies (Fig. 2A). The majority of clinically diagnosed patients with type 2 diabetes carrying IA-2ec autoantibodies tested negative for IA-2ic, GAD65, ZnT8, and micro-insulin autoantibodies (mIAA) (Fig. 1B). IA-2ec antibody index was higher in patients with type 1 diabetes compared with that of patients with type 2 diabetes (P = 0.036) and control subjects (P < 0.0001). The Venn diagram shows the frequency of islet autoantibodies (alone or in combination) in both patients with type 1 (Fig. 2A) and type 2 diabetes (Fig. 2B). There was no statistically significant difference with respect to age or ethnicity in IA-2ec antibody–positive compared with IA-2ec antibody–negative patients with type 2 diabetes.
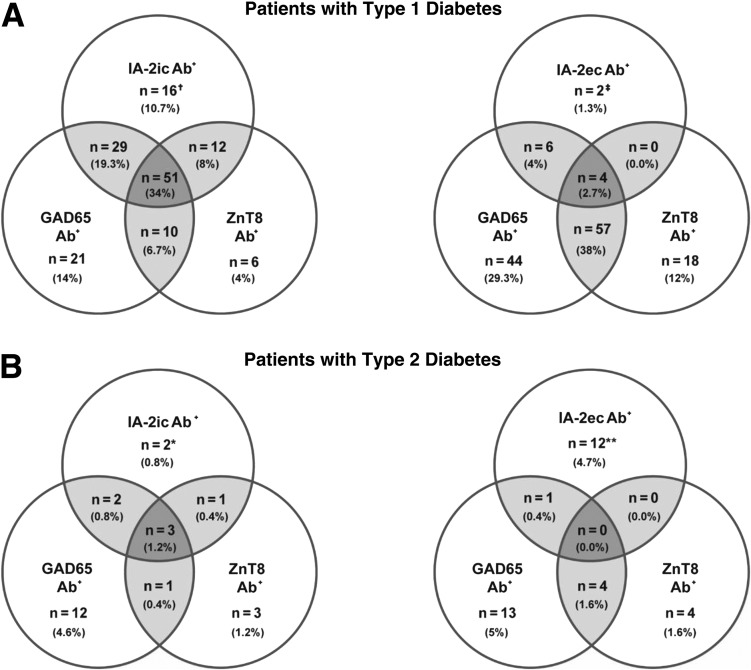
Figure 2.
Venn diagrams of autoantibody combinations in autoantibody-positive patients with type 1 and 2 diabetes. The frequency of autoantibodies in patients with type 1 (A) and type 2 (B) diabetes (IA-2ec, IA-2ic, GAD65, and ZnT8 autoantibodies). Intersecting regions (lightly shaded) indicate the number of patients positive for different combinations of islet autoantibodies. Insulin autoantibodies were not included in this analysis because many patients were on insulin therapy. *Both patients with type 2 diabetes were negative for IA-2ec antibody (Ab) autoantibodies. **All patients with type 2 diabetes were negative for IA-2ic autoantibodies. †One out of 16 patients with type 1 diabetes was IA-2ec autoantibody positive. ‡One out of two patients with type 1 diabetes was IA-2ic autoantibody positive.
Of 178 healthy volunteers, 0.6%, 1.1%, and 1.7% exceeded the thresholds for IA-2ic, GAD65, and ZnT8 antibodies, respectively.
As expected, the vast majority of patients with type 1 diabetes had at least one islet autoantibody as compared with patients with type 2 diabetes. In particular, 146 out of 150 (97.3%) patients with type 1 diabetes and 36 out of 258 (14%) patients with type 2 diabetes had at least one islet autoantibody (P = 0.0011). Four (2.7%) patients with type 1 diabetes and none of the patients with type 2 diabetes tested positive for all autoantibodies (P = 0.1958). Patients with type 1 diabetes were more frequently positive for single and multiple autoantibodies as compared with patients with type 2 diabetes.
Although the prevalence of GAD65, mIAA, ZnT8, and IA-2ic autoantibodies was significantly higher in patients with type 1 diabetes compared with that of patients with type 2 diabetes (74 vs. 6.9, 52.7 vs. 3.1, and 72 vs 3.1%, respectively; P < 0.0003), the prevalence of IA-2ec autoantibodies was higher in patients with type 1 diabetes as compared to that of patients with type 2 diabetes (8 vs. 5%).
Although the prevalence of IA-2ic autoantibodies alone was significantly higher in patients with type 1 diabetes than that of patients with type 2 diabetes (15 [10%] vs. 2 [0.8%]; P < 0.0011), the prevalence of IA-2ec autoantibodies alone was higher in patients with type 2 diabetes as compared with that of subjects with type 1 diabetes (12 [4.7%] vs. 1 [0.7%]). The most frequent single islet autoantibody in patients with type 1 diabetes was GAD65 autoantibody and IA-2ec autoantibody in patients with type 2 diabetes. Overall, the addition of the IA-2ec autoantibodies in patients with type 2 diabetes to islet autoantibodies, measured by routine assays, resulted in an increase in sensitivity from 9.3% (24 out of 258) to 14% (36 out of 258).
Table 1 shows the islet autoantibody status in patients with type 1 diabetes and clinically diagnosed patients with type 2 diabetes with diabetes duration <6 months. The vast majority of these patients was either on diet alone or oral hypoglycemic therapy. Interestingly, 1 out of 3 (33.3%) IA-2ec antibody–positive new-onset patients with type 2 diabetes was on insulin therapy (Table 1). In addition, we analyzed the data after matching patients with type 1 and type 2 diabetes for insulin use (Table 2). As expected, we found a much higher prevalence of islet autoantibodies in subjects with type 1 diabetes on insulin therapy compared with that of patients with type 2 diabetes on dietary or oral hypoglycemic therapy. Islet autoantibodies were present in ∼21% of clinically diagnosed patients with type 2 diabetes on insulin therapy (Table 2).
Table 1.
Islet autoantibody status in patients with type 1 diabetes and clinically diagnosed type 2 diabetes with diabetes duration <6 months
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| N | 147 | 50 |
| Male sex | 72 (48.9) | 33 (66) |
| Age (years) | 13.3 ± 10 | 55.6 ± 9.8 |
| IA-2ec Ab | 12 (8.2) | 3a (6) |
| IA-2ic Ab | 105 (71.4) | 0 |
| GAD65 Ab | 109 (74.1) | 0 |
| ZnT8 Ab | 78 (53.1) | 0 |
| Single islet Ab | 42 (28.6) | 3 (6) |
| At least one islet Ab | 143 (97.3) | 3 (6) |
| Multiple islet Ab | 101 (68.7) | 0 |
| Diet use | 0 | 26 (52) |
| OHA use | 0 | 18 (36) |
| Insulin use | 147 (100) | 5 (10) |
Data are mean ± SD or n (%).
Ab, antibody; OHA, oral hypoglycemic agent.
aOne patient was on insulin therapy.
Table 2.
Islet autoantibody status in patients with type 1 diabetes and clinically diagnosed type 2 diabetes on insulin therapy
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| N | 150 | 73 |
| Male sex | 74 (49.3) | 39 (53.4) |
| Age (years) | 13.4 ± 10.4 | 54.7 ± 9 |
| IA-2ec Ab | 12 (8) | 4 (5.5) |
| IA-2ic Ab | 108 (72) | 5 (6.8) |
| GAD65 Ab | 111 (74) | 11 (15) |
| ZnT8 Ab | 79 (52.7) | 4 (5.5) |
| Single islet Ab | 43 (28.7) | 9 (12.3) |
| At least one islet Ab | 146 (97.3) | 15 (20.6) |
| Multiple islet Ab | 103 (68.7) | 6 (8.2) |
| Insulin use | 150 (100) | 73 (100) |
Data are mean ± SD or n (%).
Ab, antibody.
Specific Autoantibody Binding to Novel Epitopes Within the IA-2ec
Competitive binding studies using unlabeled IA-2ec (amino acids 26–577), and IA-2ic (amino acids 605–979) proteins demonstrated specificity of IA-2ec autoantibodies (Fig. 3). Sera from patients with type 1 and 2 diabetes, positive for IA-2ec autoantibodies, were preincubated with unlabeled antigen (IA-2ec or IA-2ic). This resulted in complete inhibition of serum binding to [35S]methionine-labeled IA-2ec only after preincubation with unlabeled IA-2ec and in no inhibition after preincubation with unlabeled IA-2ic (Fig. 3). These experiments suggest that autoantibody reactivity to IA-2ec is due to specific binding.
CD4+ T-Cell Responses to IA-2ec Peptides in Subjects With Type 1 Diabetes
It is well appreciated that T and B cells undergo cognate interactions and that antigen-specific CD4 T-cell interactions with B cells aid in the eventual production of antibodies. To evaluate IA-2ec–specific T-cell responses, we stimulated PBMCs from 18 patients with type 1 diabetes and 13 HLA-matched healthy subjects with epitopes from IA-2ec followed by staining with peptide/DQ8(DQB1*03:02) tetramers. We selected four IA-2ec peptides containing a posttranslational modification (each with at least one deamidated glutamate residue) for these experiments, as deamidated epitopes from other islet antigens have been reported in type 1 diabetes (20,21). Fig. 4A depicts representative positive tetramer staining for the negative control tetramer, each of the four IA-2 peptides, and a positive control tetramer. Each IA-2 peptide elicited positive responses in many subjects with type 1 diabetes (and more rarely in control subjects), but responses were more effectively visualized by combining all four tetramers. As shown in Fig. 4B, IA-2–specific T cells were present at significantly higher magnitudes in patients with type 1 diabetes than in control subjects (P = 0.0017). These results provide direct evidence that DQ8-restricted CD4+ T cells recognize deamidated epitopes derived from the IA-2ec and suggest that such T-cell responses are more commonly present in subjects with type 1 diabetes.
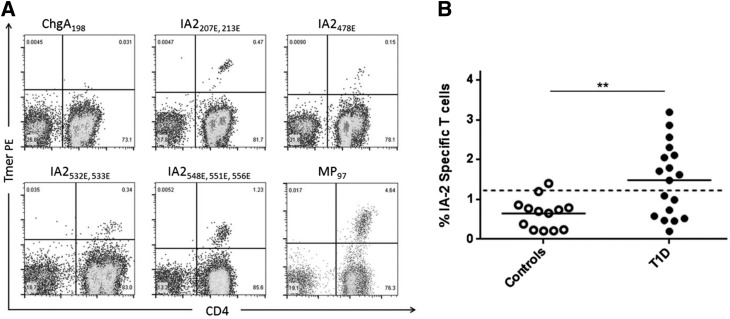
Figure 4.
CD4+ T-cell responses to IA-2ec peptides are elevated in subjects with type 1 diabetes. A: Representative tetramer staining of T cells using tetramers loaded with a negative control peptide (chromogranin A 198 [ChgA198]), DQ8-restricted IA-2 peptides (as indicated on each panel), or an influenza peptide (MP-97) after 2 weeks of in vitro expansion. B: Comparison of IA-2–specific T-cell responses measured in 18 subjects with type 1 diabetes (T1D) and 13 HLA-matched control subjects (all with DQ8+ haplotypes) by tetramer staining (combined percentages for all four epitopes). Responses >1.1% (1 SD above the mean response for controls, indicated by the dashed line) were above the positive threshold. The magnitude of responses to IA-2ec peptides were significantly higher in subjects with type 1 diabetes (P = 0.0017, two-tailed t test with Welch’s correction).
Conclusions
The specific characterization of diverse subtypes of diabetes has been a moving target and a major topic of debate (2,22). A well-documented example is the case of patients who are generally adults and present with a type 2 diabetic phenotype as well as circulating islet autoantibodies, generally GAD65 autoantibodies (23). These characteristics are defined as LADA and sometimes termed type 1.5 diabetes (24,25). The demonstration that autoimmune responses play a key role in the pathogenesis of type 1 diabetes led to the assumption that type 1 and 2 diabetes possess unique etiologies, disease courses, and, consequently, therapeutic regimens. However, solid evidence indicates that overlap exists even among the most “typical” diabetes phenotypes (2,6,22,26). Thus, the current classification of diabetes poses challenges to the diagnosis and treatment of patients.
Autoantibodies are powerful tools in translational research, biomarker identification, and diagnostic testing for autoimmune disorders. Because of their robust measurements and ease of use, immunoassays are among the preferred methods for investigation of various biological and clinical questions. We optimized a new IA-2ec antibody biomarker detecting islet autoimmunity in a subset of patients with type 1 and 2 diabetes. IA-2ec autoantibodies identified islet autoimmunity in a subset of clinically diagnosed patients with type 2 diabetes that were otherwise negative for conventional islet autoantibody testing.
We analyzed the prevalence of islet autoantibodies in recent-onset type 1 and 2 diabetes with the assumption that the degree of glycemic control is similar in those two groups according to previously published observations (27,28). The vast majority of newly diagnosed patients with type 2 diabetes evaluated in this study was either on dietary or oral hypoglycemic therapy. It is noteworthy to acknowledge that 33.3% of newly diagnosed patients with type 2 diabetes using insulin had detectable levels of IA-2ec autoantibodies with no evidence of other islet autoantibodies (Table 1).
Although canonical immune responses to IA-2ic are considered to be immunodominant (29–31), we found autoantibody and T-cell responses specifically directed to IA-2ec in individuals with or without antibodies directed to IA-2ic. Our results reinforce the notion that long-lived autoantibody responses in the natural history of autoimmune disorders, such as type 1 diabetes, are generally regarded to be polyclonal and yet not restricted to one portion of a given self-antigen (32–35).
The presence of islet autoantibodies in clinically diagnosed patients with type 2 diabetes by conventional criteria (American Diabetes Association or World Health Organization) is not uncommon among older patients with diabetes, being 5–10% or higher, especially those on insulin therapy (36–38). In the current study, we found islet autoantibodies in ∼21% of clinically diagnosed patients with type 2 diabetes on insulin therapy (Table 2). Thus, theoretically, there are at least as many islet autoantibody–positive older patients with diabetes as there are children affected by type 1 diabetes. This is a public health issue. Additional immunological markers of autoimmune diabetes, such as IA-2ec autoantibodies, may identify even larger numbers of clinically diagnosed patients with type 2 diabetes. Given its relative simplicity, larger studies are needed to measure IA-2ec antibodies to potentially identify clinically diagnosed patients with type 2 diabetes who may rapidly progress to insulin-requiring diabetes.
A limitation of this study is the different durations of diabetes between subjects with type 1 and 2 diabetes; the latter had long-standing diabetes for the most part.
We previously reported that islet autoantibody–positive long-term patients with type 2 diabetes exhibited profound impairment of insulin secretion as well as reduced β-cell mass seemingly determined by an immune-mediated injury of pancreatic β-cells (37,39). Further studies aimed at investigating the significance of autoimmunity and the pancreas immunopathology in this subset of patients with type 2 diabetes are required.
It is noteworthy to acknowledge that the presence of autoantibodies reacting with portions of both IA-2ec and IA-2ic has been associated with LADA (40,41). Brooks-Worrell et al. (42) reported islet-reactive T cells in up to 50% of seronegative patients with type 2 diabetes. As there are interactions between CD4 T cells and B cells leading to antibody production, the possibility exists that some of those autoantibody-negative patients with type 2 diabetes may carry IA-2ec autoantibodies.
We assessed IA-2ec CD4 T-cell responses and observed disease-associated responses to epitopes within the extracellular domain with tetramer staining of PBMCs. Similar to type 1 diabetic autoantibodies, T-cell responses to islet proteins may be biomarkers and important contributors of progressive decline in β-cell function observed both in patients with type 1 and 2 diabetes. We also show that peptides derived from IA-2ec are immunogenic in vitro and provide evidence that the precursor frequency of HLA-DQ8–restricted T cells in peripheral blood is higher in patients with type 1 diabetes than in healthy control subjects. Although initial studies have focused on HLA-DR4 epitopes in IA-2ic (30), recent observations demonstrated that naturally processed and posttranslationally modified IA-2 peptides within the extracellular domain proved to be targets of autoreactive CD4+ T cells in patients with type 1 diabetes (21,43). These studies demonstrated that during inflammation both human islets and dendritic cells, which process and present antigen to CD4 T cells, can activate tissue transglutaminase to deamidate islet autoantigens (44,45). Of note, the amino acid sequence of HLA-DQ8–restricted epitopes of IA-2ec described in our study are different than those identified in the latter reports.
In summary, our findings suggest that IA-2ec autoantibodies and T cells can be detected in patients with type 1 diabetes. IA-2ec autoantibodies identify a subgroup of adult autoimmune phenotypic patients with type 2 diabetes who test negative for conventional islet autoantibodies. Autoimmunity to new IA-2 epitopes within the extracellular domain will provide impetus for efforts in developing mechanistic biomarkers, which are embedded in disease pathogenesis, as well as new IA-2 peptide-based immunotherapies for autoimmune diabetes and possibly assessing responsiveness to therapy.
Article Information
Acknowledgments. The authors thank Dr. Bill Kwok and I-Ting Chow, Benaroya Research Institute, for providing the DQ8 tetramers and Curt Baldwin, a freelance graphic designer, for assistance with digital figures.
Funding. This work was supported by the National Institutes of Health (grants R01-DK-53456 and R01-DK-56200), the Robert and Janice McNair Foundation, JDRF (17-2012-688), JDRF Biomarker Working Group, the Michigan Institute for Clinical & Health Research, the Clinical and Translational Science Award program (UL1RR024986 to M.P.), and the National Institute of Diabetes and Digestive and Kidney Diseases (grants P30-DK-020572 to the Michigan Diabetes Research Center and P30-DK-092926 to the Michigan Center for Diabetes Translational Research).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.-C., E.A.J., M.P.M., Q.O., L.Y., A.M., R.G., and M.P. generated data, wrote the manuscript, and contributed to discussion. S.L.P. and D.A.-L. generated data. M.S., M.L., A.C., and R.J.A. reviewed and edited the manuscript and contributed to discussion. M.A.-C., S.H., and M.P. performed statistical analysis. M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: prediction, significance, and detection of distinct disease subtypes. Cold Spring Harb Perspect Med 2012;2:a012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks-Worrell B, Narla R, Palmer JP. Biomarkers and immune-modulating therapies for type 2 diabetes. Trends Immunol 2012;33:546–553 [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Worrell B, Narla R, Palmer JP. Islet autoimmunity in phenotypic type 2 diabetes patients. Diabetes Obes Metab 2013;15(Suppl. 3):137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes 2000;49:32–38 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie RD, Williams R, Pozzilli P. Clinical review: type 1 diabetes and latent autoimmune diabetes in adults: one end of the rainbow. J Clin Endocrinol Metab 2006;91:1654–1659 [DOI] [PubMed] [Google Scholar]
- 8.Leslie RD, Kolb H, Schloot NC, et al. Diabetes classification: grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev 2008;24:511–519 [DOI] [PubMed] [Google Scholar]
- 9.Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 1994;13:505–514 [DOI] [PubMed] [Google Scholar]
- 10.Mziaut H, Kersting S, Knoch KP, et al. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci U S A 2008;105:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai T, Hirai H, Zhang G, et al. Deletion of Ia-2 and/or Ia-2β in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia 2011;54:2347–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai T, Notkins AL. Pathophysiologic changes in IA-2/IA-2β null mice are secondary to alterations in the secretion of hormones and neurotransmitters. Acta Diabetol 2016;53:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torkko JM, Primo ME, Dirkx R, et al. Stability of proICA512/IA-2 and its targeting to insulin secretory granules require β4-sheet-mediated dimerization of its ectodomain in the endoplasmic reticulum. Mol Cell Biol 2015;35:914–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morran MP, Casu A, Arena VC, et al. Humoral autoimmunity against the extracellular domain of the neuroendocrine autoantigen IA-2 heightens the risk of type 1 diabetes. Endocrinology 2010;151:2528–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 16.Wasmeier C, Hutton J. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem 1996;271:18161–18170 [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Liu Y, Miao D, et al. Triple chimeric islet autoantigen IA2-ZnT8WR to facilitate islet autoantibody determination. J Immunol Methods 2010;353:20–23 [DOI] [PubMed] [Google Scholar]
- 18.Grubin CE, Daniels T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 1994;37:344–350 [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James EA, Mallone R, Schloot NC, et al.; T-Cell Workshop Committee, Immunology of Diabetes Society . HLA class II tetramer-directed epitope validation initiative. Diabetes Metab Res Rev 2011;27:727–736 [DOI] [PubMed] [Google Scholar]
- 21.van Lummel M, van Veelen PA, de Ru AH, et al. Discovery of a selective islet peptidome presented by the highest-risk HLA-DQ8trans molecule. Diabetes 2016;65:732–741 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell–centric classification schema. Diabetes Care 2016;39:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmet PZ, Tuomi T, Mackay IR, et al. Latent autoimmune diabetes mellitus in adults (LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med 1994;11:299–303 [DOI] [PubMed] [Google Scholar]
- 24.Palmer JP, Hirsch IB. What’s in a name: latent autoimmune diabetes of adults, type 1.5, adult-onset, and type 1 diabetes. Diabetes Care 2003;26:536–538 [DOI] [PubMed] [Google Scholar]
- 25.Redondo MJ. LADA: time for a new definition. Diabetes 2013;62:339–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietropaolo M, Barinas-Mitchell E, Kuller LH. The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity? Diabetes 2007;56:1189–1197 [DOI] [PubMed] [Google Scholar]
- 27.Clements MA, Lind M, Raman S, et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care 2014;2:e000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiteerapong N, Karter AJ, Moffet HH, Cooper JM, Gibbons RD, Liu JY, Gao Y, Huang ES. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: The Diabetes & Aging Study. J Diabetes Complications 2017;31:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verge CF, Stenger D, Bonifacio E, Colman PG, Pilcher C, Bingley PJ, Eisenbarth GS. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 1998;47:1857–1866 [DOI] [PubMed] [Google Scholar]
- 30.Peakman M, Stevens EJ, Lohmann T, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest 1999;104:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin KA, Richardson CC, Williams S, et al. Relationships between major epitopes of the IA-2 autoantigen in type 1 diabetes: implications for determinant spreading. Clin Immunol 2015;160:226–236 [DOI] [PubMed] [Google Scholar]
- 32.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev 2010;236:139–150 [DOI] [PubMed] [Google Scholar]
- 33.Skärstrand H, Lernmark A, Vaziri-Sani F. Antigenicity and epitope specificity of ZnT8 autoantibodies in type 1 diabetes. Scand J Immunol 2013;77:21–29 [DOI] [PubMed] [Google Scholar]
- 34.Di Zenzo G, Thoma-Uszynski S, Calabresi V, et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol 2011;131:2271–2280 [DOI] [PubMed] [Google Scholar]
- 35.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barinas-Mitchell E, Pietropaolo S, Zhang YJ, et al. Islet cell autoimmunity in a triethnic adult population of the Third National Health and Nutrition Examination Survey. Diabetes 2004;53:1293–1302 [DOI] [PubMed] [Google Scholar]
- 37.Barinas-Mitchell E, Kuller LH, Pietropaolo S, Zhang YJ, Henderson T, Pietropaolo M. The prevalence of the 65-kilodalton isoform of glutamic acid decarboxylase autoantibodies by glucose tolerance status in elderly patients from the cardiovascular health study. J Clin Endocrinol Metab 2006;91:2871–2877 [DOI] [PubMed] [Google Scholar]
- 38.Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subauste A, Gianani R, Chang AM, et al. Islet autoimmunity identifies a unique pattern of impaired pancreatic beta-cell function, markedly reduced pancreatic beta cell mass and insulin resistance in clinically diagnosed type 2 diabetes. PLoS One 2014;9:e106537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzzetti R, Spoletini M, Zampetti S, et al.; NIRAD Study Group (NIRAD 8) . Tyrosine phosphatase-related islet antigen 2(256-760) autoantibodies, the only marker of islet autoimmunity that increases by increasing the degree of BMI in obese subjects with type 2 diabetes. Diabetes Care 2015;38:513–520 [DOI] [PubMed] [Google Scholar]
- 41.Tiberti C, Giordano C, Locatelli M, et al. Identification of tyrosine phosphatase 2(256-760) construct as a new, sensitive marker for the detection of islet autoimmunity in type 2 diabetic patients: the non-insulin requiring autoimmune diabetes (NIRAD) study 2. Diabetes 2008;57:1276–1283 [DOI] [PubMed] [Google Scholar]
- 42.Brooks-Worrell BM, Reichow JL, Goel A, Ismail H, Palmer JP. Identification of autoantibody-negative autoimmune type 2 diabetic patients. Diabetes Care 2011;34:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Lummel M, van Veelen PA, de Ru AH, et al. Dendritic cells guide islet autoimmunity through a restricted and uniquely processed peptidome presented by high-risk HLA-DR. J Immunol 2016;196:3253–3263 [DOI] [PubMed] [Google Scholar]
- 44.van Lummel M, Zaldumbide A, Roep BO. Changing faces, unmasking the beta-cell: post-translational modification of antigens in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2013;20:299–306 [DOI] [PubMed] [Google Scholar]
- 45.Yang J, James EA, Sanda S, Greenbaum C, Kwok WW. CD4+ T cells recognize diverse epitopes within GAD65: implications for repertoire development and diabetes monitoring. Immunology 2013;138:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]