Abstract
OBJECTIVE
To evaluate patient-level glycemic control and facility variation of a proposed out-of-range (OOR) measure (overtreatment [OT] [HbA1c <7% (53 mmol/mol)] or undertreatment [UT] [>9% (75 mmol/mol)]) compared with the standard measure (SM) (HbA1c <8% [64 mmol/mol]) in high-risk older adults.
RESEARCH DESIGN AND METHODS
Veterans Health Administration patients ≥65 years of age in 2012 who were taking antihyperglycemic agents in 2013 were identified. Patient-level rates and facility-level rates/rankings were calculated by age and comorbid illness burden.
RESULTS
We identified 303,097 patients who were taking antiglycemic agents other than metformin only. The study population comprised 193,689 patients with at least one significant medical, neurological, or mental health condition; 98.2% were taking a sulfonylurea and/or insulin; 55.2% were aged 65–75 years; and 44.8% were aged >75 years. The 47.4% of patients 65–75 years met the OOR measure (33.4% OT, 14% UT), and 65.7% met the SM. For patients aged >75 years, rates were 48.1% for OOR (39.2% OT; 8.9% UT) and 73.2% for SM. Facility-level rates for OOR for patients aged 65–75 years ranged from 33.7 to 60.4% (median 47.4%), with a strong inverse correlation (ρ = −0.41) between SM and OOR performance rankings. Among the best-performing 20% facilities on the SM, 14 of 28 ranked in the worst-performing 20% on the OOR measure; 12 of 27 of the worst-performing 20% facilities on the SM ranked in the best-performing 20% on the OOR measure.
CONCLUSIONS
Facility rankings that are based on an SM (potential benefits) and OOR measure (potential risks) differ substantially. An OOR for high-risk populations can focus quality improvement on individual patient evaluation to reduce the risk for short-term harms.
Introduction
According to the most recent National Diabetes Statistics Report, 11.2 million people ≥65 years of age have diabetes (1). Individualized target values for glycemic control for older adults with diabetes, based on comorbid conditions and other factors, are recommended by the American Diabetes Association (ADA) and the American Geriatrics Society (2), the ADA/European Association for the Study of Diabetes (3), and the Department of Veterans Affairs (VA)/Department of Defense (4). Two ongoing national diabetes medication safety programs—the American Board of Internal Medicine Foundation/American Geriatrics Society Choosing Wisely campaign (5) and the Department of Health and Human Services National Action Plan for Prevention of Adverse Drug Events–Diabetes Agents (DHHS-NAP) federal interagency partnership (6)—emphasize the importance of setting and reevaluating individualized glycemic goals on the basis of a process of shared decision making that incorporates health literacy and patient preferences. The DHHS-NAP focuses on prevention of hypoglycemia (6), which has been increasingly recognized as a public health concern (7). Indeed, hypoglycemia now exceeds hyperglycemia as a cause of hospitalization among Medicare beneficiaries (8) and is a common cause for emergency department visits among patients who take insulin (9).
The current National Quality Forum (NQF)–endorsed glycemic control performance measures applicable to patients 65–75 years of age include HbA1c <8% (64 mmol/mol) and >9% (75 mmol/mol) (10). Shortcomings of one-size-fits-all dichotomous measures include not rewarding physicians for achieving marked improvement in glycemic control that has not achieved the target level (11) and an inability to simultaneously address the balance of benefits and harms for individual patients.
We have proposed a potential overtreatment (OT) measure (HbA1c <7% [53 mmol/mol]) for older adults with serious comorbid conditions who take antihyperglycemic agents that carry a higher risk of hypoglycemia (insulin and sulfonylureas) (12). However, an increased focus on OT could similarly result in a decreased emphasis on undertreatment (UT) (HbA1c >9% [75 mmol/mol]) (13). Therefore, we propose an out-of-range (OOR) accountability measure that combines potential OT and UT for patients aged ≥65 years with diabetes and significant comorbid conditions taking antihyperglycemic agents other than metformin alone. The principal study objective was to compare the OOR measure with the NQF-endorsed measure (HbA1c <8% [64 mmol/mol]) and to evaluate facility variation and rankings among Veterans Health Administration (VHA) facilities. In addition to evaluating patients aged 65–75 years who are included in the current NQF measure, we evaluated patients aged >75 years. Finally, we evaluated a companion in-range (IR) (HbA1c 7.5–8.5% [58–69 mmol/mol]) quality improvement measure.
Research Design and Methods
Study Population
We identified patients with diabetes aged ≥65 years as of 1 October 2012 who used VHA care in fiscal years (FYs) 2012 and 2013 (1 October 2011 to 30 September 2013). Diabetes was defined based on two or more ICD-9 Clinical Modification (CM) codes for diabetes (250.xx) associated with clinical face-to-face outpatient care on separate calendar days or receipt of any antihyperglycemic medication prescription (insulin, sulfonylurea, biguanide, α-glucosidase inhibitor, dipeptidyl peptidase 4 inhibitor, incretin mimetics, meglitinide, or thiazolidinedione). We then retained those with an HbA1c test performed in VHA laboratories in FY 2013 who received antihyperglycemic agents other than metformin only, consistent with its recommendation as first-line therapy and favorable safety profile (2–5). Patients were included only if they had active prescriptions for antihyperglycemic medications within 60 days of their last HbA1c value in FY 2013 to increase the likelihood that they were receiving active therapy. Finally, patients with at least one specified comorbidity were included.
Data Sources
We used VHA inpatient and outpatient encounter files and laboratory and medications data.
Outcome Measures
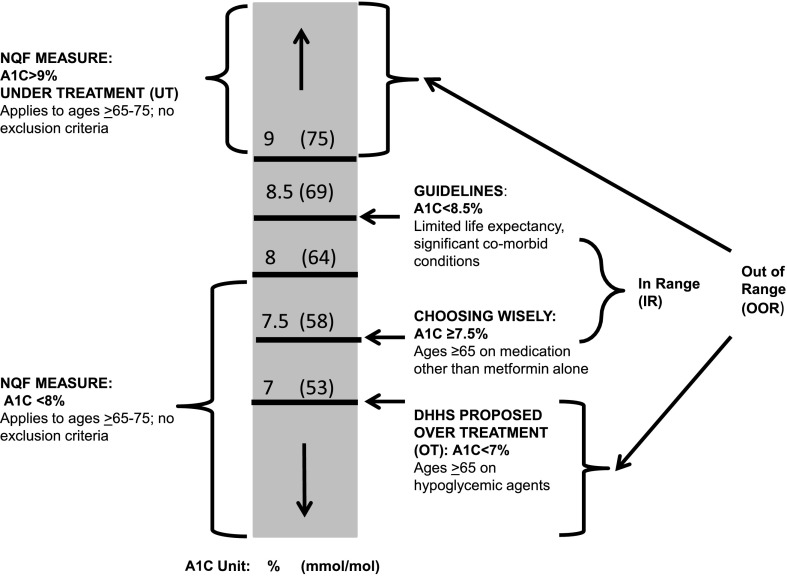
Patient-level rates and facility-level rankings on OOR (HbA1c <8% [64 mmol/mol]) measures and IR control were determined in specified subpopulations of increasing comorbid illness burden. Our conceptual framework (Fig. 1) maps the proposed OOR accountability measure to existing and proposed performance measures, whereas the IR quality improvement component is consistent with guideline recommendations to individualized target goals.
Figure 1.
Conceptual framework for the development of OOR and IR glycemic measures for adults aged ≥65 years. The framework incorporates existing national performance measures with federal agency and professional society recommendations.
For the OOR measure, we used the NQF-endorsed measures that apply to the 65–75-year-old population (10). We chose the HbA1c <8% (64 mmol/mol) threshold as the comparator because that is the glycemic performance included in the NQF Optimal Diabetes Care composite measure (NQF 0729), which requires that all component indicators be achieved to receive credit for passing (10). We chose the current HbA1c >9% (75 mmol/mol) poor control measure (NQF 0059) as UT.
Our justification for defining OT as HbA1c <7% (53 mmol/mol) for older adults with clinically significant conditions who take antihyperglycemic medications, especially those that are hypoglycemia prone, is twofold. First, the NQF measure assessing the percentage of patients aged <65 years who achieve this level of control excludes those with specific comorbid conditions, including cardiovascular disease, advanced diabetes complications, and cognitive impairment (NQF 0731) (10). Parenthetically, medication use is not part of the NQF measure. Second, the DHHS-NAP recommends this threshold as a balancing measure for patients on antihyperglycemic agents with an increased risk of hypoglycemia (6).
We defined an IR glycemic control quality improvement component as an HbA1c between 7.5 and 8.5% (58–69 mmol/mol). This range is consistent with conclusions from multiple guidelines that intensive antihyperglycemic therapy, especially the use of insulin, to achieve A1C values <7% (58 mmol/mol) is not warranted in older adults with substantial comorbid illness burden (2–5,14,15). An upper threshold of up to 8.5% (69 mmol/mol) for individuals with limited life expectancy and complex comorbid conditions as well prior episodes of or high risk for severe hypoglycemic events is consistent with current guideline recommendations from the 2016 ADA Standards of Care (14) and the 2015 ADA/European Association for the Study of Diabetes position statement (15).
A range can accommodate the inability of administrative data to assess disease severity, including activities of daily living. Additionally, it is not possible to assess social determinants that increase the risk for hypoglycemia, including patient preferences for medications and lifestyle, food insecurity, and intercurrent illnesses. Furthermore, the true value of an HbA1c test result is within a range in which the magnitude of the upper and lower bounds depends on the performance quality of the individual laboratory (16). Indeed, sequential HbA1c values that are <0.5% units (5.5 mmol/mol) of each other may not be statistically significant (17). We further note that the proposed IR also encompasses the upper and lower limits of an A1C value of 8% (64 mmol/mol).
We determined comorbidity categories for the denominator of the OOR measure based on specified baseline (FY 2012) diagnoses of chronic kidney disease (serum creatinine >1.7 mg/dL) and significant comorbid conditions using previously published ICD-9-CM taxonomies (18). Significant medical health conditions included four major categories: 1) conditions associated with diminished life expectancy (end-stage hepatic disease and cancer, excluding basal and squamous skin cancers), 2) advanced complications of diabetes (advanced/proliferative retinopathy, end-stage renal disease, lower-extremity amputation), 3) neurological conditions that could increase the risk for or impair response to symptomatic hypoglycemia (gastroparesis, Parkinson disease, aphasia, dysphasia, hemiplegia, apraxia, epilepsy, transient ischemic attack, cognitive impairment, and dementia), and 4) cardiovascular diseases (congestive heart failure, myocardial infarction, and ischemic vascular disease). We had previously reported that among patients <65 years of age, the 5-year unadjusted mortality rate within these stratifications was 45.0% for patients characterized as having decreased life expectancy, 35.7% for those with advanced complications, and 24.2% for those with neurological conditions (19). We also included two mental health conditions, major depression and alcohol and/or substance abuse, that could affect the risk for and/or response to hypoglycemia. To standardize the facility population for evaluation of facility-level variation, we assigned patients to the VHA facility where their last HbA1c test in FY 2013 was performed.
Statistical Analysis
We conducted statistical analyses by age-group for the study population. We calculated the rates of all studied glycemic control measures for various nested subpopulations based on comorbid conditions. To evaluate facility variation in glycemic control, we reported variation in descriptive statistics for facility-level rates for all measures. We also evaluated variation according to the coefficient of variation (CV), which measures relative variation to the mean.
We ranked facility-level rates according to the OOR and <8% (64 mmol/mol) measures. We selected three groups from 139 facilities based on the OOR ranking results: the best-performing 10%, the median-performing 10%, and the worst-performing 10%. Lower rates of OOR correspond to better performance, and higher rates indicate worse performance. In addition to graphic visualization of facility variation and relationships among the HbA1c measures, we obtained Spearman rank correlation coefficients (ρ) between HbA1c measures. Finally, we assessed the movements of facilities in ranking deciles on the basis the OOR and <8% (64 mmol/mol) measures. The study was approved by the VA New Jersey Health Care System institutional review board.
Results
We identified 1,036,912 patients with diabetes who were alive as of 1 October 2012 and used the VHA for clinical outpatient care during FYs 2012 and 2013. There were 677,045 (65.3%) who were aged ≥65 years, of whom 574,492 (84.6%) had an HbA1c test performed in VHA laboratories in FY 2013. We excluded 190,920 who had no prescriptions for diabetes medications (33.2%) within 60 days of the last HbA1c test in FY 2013 as well as an additional 80,475 (14%) who were taking metformin only during that time; we retained the remaining 303,097 (52.85%) patients. Overall, 98.2% were prescribed insulin and/or sulfonylureas; use of insulin alone and sulfonylureas alone was observed in 30.1% and 17.5%, respectively.
The characteristics of these 303,097 patients are shown in Supplementary Table 1. There were 58.3% aged 65–75 years, and 41.7% were >75 years. The population was 98.7% male with high degrees of comorbidity. At least one comorbid condition, including elevated serum creatinine, was present in 60.4% of patients 65–75 years, and 68.7% of those were >75 years. These conditions included diminished life expectancy (11.5 and 15%), advanced diabetes complications (9.1 and 11.0%), cognitive impairment/dementia (5.4% and 11.5%), and cardiovascular disease (39.0 and 46.6%). There were 61.1 and 53.8% who received insulin in their respective age-groups.
We excluded 110,608 patients without a specified comorbid illness. The final study population comprised 106,866 patients aged 65–75 years and 86,823 aged >75 years who had at least one specified comorbid condition (Table 1, group H). The numbers of patients in the nested high-risk subpopulations and the incremental increase resulting from adding an additional category of diseases are presented for groups A–H. For example, the incremental change of adding cognitive impairment to those with elevated serum creatinine (group B) was 14.3% of the study population for those aged ≥75 years and 7.9% for those aged 65–75 years, whereas the addition of major depression and substance use (groups F and G) had higher incremental change in younger (11.0%) than in older (1.8%) individuals. Even though cardiovascular disease was the last comorbid condition added, it had the greatest incremental change in the study population (group H) (37.2% in those 65–75 years and 35.1% for those >75 years).
Table 1.
Rates of various HbA1c measures in nested high-risk groups of the study population* based on comorbidities
| Rates of HbA1c measures for each denominator |
||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Incremental change | Denominator | OT¶ | NQF# | UT** | OOR†† | IR‡‡ | |
| Aged 65–75 years | ||||||||
| A | Creatinine ≥1.7 mg/dL | 16,395 (15.3) | 36.5 | 67 | 13.2 | 49.7 | 27.3 | |
| B | A + cognitive impairment or dementia | 8,441 (7.9) | 24,836 (23.2) | 36.3 | 66.7 | 13.7 | 50 | 26.9 |
| C | B + advanced diabetes complications† | 11,983 (11.2) | 36,819 (34.5) | 33.6 | 64.8 | 14.6 | 48.2 | 28 |
| D | C + diminished life expectancy‡ | 15,802 (14.8) | 52,621 (49.2) | 34.7 | 66.1 | 13.8 | 48.5 | 27.5 |
| E | D + other neurological conditions§ | 2,710 (2.5) | 55,331 (51.8) | 34.8 | 66.2 | 13.8 | 48.6 | 27.5 |
| F | E + major depression | 7,303 (6.8) | 62,634 (58.6) | 34.6 | 66.1 | 14.0 | 48.5 | 27.5 |
| G | F + alcohol/drug abuse | 4,503 (4.2) | 67,137 (62.8) | 34.8 | 66.1 | 14.1 | 48.9 | 27.3 |
| H | G + cardiovascular diseases‖ (study population*) | 39,729 (37.2) | 106,866 (100) | 33.4 | 65.7 | 14.0 | 47.4 | 28.2 |
| Aged >75 years | ||||||||
| A | Creatinine ≥1.7 mg/dL | 18,830 (21.7) | 41.4 | 73.6 | 8.5 | 49.9 | 26.5 | |
| B | A + cognitive impairment or dementia | 12,433 (14.3) | 31,263 (36.0) | 40.5 | 72.6 | 9.4 | 49.9 | 26.3 |
| C | B + advanced diabetes complications† | 9,526 (11.0) | 40,789 (47.0) | 39.7 | 72.3 | 9.6 | 49.3 | 26.6 |
| D | C + diminished life expectancy‡ | 12,439 (14.3) | 53,228 (61.3) | 40.2 | 73.1 | 9.3 | 49.5 | 26.3 |
| E | D + other neurological conditions§ | 1,524 (1.8) | 54,752 (63.1) | 40.2 | 73.1 | 9.2 | 49.4 | 26.4 |
| F | E + major depression | 1,077 (1.2) | 55,829 (64.3) | 40.1 | 73.1 | 9.3 | 49.4 | 26.4 |
| G | F + alcohol/drug abuse | 539 (0.6) | 56,368 (64.9) | 40.2 | 73.1 | 9.3 | 49.5 | 26.4 |
| H | G + cardiovascular diseases‖ (study population*) | 30,455 (35.1) | 86,823 (100) | 39.2 | 73.2 | 8.9 | 48.1 | 27.1 |
Data are n (%) or %. To convert serum creatinine to micromoles per liter, multiply by 88.4.
*The study population comprised patients with diabetes and an HbA1c value available in FY 2013 who were taking any antiglycemic agent other than metformin alone within 60 days before their last HbA1c in FY 2013 and who had at least one of the studied comorbidities.
†Advanced diabetic complications include end-stage renal disease, amputation, and advanced retinopathy.
‡Diminished life expectancy includes end-stage hepatic disease and cancer (excluding basal and squamous skin cancers).
§Other neurological conditions include gastroparesis, Parkinson disease, aphasia, dysphasia, hemiplegia, apraxia, epilepsy, and transient ischemic attack.
‖Cardiovascular diseases include myocardial infarction, chronic heart failure, and ischemic vascular disease.
¶OT, <7% (53 mmol/mol).
#NQF, <8% (64 mmol/mol).
**UT, >9% (75 mmol/mol).
††OOR, <7% (53 mmol/mol) or >9% (75 mmol/mol).
‡‡IR, 7.5–8.5% (58–69 mmol/mol).
The percentage of patients in the study population (group H) with HbA1c thresholds of <6.5% (47.5 mmol/mol) (data not shown), <7% (53 mmol/mol), <8% (64 mmol/mol), and >9% (75 mmol/mol) were 17.5, 33.4, 65.7, and 14.0%, respectively, for those aged 65–75 years and 20.9, 39.2, 73.2, and 8.9% for those aged >75 years. There were 47.4% of patients 65–75 years with an HbA1c <7% (53 mmol/mol) or >9% (75 mmol/mol) and 28.2% with an HbA1c in the range of 7.5–8.5 (58–69 mmol/mol). For patients >75 years, the respective percentages were 48.1% and 27.1%.
Table 2 shows the distributions of facility-level rates for various HbA1c measures based on patients with at least one of the studied comorbidities (Table 1, group H) Consistent with the overall findings from the patient-level analysis (Table 1), there were higher OT rates (mean 39.3 vs. 33.6% for HbA1c <7% [53 mmol/mol]) and lower UT rates (8.9 vs. 13.9% for HbA1c >9% [75 mmol/mol]) for patients aged ≥75 years than their younger counterparts.
Table 2.
Distribution of facility-level rates of various HbA1c measures among the study population by age-group
| 65–75 Years |
>75 Years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c measure | Median | Min. | Max. | Mean | SD | CV | Median | Min. | Max. | Mean | SD | CV |
| <7% (53 mmol/mol) | 33.0 | 23.4 | 49.9 | 33.6 | 5.0 | 14.8 | 38.6 | 24.1 | 59.3 | 39.3 | 2.5 | 6.3 |
| <8% (64 mmol/mol) | 66.0 | 54.5 | 77.3 | 65.8 | 4.3 | 6.6 | 73.2 | 60.0 | 83.2 | 73.2 | 1.4 | 1.9 |
| >9% (75 mmol/mol) | 13.8 | 7.6 | 21.6 | 13.9 | 2.6 | 18.6 | 8.7 | 4.2 | 17.4 | 8.9 | 4.2 | 46.4 |
| 7.5–8.5% (58–69 mmol/mol) | 28.0 | 18.4 | 36.5 | 28.2 | 3.0 | 10.7 | 27.0 | 18.2 | 38.5 | 27.0 | 2.1 | 7.8 |
| <7% (53 mmol/mol) or >9% (75 mmol/mol) | 47.4 | 33.7 | 60.4 | 47.4 | 4.2 | 8.8 | 47.9 | 35.3 | 64.3 | 48.2 | 1.8 | 3.8 |
Data are percentage A1C unless otherwise indicated. The study population comprised patients with diabetes with an HbA1c value available in FY 2013 who were taking any antiglycemic agent other than metformin alone within 60 days before their last HbA1c in FY 2013 and who had at least one of the studied comorbidities. Max., maximum; Min., minimum.
Overall, marked facility-level variation was observed. For example, for patients 65–75 years of age, facility-level OOR rates varied from 33.7 to 60.4%. HbA1c <7% (53 mmol/mol) rates varied from 23.4 to 49.9%, IR rates varied from 18.4 to 36.5%, and HbA1c >9% (75 mmol/mol) rates varied from 7.6 to 21.6%. The maximum rate was nearly threefold of the minimum rate for HbA1c >9% (75 mmol/mol) and close to twofold for other measures. Use of a lower (more stringent) threshold of HbA1c (<6.5% [47.5 mmol/mol]) had only a modest effect. Facility OOR rates varied from 19.7 to 42.4% for patients 65–75 years and from 11.2 to 40.7% for patients ≥75 years. The variation to the mean was greatest for HbA1c >9% (75 mmol/mol) (CV 18.6 in the 65–75-year and 46.4 in the ≥75-year age-groups). Facility variation was generally greater for patients 65–75 years (except for HbA1c >9% [75 mmol/mol]).
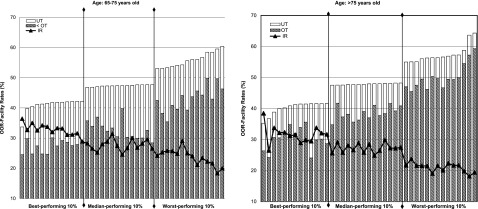
We assessed the relationships among facility-level performance rankings based on various HbA1c measures. Figure 2 shows that within each performance group (based on facility-level OOR measure) and across the selected groups, IR rates decreased along with increased OOR rates, indicating an inverse relationship (ρ = −0.83 [P < 0.01] for both age-groups based on all facilities). Facility-level rates for OT (HbA1c <7% [53 mmol/mol]) increased with increased OOR rates, indicating a positive relationship of the rates but a negative relationship between performance rankings (ρ = −0.82 for the 65–75-year and −0.91 for the >75-year age-groups; P < 0.01). However, no obvious pattern for the relationship was found between UT and OOR (ρ = 0.03 [P = 0.70] and 0.08 [P = 0.33], respectively).
Figure 2.
Facility-level rates of HbA1c measures for selected performance groups based on facility rankings from the OOR measure. The study population included patients with diabetes and an HbA1c value available in FY 2013 who were taking any antihyperglycemic agent other than metformin alone within 60 days before their last HbA1c in FY 2013 and who had at least one of the studied comorbidities. Facilities were ranked based on the OOR measure (HbA1c <7% [53 mmol/mol]; HbA1c >9% [75 mmol/mol]). Lower rates correspond to better performance. Three groups were presented: the best-performing 10%, median-performing 10%, and worst-performing 10%.
We found that the facility-level rankings between HbA1c <8% (64 mmol/mol) and OOR measures were moderately correlated (ρ = −0.41 and −0.54; P < 0.01 for both) for the two age-groups. We further assessed the movements of facilities in ranking deciles on the basis of these two measures and observed that 14 of the 28 facilities ranked in the best-performing 20% based on HbA1c <8% (64 mmol/mol) were ranked in the worst-performing 20% based on their OOR rates (data not shown). On the other hand, 12 of the 27 facilities ranked in the worst-performing 20% based on HbA1c <8% (64 mmol/mol) were ranked in the best-performing 20% based on their ORR rates (data not shown).
Conclusions
The findings support two conclusions with differing implications regarding glycemic control for patients 65–75 years of age, all of whom had at least one significant comorbid condition and almost all of whom received agents that are hypoglycemia prone (i.e., insulin, sulfonylureas). On the basis of the NQF-endorsed HbA1c <8% (64 mmol/mol) measure (10), nearly two-thirds (65.7%) of all patients were receiving quality care. Alternatively, on the basis of the OOR measure, about one-half (47.4%) were significantly UT or OT according to current guideline recommendations. The magnitude is comparable for patients >75 years of age, who are not included in NQF-endorsed glycemic measures. Only 28.4% of patients aged 65–75 years and 27.1% of those >75 years had HbA1c results between 7.5 and 8.5% (58–69 mmol/mol), which we defined as IR. In a sensitivity analysis, we noted higher rates of an even-lower HbA1c threshold <6.5% (47.5 mmol/mol) than poor A1C (>9% [75 mmol/mol]) control.
These results highlight that the determination of quality rankings depends on the perspective taken and technical specifications of measures. Our perspective is that a major clinical focus of glycemic management for an older population, especially those with significant disease burden, should address the risk for and/or presence of symptoms and adverse events related to either glycemic control or medications. Therefore, we propose designating the OOR measure as an accountability patient safety measure to address a population health need not currently addressed by endorsed measures. Our proposal also is consistent with the need to increase clinician and patient awareness that an A1C test result is within a range (16,17).
The emphasis on prevention of short-term medication harms is consistent with the NQF-endorsed patient safety measure, which has no upper age limit and is used by the Centers for Medicare & Medicaid Services to monitor the anticoagulant effect of Coumadin using the international normalized ratio (20). Maintaining the international normalized ratio within a range is the goal for maximizing benefit (i.e., protect patients from blood clots) while minimizing risk (i.e., risk of hemorrhage attributable to excessive anticoagulation).
However, it is important from both an individual patient and a population health perspective to balance avoidance of harms with decreasing long-term risk of microvascular complications. Therefore, the availability of an IR quality improvement measure would enable most high-risk patients to be managed safely over time within a range appropriate for and agreed to by the individual patient in the context of shared decision making (2–6).
This approach is consistent with the major randomized controlled trials of intensive treatment of patients with a longer duration of diabetes (21–23). These trials demonstrated that the absolute risk reduction (benefits) of HbA1c lowering on microvascular complications are modest over 5–10-year periods and were most significant for the onset and progression of renal disease. Although major guidelines differ about how comorbid severity is defined, all recommend that glycemic targets reflect illness burden, life expectancy, and individual risk for hypoglycemic events.
The prevalence of significant comorbid illness in older adults with diabetes in the VA is similar in magnitude, given methodological differences in illness severity ascertainment, to the comorbid illness burden in the U.S. A recent study that used the National Health and Nutrition Examination Survey (NHANES), which is representative of the nation’s noninstitutionalized civilians, reported that of older adults (>65 years; mean 74 years) with diabetes, 50.7% were relatively healthy, 28.1% had complex/intermediate health, and 21.2% had very complex/poor health (24). Very complex health status was defined as having two or more impairments in activities of daily living or receiving dialysis. A study limitation was that the prevalence of poor health was underestimated because conditions such as end-stage (stage III–IV) congestive heart failure, oxygen-dependent lung disease, and metastatic cancer could not be ascertained. Complex health status was defined as three or more chronic conditions, including congestive heart failure, lung disease, significant-stage chronic kidney disease, coronary heart disease, stroke, urinary incontinence, major depression, active cancer, and substance abuse.
Additionally, substudies from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that hypoglycemic risk is not limited to severe illnesses or even to patients with very low A1C levels. Even modest elevations of serum creatinine levels (>1.3 mg/dL) and mildly impaired cognitive impairment were associated with an increased risk of severe hypoglycemia in both the control and the treatment arms (25,26). Such factors may contribute to episodes of severe hypoglycemia in patients with higher HbA1c values in clinical practice (27).
Lower socioeconomic status (28) and decreased health literacy (29) were associated with an increased risk of hypoglycemia, even in members of a large regional health maintenance organization. The risk for hypoglycemia-related hospital admissions was 27% higher in the last week of the month relative to the first week in low-income patients compared with high-income patients in California (29). One proposed explanation for this finding was food insufficiency (30). The NQF is studying the use of socioeconomic factor risk adjustment for plan comparisons (31). However, clinicians must make treatment decisions that take into account factors not easily ascertained, including the capacity for diabetes self-management, social support, and finances.
Given the complexity of glycemic management in a high-risk adult population, it is perhaps not surprising that practicing physicians’ incorporation of guidelines into practice varies considerably. In a recent scenario-based survey evaluating endocrinologists’ perspectives on individualized glycemic target goals based on clinical factors, wide variation existed, with many choosing HbA1c targets both lower and greater than 8% (64 mmol/mol), even in patients with significant comorbid conditions (32). In addition, individual patient preferences differ. For example, patients have ranked gastrointestinal effects as more important than hypoglycemia (33). Some older adults express a preference to forego insulin therapy even if this decision would shorten their life expectancy (34).
We recognize that management of diabetes continues to evolve and that we can expect the development of new and more effective medications. These medications include those with lower risks of hypoglycemia (e.g., dipeptidyl peptidase 4 inhibitors, glucagon-like peptide 1 receptor agonists, and sodium–glucose cotransporter 2 inhibitors) and/or those that may have additional benefits above and beyond their antihyperglycemic effects. A recent Food and Drug Administration safety review relaxed the restrictions on metformin usage in chronic kidney disease (35). Therefore, consistent with evolving recommendations for antihyperglycemic medications (14,15,36), future measures of glycemic control should exclude patients who are not receiving hypoglycemia-prone drugs. Reflecting prescribing practices in 2012–2013, >98% of our study population received sulfonylurea agents and/or insulin.
However, there will always be existing and new safety concerns. Balancing the benefits and risks of polypharmacy in older adults with diabetes, therefore, is important (37). In addition to hypoglycemia, other adverse outcomes could include nausea, dehydration, urinary tract infection, and worsening renal function that could result in potentially preventable emergency department visits or hospitalizations in vulnerable populations.
This study has significant strengths, including linked demographic, laboratory, pharmacy, and administrative data, which have enabled us to identify patients taking any combination of antihyperglycemic medications as well as to identify whether the prescription was current. We also could identify serum creatinine values in the population. We note as limitations an inability to assess the severity of comorbid conditions. Although the use of ICD-10-CM taxonomies is expected to permit better discrimination of more severe stages of common conditions, including cardiac, pulmonary, and liver disease, than is possible with ICD-9-CM coding, significant differences exist in comparability (38). Consequently, our taxonomy, technical specifications, and findings will need further validation. We also note a lack of prospective outcome data to know whether OOR measures identify patients who subsequently experience significant adverse events.
In conclusion, our proposed OOR measure attempts to align the concepts of quality (individualizing targets on the basis of the principle of absolute risk reduction), safety (potential reduction in medication harms), and value to health care systems and payers (potential decreased costs of both OT and UT) and to patients (improved quality of life, satisfaction). Our approach is consistent with the recent Centers for Medicare & Medicaid Services proposed rule for addressing medication safety for beneficiaries taking hypoglycemic agents in support of the Merit-based Incentive Payment System and Alternative Payment Models (39). The proposed measure would focus on patients prescribed antidiabetic agents (e.g., insulin, sulfonylureas) and require physicians to document an individualized glycemic treatment goal that takes into account patient-specific factors, including age, comorbidities, and risk for hypoglycemia at least annually. This approach also emphasizes, in an increasingly data driven, technological medical age, that the needs, preferences, and safety of the patient must always be individualized and should always be paramount (40).
Supplementary Material
Article Information
Acknowledgments. The authors thank Mazhgan Rowneki, VA New Jersey Health Care System, East Orange, NJ, for assistance in manuscript submission.
Funding. This work was funded by a VHA Health Services Research & Development (HSRD) Diabetes Quality Enhancement Research Initiative grant RRP 12-492 to L.P. and a VA HSRD grant IIR 11-077 to C.-L.T. The VHA played no role in the analyses or decision to submit the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.P. initiated the study, contributed to the study design, researched data, and wrote, reviewed, and edited the manuscript. C.-L.T. contributed to the study design, researched data, and wrote, reviewed, and edited the manuscript. O.S. researched data, contributed to the discussion, and reviewed/edited the manuscript. M.M. researched data, contributed to the discussion, and reviewed/edited the manuscript. D.A. contributed to the study design and wrote, reviewed, and edited the manuscript. L.P. and C.-L.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015; Preventing Overdiagnosis Conference, Bethesda, MD, 1–3 September 2015; Academy Health Research Meeting, Boston, MA, 25–28 June 2016; and the Guideline International Network Conference, Philadelphia, PA, 27–30 September 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0953/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Centers for Disease Control and Prevention. National diabetes statistics report [Internet], 2014. Available from http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html. Accessed 18 September 2016
- 2.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association; European Association for the Study of Diabetes . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Veterans Affairs/Department of Defense. Management of diabetes mellitus (DM): guideline summary [Internet], 2010. Available from http://www.healthquality.va.gov/guidelines/CD/diabetes/DM2010_SUM-v4.pdf. Accessed 10 August 2016
- 5.AGS Choosing Wisely Workgroup. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc 2014;62:950–960 [DOI] [PubMed]
- 6.Department of Health and Human Services. National action plan for adverse drug event prevention: diabetes agents [Internet], 2014. Available from http://www.health.gov/hcq/pdfs/ADE-Action-Plan-Diabetes-Agents.pdf. Accessed 18 September 2016
- 7.Pogach L, Aron D. Balancing hypoglycemia and glycemic control: a public health approach for insulin safety. JAMA 2010;303:2076–2077 [DOI] [PubMed] [Google Scholar]
- 8.Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014;174:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014;174:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Quality Forum. National quality forum-endorsed measures for endocrine conditions, 2013–2015: final report. Available from http://www.qualityforum.org/Publications/2015/11/NQF-Endorsed_Measures_for_Endocrine_Conditions_Final_Report.aspx. Accessed 10 August 2016
- 11.Aron D, Pogach L. One size does not fit all: a continuous measure for glycemic control in diabetes: the need for a new approach to assessing glycemic control. Jt Comm J Qual Improv 2007;33:636–643 [DOI] [PubMed] [Google Scholar]
- 12.Pogach L, Aron D. The other side of quality improvement in diabetes for seniors: a proposal for an overtreatment glycemic measure. Arch Intern Med 2012;172:1510–1512 [DOI] [PubMed] [Google Scholar]
- 13.Aron DC. No “black swan”: unintended but not unanticipated consequences of diabetes performance measurement. Jt Comm J Qual Patient Saf 2013;39:106–108 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S52–S5926696682 [Google Scholar]
- 15.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Diabetes and Digestive and Kidney Diseases. Comparing tests for diabetes and prediabetes: a quick reference guide [Internet], 2014. Available from https://www.niddk.nih.gov/health-information/health-topics/diagnostic-tests/comparing-tests-diabetes-prediabetes/Documents/Comparing_Tests_for_DM_508.pdf. Accessed 18 September 2016
- 17.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011;57:e1–e47 [DOI] [PubMed] [Google Scholar]
- 18.Tseng CL, Soroka O, Maney M, et al. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med 2014;174:259–268 [DOI] [PubMed] [Google Scholar]
- 19.Pogach LM, Tiwari A, Maney M, Rajan M, Miller DR, Aron D. Should mitigating comorbidities be considered in assessing healthcare plan performance in achieving optimal glycemic control? Am J Manag Care 2007;13:133–140 [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. Quality measures [Internet], 2016. Available from https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html?redirect=/QUALITYMEASURES/. Accessed 18 September 2016
- 21.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 22.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 24.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller ME, Bonds DE, Gerstein HC, et al.; ACCORD Investigators . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punthakee Z, Miller ME, Launer LJ, et al.; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathak RD, Schroeder EB, Seaquist ER, et al.; SUPREME-DM Study Group . Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. Integrated Health Care Delivery Systems: 2005-2011. Diabetes Care 2016;39:33–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkowitz SA, Karter AJ, Lyles CR, et al. Low socioeconomic status is associated with increased risk for hypoglycemia in diabetes patients: the Diabetes Study of Northern California (DISTANCE). J Health Care Poor Underserved 2014;25:478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar U, Karter AJ, Liu JY, Moffet HH, Adler NE, Schillinger D. Hypoglycemia is more common among type 2 diabetes patients with limited health literacy: the Diabetes Study of Northern California (DISTANCE). J Gen Intern Med 2010;25:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood) 2014;33:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Quality Forum. Risk adjustment for socioeconomic status or other sociodemographic factors [Internet], 2014. Available from http://www.qualityforum.org/Publications/2014/08/Risk_Adjustment_for_Socioeconomic_Status_or_Other_Sociodemographic_Factors.aspx. Accessed 18 September 2016
- 32.Cahn A, Raz I, Kleinman Y, et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care 2015;38:2293–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purnell TS, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care 2014;37:2055–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care 2008;46:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function [Internet]. Available from http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed 18 September 2016
- 36.American Association of Clinical Endocrinologists; American College of Endocrinology. AACE/ACE comprehensive type 2 diabetes management algorithm 2016 [Internet], 2016. Available from https://www.aace.com/publications/algorithm. Accessed 10 August 2016
- 37.Lipska KJ, Krumholz H, Soones T, Lee SJ. Polypharmacy in the aging patient: a review of glycemic control in older adults with type 2 diabetes. JAMA 2016;315:1034–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenton SH, Benigni MS. Projected impact of the ICD-10-CM/PCS conversion on longitudinal data and the Joint Commission Core Measures. Perspect Health Inf Manag 2014;11:1g. Available from http://perspectives.ahima.org/projected-impact-of-the-icd-10-cmpcs-conversion-on-longitudinal-data-and-the-joint-commission-core-measures/#.VxzO6jHkoXg. Accessed 18 September 2016 [PMC free article] [PubMed]
- 39.Department of Health and Human Services; Centers for Medicare & Medicaid Services. 42 CFR Parts 414 and 495: Medicare program; Merit-based Incentive Payment System (MIPS) and Alternative Payment Model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models [proposed rule]. 81 Fed Reg 28162 (9 May 2016). Available from https://www.gpo.gov/fdsys/pkg/FR-2016-05-09/pdf/2016-10032.pdf. Accessed 18 September 2016 [PubMed]
- 40.Sarosi GA. The tyranny of guidelines. Ann Intern Med 2015;163:562–563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.