Abstract
Dynamic adjustment of insulin secretion to compensate for changes of insulin sensitivity that result from alteration of nutritional or metabolic status is a fundamental aspect of glucose homeostasis. To investigate the role of the brain in this coupling process, we used cold exposure as an experimental paradigm because the sympathetic nervous system (SNS) helps to coordinate the major shifts of tissue glucose utilization needed to ensure that increased thermogenic needs are met. We found that glucose-induced insulin secretion declined by 50% in rats housed at 5°C for 28 h, and yet, glucose tolerance did not change, owing to a doubling of insulin sensitivity. These potent effects on insulin secretion and sensitivity were fully reversed by returning animals to room temperature (22°C) for 4 h or by intravenous infusion of the α-adrenergic receptor antagonist phentolamine for only 30 min. By comparison, insulin clearance was not affected by cold exposure or phentolamine infusion. These findings offer direct evidence of a key role for the brain, acting via the SNS, in the rapid, highly coordinated, and reciprocal changes of insulin secretion and insulin sensitivity that preserve glucose homeostasis in the setting of cold exposure.
Introduction
The capacity to adjust insulin secretion to compensate for changes of systemic insulin sensitivity is an essential but poorly understood aspect of glucose homeostasis (1–3). In insulin-resistant conditions such as obesity (3), pregnancy (4), or adolescence (5), for example, insulin secretion must increase to maintain euglycemia, and the reverse applies to conditions of increased insulin sensitivity (1–3,6). Failure to mount an appropriate pancreatic β-cell response to worsening insulin resistance is a hallmark of the progression from normal glucose tolerance to type 2 diabetes (T2D) (7).
The concept that insulin sensitivity and insulin secretion are reciprocally regulated stems from human studies in which plasma glucose and insulin levels measured during a frequently sampled intravenous glucose tolerance test (FSIGT) were analyzed by the minimal model method. This approach generates reliable estimates of key determinants of glucose tolerance: insulin secretion (typically measured as the acute insulin response to glucose [AIRG]), insulin sensitivity (based on the insulin sensitivity index [SI]), and glucose effectiveness (SG), a measure of glucose disposal at basal insulin (independent of an increment in insulin secretion), which can be further separated into the basal insulin effect (BIE) and glucose effectiveness at zero insulin (GEZI). The relationship between SI and AIRG is hyperbolic in humans, such that the product of the two, referred to as the “disposition index” or DI, a measure of the net contribution made by insulin on glucose levels during the FSIGT, tends to remain constant irrespective of the prevailing level of insulin sensitivity (3), which provides for stable glucose homeostasis in the face of diverse internal and external challenges to tissue glucose utilization (6).
The coupling of insulin secretion to insulin sensitivity cannot be explained by changes of plasma glucose levels (8). Although rising nocturnal plasma levels of nonesterified fatty acids (NEFA) were identified as a potential mechanism linking increased insulin secretion to obesity-induced insulin resistance (9), the extent to which this mechanism contributes to the hyperbolic relationship between insulin secretion and insulin sensitivity remains unknown. To investigate the role of the brain in this coupling mechanism, we sought to interrogate its contribution to the adaptive metabolic response to a physiological challenge. To this end, we used cold exposure as a provocative intervention, based on the following considerations: 1) maintenance of core body temperature in cold environments depends on temperature-sensitive hypothalamic neurocircuits that increase sympathetic nervous system (SNS) outflow to thermogenic tissues (10,11); 2) cold exposure increases SNS outflow to the pancreas (12), which likely explains the associated reduction of insulin secretion (via a mechanism involving activation of α-adrenergic receptors on pancreatic β-cells [13–15]); and 3) glucose tolerance does not change or is improved despite the effect of cold exposure to decrease insulin secretion (16,17). Although the latter observation can be accounted for by the known effect of cold exposure to increase insulin sensitivity in thermogenic tissues (17–19), the goal of the current work was to determine whether the brain serves to ensure that insulin secretion declines in a manner that preserves glucose homeostasis and averts hypoglycemia.
To this end, we measured the effect of cold exposure on determinants of glucose tolerance in rats using the FSIGT/minimal model approach in the presence and absence of an intravenous (i.v.) infusion of the α-adrenergic receptor (α-AR) blocker phentolamine. Because the proper interpretation of our data hinges on whether the relationship between insulin secretion and insulin sensitivity in rats comports with the hyperbolic relationship characteristic of humans, we also sought to create a normative rat database with which to interrogate this relationship. This goal was achieved by analyzing pooled data from FSIGT studies conducted in either of two strains of rat (Wistar or Long-Evans) fed a standard chow diet or a high-fat diet (HFD), some of which were included as normal control data in a previous publication (20).
We report that, as expected, the relationship between insulin sensitivity and insulin secretion in rats conforms to the hyperbolic function characteristic of humans. We further demonstrate that the potent effect of cold exposure to the increase of insulin sensitivity is perfectly offset by a proportionate decline of glucose-induced insulin secretion such that glucose tolerance remains unchanged. Moreover, the effects of cold exposure on insulin secretion and insulin sensitivity are rapidly reversed by systemic α-AR blockade. Together, these findings offer direct evidence of a key role for the brain in the highly coordinated, potent, and reciprocal adjustments of insulin sensitivity and insulin secretion that maintain stable glucose homeostasis during cold exposure.
Research Design and Methods
All procedures were performed in accordance with National Institutes of Health Guidelines for the Care and Use of Animals and were approved by the University of Washington Animal Care Committee.
Experimental Animals
Adult male Wistar and Long Evans rats (Harlan Laboratories, Indianapolis, IN) were individually housed under specific pathogen-free conditions in a temperature-controlled room with a 12:12 h light/dark cycle and provided ad libitum access to water and standard laboratory chow (PMI Nutrition, St. Louis, MO) or an HFD containing 60% kcal fat (D12492; Research Diets, Inc., New Brunswick, NJ), unless otherwise stated.
Surgery
Rats underwent implantation of catheters into the carotid artery and jugular vein and received buprenorphine hydrochloride (Reckitt Colman Pharmaceuticals, Richmond, VA) at the completion of the surgery as previously described (20–22).
FSIGT and Minimal Model Analysis
After an overnight fast, multiple blood samples were obtained from unrestrained, conscious animals via an arterial catheter as previously described (20–23). Plasma insulin and blood glucose levels generated from the FSIGTs were analyzed using MinMod software to quantify SG and SI as previously described (20,23,24). AIRG, the incremental area under the insulin curve during the FSIGT (AUCinsulin), the BIE, GEZI, and the glucose disappearance rate constant (KG) were calculated as previously described (20,23,24). A detailed description of the FSIGT and minimal model analysis is provided in the Supplementary Data.
Euglycemic-Hyperinsulinemic Clamp
To independently assess the effect of phentolamine on insulin sensitivity in cold-exposed rats, a euglycemic-hyperinsulinemic clamp was performed as previously described (21,22). A detailed description of the clamp procedure is provided in the Supplementary Data.
Analysis of Rates of Insulin Secretion and Insulin Clearance
Insulin clearance was estimated by first calculating the insulin secretion rate using deconvolution of the measured C-peptide concentrations, followed by calculation of the rate of insulin clearance as AUC(insulin secretion)/AUC(plasma insulin). The parameters for rat C-peptide kinetics were obtained from a kinetic study using 5 nmol/kg injections of rat C-peptide 2 in Sprague-Dawley rats. The C-peptide concentration data after the injection were well described by a one-compartment model with clearance = 29.7 mL/min/kg and volume = 656 mL/kg (25).
A Normative Database for Analysis of Relationships Between Insulin Sensitivity, Insulin Secretion, SG, and Glucose Tolerance in Rats
To create a normative database with which to analyze relationships between the determinants of glucose tolerance in rats, we assembled data from studies previously conducted in adult male rats in our laboratory that used the FSIGT and minimal model analysis protocol described above that included two different rat strains (Wistar [n = 54] or Long-Evans [n = 25]), provided ad libitum access to chow (n = 62) or an HFD (n = 17) for 5 days or 3 months before study, vehicle-treated animals from a previous study (20), and animals from the current studies.
A key goal was to determine whether the relationship between insulin sensitivity and insulin secretion conforms to a rectangular hyperbola in rats as it does in humans (2,3,26,27). To test this hypothesis, we used reduced major axis regression (RMA) with bootstrapped estimates of the SEs (28) (http://www.exetersoftware.com/cat/biomstat/book_biometry.html) to fit linear models for the relationship between a measure of insulin secretion (AIRG) or an integrated measure of the circulating insulin level during the FSIGT (AUCinsulin), which reflects insulin secretion and its clearance from plasma, and the inverse of insulin sensitivity (i.e., as a function of [1/SI]). For completeness, we also modeled insulin secretion equaling a regression slope times (1/sensitivity) without an intercept term using ordinary least squares regression (29), such that the product of insulin secretion and insulin sensitivity equals a constant. Comparison of the RMA versus intercept-excluded hyperbolic fits enabled a comprehensive qualitative and quantitative assessment of the extent to which the “hyperbolic law” applies to rats.
Effect of Cold Exposure on Determinants of Glucose Tolerance
Animals matched by body weight were housed at room temperature (22°C, n = 13) or in a cold environment (5°C, n = 13) for 28 h without access to food before a FSIGT conducted at these same temperatures. Experiments were performed using a crossover design, such that each animal was studied at each temperature separated by at least 10 days. To assess the durability of cold-induced changes in determinants of glucose tolerance, separate cohorts of rats (n = 9/group) were housed for 24 h at room temperature or at 5°C, and then housed at room temperature for an additional 4 h before and during an FSIGT.
Role of the SNS on Determinants of Glucose Tolerance in Cold-Exposed Rats
To determine whether α-AR signaling is required for the effects of cold exposure on AIRG and SI, rats with chronic indwelling catheters were exposed to the cold (5°C) for 28 h without access to food. At 30 min before the FSIGT, a continuous i.v. infusion of the nonselective α-AR blocker phentolamine at a dose of 8 μg/kg/min (identified in pilot studies as a dose that has minimal effects on mean arterial pressure and heart rate) (Supplementary Fig. 1) or vehicle (n = 9/group) was commenced, with infusion continuing throughout the FSIGT.
Effect of Phentolamine on Determinants of Insulin Action in Cold-Exposed Rats
After implantation of catheters, adult male Wistar rats were exposed to the cold (5°C) for 28 h without access to food, and a euglycemic-hyperinsulinemic clamp was performed (21,22). Although tracer dilution analysis using tritiated glucose was included in the study design, the data are not included owing to technical problems that confound data interpretation. Animals received a continuous i.v. infusion beginning at t = −30 min of phentolamine (8 μg/kg/min) or its vehicle. A primed continuous infusion of regular human insulin (16 mU/kg bolus, followed by 2 mU/kg/min HumulinR; Eli Lilly, Indianapolis, IN) was initiated at t = 0 min. A 50% dextrose solution was infused as required to maintain euglycemia, and serial blood samples were obtained for determination of plasma glucose and insulin levels.
Blood Collection and Assay
Whole blood samples for plasma hormonal measures were collected in appropriately treated tubes (22) and centrifuged, and the plasma was removed and stored at −80°C for subsequent assay. Plasma glucose was measured using a GM9D glucose direct analyzer (Analox Instruments, Stourbridge, U.K.), plasma insulin (Crystal Chem, Downers Grove, IL), glucagon (Mercodia, Uppsala, Sweden), and corticosterone (Alpco, Salem, NH) by ELISA and NEFAs by colorimetry (Wako Chemicals, Richmond, VA).
Statistical Analysis
Results are expressed as mean ± SEM. Significance was established at P < 0.05 (two-tailed). Statistical analyses of effects of cold exposure or phentolamine on metabolic parameters were performed using Statistica 7.1 software (StatSoft, Inc., Tulsa, OK). A one-way ANOVA with a least significant difference post hoc test was used to compare mean values among multiple groups, a two-sample unpaired Student t test was used for two-group comparisons, and a paired Student t test was used for within-group comparisons. RMA regression was performed via constrained nonlinear regression in SPSS 23 software (IBM Corp., Armonk, NY) using sequential quadratic programming and the loss function specified in the IBM technical note available at http://www-01.ibm.com/support/docview.wss?uid=swg21476031. The general linear model in SPSS was used to model the relationships among measures of glucose tolerance and measures of insulin- and noninsulin-mediated determinants of glucose homeostasis.
Results
Relationship Between Insulin Sensitivity and the Insulin Response to Glucose in Rats
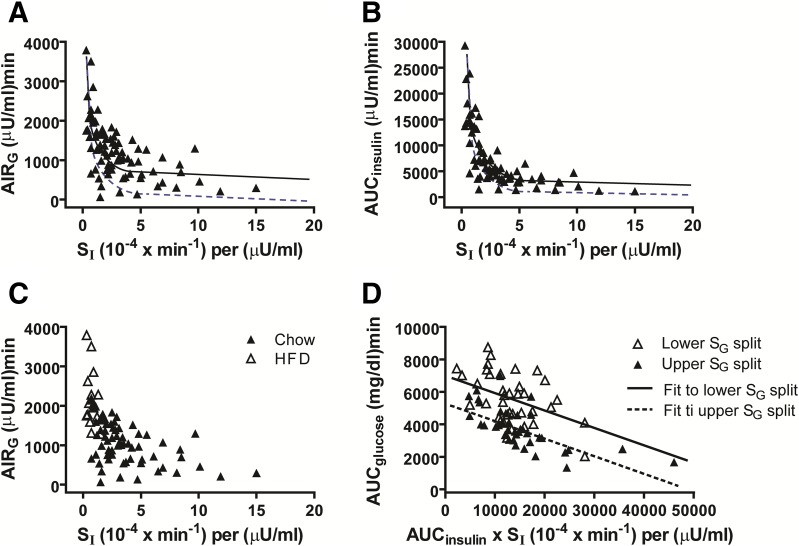
Analysis of data compiled from a cohort of 79 normal rats revealed the predicted hyperbolic relationship between insulin sensitivity (SI) and the insulin response to glucose, whether the latter was measured as AIRG or as AUCinsulin (Fig. 1A and B). Although RMA regression identified asymptotic values (y-intercepts) with bootstrap estimated 95% CIs that did not include zero (383, 689 for AIRG; 889, 2673 for AUCinsulin), indicating that the intercepts are statistically significant, inspection of Fig. 1 suggests that the relationship between SI and either measure of the insulin response is fundamentally hyperbolic in nature. We suspect that because AUCinsulin reflects the exposure of body tissues to insulin during the FSIGT more effectively than does AIRG, the relationship between SI and AUCinsulin conforms to a hyperbola more closely than does that between SI and AIRG (Fig. 1A and B). Moreover, the insulin sensitivity of rats fed the HFD was reduced relative to that of chow-fed controls (mean SI: 0.80 ± 0.39 vs. 3.80 ± 2.84 [0−4 × min−1]/µU/mL; P < 0.0001), whereas insulin secretion was increased (mean AIRG: 2,157 ± 700 vs. 1,003 ± 480 µU/mL × min; P < 0.0001) (Fig. 1C).
Figure 1.
Relationship between insulin sensitivity and insulin secretion is hyperbolic in nature in rats. Data are from control rats tested at room temperature (22°C; n = 79) depicting the relationships between insulin sensitivity (SI) measured via the minimal model and first-phase insulin secretion measured as AIRG (A); incremental AUCinsulin measured as total AUC during the FSIGT minus basal insulin AUC (B); and AIRG in HFD- and chow-fed rats shown separately (C). D: Simplified depiction of model 4 of Table 1 shows an ANCOVA model for the dependence of glucose tolerance (measured as the net AUCglucose during the FSIGT) on insulin action (measured as SI × net AUCinsulin) in groups categorized by median splits of SG. Solid curves are equations of the form: insulin response = a + b × (1/insulin sensitivity), where the optimal model fit was achieved using RMA regression to account for error in both the x and y variables (research design and methods). Dashed curves are equations of the form: insulin response = c × (1/sensitivity) fit by ordinary least squares optimization using a model that excluded the intercept term in accordance with the concept that insulin secretion × sensitivity ≈ constant for subjects with normal glucose tolerance (the hyperbolic law of glucose homeostasis). For AUCinsulin, the agreement of the optimal RMA fit with the hyperbolic-law curve is excellent, consistent with the fact that insulin acts across the entire glucose tolerance test to promote a return to normoglycemia.
To assess the relative contributions of insulin-dependent and insulin-independent mechanisms to glucose disposal during the FSIGT, we performed multiple regression analyses in which the dependent variables were either of two measures of glucose tolerance (KG or AUCglucose) during the FSIGT. These models included as predictor variables the interaction of the insulin response (measured as AIRG or AUCinsulin) and insulin sensitivity, along with SG (Table 1 and Fig. 1D). As indicated in Table 1, SG makes a substantial contribution to glucose tolerance whether the latter is measured as KG (models 1 and 2) or AUCglucose (models 3 and 4), as in humans (30). Relatedly, the contribution made by overall insulin action (represented by the DI or by the product of SI × AUCinsulin) to glucose tolerance appears to be larger when AUCglucose is used instead of KG.
Table 1.
Multiple regression analysis of the contribution made by insulin-dependent and noninsulin-dependent mechanisms to glucose tolerance in normal rats (n = 79) housed at room temperature (22°C)
| Model, outcome | Parameter | Coefficient | SE | t statistic | P | Partial η2* |
|---|---|---|---|---|---|---|
| 1, KG | Intercept | −0.035 | 0.003 | −10.74 | <0.0001 | 0.62 |
| (R2 = 0.43) | AIRG × SI | −2.20E-10 | 5.82E-11 | −3.79 | 0.0003 | 0.17 |
| SG | −0.291 | 0.055 | −5.33 | <0.0001 | 0.29 | |
| 2, KG | Intercept | −0.032 | 0.004 | −8.21 | <0.0001 | 0.49 |
| (R2 = 0.40) | AUCinsulin × SI | −5.24E-11 | 1.69E-11 | −3.11 | 0.003 | 0.12 |
| SG | −0.328 | 0.055 | −5.99 | <0.0001 | 0.34 | |
| 3, AUCglucose | Intercept | 7,801.26 | 299.85 | 26.02 | <0.0001 | 0.90 |
| (R2 = 0.67) | AIRG × SI | −4.74E-05 | 5.40E-06 | −8.86 | <0.0001 | 0.52 |
| SG | −31,648.43 | 4,720.64 | −6.70 | <0.0001 | 0.38 | |
| 4, AUCglucose | Intercept | 8,505.76 | 386.25 | 22.02 | <0.0001 | 0.87 |
| (R2 = 0.60) | AUCinsulin × SI | −1.22E-05 | 1.70E-06 | −7.23 | <0.0001 | 0.42 |
| SG | −38,162.57 | 5,126.08 | −7.45 | <0.0001 | 0.44 |
Regression models estimate the contributions made by measures of insulin action, represented by the product of sensitivity (SI) and the insulin response (measured either as AIRG or AUCinsulin), together with the largely insulin-independent effect of glucose to promote its own disappearance (SG). Glucose tolerance is measured either as KG or AUCglucose. Interpretation: At any fixed value of SG, the predicted mean glucose tolerance improves significantly with increases of insulin action. At any fixed value of insulin action, the predicted mean glucose tolerance increases significantly with increases of SG. The t statistic and effect size statistic (partial η2 values) suggest that SG plays the dominant role in the acute phase of glucose disappearance (measured as KG) in models 1 and 2. Models 3 and 4 suggest that although SG strongly also affects glucose tolerance when measured throughout the glucose tolerance test (AUCglucose), insulin action is of markedly greater importance over the entire glucose tolerance test than at the beginning of the glucose tolerance test (when KG is measured), consistent with the concept that the contribution made by insulin to glucose tolerance increases over the course of the study. *A measure of effect size related the proportion of variance accounted for by the variable.
To analyze the relationship between insulin action and glucose tolerance among rats with relatively high or low values of SG, regression lines were fit by ANCOVA for each cohort (Fig. 1D). Although the slopes of the lines fit to each of the two groups did not differ significantly (P = 0.54), for any given value of insulin action, the rats in the upper SG split had significantly better (P < 0.0001) mean overall glucose tolerance than did rats in the lower SG split. These observations suggest that the effect of insulin action on glucose tolerance in rats is influenced by variation in SG, a conclusion consistent with data obtained from three other models (data not shown). Similar outcomes were obtained using the models that replaced SG with GEZI (data not shown), consistent with human data (30). Indeed, GEZI (but not BIE) appears to be a substantial determinant of glucose tolerance in rats, given that the Pearson correlation (r) between KG and GEZI was 0.56 (P < 0.0001).
Overall, our results indicate that either AIRG or AUCinsulin is a useful index of the insulin response to glucose and that together they support the conclusion that variation in insulin sensitivity across animals is effectively compensated by a proportionate change of insulin secretion.
Effect of Cold Exposure on Determinants of Glucose Tolerance
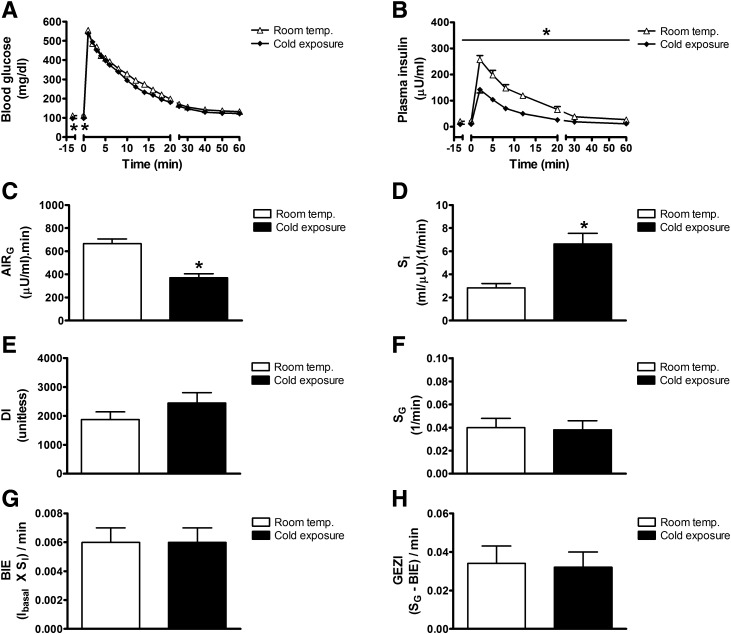
To investigate mechanisms underlying the coordinate regulation of insulin sensitivity and insulin secretion, we sought to validate previous evidence that cold exposure inhibits insulin secretion in rats (16,19). As expected (16,26), fasting levels of blood glucose (112.6 ± 3.5 vs. 97.5 ± 2.3 mg/dL; P < 0.05) and plasma insulin (22.3 ± 2.9 vs. 10.0 ± 1.1 μU/mL; P < 0.05) were lower in fasted rats housed in a cold environment (5°C for 28 h) than at room temperature (22°C) (Fig. 2A and B). Moreover, in rats that underwent a FSIGT, followed by minimal model analysis (20,23,24,31), as expected, AIRG was potently inhibited by cold exposure by ∼50% (AIRG: 667 ± 41 for room temperature vs. 372 ± 33 μU/mL × min for cold exposure; P < 0.001), despite glucose tolerance remaining virtually unchanged (AUCglucose: 5,869 ± 233 for room temperature vs. 5,848 ± 442 for cold exposure; P = NS) (Fig. 2A and B) (16). To explain this outcome, we hypothesized that a compensatory increase of insulin sensitivity must have occurred in the cold-exposed group. As predicted, we found that the potent effect of cold exposure to inhibit insulin secretion was offset by a near doubling of insulin sensitivity (SI) (Fig. 2C and D). Consequently, neither the DI nor glucose tolerance was substantially altered (Fig. 2E). In addition, cold exposure had no significant effect on SG, the BIE, or GEZI (Fig. 2F–H).
Figure 2.
Effect of cold exposure on determinants of glucose tolerance. Blood glucose levels (A), plasma insulin levels (B), AIRG (C), insulin sensitivity (SI) (D), disposition index (DI) (E), SG (F), BIE (G), and the insulin-independent parameter, GEZI (H ), in fasted adult male Wistar rats that were exposed to room temperature (22°C) or the cold (4°C) for 28 h and underwent a FSIGT. Mean ± SEM. *P < 0.05 vs. room temp.
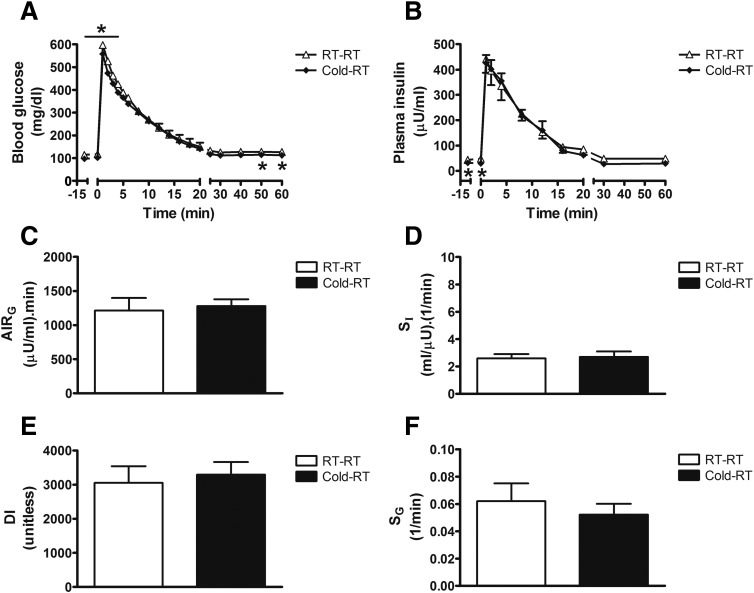
Reversibility of the Effect of Cold Exposure on Determinants of Glucose Tolerance
To assess the durability of the potent and highly coordinated (and offsetting) changes of insulin secretion and insulin sensitivity elicited by cold exposure, we performed an FSIGT on separate cohorts of fasted rats that were maintained at room temperature or were housed in the same cold environment (5°C) for 24 h and then returned to room temperature (22°C) for 4 h before study. Although fasting blood glucose and plasma insulin levels remained slightly lower in cold-exposed animals that were then returned to room temperature (Fig. 3A and B), the effect of cold exposure to inhibit glucose-induced insulin secretion (AIRG) (Fig. 3B) and/or increase SI (Fig. 2D) was no longer evident 4 h after returning to room temperature (Fig. 3D). As before, neither DI nor SG (Fig. 3E and F), GEZI, or BIE (data not shown) differed between groups (Fig. 3E). Thus, the potent and highly coordinated effects of cold exposure on insulin secretion and insulin sensitivity are reversed within 4 h after the return to room temperature.
Figure 3.
Effect of cold exposure to reduce insulin secretion and increase insulin sensitivity is rapidly reversible. Blood glucose levels (A), plasma insulin levels (B), AIRG (C), insulin sensitivity (SI) (D), DI (E), and SG (F) in fasted adult male Wistar rats that remained at room temperature (RT-RT; 22°C) or were exposed to the cold (4°C) for 24 h before returning to room temperature for 4 h (Cold-RT) and undergoing a FSIGT. Mean ± SEM. *P < 0.05 vs. RT-RT.
Effect of Phentolamine on Determinants of Glucose Tolerance in Cold-Exposed Rats
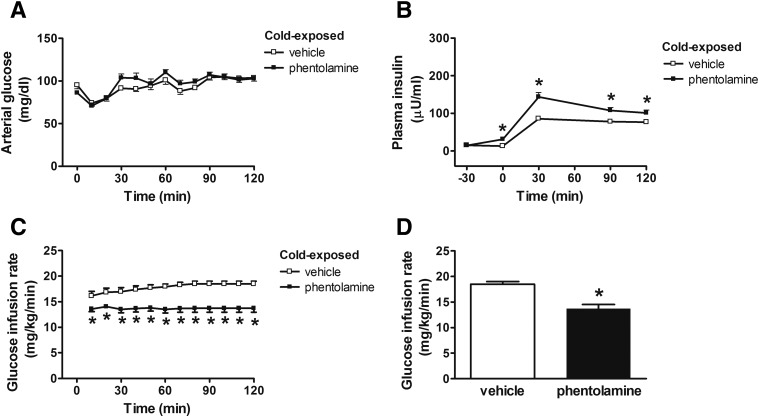
To determine whether a change in SNS output contributes to the rapidly reversible effect of cold exposure on insulin secretion and insulin sensitivity, fasted rats housed at 5°C for 28 h received a continuous i.v. infusion of the α-AR blocker phentolamine or its vehicle for 30 min before and then during an FSIGT. As predicted, the effect of cold exposure to suppress basal- and glucose-induced insulin secretion was reversed by i.v. phentolamine, such that the low plasma insulin levels typical of animals housed in the cold were restored to values observed in animals housed at room temperature (13.9 ± 2.4 vs. 28.4 ± 1.9 μU/mL; P < 0.05). Despite this marked increase of insulin secretion, glucose tolerance was once again largely unaffected (AUCglucose: 5,151 ± 351 for vehicle vs. 5,078 ± 378 for phentolamine; P = NS) (Fig. 4B and C). In addition, the potent effect of cold exposure to increase SI was also rapidly reversed by phentolamine-induced α-AR blockade, such that the DI did not change (Fig. 4D and E). Interestingly, SG was also increased in phentolamine-treated animals (Fig. 4F), an effect due largely to an increase of GEZI with no change in BIE (data not shown). Collectively, these data show that the potent, highly coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity induced by cold exposure are both reversible within 30 min and dependent on intact α-AR signaling.
Figure 4.
Role of the SNS on determinants of glucose tolerance in cold-exposed rats. Blood glucose levels (A), plasma insulin levels (B), AIRG (C), insulin sensitivity (SI) (D), DI (E), and SG (F) in fasted adult male Wistar rats that were exposed to the cold at 4°C for 28 h and given a continuous i.v. infusion of the α-AR blocker phentolamine (8 μg/kg/min) or vehicle, beginning 30 min before and continuing throughout a FSIGT. Mean ± SEM. *P < 0.05 vs. vehicle.
Effect of Phentolamine on Insulin Sensitivity Measured by Euglycemic-Hyperinsulinemic Clamp in Cold-Exposed Rats
To confirm our finding that α-AR blockade reverses the effect of cold exposure to increase insulin sensitivity, we measured the effect of i.v. phentolamine infusion on the glucose infusion rate (GIR), a direct measure of whole-body insulin sensitivity, in cold-exposed rats during a euglycemic-hyperinsulinemic clamp. By design, arterial blood glucose levels were similar between groups during the clamp period (Fig. 5A), although plasma insulin levels during the clamp were significantly increased in cold-exposed animals receiving phentolamine (Fig. 5B), raising the possibility that phentolamine infusion affected insulin clearance as well as insulin secretion. Yet despite higher plasma insulin levels, the GIR required to maintain euglycemia was decreased by ∼25% (P < 0.05) in cold-exposed rats that received i.v. phentolamine (Fig. 5C and D). This finding offers direct, independent confirmation of the effect of systemic α-AR blockade to reduce insulin sensitivity in cold-exposed rats.
Figure 5.
Effect of α-AR blockade on insulin sensitivity in cold-exposed rats during a euglycemic-hyperinsulinemic clamp. Arterial blood glucose (A), plasma insulin (B), GIR (C), and mean GIR (D) during the last 30 min required to maintain euglycemia during a euglycemic-hyperinsulinemic clamp in cold-exposed rats (4°C for 28 h) given a continuous i.v. infusion of phentolamine (8 μg/kg/min) or vehicle. Mean ± SEM. *P < 0.05 vs. vehicle.
Effects of Cold Exposure With or Without Phentolamine Infusion on Insulin Clearance From Plasma and on Other Humoral Determinants of Glucose Homeostasis
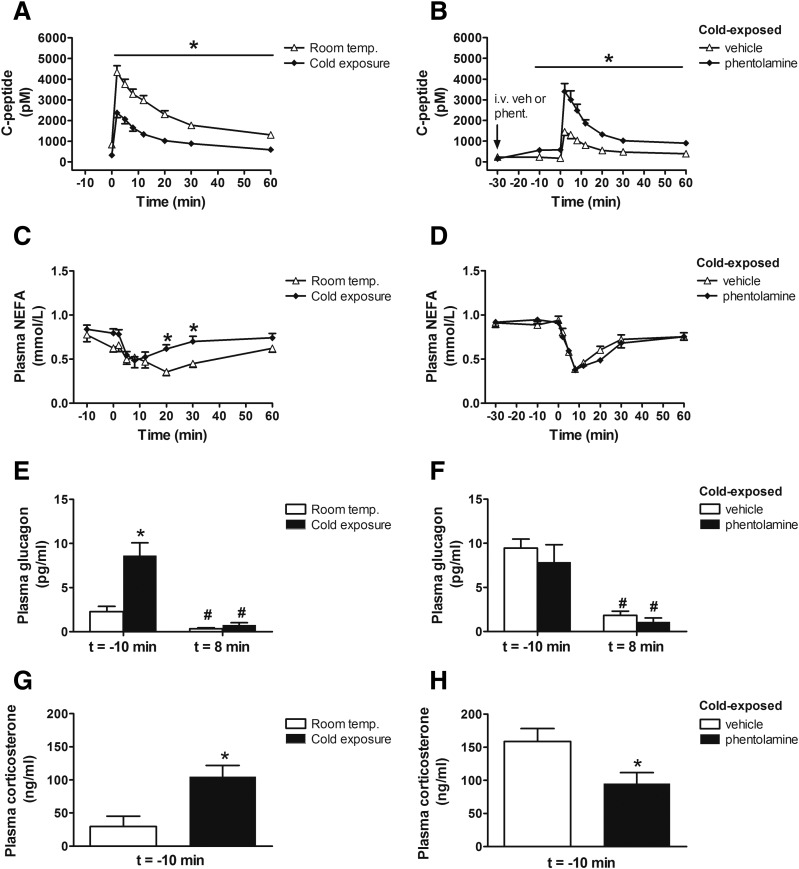
To determine whether either cold exposure or phentolamine administration affected insulin clearance from plasma as well as insulin secretion, we measured C-peptide levels in plasma samples obtained during the FSIGT studies reported above and subjected these data to a model-based deconvolution analysis (25). We found that changes in the plasma level of C-peptide closely paralleled those of insulin from both studies (Fig. 6A and B). Further analysis also revealed no difference in rates of insulin clearance between animals housed at room temperature versus the cold (0.261 vs. 0.279; P = 0.59) or between cold exposed animals treated with i.v. vehicle versus phentolamine (0.184 vs. 0.199; P = 0.31). Both the effect of cold exposure to reduce the plasma insulin response to glucose and the effect of phentolamine to reverse this effect, therefore, resulted from changes of insulin secretion rather than insulin clearance.
Figure 6.
Effect of cold exposure and the SNS on insulin clearance and hormonal measures. Plasma C-peptide (A and B), plasma NEFA (C and D), plasma glucagon before (t = −10 min) and after (t = 8 min) i.v. glucose bolus (E and F), and plasma corticosterone levels before (t = −10 min) i.v. glucose bolus (G and H) in fasted adult male Wistar rats 1) exposed at room temperature (22°C) or the cold (4°C) for 28 h or 2) to the cold at 4°C for 28 h and given a continuous i.v. infusion of phentolamine (8 μg/kg/min) or vehicle, beginning 30 min before and continuing throughout a FSIGT. Mean ± SEM. *P < 0.05 vs. room temperature (left panels), *P < 0.05 vs. vehicle (right panels); #P < 0.05 vs. t = −10 min.
An increase in nocturnal NEFA levels has been reported to contribute to the compensatory increase of insulin secretion in a dog model of insulin resistance induced by HFD feeding (8,9). However, we found no difference of fasting NEFA levels between rats housed at room temperature and cold-exposed animals. Further, NEFA levels tended to increase in cold-exposed animals during the FSIGT, whereas insulin secretion was reduced (Fig. 6C). Combined with the observation that plasma NEFA levels dropped comparably in the first 10 min after administration of glucose in cold-exposed animals, irrespective of whether they received i.v. saline or phentolamine (P = NS) (Fig. 6D), our data suggest that the effect of phentolamine to acutely increase insulin secretion in cold-exposed rats was not a consequence of increased plasma NEFA levels.
In addition, we found that plasma glucagon levels were elevated in cold-exposed animals relative to those housed at room temperature (Fig. 6E) and were similarly suppressed after an i.v. glucose bolus during the FSIGT, irrespective of the temperature at which they were housed (Fig. 6F). Similarly, plasma corticosterone levels were elevated in cold-exposed rats, and phentolamine treatment, if anything, lowered plasma corticosterone levels (Fig. 6G and H), despite reversing the effect of cold to improve insulin sensitivity (Figs. 4 and 5). Taken together, these data suggest that neither changes in circulating NEFA levels nor the neuroendocrine response to cold is likely to explain the robust and highly coordinated changes of insulin secretion and insulin sensitivity.
Discussion
That cold exposure elicits increased whole-body glucose utilization (to support the increased demand for heat production) (19) and decreased insulin secretion (16,32) with little or no change of glucose tolerance points to a highly coordinated metabolic response that enables thermogenic needs to be met while preserving glucose homeostasis. Our data suggest that these adaptive changes of insulin secretion and sensitivity to cold exposure occur quickly via a mechanism involving the SNS. Specifically, we found that the effect of cold exposure to inhibit the insulin response to glucose (whether measured as AIRG or AUCinsulin) in rats was precisely offset by a proportionate increase of insulin sensitivity (measured as SI), such that glucose tolerance was unchanged. Moreover, this reduced insulin response to glucose was due entirely to reduced insulin secretion with no change of plasma insulin clearance, was fully established within 28 h of exposure to a cold environment, and returned to baseline within just 4 h after the return to room temperature. Our finding that the effect of cold exposure on these parameters was fully reversed by α-AR blockade implicates the SNS in the coordinate regulation of insulin secretion and sensitivity in this setting. That this reversal was achieved within just 30 min of the onset of phentolamine infusion attests to the remarkable rapidity with which this coordinate regulation takes place.
The proper interpretation of these findings partly hinges on the extent to which the well-documented hyperbolic relationship between insulin secretion and insulin sensitivity in humans (3) applies to rats as well. To address this question, we analyzed the relationship between SI and two different measures of the insulin response to a glucose challenge (AIRG and AUCinsulin) across a large cohort of normal rats housed at room temperature (22°C). To ensure a sufficiently broad distribution of these parameters, we included data from two different rat strains (Wistar and Long-Evans) fed either of two diets (standard chow or a 60% kcal HFD). As predicted, the relationship between SI and each of the two measures of insulin response was largely hyperbolic. Although we also found that SG makes a major contribution to glucose tolerance, which is consistent with prior evidence (33) and may help explain why the RMA regression hyperbolic fits included significant nonzero asymptotic values (intercept terms), our data establish that the relationship between insulin sensitivity and insulin secretion in rats is quite similar to that in humans. By recapitulating the relationship observed in humans, our data also support the validity of the FSIGT/minimal model method for metabolic studies in rats. Extending this validation is our findings that 1) consuming an HFD reduces insulin sensitivity in otherwise normal rats and that this effect is offset by a proportionate increase of insulin secretion (Fig. 1C), and 2) in cold-exposed rats, the effect of i.v. phentolamine to rapidly reduce insulin sensitivity when measured as SI was replicated using the euglycemic-hyperinsulinemic clamp technique.
By investigating the role played by the brain (acting via the SNS) in the adaptive response to a physiological challenge, our approach is a departure from more conventional strategies that rely on administration of peptides or drugs into the brain to investigate its role in glucose homeostasis. Indeed, metabolic studies are usually conducted under constant environmental conditions to avoid the potentially confounding effect of commonly encountered environmental challenges. This strategy reduces variability in measured end points but also precludes investigation into how animals adapt to such challenges. Should the brain’s role in glucose homeostasis take on greater importance when animals are confronted with a real-world environmental challenge, therefore, housing them in an unchanging (and unchallenging) environment by definition places major limits on the insights that can be gained. In this context, we note that the current study paradigm involved exposure to temperatures comparable to what rodents throughout much of the world experience daily.
To understand how cold exposure effects these highly coordinated changes of insulin secretion and insulin sensitivity, we consider three possibilities: 1) adaptive changes of insulin sensitivity are secondary to the change of insulin secretion, 2) adaptive changes of insulin secretion are secondary to the change of insulin sensitivity, or 3) adaptive changes of both insulin sensitivity and insulin secretion are part of an integrative regulatory process involving the SNS. With respect to the first possibility, we note previous evidence demonstrating that insulin sensitivity does not increase when insulin secretion is reduced, whether by a pancreatic β-cell toxin (e.g., streptozotocin) (34), inhibitory hormones (e.g., somatostatin) (35), partial pancreatectomy (36), or disease (e.g., T2D) (37). The alternative possibility that reduced insulin secretion is a consequence of a cold-induced increase of insulin sensitivity is consistent with the known effect of cold exposure to increase insulin-mediated glucose uptake into thermogenic tissues, including skeletal muscle, heart, and white and brown adipose tissue (17–19). One potential mechanism to explain how this effect is coupled to a proportionate decrease of insulin secretion is through reduced nutrient stimulation of β-cells via a mechanism involving, for example, reduced circulating glucose or NEFA levels (8–10). Such a mechanism is inconsistent with both the modest effect of cold exposure on plasma levels of these nutrients and the lack of any effect of phentolamine on these plasma values (despite its ability to fully reverse cold-induced inhibition of insulin secretion). Similarly, although cold exposure increased circulating levels of glucagon and corticosterone via a mechanism that was partially reversed by phentolamine, such changes do not offer a viable explanation for the associated changes of insulin secretion and sensitivity.
Could cold-induced changes of insulin secretion and insulin sensitivity be mediated via the SNS? That the effect of cold exposure to increase insulin sensitivity was reversed by α-AR blockade offers direct evidence that increased SNS outflow is required for this effect, consistent with an extensive literature implicating the brain in the control of insulin sensitivity (38) via mechanisms involving the SNS (39). The hypothesis that the cold-induced decline of insulin secretion was also mediated via the SNS is supported by pioneering work documenting autonomic control of pancreatic islet function some 40 years ago (15) and by evidence that cold exposure increases sympathetic tone to the pancreas (12). The mechanism underlying this effect was subsequently shown to involve α-AR signaling in pancreatic islets (13–15), a possibility reinforced by our finding that the effect of cold exposure to reduce insulin secretion was rapidly reversed (within 30 min) by phentolamine administration.
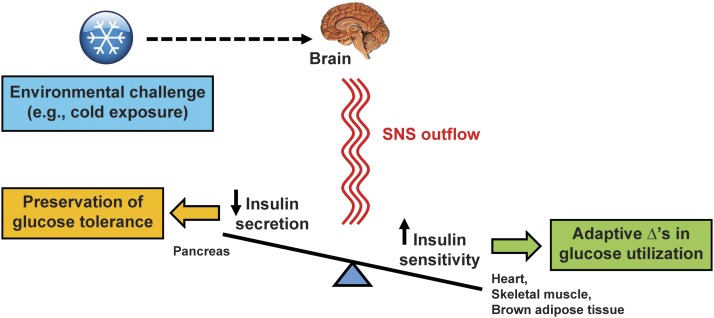
These observations collectively support a model in which SNS outflow to both pancreatic islets and thermogenic tissues underlies the highly coordinated and proportionate changes of insulin sensitivity and insulin secretion that effectively redirect substrate to thermogenic tissues without altering glucose tolerance (Fig. 7). Future studies are warranted to clarify the cellular basis for these effects and to further delineate the brain’s role in these metabolic responses. For example, the specific role played by islet sympathetic nerves could be investigated by sympathetic denervation of the pancreas or by targeted deletion of α-AR in pancreatic β-cells. An additional priority is to determine whether reciprocal adjustment of insulin secretion and insulin sensitivity is triggered by activation of hypothalamic cold-sensitive neurons involved in thermoregulation.
Figure 7.
Model for central nervous system regulation of insulin secretion and insulin sensitivity via the SNS during cold exposure. In response to thermal sensory input from the periphery during cold exposure, the brain engages a series of adaptive responses that increase SNS outflow to thermogenic tissues (i.e., brown adipose tissue, skeletal muscle, heart) as needed to meet the increased demands for heat production in order to maintain core temperature. At the same time, cold exposure increases SNS outflow to the pancreas, which potently inhibits insulin secretion via activation of α-ARs on pancreatic β-cells. Our findings demonstrate that the effect of cold exposure to increase insulin sensitivity is offset by a proportionate decline in glucose-induced insulin secretion such that glucose tolerance remains unchanged. Moreover, the effects of cold exposure on insulin sensitivity and insulin secretion are rapidly reversed by systemic α-AR blockade and by returning animals to room temperature. These findings implicate a key role for the brain, via the SNS, in the rapid, highly coordinated, and reciprocal changes of insulin secretion and insulin sensitivity that preserve glucose homeostasis in the setting of cold exposure.
In conclusion, we report that the hyperbolic relationship between insulin secretion and insulin sensitivity characteristic of humans is operational in rats as well. We demonstrate further that in rats, the rapid, potent, and highly coordinated changes of insulin sensitivity and insulin secretion elicited by cold exposure are critically dependent on the SNS. We speculate that these metabolic adjustments are components of a larger and highly integrated set of responses elicited by the brain to enable effective thermogenesis while preserving metabolic homeostasis and thereby promote survival during cold exposure.
Supplementary Material
Article Information
Acknowledgments. The authors greatly acknowledge valuable guidance provided by Dr. David Wasserman, Vanderbilt University, and technical assistance provided by Amy Martinson, from the laboratory of Charles E. Murry, University of Washington, for measures of heart rate and mean arterial pressure.
Funding. This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK-089056 (G.J.M.), DK-27619 and DK-29867 (R.N.B.), DK-50154 (G.J.T.), DK-083042, DK-090320, and DK-101997 (M.W.S.); the National Institute of Diabetes and Digestive Kidney Diseases–funded Nutrition Obesity Research Center (DK-035816) and Diabetes Research Center (DK-017047); the Nutrition, Obesity and Atherosclerosis Training Grant from the National Heart, Lung, and Blood Institute (T32-HL-007028) and the Diabetes and Metabolism Training Grant (T32-DK-0007247) at the University of Washington; and the Department of Veterans Affairs grant BX001060 (S.E.K.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.J.M., K.M., K.J.K., F.P., D.S., R.N.B., G.J.T., S.E.K., and M.W.S. analyzed data and edited the manuscript. G.J.M., K.M., J.M.R., J.M.S., M.E.M., J.T.N., and N.K.A. conducted experiments and acquired data. G.J.M., K.M., and M.W.S. designed research studies and wrote the manuscript. M.W.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1351/-/DC1.
J.M.R. is currently affiliated with Early Regulatory Toxicology, Non-clinical Development, Novo Nordisk A/S, Måløv, Denmark.
References
- 1.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Prigeon RL, McCulloch DK, et al. . Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol 1990;162:1008–1014 [DOI] [PubMed] [Google Scholar]
- 5.Moran A, Jacobs DR Jr., Steinberger J, et al. . Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 7.Lorenzo C, Wagenknecht LE, Rewers MJ, et al. . Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2098–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 2007;292:E1590–E1598 [DOI] [PubMed] [Google Scholar]
- 9.Broussard JL, Kolka CM, Castro AV, et al. . Elevated nocturnal NEFA are an early signal for hyperinsulinaemic compensation during diet-induced insulin resistance in dogs. Diabetologia 2015;58:2663–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maickel RP, Matussek N, Stern DN, Brodie BB. The sympathetic nervous system as a homeostatic mechanism. I. Absolute need for sympathetic nervous function in body temperature maintenance of cold-exposed rats. J Pharmacol Exp Ther 1967;157:103–110 [PubMed] [Google Scholar]
- 11.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014;19:741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young JB, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol 1979;236:E524–E533 [DOI] [PubMed] [Google Scholar]
- 13.Ahrén B, Taborsky GJ Jr., Porte D Jr. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 1986;29:827–836 [DOI] [PubMed] [Google Scholar]
- 14.Fagerholm V, Haaparanta M, Scheinin M. α2-adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol 2011;108:365–370 [DOI] [PubMed] [Google Scholar]
- 15.Porte D Jr., Robertson RP. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc 1973;32:1792–1796 [PubMed] [Google Scholar]
- 16.Vallerand AL, Lupien J, Bukowiecki LJ. Interactions of cold exposure and starvation on glucose tolerance and insulin response. Am J Physiol 1983;245:E575–E581 [DOI] [PubMed] [Google Scholar]
- 17.Vallerand AL, Pérusse F, Bukowiecki LJ. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol 1990;259:R1043–R1049 [DOI] [PubMed] [Google Scholar]
- 18.Smith SA, Young P, Cawthorne MA. Quantification in vivo of the effects of insulin on glucose utilization in individual tissues of warm- and cold-acclimated rats. Biochem J 1986;237:789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallerand AL, Pérusse F, Bukowiecki LJ. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol 1987;253:E179–E186 [DOI] [PubMed] [Google Scholar]
- 20.Rojas JM, Matsen ME, Mundinger TO, et al. . Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol Metab 2015;4:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.German J, Kim F, Schwartz GJ, et al. . Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German JP, Thaler JP, Wisse BE, et al. . Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 2011;152:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton GJ, Matsen ME, Bracy DP, et al. . FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013;123:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso LC, Watanabe Y, Stefanovski D, et al. . Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 2012;20:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahrén B, Thomaseth K, Pacini G. Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 2005;48:2140–2146 [DOI] [PubMed] [Google Scholar]
- 26.Beck LV, Zaharko DS, Kalser SC. Variation in serum insulin and glucose of rats with chronic cold exposure. Life Sci 1967;6:1501–1506 [DOI] [PubMed] [Google Scholar]
- 27.Bergman RN. Minimal model: perspective from 2005. Horm Res 2005;64(Suppl. 3):8–15 [DOI] [PubMed] [Google Scholar]
- 28.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 4th ed. New York, W.H. Freeman, 2012 [Google Scholar]
- 29.Boston RC, Moate PJ, Stefanovski D, Sumner AE, Bergman RN. AKA-glucose: a program for kinetic and epidemiological analysis of frequently sampled intravenous glucose tolerance test data using database technology. Diabetes Technol Ther 2005;7:298–307 [DOI] [PubMed] [Google Scholar]
- 30.Kahn SE, Prigeon RL, McCulloch DK, et al. . The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 1994;43:587–592 [DOI] [PubMed] [Google Scholar]
- 31.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 [DOI] [PubMed] [Google Scholar]
- 32.Vallerand AL, Frim J, Kavanagh MF. Plasma glucose and insulin responses to oral and intravenous glucose in cold-exposed humans. J Appl Physiol (1985) 1988;65:2395–2399 [DOI] [PubMed]
- 33.Bunner AE, Chandrasekera PC, Barnard ND. Knockout mouse models of insulin signaling: relevance past and future. World J Diabetes 2014;5:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobin BL, Finegood DT. Reduced insulin secretion by repeated low doses of STZ impairs glucose effectiveness but does not induce insulin resistance in dogs. Diabetes 1993;42:474–483 [DOI] [PubMed] [Google Scholar]
- 35.Kahn SE, Klaff LJ, Schwartz MW, et al. . Treatment with a somatostatin analog decreases pancreatic B-cell and whole body sensitivity to glucose. J Clin Endocrinol Metab 1990;71:994–1002 [DOI] [PubMed] [Google Scholar]
- 36.Ward WK, Wallum BJ, Beard JC, Taborsky GJ Jr., Porte D Jr. Reduction of glycemic potentiation. Sensitive indicator of beta-cell loss in partially pancreatectomized dogs. Diabetes 1988;37:723–729 [DOI] [PubMed] [Google Scholar]
- 37.Utzschneider KM, Prigeon RL, Carr DB, et al. . Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 2006;29:356–362 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MW, Seeley RJ, Tschöp MH, et al. . Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 2013;503:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 1999;48:1706–1712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.