Abstract
Islet cell transplantation has limited effectiveness because of an instant blood-mediated inflammatory reaction (IBMIR) that occurs immediately after cell infusion and leads to dramatic β-cell death. In intraportal islet transplantation models using mouse and human islets, we demonstrated that α-1 antitrypsin (AAT; Prolastin-C), a serine protease inhibitor used for the treatment of AAT deficiency, inhibits IBMIR and cytokine-induced inflammation in islets. In mice, more diabetic recipients reached normoglycemia after intraportal islet transplantation when they were treated with AAT compared with mice treated with saline. AAT suppressed blood-mediated coagulation pathways by diminishing tissue factor production, reducing plasma thrombin-antithrombin complex levels and fibrinogen deposition on islet grafts, which correlated with less graft damage and apoptosis. AAT-treated mice showed reduced serum tumor necrosis factor-α levels, decreased lymphocytic infiltration, and decreased nuclear factor (NF)-κB activation compared with controls. The potent anti-inflammatory effect of AAT is possibly mediated by suppression of c-Jun N-terminal kinase (JNK) phosphorylation. Blocking JNK activation failed to further reduce cytokine-induced apoptosis in β-cells. Taken together, AAT significantly improves islet graft survival after intraportal islet transplantation by mitigation of coagulation in IBMIR and suppression of cytokine-induced JNK and NF-κB activation. AAT-based therapy has the potential to improve graft survival in human islet transplantation and other cellular therapies on the horizon.
Introduction
Allogeneic islet transplantation is a promising approach for treating patients with type 1 diabetes (T1D) (1). Islet autotransplantation is used to avoid pancreatogenic diabetes in patients who undergo total pancreatectomy for chronic pancreatitis intractable to medical management (2). In both situations, islet engraftment after transplantation is compromised, and β-cell death is problematic. Stresses induced during islet harvesting and posttransplantation (PT) may lead to up to 60% islet death within 2–3 days after transplantation (3,4). Many factors, including instant blood-mediated inflammatory reaction (IBMIR), proinflammatory cytokines, hypoxia, and nutritional deprivation, contribute to β-cell death. Strategies that maintain normal islet cell function and reduce cell death after transplantation would improve patient outcomes and have ready clinical applicability.
α-1 Antitrypsin (AAT) is a serine protease inhibitor that belongs to the serpin superfamily. It has a high concentration in serum and is available for clinical use as a purified human product (5). AAT inhibits various enzymes, including neutrophil elastase, cathepsin G, proteinase 3, thrombin, trypsin, and chymotrypsin (6). Patients with genetic deficiency of AAT have higher risks for emphysema from alveolar destruction (7). Beside inhibition of elastase, AAT exerts anti-inflammatory effects via suppressing cytokine production, complement activation, and immune cell infiltration. AAT also functions as an antiapoptotic factor for endothelial cells and vascular smooth muscle cells (8,9). AAT has beneficial effects in the treatment of diabetes. AAT protects β-cells from apoptosis induced by proinflammatory cytokines and streptozotocin (10). AAT injection in NOD mice reduces the intensity of insulitis, preserves β-cell mass, and prevents the onset of diabetes via modulating T regulatory cells (11,12). AAT also protects islets from graft failure and immune rejection in mouse transplantation models (13,14). Adding AAT into islet digestion medium improves porcine islet isolation by inhibiting trypsin activity during pancreas digestion (15). AAT monotherapy prolongs allograft survival in mice by elevating vascular endothelial growth factor expression and promotion of islet revascularization (14). Short-term treatment with AAT in the peritransplant period protects a marginal mass of monkey islet autografts from long-term, nonimmunological graft loss through effects on expression of transforming growth factor-β, nuclear factor (NF)-κB, and AKT (16). Thus, the beneficial effects of AAT in the islet transplantation setting may be mediated by its antiapoptotic and anti-inflammatory properties and promotion of islet revascularization.
In many animal studies, islets are transplanted under the kidney capsule for easy analysis. In clinical practice, islets are transplanted into the liver by portal vein infusion. The major difference between these two methods is that in portal vein infusion, islets are directly exposed to blood after transplantation, which leads to IBMIR, a thrombotic/inflammatory reaction mediated by the innate immune system (17–19). IBMIR involves activation of the coagulation cascade and the inflammatory pathway and eventually leads to clot formation and infiltration of leukocytes into the islets that cause islet destruction and failure of engraftment (20). IBMIR is a significant factor in the damage to allogeneic, xenogeneic, and autologous islet transplants (18,21), which results in a prothrombotic state and secretion of proinflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8, and interferon-inducible protein-10 (18). IBMIR also happens after hepatocyte and mesenchymal stem cell transplantation (22,23). Therefore, IBMIR may represent a major hurdle for all cell therapies and is being targeted by NF-κB inhibitors or anticoagulants such as low-molecular-weight dextran sulfate (24,25). Furthermore, Hering et al. (26) showed that a TNF-α inhibitor, etanercept, in addition to prolonged heparin treatment, contributed to improved islet engraftment in their clinical trial on single-donor, marginal dose islet transplants in patients with T1D. Suppression of IBMIR may therefore contribute to a better outcome in islet transplantation (26).
Although the protective effect of AAT in the kidney capsule islet transplantation model has been reported (13,14,16,17,27), whether AAT protects intrahepatic islet grafts that mimic the clinical islet transplantation setting remains unknown. The focus of this study was to evaluate the effect of AAT on IBMIR-induced islet death and understand the molecular mechanisms of the anti-inflammatory effects of AAT.
Research Design and Methods
The Medical University of South Carolina Animal Care and Use Committee approved all mouse experiments.
Mice
Male C57BL/6 and NOD-SCID mice at 7–8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME).
Mouse Islets Isolation, Diabetes Induction, and Islet Transplantation
Islets were isolated by collagenase digestion as described previously (28). Diabetes was induced in mice by one injection of streptozotocin (225 mg/kg; Sigma-Aldrich). Mice were considered diabetic when nonfasting blood glucose >300 mg/dL for at least 2 consecutive days. For islet transplantation, 200–250 mouse or 500 human islets were infused in a total volume of 200 µL Hanks’ balanced salt solution and 0.5% BSA into the recipient liver as previously described (29). Mice with blood glucose levels <200 mg/dL were considered normoglycemic.
In Vitro Miniature Tube Model for IBMIR
Miniaturized in vitro tube models were used as previously described (18,25). Briefly, mice blood was collected in heparin-lithium coated 1.5 mL Eppendorf tubes (Fisher Scientific). Islets were mixed with blood (500 islets/500 μL blood) in the presence or absence of AAT (0.5 mg/mL) in an incubator on a hematology mixer for 6 h. Supernatant samples were collected at 0, 15, and 60 min and at 3 and 6 h.
AAT Injection and Concentration in Serum
C57BL/6 mice were injected with AAT (2 mg/mouse, i.p.; Prolastin-C, Grifols) or saline every 2 (Q2D) or 3 days (Q3D) for a total of eight doses. Control mice received saline at the same schedule as the Q2D group. Serum concentrations of human AAT were determined before the last dose using a human AAT-specific ELISA kit (Abcam) according to the manufacturer’s instructions. In the islet transplantation model, the first dose of AAT was given 1 day before islet transplantation.
Immunohistochemical Staining
Livers were harvested 6 h after transplantation. Sections of 5 μm at 100-μm intervals were incubated with anti-F4/80, anti-tissue factor (TF) (Abcam) and anti-insulin antibodies (Thermo Fisher Scientific) overnight at 4°C. For miniature tube assays, islets in blood were spun down, washed on a cell strainer with cold PBS, and subjected to frozen sections. Anti-phosphorylated c-Jun N-terminal kinase (p-JNK) (1:300; Cell Signaling) and anti-insulin antibodies were applied. A ZEISS AxioImager M2 microscope and Leica SP5 confocal microscope were used. Biotinylated goat anti-mouse fibrinogen antibody (Accurate Chemical & Scientific Corporation) was used to stain fibrin deposition (28).
Neutrophil Infiltration
The Naphthol AS-D Chloroacetate (Specific Esterase) Kit (Sigma-Aldrich) was used to stain for polymorphonuclear cells according to the manufacturer's instructions.
Preparation of Nuclear Extract and Determination of NF-κB Activation
Liver tissues were collected 24 h after transplantation. A nuclear extraction kit, followed by a NF-κB (p65) transcription factor assay kit (Cayman), was used as described by the manufacturer. Protein concentrations were determined by Pierce BCA protein assay (Thermo Fisher Scientific).
Intravenous Glucose Tolerance Test
Recipient mice were fasted overnight and injected with glucose solution at 1 g/kg via tail vein. Blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min after injection.
Measurements of Coagulation Factors and Plasma Cytokines
Serum thrombin-antithrombin (TAT), mouse TF (Abcam ELISA), and mouse TNF-α (RayBio ELISA) were measured according to the manufacturers’ instructions.
Cytokine Treatment and Western Blot
βTC3 cells were preincubated with AAT for 2 h before the addition of 100 units/mL IL-1β and 1,000 units/mL interferon-γ (IFN-γ). Human islets were treated with 50 units/mL TNF-α, 50 units/mL IL-1β, and 1,000 units/mL INF-γ until cells were collected at indicated times. Proteins were analyzed by immunoblotting with antibodies against p-JNK and total JNK. Histone H3 was used as a loading control. This experiment was repeated five times. Western blots were further quantified using standard densitometric analysis with ImageJ software (National Institutes of Health). Briefly, the bands on the films were digitized on a flatbed photo scanner. Each band was manually selected, and the intensities were measured using the “gel analyzer” option in ImageJ software. The integrated band densities were analyzed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA).
Statistical Analysis
Graft survival was plotted using StatView software, and differences were compared by a log-rank test. Data are expressed as mean ± SD. Differences between groups were compared for statistical significance by ANOVA or Student t test. P < 0.05 denoted significance.
Results
Effect of AAT on Functional Outcome of Intrahepatic Islet Grafts
We first examined the protective effects of AAT in a syngeneic islet transplantation model in which a marginal mass of islets (n = 200–250) from C57BL/6 mice were infused into the liver of syngeneic diabetic recipients. At 60 days PT, only 2 of 18 mice (11%) in the control group reached normoglycemia compared with 4 of 12 (33%) in the AAT Q3D group (n = 12, P = 0.05 vs. control by log-rank test) (Fig. 1A) and 11 of 19 (58%) in the AAT Q2D group (n = 19, P = 0.001 vs. control). In mice with normoglycemia, average days needed to reach normoglycemia was 54.5 ± 0.5 in control, 21.8 ± 8.1 in AAT Q3D, and 5.0 ± 1.9 in AAT Q2D groups (Fig. 1B). At 30 days PT, we performed intravenous glucose tolerance test (IVGTT) in AAT-treated mice and controls that were normoglycemic. Mice that received AAT treatment (Q2D) showed faster glucose clearance (Fig. 1C) and a significantly smaller area under the curve (AUC) after the glucose challenge compared with controls (Fig. 1C inset). These data provided strong evidence that AAT treatment improved graft survival and function in the intrahepatic islet transplantation model. We measured trough serum AAT levels before the last AAT injection for the Q2D and Q3D regimens. A significantly higher plasma AAT level was observed in the Q2D group (3.52 ± 0.515 mg/mL, n = 8) than in the Q3D group (2.03 ± 0.103 mg/mL, n = 11) (Fig. 1D).
Figure 1.
AAT treatment improves islet survival and function in a syngeneic mouse intraportal islet transplantation model. A: Kaplan-Meier analysis shows significantly higher percentages of PT normoglycemia in diabetic mice that received AAT (2 mg/kg) Q2D or Q3D compared with control (Ctrl), which received vehicle. B: AAT-treated mice reached normoglycemia faster than control mice. C: IVGTT shows that AAT treatment (Q2D) enhances blood glucose disposal 30 days after transplantation. Inset, AUC of IVGTT. D: Serum concentrations of human AAT before the last dose in each group (n = 8–9 in each group). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 by Student t test.
Effects of AAT on Plasma C-Peptide and Islet Apoptosis
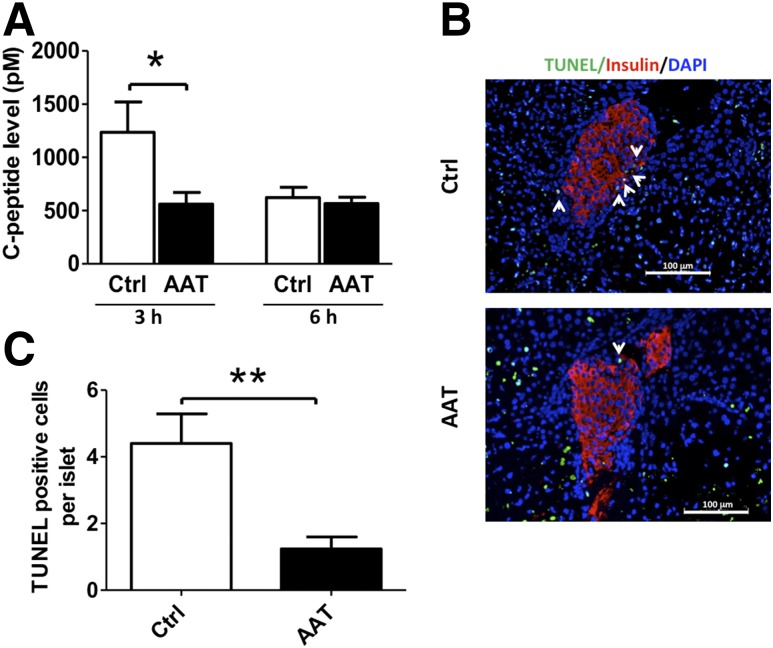
A rapid rise of plasma C-peptide levels is usually seen in patients with islet transplantation, likely the result of insulin release from damaged islet grafts caused by IBMIR. We found that in mice transplanted with 500 islets in the liver, the C-peptide level elevated 3 h PT and declined 6 h PT. AAT treatment significantly reduced the plasma C-peptide level 3 h PT compared with the control group (Fig. 2A). Furthermore, the AAT-treated mice had lower islet graft apoptosis as analyzed by TUNEL assay at 24 h PT compared with controls (Fig. 2B and C).
Figure 2.
AAT reduces immediate release of C-peptide and inhibits islet cell death after transplantation. A: Serum C-peptide levels in AAT-treated and control (Ctrl) recipients 3 and 6 h PT as measured by ELISA. B: Islets in liver sections from AAT-treated mice showed fewer TUNEL+ dead cells compared with islets from control mice 24 h after islet transplantation. Nuclei are stained with DAPI (blue) and β-cells with anti-insulin antibody (red). Arrowheads point to TUNEL+ cells (green) within an islet. C: Percentage of TUNEL+ cells per islet graft as determined after microscopic evaluation of intrahepatic islets randomly selected from three different recipients in Ctrl (25 islets with sizes of 10,900.5 ± 3,700.7 µm2) and AAT (20 islets with sizes of 9,480.4 ± 4,840.2 µm2) groups. Results are expressed as means ± SD. *P < 0.05, **P < 0.01 by Student t test.
To confirm the protective effects of AAT on islets undergoing IBMIR, C-peptide levels were measured in vitro in a miniature tube model as previously described (18,25). Mouse islets were incubated with blood from the same strain for 6 h. C-peptide release was significantly elevated in the islets plus blood group compared with the islets plus medium group (Supplementary Fig. 1), indicating the presence of IBMIR-induced damage in islets incubated with blood. Importantly, the presence of AAT significantly suppressed the acute release of C-peptide 3 h after incubation (Supplementary Fig. 1A).
Effect of AAT on Neutrophil and Macrophage Infiltration
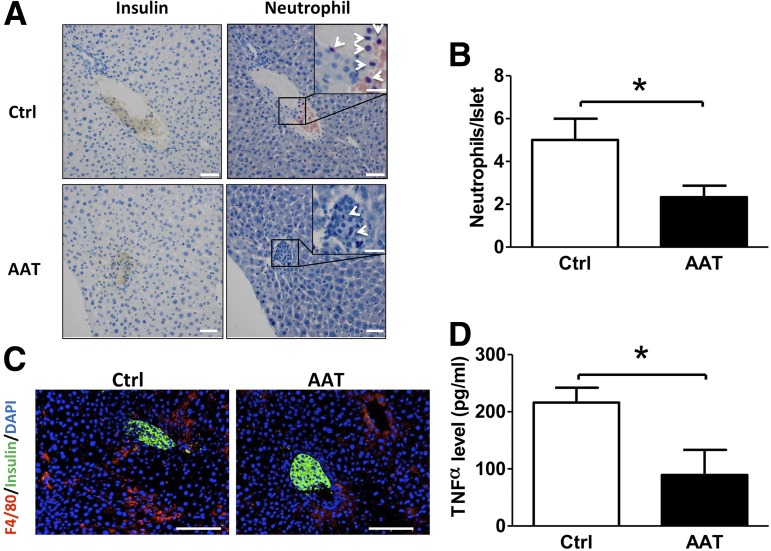
One of the major characteristics of IBMIR after intraportal transplantation is the recruitment of neutrophils and macrophages to sites of islet engraftment (30). To test our hypothesis that AAT protects islet grafts from IBMIR, livers of recipients were collected 6 h PT for histological analysis. We observed a substantial neutrophil infiltration in livers from control animals, which was consistent with previous reports of a similar assay (28). In contrast, treatment with AAT significantly reduced neutrophil infiltration into islets compared with controls (Fig. 3A and B). Similarly, fewer macrophages (F4/80+ cells) were seen at the site of islet grafts in AAT-treated mice compared with those from controls (Fig. 3C). Overall, AAT treatment inhibited neutrophil and macrophage infiltration in transplanted islets.
Figure 3.
AAT suppresses neutrophil and macrophage infiltration and serum TNF-α production. A: Accumulation of neutrophils identified by naphthol AS-D chloroacetate esterase staining in AAT (n = 3) or control (Ctrl; n = 3) grafts 6 h PT. Serial paraffin sections of an islet graft were stained with anti-insulin (left, brown staining) and naphthol AS-D chloroacetate esterase staining (right, purple staining). Arrowheads point to neutrophils. B: Numbers of neutrophils within the islet were lower in the AAT group than in the control group 6 h after islet transplantation. A total of 25 Ctrl islets (average size: 8,500.9 ± 6,816.6 µm2) and 27 AAT islets (average size: 10,549.2 ± 6,596.9 µm2) were counted. C: Representative photomicrographs show fewer infiltrated macrophages surrounding transplanted islets identified by the anti-F4/80 antibody in AAT compared with Ctrl mice 6 h after islet transplantation. Red staining identifies macrophages, green staining identifies β-cells, and blue represents nuclear material from all cells. D: Serum TNF-α levels measured by ELISA are higher in serum of Ctrl (n = 6) than AAT-treated (n = 4) recipients 6 h after islet transplantation. Scale bars are 100 μm for panels A and C and 20 μm for insets in panel A. *P < 0.05 AAT vs. Ctrl by Student t test.
We further measured serum TNF-α in mice treated with AAT or vehicle. The level of serum TNF-α was not detected in mice before the transplantation, whereas a significant rise of TNF-α was observed in the control animals 6 h PT. Administration of AAT significantly decreased the serum TNF-α level (Fig. 3D).
AAT Alters TF Expression, Plasma TAT Levels, and Fibrin Deposition
TF expressed on islets is considered the trigger for IBMIR. To determine whether AAT inhibits IBMIR through the suppression of TF, immunofluorescent staining was performed to analyze the expression of TF by islet grafts after transplantation. At 6 h PT, a dramatic expression of TF was observed in control grafts. In contrast, AAT administration significantly repressed expression of TF by islets, as indicated by immunohistochemistry and the relative intensity calculated by dividing the intensity of insulin staining (Fig. 4A and B).
Figure 4.
AAT inhibits activation of coagulation after intraportal transplantation. A: Reduced TF expression in AAT-treated grafts compared with control (Ctrl) grafts. Immunohistochemistry staining of TF (green) in transplanted islets (red for insulin) from AAT or Ctrl mice. Arrows point to TF+ cells. B: Relative fluorescence intensity of TF divided by intensity of insulin in grafts from AAT or Ctrl mice. C: Change of plasma TAT levels in AAT and Ctrl mice at 3 and 6 h PT. D: Fibrinogen deposition in transplanted islets 6 h after intraportal transplantation in Ctrl and AAT mice. Serial paraffin sections of an islet graft were stained with anti-insulin (left) and anti-fibrinogen (right). The dashed line outlines islet grafts based on the insulin staining. The arrow indicates fibrin deposition on islet graft. Scale bars = 100 μm. Data are expressed as mean ± SD. *P < 0.05 AAT vs. Ctrl by Student t test.
TAT is a sensitive indicator for the activation of blood coagulation. To test for the effect of AAT on the thrombotic reaction in IBMIR, plasma TAT levels were monitored. AAT treatment effectively suppressed the elevated plasma TAT level observed in the control group within 6 h PT (Fig. 4C). In the in vitro miniature tube model, TAT was not detected in islets incubated with medium but was markedly elevated in islets incubated with blood. In contrast, AAT effectively blocked this elevation of TAT (Supplementary Fig. 1B). We also measured the generation of fibrinogen deposition, which is normally caused by the activation of thrombin and is detrimental to islet grafts. As shown by immunohistochemistry, an apparent decrease of fibrin staining was observed in islet grafts from the AAT group compared with grafts from the control group 6 h PT (Fig. 4D). These data further confirmed the antithrombotic effect of AAT in IBMIR.
Cytokine-Induced JNK Phosphorylation In Vitro in βTC3 Cells
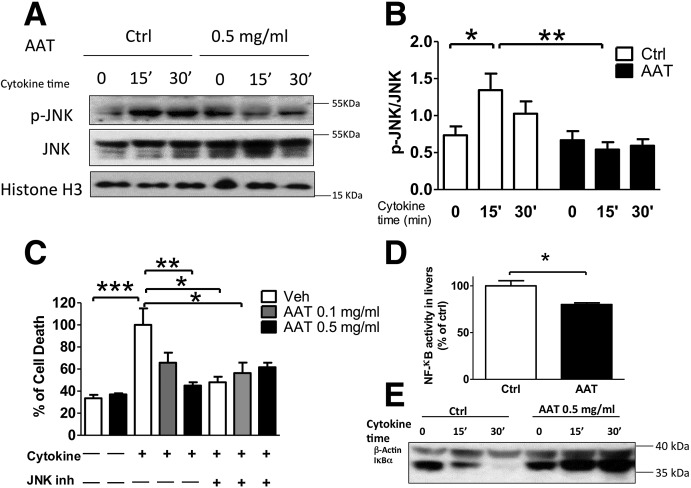
IBMIR produces systemic and local proinflammatory cytokine cascades. Proinflammatory cytokines induce pancreatic β-cell death via JNK phosphorylation and NF-κB activation (20,31). Whether AAT has an effect on the JNK pathway is not currently known. Transcriptional profiles of islet grafts and surrounding liver tissues from control and AAT-treated mice revealed a significant downregulation of the mRNA level of Jun, the substrate of JNK, by AAT treatment. Western blot analysis revealed that AAT significantly suppressed JNK phosphorylation in βTC3 cells 15 and 30 min after cytokine stimulation (Fig. 5A and B). By measuring cell death, we found that AAT treatment suppressed cytokine-induced β-cell death in a dose-dependent manner, an effect comparable to that of the JNK inhibitor SP600125 (Fig. 5C). Moreover, the addition of SP600125 failed in further reducing the AAT-suppressed cell death, implying the possibility that the potent anti-inflammatory effect of AAT is mediated by suppression of JNK phosphorylation.
Figure 5.
AAT protects islets from cytokine-induced cell death by suppression of JNK phosphorylation and NF-κB activation. AAT treatment reduces p-JNK after cytokine treatment as measured by Western blot. A: Representative immunoblot of p-JNK and total JNK and histone H3 by Western blot. B: Ratio of p-JNK to total JNK from immunoblots quantified by densitometry. Data are presented as mean ± SD for five individual experiments. C: Cell death measured in βTC3 cells pretreated with AAT at 0.1 mg/mL or 0.5 mg/mL and/or 20 nmol/L SP600125, the JNK phosphorylation blocker (JNK inh), for 1 h and stimulated with cytokines for 48 h using the Cell Death ELISA kit. Experiments were repeated four times. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 using one-way ANOVA, followed by Tukey post hoc analysis. D: NF-κB activity (percentage to control [Ctrl]) as measured by p65 level in the nuclear fraction by ELISA in livers bearing islet grafts. Experiments were repeated three times. E: Representative immunoblot of inhibitor of κB-α (IκBα) and β-actin in βTC3 cells pretreated with vehicle or 0.5 mg/mL AAT, and stimulated with cytokine for 0–30 min as analyzed by Western blot. *P < 0.05 AAT vs. Ctrl by Student t test.
AAT Attenuates NF-κB Activation in Liver Tissue and in βTC3 Cells
Activation of NF-κB was studied in liver tissues harvested 24 h PT. A significant suppression of NF-κB activation (p65 nuclear translocation) was observed in livers of mice treated with AAT compared with the control (Fig. 5D), indicating that AAT attenuated the NF-κB activation triggered by IBMIR. Consistent with this, we observed that AAT treatment prevented cytokine-induced inhibitor of κB-α (IκBα) degradation, indicating that NF-κB activation was also suppressed by AAT treatment on βTC3 cells (Fig. 5E). These experiments show that AAT protects β-cells from cytokine-induced apoptosis by suppression of JNK phosphorylation and NF-κB activation.
AAT Protects Human Islet Grafts Transplanted Into Diabetic NOD-SCID Mice Livers
Because there are major differences between mouse and human islets (32,33), testing the effects of AAT on human islets in IBMIR is necessary to confirm the potential clinical relevance of this drug. To this end, a marginal mass of human islets were infused into diabetic NOD-SCID mice livers in the presence or absence of one injection of AAT (2 mg/mL, i.p.). We found that human C-peptide level peaked at 1 h PT in the control recipient serum samples and that AAT treatment attenuated the rapid release of C-peptide from the islet grafts (Fig. 6A). The infusion of human islets triggered a substantial increase of plasma TAT, and in accordance with our data in the C57BL/6 mice, AAT significantly lowered plasma TAT levels in recipients (Fig. 6B). Furthermore, we found that neutrophilic infiltration to the human islet grafts was blocked by AAT (Fig. 6C and D). Immunohistochemistry showed reduced macrophage accumulation at the locations of islet grafts in the AAT-treated livers (Fig. 6E), suggesting that AAT treatment protected human islet grafts from the damage of IBMIR.
Figure 6.
AAT reduces serum C-peptide and TAT levels and inhibits inflammatory cells infiltration to human islet grafts. A: Serum C-peptide levels in AAT-treated and control (Ctrl) recipients 1, 3, and 6 h PT as measured by ELISA. B: Change of plasma TAT levels in AAT and Ctrl mice. *P < 0.05, **P < 0.01 vs. Ctrl by two-way repeated-measures ANOVA, followed by Bonferroni correction. C: Less neutrophil infiltration was identified in AAT grafts compared with control grafts 6 h PT. Serial paraffin sections of an islet graft were stained with anti-insulin (left) and naphthol AS-D chloroacetate esterase staining (right). The dashed line illustrates islets with positive insulin staining, and arrowheads point to neutrophils. Scale bars = 100 μm. D: Number of neutrophils per islets in AAT and control mice (n = 20 per group) 6 h after islet transplant. E: Representative photomicrographs show more infiltrated macrophages surrounding Ctrl vs. AAT islet grafts as identified by staining with the anti-F4/80 antibody 6 h after islet transplantation. Green staining identifies macrophages, red staining identifies insulin+ cells, and blue represents nuclei. *P < 0.05 AAT vs. Ctrl by Student t test.
AAT Reduces TF Expression and Inhibits Neutrophil Infiltration in a Human Islet In Vitro Miniature Tube Model
To further confirm the protective effects of AAT on human islets in IBMIR, we incubated human islets in vitro in NOD-SCID mouse blood for 15 min with constant disturbance. We observed less TF expression in islets pretreated with AAT for 2 h before the exposure to blood. Compared with islets cultured in medium, addition of AAT significantly decreased TF (Fig. 7A and B), without other exogenous stimulation, indicating that AAT suppresses TF expression not only under pathological stress, such as IBMIR, but also under relatively normal conditions like an in vitro culture. The exposure to blood induced a significant number of neutrophils infiltrating into human islets, with morphological damage. In contrast, the islets obtained from the AAT-treated group exhibited fewer neutrophils with preserved morphology (Fig. 7C and D). Thus, AAT could inhibit TF expression and neutrophilic damage to human islets in a miniature tube assay.
Figure 7.
AAT reduces TF expression and inhibits neutrophil infiltration to human islet in miniature tube assay. A: Reduced TF expression was observed in human islets (Hi) transplanted to AAT-treated recipients compared with controls 15 min after exposure to NOD-SCID blood. GM, growth medium. B: Relative fluorescence intensity of TF divided by intensity of insulin from the AAT or control (Ctrl) group. ImageJ software was used to measure three to nine islets in each group. More polymorphonuclear cell infiltrations were observed in Ctrl islets compared with AAT-treated islets as determined by naphthol AS-D chloroacetate esterase staining 15 min after exposure to NOD-SCID blood as measured by immunohistochemistry staining (C) and cell counting (D). Scale bars = 50 μm. More than 30 islets were counted in each group. *P < 0.05 AAT vs. Ctrl, ***P < 0.001 by Student t test.
AAT Suppresses JNK Phosphorylation in Human Islets In Vitro
To determine whether AAT inhibits JNK phosphorylation in human islets, islets were exposed to NOD-SCID mouse blood or a cytokine cocktail consisting of TNF-α, IL-1β, and IFNγ. By immunohistochemistry, we observed that pretreatment of AAT significantly reduced p-JNK+ staining after incubation with blood for 15 min (Fig. 8A and B). In addition, cytokine-induced islet cell apoptosis, as detected by TUNEL at 72 h after stimulation, was significantly decreased by AAT pretreatment (Supplementary Fig. 2). Furthermore, AAT inhibited JNK phosphorylation in a dose-dependent manner in these samples at 15, 30, and 60 min of cytokine exposure (Fig. 8C). These results confirmed that AAT could inhibit JNK phosphorylation in human islets.
Figure 8.
AAT suppressed JNK phosphorylation in human islets in vitro. A: Immunohistochemistry staining of TF in human islets 15 min after exposure to NOD-SCID blood. B: Relative fluorescence intensity of p-JNK divided by intensity of insulin from AAT or control (Ctrl) group. In each group, 10 islets were measured using ImageJ software. C: Human islets were treated with cytokines with or without preincubation with AAT for the indicated times. Representative immunoblot of p-JNK is shown. Ratio of p-JNK to total JNK from immunoblots was quantified by densitometry. Scale bars = 50 μm. *P < 0.05 AAT vs. Ctrl by Student t test.
Discussion
More than half of transplanted islets may be lost in the immediate PT period (34). Strong evidence shows that IBMIR is the major cause of early islet graft loss in autologous and in allogeneic islet transplantation. In this study, we found that AAT protects islet grafts from IBMIR-induced damage and thus improves the outcome of syngeneic intraportal islet transplantation in mice. We showed that AAT treatment significantly reduces the activation of coagulation and inflammation pathways in IBMIR. This leads to less islet cell death 24 h PT, prolongs islet graft survival, and improves islet function 60 days PT.
We demonstrate for the first time that AAT suppresses IBMIR in the intraportal islet transplantation model with both mouse and human islets. Previous studies have shown that AAT administration prolonged islet allograft survival when islets were transplanted under the kidney capsule (13,14,17). In the clinic, however, islets are infused to the patient’s liver through the portal vein. The major difference between intraportal and subcapsular kidney islet transplantation is that IBMIR is only present in the former where islets are directly exposed to the recipients’ blood circulation (19).
A short-term AAT treatment on diabetic cynomolgus monkeys that underwent intraportal autologous islet transplantation improved blood glucose levels and preserved graft morphology at day 468 PT (16). Given that IBMIR begins immediately after islet infusion and peaks in less than 3 h (18), our group focused on investigating the short-term effects of AAT with regard to the inhibition of coagulation and inflammation. We found that AAT significantly suppressed elevated plasma TAT levels, reduced TF expression, and reduced fibrin deposition within 6 h PT in recipients that received mouse or human islets. AAT also decreased circulating TNF-α levels and leukocyte infiltration in the islet grafts compared with the control group. All of these results translated into less islet cell damage and better blood glucose control 30 days PT in recipients treated with AAT. The evidence shows that AAT inhibits IBMIR, which results in improved outcome of intraportal islet transplantation in mice.
TF is produced and secreted by the endocrine cells of the islets. Exposure of islet cell–expressed TF to the recipient’s blood circulation is thought to initiate IBMIR (6). The interaction of TF and factor VII results in the activation of the extrinsic pathway, leading to the rapid elevation of plasma TAT regularly seen in patients undergoing islet transplantation (18). These thrombotic reactions cause fibrin deposition on islet grafts, as was observed in our experimental setting (28). Therefore, methods that can suppress the thrombotic reaction become intriguing therapeutic strategies in the prevention of IBMIR. Currently, most centers use systemic administration of heparin to prevent blood clots in autologous and allogeneic islet transplantations. However, the potential therapeutic efficacy of heparin is largely limited by its short half-life and the risk of systemic bleeding complications. Furthermore, heparin only at a clinically used concentration has failed to prevent infiltration of CD11b+ cells (28,35), indicating that additional anti-inflammatory reagents are needed. Indeed, in the Clinical Islet Transplantation 07 (CIT07) protocol, an extension of anticoagulation beyond the islet infusion procedure was adopted in combination with peritransplant administration of a TNF-α inhibitor, which contributed to improved islet engraftment in patients with T1D (36).
Low-molecular-weight dextran sulfate (24) and a combination of Tirofiban and activated protein C (37) have also been tested in an ex vivo loop model and proved effective in ameliorating the thrombotic reaction in IBMIR. However, to date, neither of these treatment strategies has been reported. There is no current literature that describes the use of these treatments in the in vivo islet transplant model. To address the inflammatory reactions in IBMIR, Withaferin A, an NF-κB inhibitor, was applied in a miniature tube model and demonstrated significant inhibitory effects on cytokine secretion and neutrophil infiltration but no effect on elevated TAT level in the tube model (25). In this study, we found that AAT markedly inhibited TF expression and suppressed elevated plasma TAT levels caused by IBMIR in addition to its inhibitory effects on inflammatory cell infiltration and cytokine release, both in mouse and human islets. Because AAT has well-established anti-inflammatory effects in a large number of other studies (13,17,27,38,39), we suspected that the effect was not specific to the coagulation cascade.
Cytokines produced by infiltrated leukocytes and endocrine islet cells in IBMIR play a crucial role in the destruction of islet grafts (17,21,30,40). Cytokine-induced β-cell failure and death are key events that lead to diabetes (41). Cytokines rapidly activate stress-activated protein kinase/JNK, as well as other proinflammatory kinases. Prolonged and pronounced activation of JNK leads to β-cell death (42,43). According to our expression profile–based analysis, the mRNA level of Jun, which is the substrate of JNK, was significantly downregulated by AAT treatment. We hypothesized that inhibition of the JNK pathway served as one underlying mechanism of the protective effect of AAT on cytokine-induced β-cell death seen in our experimental setting of IBMIR and also in other models with T1D (12) and cytokine stimulation (13). The data that AAT significantly suppressed cytokine-induced JNK phosphorylation and cell death and that blocking JNK phosphorylation showed similar protective effects of AAT indicated for the first time that AAT exerts its protective function via suppression of JNK phosphorylation in the intrahepatic islet transplantation model. Feng et al. (44) demonstrated that overexpression of AAT decreased hypoxia/reperfusion–induced p38 expression in human umbilical vein endothelial cells, supporting the hypothesis that normal AAT is able to regulate the intracellular mitogen-activated protein kinase pathway, in addition to its well-known protease inhibitor function.
This study has some limitations. By implicating multiple mechanisms by which AAT protects islets from short- and long-term injury and apoptotic death, we run the risk that these mechanisms may be interdependent. More elaborate experiments could determine which of the protective pathways has more effect than others. More work is also justified to determine whether AAT directly suppresses JNK activation or the upstream factors in the JNK pathway in islet β-cells. It is fitting and proper that this work be done in a human islet transplantation setting because the demonstrated benefits of AAT are strong and the toxicities of this medication to humans are minimal.
By careful examination of a number of cellular pathways, we have identified a critical mechanism by which AAT exerts protective effects in a mouse intrahepatic islet transplantation model. These data support the feasibility of using AAT in clinical islet transplantation and other cell therapies that involve IBMIR.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Bashoo Naziruddin (Baylor University Medical Center, Dallas, TX) for help setting up the in vitro miniature tube model for IBMIR and the Reeves family (Bentonville, AR) for their generous donation.
Funding. This study was supported by the Foundation for the National Institutes of Health grants 1R01-DK-105183, DK-099696, and DK-097544 to H.W. and National Institutes of Health/National Center for Advancing Translational Sciences grant UL1-TR-001450.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.W. performed ex vivo experiments, analyzed data, and wrote the manuscript. Z.S. and W.G. performed in vivo experiments. D.B.A. and K.A.M. participated in writing the manuscript. W.C. participated in the human islet study. C.S. participated in data discussion and in writing the manuscript. H.W. designed the study, performed some in vivo experiments, analyzed the data, and wrote the manuscript. H.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1036/-/DC1.
References
- 1.Ichii H, Ricordi C. Current status of islet cell transplantation. J Hepatobiliary Pancreat Surg 2009;16:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Desai KD, Dong H, et al. . Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation 2013;95:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaña E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 1993;91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996;45:1161–1167 [DOI] [PubMed] [Google Scholar]
- 5.Brantly ML, Wittes JT, Vogelmeier CF, Hubbard RC, Fells GA, Crystal RG. Use of a highly purified alpha 1-antitrypsin standard to establish ranges for the common normal and deficient alpha 1-antitrypsin phenotypes. Chest 1991;100:703–708 [DOI] [PubMed] [Google Scholar]
- 6.Breit SN, Wakefield D, Robinson JP, Luckhurst E, Clark P, Penny R. The role of alpha 1-antitrypsin deficiency in the pathogenesis of immune disorders. Clin Immunol Immunopathol 1985;35:363–380 [DOI] [PubMed] [Google Scholar]
- 7.Mordwinkin NM, Louie SG. Aralast: an alpha 1-protease inhibitor for the treatment of alpha-antitrypsin deficiency. Expert Opin Pharmacother 2007;8:2609–2614 [DOI] [PubMed] [Google Scholar]
- 8.Petrache I, Fijalkowska I, Medler TR, et al. . alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol 2006;169:1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc 2006;3:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Lu Y, Campbell-Thompson M, et al. . Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007;56:1316–1323 [DOI] [PubMed] [Google Scholar]
- 11.Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997;88:561–572 [DOI] [PubMed] [Google Scholar]
- 12.Koulmanda M, Bhasin M, Hoffman L, et al. . Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A 2008;105:16242–16247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A 2005;102:12153–12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis EC, Mizrahi M, Toledano M, et al. . alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A 2008;105:16236–16241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimoda M, Noguchi H, Fujita Y, et al. . Improvement of porcine islet isolation by inhibition of trypsin activity during pancreas preservation and digestion using α1-antitrypsin. Cell Transplant 2012;21:465–471 [DOI] [PubMed] [Google Scholar]
- 16.Koulmanda M, Sampathkumar RS, Bhasin M, et al. . Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant 2014;14:1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abecassis A, Schuster R, Shahaf G, et al. . α1-antitrypsin increases interleukin-1 receptor antagonist production during pancreatic islet graft transplantation. Cell Mol Immunol 2014;11:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014;14:428–437 [DOI] [PubMed] [Google Scholar]
- 19.Sakata N, Tan A, Chan N, et al. . Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc 2009;41:346–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 2011;16:620–626 [DOI] [PubMed] [Google Scholar]
- 21.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci 2000;105:125–133 [DOI] [PubMed] [Google Scholar]
- 22.Moll G, Rasmusson-Duprez I, von Bahr L, et al. . Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012;30:1565–1574 [DOI] [PubMed] [Google Scholar]
- 23.Gustafson EK, Elgue G, Hughes RD, et al. . The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation 2011;91:632–638 [DOI] [PubMed] [Google Scholar]
- 24.Johansson H, Goto M, Dufrane D, et al. . Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant 2006;6:305–312 [DOI] [PubMed] [Google Scholar]
- 25.Kanak MA, Takita M, Itoh T, et al. . Alleviation of instant blood-mediated inflammatory reaction in autologous conditions through treatment of human islets with NF-κB inhibitors. Transplantation 2014;98:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hering BJ, Kandaswamy R, Ansite JD, et al. . Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835 [DOI] [PubMed] [Google Scholar]
- 27.Koulmanda M, Bhasin M, Fan Z, et al. . Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci U S A 2012;109:15443–15448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui W, Angsana J, Wen J, Chaikof EL. Liposomal formulations of thrombomodulin increase engraftment after intraportal islet transplantation. Cell Transplant 2010;19:1359–1367 [DOI] [PubMed] [Google Scholar]
- 29.Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature 1973;244:447. [DOI] [PubMed] [Google Scholar]
- 30.Martin BM, Samy KP, Lowe MC, et al. . Dual islet transplantation modeling of the instant blood-mediated inflammatory reaction. Am J Transplant 2015;15:1241–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphrey RK, Newcomb CJ, Yu SM, et al. . Mixed lineage kinase-3 stabilizes and functionally cooperates with TRIBBLES-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells. J Biol Chem 2010;285:22426–22436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 2012;61:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 2014;63:819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 35.Bennet W, Sundberg B, Groth CG, et al. . Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 1999;48:1907–1914 [DOI] [PubMed] [Google Scholar]
- 36.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. . Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akima S, Hawthorne WJ, Favaloro E, et al. . Tirofiban and activated protein C synergistically inhibit the instant blood mediated inflammatory reaction (IBMIR) from allogeneic islet cells exposure to human blood. Am J Transplant 2009;9:1533–1540 [DOI] [PubMed] [Google Scholar]
- 38.Churg A, Dai J, Zay K, et al. . Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Invest 2001;81:1119–1131 [DOI] [PubMed] [Google Scholar]
- 39.Pott GB, Chan ED, Dinarello CA, Shapiro L. α-1-Antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol 2009;85:886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SoRelle JA, Itoh T, Peng H, et al. . Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia 2013;56:814–824 [DOI] [PubMed] [Google Scholar]
- 41.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54(Suppl. 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem 2003;278:52810–52819 [DOI] [PubMed] [Google Scholar]
- 43.Abdelli S, Ansite J, Roduit R, et al. . Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004;53:2815–2823 [DOI] [PubMed] [Google Scholar]
- 44.Feng Y, Hu L, Xu Q, et al. . Cytoprotective role of alpha-1 antitrypsin in vascular endothelial cell under hypoxia/reoxygenation condition. J Cardiovasc Pharmacol 2015;66:96–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.