Abstract
Misregulated hormone secretion from the islet of Langerhans is central to the pathophysiology of diabetes. Although insulin plays a key role in glucose regulation, the importance of glucagon is increasingly acknowledged. However, the mechanisms that regulate glucagon secretion from α-cells are still unclear. We used pseudoislets reconstituted from dispersed islet cells to study α-cells with and without various indirect effects from other islet cells. Dispersed islet cells secrete aberrant levels of glucagon and insulin at basal and elevated glucose levels. When cultured, murine islet cells reassociate to form pseudoislets, which recover normal glucose-regulated hormone secretion, and human islet cells follow a similar pattern. We created small (∼40-µm) pseudoislets using all of the islet cells or only some of the cell types, which allowed us to characterize novel aspects of regulated hormone secretion. The recovery of regulated glucagon secretion from α-cells in small pseudoislets depends upon the combined action of paracrine factors, such as insulin and somatostatin, and juxtacrine signals between EphA4/7 on α-cells and ephrins on β-cells. Although these signals modulate different pathways, both appear to be required for proper inhibition of glucagon secretion in response to glucose. This improved understanding of the modulation of glucagon secretion can provide novel therapeutic routes for the treatment of some individuals with diabetes.
Introduction
Glucose homeostasis depends on hormones secreted by the islet of Langerhans. Insufficient levels of the main islet hormone insulin leads to diabetes, and the use of insulin to treat this disease ranks among the most important medical therapies. The success of insulin as a clinical treatment of diabetes has focused most islet research onto the insulin-secreting β-cells. Glucagon, another islet hormone, is known to prevent hypoglycemia. Although we now understand that aberrant glucagon secretion from pancreatic islet α-cells can exacerbate hyperglycemia, the role of glucagon in diabetes remains controversial and poorly understood. In healthy individuals, glucagon secretion from islet α-cells decreases in response to elevated glucose, but paradoxically, dispersed α-cells increase their glucagon secretion under the same conditions (1–3). The mechanistic origin of this paradox is currently unknown, but recent publications (4,5) suggest that multiple signaling pathways within α-cells and between islet cells likely simultaneously regulate glucagon secretion.
Published data support several models to explain how α-cells regulate glucagon secretion (6–8). These models fall into three classes; α-cell–intrinsic, paracrine-signaling, and juxtacrine-signaling models. In the intrinsic α-cell model, α-cells regulate their own secretion through changes in intracellular metabolism and electrical signaling in response to glucose (9,10). In the paracrine-signaling model, glucose indirectly inhibits glucagon secretion through factors secreted by the other islet cell types, including insulin from β-cells and somatostatin from δ-cells (4,11–13). In the juxtacrine-signaling model, specific cell-to-cell contacts impinge upon EphA receptors in the α-cells, putatively by ephrinA ligands on the neighboring β-cells, to regulate glucagon secretion (5). On their own, each of these models fails to completely explain the published data concerning glucose inhibition of glucagon secretion from islet and dispersed α-cells. Thus, we hypothesize that a combination of signaling pathways, both intracellular and intercellular, are required to inhibit glucagon secretion from α-cells in response to glucose.
To enable determination of the direct impacts and interactions of multiple signaling pathways on glucagon secretion, we created and characterized pseudoislets composed of either all islet cell types or specific purified combinations of individual cell types. Pseudoislets can be created by dissociating islets and allowing them to reassociate in culture over time (14–20). Pseudoislets from murine and human islet cells demonstrate isletlike characteristics, including morphology and glucose-stimulated insulin secretion from β-cells (GSIS). However, α-cell function and glucagon secretion in pseudoislets have not been fully characterized.
We show that small pseudoislets self-assemble from dispersed cells in culture from mouse islet cells with a time course of 3 days and from human donor islet cells over 14 days. Pseudoislets from mice reestablish normal insulin and glucagon secretion, and the measured changes in hormone secretion correlate with changes in multiple intracellular signaling pathways. Human pseudoislets, although more variable, follow a similar trend of recovery for both insulin and glucagon secretion. Additionally, pseudoislets of specific mouse islet cell-type combinations, created using FACS of labeled islet cells, demonstrate the relative importance of specific islet paracrine factors and cell-to-cell contacts on the regulation of glucagon secretion from α-cells.
Research Design and Methods
Islet Isolation
All animal studies were completed under approval by the Vanderbilt Institutional Animal Care and Use Committee (Nashville, TN) and the Washington University Animal Studies Committee (St. Louis, MO). Male mice, aged 8–16 weeks on the C57BL/6 background, were used. Islets were isolated using a 0.075% collagenase digestion at 34°C and allowed to recover overnight in mouse media (RPMI 1640 with 10% FBS, penicillin–streptomycin, and 11 mmol/L glucose) prior to experimentation. Human islets were obtained through the Integrated Islet Distribution Program. Upon arrival, human islets were cultured in human media (RPMI 1640 with 20% FBS, penicillin–streptomycin, and 11 mmol/L glucose) for 2 h prior to experimentation.
Transgenic Animals
To obtain fluorescently labeled β-cells, mouse insulin promoter GFP–expressing mice were used. To obtain fluorescently labeled α-cells and δ-cells, mROSA tandem-dimer red fluorescent protein (RFP) mice were crossed with a glucagon CRE or somatostatin CRE recombinase, resulting in animals containing islets with red fluorescent α-cells or δ-cells, respectively (1,21).
Islet Dispersion and Cell Sorting
Islets were washed in Dulbecco's PBS (no Ca2+ or Mg2+ [pH 7.4]) and then dispersed using Accutase (Life Technologies) for 6 min at 37°C under gentle pipetting. Cells were resuspended in either islet media for further culture or Krebs-Ringer bicarbonate HEPES (KRBH) buffer (128.8 mmol/L NaCl, 4.8 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4 7H2O, 2.5 mmol/L CaCl2, 20 mmol/L HEPES, 5 mmol/L NaHCO3, and 0.1% BSA [pH 7.4]) for flow sorting. An FACS Aria (BD Biosciences) was used to sort GFP- or RFP-positive cells. Purity and viability were verified after sorting using endogenous fluorescence and uptake of DAPI. Dispersion resulted in >75% viable cells, and the resulting fields of plated cells were examined by digital-phase contract microscopy to ensure that no clusters remained. This ensured that >95% in a single-cell state, with only a minimal number of cell douplets, but no other clusters visible. This was verified by FACS, which showed that the removal of the <5% of doublet cells does not affect pseudoislet formation or glucagon secretion recovery (Supplementary Fig. 1). Content samples of purified cells had undetectable levels of opposing cell types and their hormones when analyzed by ELISA.
Pseudoislets Creation
Dispersed islet cells or FACS cells were plated on 30 µg Bovine T1 collagen (BD Biosciences) and 20 µg human fibronectin–coated (Thermo Fisher Scientific) MatTek glass-bottom dishes with islet media (22). The dispersed cells from 25 islets were plated for all cell-type pseudoislets. Selective pseudoislets of α-cells with β-cells and α-cells with δ-cells were plated in a ratio indicative of islet ratios (∼8:1, β:α; and 2:1, α:δ) (23). Islet media was refreshed every 24 h. Data were collected from pseudoislets at 72 h, unless otherwise indicated.
Static Hormone Secretion Assay
Islets, dispersed cells, or pseudoislets were equilibrated in KRBH buffer at 2.8 mmol/L glucose for 1 h at 37°C and then transitioned to 1 or 11 mmol/L glucose for 1 h at 37°C with or without the following conditions: 100 μmol/L 3-isobutyl-1-methylxanthine (IBMX), 50 μmol/L forskolin (Fsk), 4 µg/mL ephrinA5-Fc (R&D Systems), 100 nmol/L somatostatin, 12.5 μmol/L 4-(2,5-dimethyl-pyrrol-1-yl)-2-hydroxy-benzoic acid (DPHBA; Santa Cruz Biotechnology), 1 μmol/L S961 (Novo Nordisk), and 1 μmol/L insulin. The supernatant was collected, and then the cells were lysed for hormone content using 1.5% 12 N HCl in 70% ethanol. Secretion and content samples were measured by ELISA (glucagon from RayBiotech, Inc., Norcross, GA; insulin from Alpco, Salem, NH). Secretion data are presented as percent of total hormone content.
Calcium Imaging
Islets, dispersed cells, and pseudoislets were equilibrated in KRBH at 2.8 mmol/L glucose with 5 μmol/L cell-permeant calcium indicator Fluo-4 AM for 30 min and then transitioned to 1 or 11 mmol/L glucose at 37°C. Samples were allowed to equilibrate in a CO2-controlled 37°C stage for 10 min prior to imaging. Fluo-4 signal was measured using an LSM 880 confocal microscope (Carl Zeiss) with a 488-nm laser, whereas RFP-labeled cells were imaged using 561-nm excitation. Fluo-4 intensity, an indicator for free intracellular calcium, was measured, and changes in intensity over baseline were analyzed for oscillations in order to determine if a cell is active, as previously described (24). To control for unevenness of indicator labeling among cells, the fluorescent signal was normalized to the average basal intensity for each cell. Data are reported as the percentage of cells active under each condition.
cAMP and F-actin Imaging
For immunofluorescence imaging, islets, dispersed α-cells, and pseudoislets were treated like secretion assays, but fixed in 2% paraformaldehyde in PBS at 4°C for 30 min, permeabilized, and blocked overnight in 0.3% Triton X-100, 5 mmol/L sodium azide, 1% BSA, and 5% goat serum. Samples were incubated with the primary antibodies, mouse anti-cAMP (Cell Signaling Technology), and guinea pig anti-glucagon (EMD Millipore) for 72 h at 4°C, washed three times, and then incubated with the secondary antibodies labeled with Alexa Fluor 488 and 546 (Life Technologies) and the F-actin–binding phalloidin, conjugated to Alexa Fluor 633 (Invitrogen) for 72 h at 4°C. Cells were mounted with DAPI Fluoromount-G (Southern Biotechnology Associates). Data analysis used background subtracted raw images, whereas representative images included were linearly adjusted for presentation purposes only. Fluorescence intensities were normalized to cAMP or F-actin fluorescence levels in the islet at 1 mmol/L glucose.
Data Analysis and Statistics
Data were analyzed and prepared using ImageJ (National Institutes of Health), MATLAB (MathWorks), Excel (Microsoft), or Prism 5 (GraphPad Software). Error bars represent SEM, with P < 0.05 considered statistically significant, as determined by one-way ANOVAs. To designate comparisons, an asterisk (*) labels a difference between 1 and 11 mmol/L glucose, whereas a hash mark (#) labels a difference between control (islet or pseudoislet sample used) and experimental conditions at the same glucose concentration.
Results
Formation of Pseudoislets From Dispersed Mouse and Human Islet Cells
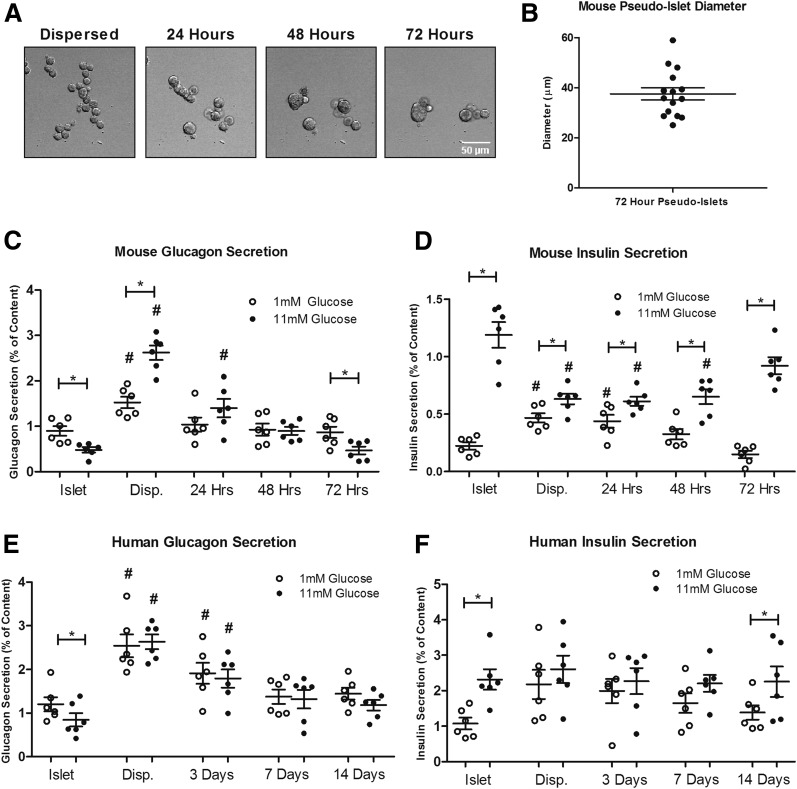
Cells from dispersed mouse islets reassociate to form pseudoislets. These pseudoislets, containing all islet cell types, begin forming within an hour postdispersion and continue forming larger aggregates over a period of 72 h (Fig. 1A). The resulting pseudoislets are smaller than native islets, and they average ∼40 µm in diameter (Fig. 1B). These pseudoislets typically contain <50 cells. Glucagon secretion, which is normally inhibited in response to 11 mmol/L glucose in the islet, is significantly increased in the dispersed state, but pseudoislets recapitulate isletlike levels of glucagon secretion in response to glucose by 72 h (Fig. 1C). Concurrently, GSIS, which is lost in dispersed β-cells, is reestablished in pseudoislets by 72 h (Fig. 1D). This change in regulated secretion does not appear to be because of nonspecific extracellular damage or time-dependent recovery from dispersion because α-cells remain responsive to stimulation with 1 µmol epinephrine during pseudoislet formation, and sparsely plated islet cells remaining at a single-cell suspension fail to recover regulated glucagon secretion over time (Supplementary Fig. 2). To determine the efficiency of our dispersion protocol, cells were passed through FACS, and successful dispersion of single cells was verified empirically by FACS scatter data (>95% single cells). To control for possible cellular damage from the FACS and determine any effects on pseudoislet formation and function from residual cell doublets or clusters, we recovered all live single-islet cells from a basic flowthrough (no fluorescence sorting) and allowed them to recover in culture and form pseudoislets. These FACS-purified samples lacked any cell doublets and clusters, yet they formed pseudoislets and exhibited temporal profiles and amplitudes of reestablished glucagon secretion similar to cells that have not undergone FACS (Supplementary Fig. 1). These data indicate that any residual doublets or possible small clusters left after dispersion or the FACS process do not interfere with pseudoislet formation and function. These data were further confirmed by treatment with a known stimulator and inhibitor of α-cell juxtacrine signaling, which showed similar results on both dispersed and FACS-sorted cells (Supplementary Fig. 3A and B).
Figure 1.
Formation of mouse and human pseudoislets in vitro. A: Representative images of mouse pseudoislets forming in vitro over 72 h after dispersion. Islet cells begin to aggregate within an hour of dispersion, form elongated structures at 24 h, and continue to round at 72 h. B: Islet cells, under these culture conditions, formed pseudoislets that averaged ∼40 µm at 72 h. C and D: Glucagon and insulin secretion in response to 1 and 11 mmol/L glucose was measured from mouse intact islets, dispersed (Disp.) islets, and pseudoislets at 24, 48, and 72 h (islets, dispersed cells, and pseudoislets each from six mice). E and F: Glucagon and insulin secretion in response to 1 and 11 mmol/L glucose was measured from human donor islets, dispersed islets, and pseudoislets at 3, 7, and 14 days (islets and pseudoislets from six donors) (Supplementary Table 1). Error bars represent SEM. *Differences between 1 and 11 mmol/L glucose. #Differences between control and conditions at the same glucose concentration (P < 0.05 by one-way ANOVA).
Similar to what is seen in the murine cells, dispersed human islets reassociate to form pseudoislets (19,25). Glucagon secretion, which is normally inhibited in the islet in response to 11 mmol/L glucose, is significantly increased in the dispersed state (Fig. 1E). The time course of pseudoislet formation with human cells, though, appears to be somewhat slower than for murine cells. Human pseudoislets secrete glucagon at near isletlike levels trending toward normal inhibition of glucagon secretion in response to glucose after ∼14 days (P = 0.18). The glucagon secretion change between low and high glucose goes from positive immediately after dispersion to negative (as it is in islets) after pseudoislet formation (Fig. 1E). Additionally, GSIS, which is lost in dispersed β-cells, is reestablished in human pseudoislets after ∼14 days (Fig. 1F). The difference in recovery time between mice and humans pseudoislets could be influenced by a variety of factors, including donor age, culture time, and species. Despite this temporal difference, mouse and human pseudoislets provide a novel tool to study the recovery of normal hormone secretion.
Changes in Islet Cell Calcium Dynamics During Pseudoislet Formation
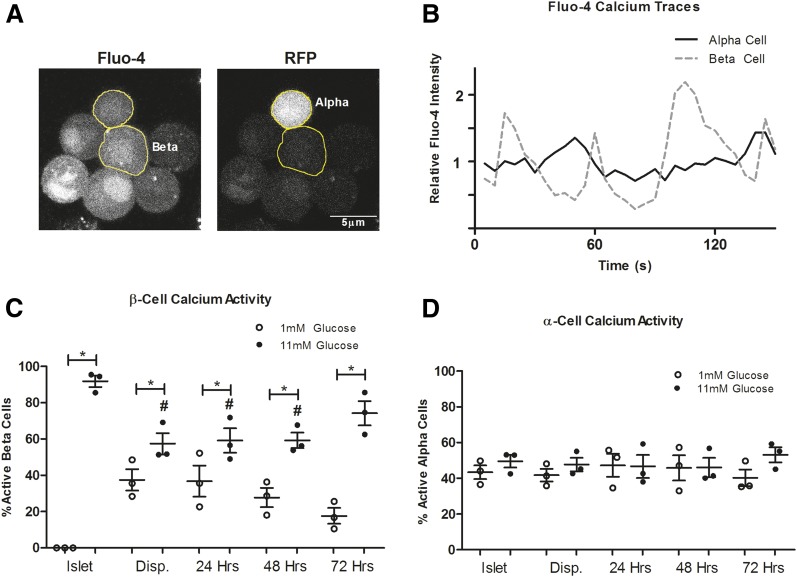
The regulation of intracellular calcium is critical for the proper release of hormones from islet (26,27). In β-cells, the complete inhibition of insulin secretion at low glucose depends upon islet electrical coupling, which is absent in dispersed β-cells (26,28). In contrast, α-cells show heterogeneous calcium dynamics at all glucose levels (1,27,29–31). We hypothesized that normal calcium dynamics, which are lost upon dispersion, would be reestablished in pseudoislet β-cells, whereas the α-cells would remain unchanged. To test this hypothesis, we labeled islets, dispersed cells, and pseudoislets with the calcium indicator dye Fluo-4 and measured intracellular free calcium activity in response to glucose (Fig. 2A and B). In islets, β-cells are electrically coupled, resulting in no measurable calcium activity at low glucose and coordinated oscillatory activity at high glucose (Fig. 2C) (26). This electrical coupling that regulates calcium activity is lost in the dispersed state, resulting in aberrant calcium activity in some β-cells at low glucose and decreased glucose-stimulated calcium oscillations. The β-cells in murine pseudoislets continue to recover decreased calcium activity at low glucose and increased calcium activity in high glucose by 72 h. In contrast, calcium activity in α-cells does not appear to change among the islet, dispersed, and pseudoislet states (Fig. 2D). Although the role of α-cell calcium dynamics in glucagon release remains unclear, the restoration of tightly regulated calcium dynamics in β-cells quantitatively parallels the recovery of insulin secretion in pseudoislets.
Figure 2.
Changes in calcium dynamics during pseudoislet formation. A: Representative image of a Fluo-4–labeled pseudoislet. α-Cells were identified by the presence of transgenic RFP expression. B: Representative traces of Fluo-4 intensity changes in an α-cell and one β-cell within a pseudoislet at 11 mmol/L glucose. The percent of oscillating β- (C) or α- (D) cells represent data from three mice (>25 β-cells/mouse or >10 α-cells/mouse). Disp., dispersed cells. Error bars represent SEM. *Differences between 1 and 11 mmol/L glucose. #Differences between control and conditions at the same glucose concentration (P < 0.05 by one-way ANOVA).
Changes in α-Cell cAMP Levels, F-actin Density, and Glucagon Secretion in Pseudoislets
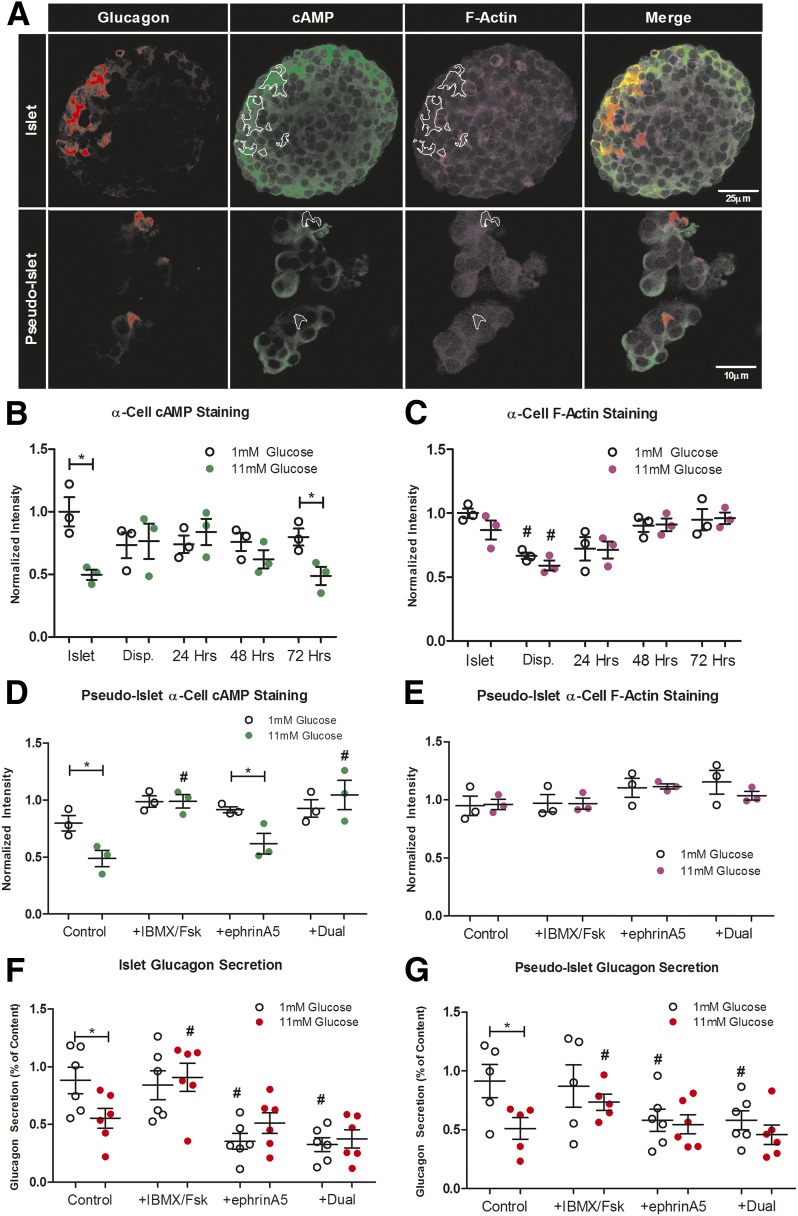
The levels of cAMP in α-cells is decreased by paracrine factors like insulin and somatostatin and has been proposed to play a key role in the inhibition of glucagon secretion in response to glucose (4,12,30). Juxtacrine signaling between EphA4/7 on α-cells and ephrins on β-cell has also been proposed to reduce glucagon secretion by modulating F-actin density (5). Although this signaling is normally dominated by EphA4, EphA7 is also expressed in α-cells and has been shown to partially compensate for the loss of EphA4 by genetic deletion (5). We hypothesize that both α-cell cAMP and F-actin levels are misregulated in the dispersed state, but recover in pseudoislets by 72 h. As previously described (4,5), we used semiquantitative immunofluorescence to measure the static levels of cAMP and F-actin in α-cells from islet, dispersed cells, and forming pseudoislets (Fig. 3A and B). Unlike intact isolated islets, pseudoislets are not encased in a collagen capsule, and although the cells of the pseudoislet remain contiguous, we find that they lose their round shape during the fixation procedure. In islet and pseudoislet α-cells, the levels of cAMP decrease in response to elevated glucose. As was seen for glucagon secretion, this glucose-dependent decrease is lost upon islet dispersion, but returns in pseudoislets within 72 h (Fig. 3B). The levels of cAMP do not appear to depend on pseudoislet diameter (Supplementary Fig. 4). In α-cells within intact islets, F-actin levels show minimal changes between 1 and 11 mmol/L glucose (Fig. 3C). However, F-actin levels are significantly decreased upon dispersion, similar to what we observe from other parameters, and F-actin reaches isletlike levels by 48 h during pseudoislet formation.
Figure 3.
Changes in cAMP levels, F-actin density, and glucagon secretion in pseudoislets. A: Fixed samples were stained for cAMP (green) and F-actin (magenta), and fluorescence intensities were measured in glucagon-positive regions (white overlay). Representative images show staining for an islet and pseudoislet. The observed changes in fixed pseudoislet morphology compared with living samples is the result of fixation. The fixed pseudoislets remain contiguous. Quantification of α-cell–specific cAMP (B) and F-actin (C) levels in intact islets, dispersed (Disp.) cells, and pseudoislets at 1 and 11 mmol/L glucose. Quantification of α-cell–specific cAMP and F-actin levels in pseudoislets at 1 and 11 mmol/L glucose in response to 100 μmol/L IMBX and 50 μmol/L Fsk, 4 µg/mL ephrinA5-Fc, or both (Dual), normalized to islet cAMP (D) or F-actin (E) levels at 1 mmol/L glucose (n = 3 mice with >15 α-cells/mouse). Glucagon secretion at 1 and 11 mmol/L glucose was measured from mouse intact islets (F) and pseudoislets at 72 h (G) in response to 100 μmol/L IMBX and 50 μmol/L Fsk, 4 µg/mL ephrinA5-Fc, or both (n = 6 mice). Error bars represent SEM. *Differences between 1 and 11 mmol/L glucose. #Differences between control and conditions at the same glucose concentration (P < 0.05 by one-way ANOVA).
To determine if these pseudoislets exhibit cAMP and juxtacrine signaling similar to intact islets, we treated pseudoislets with known stimulators of each pathway: the phosphodiesterase inhibitor IMBX combined with the adenylyl cyclase stimulator Fsk and the EphA stimulator ephrinA5-fc (Fig. 3D and E). In pseudoislets, IBMX/Fsk stimulates the formation of cAMP in α-cells at 11 mmol/L glucose, whereas ephrinA5-Fc does not alter cAMP levels (Fig. 3D). IBMX/Fsk, ephrinA5-Fc, nor the combination of both alters F-actin density in pseudoislet α-cells (Fig. 3E). EphrinA5-Fc treatment does increase F-actin density in dispersed α-cells, which correlates with a decrease in glucagon secretion (Supplementary Fig. 3B and C). The ability of ephrinA5-Fc to stimulate the formation of F-actin density in dispersed α-cells but not pseudoislets suggests that α-cells in pseudoislets have reestablished EphA4/7 forward signaling with neighboring β-cells.
Regulated glucagon secretion appears to be modulated by both the levels of cAMP and F-actin in α-cells. It is unclear how or whether these pathways interact in order to regulate glucagon secretion in response to glucose. We hypothesize that although IMBX/Fsk can potently stimulate glucagon secretion, EphA4/7 forward signaling can block glucagon secretion downstream of cAMP-dependent regulators. To test this hypothesis, we measured glucagon secretion from islets and pseudoislets treated with IMBX/Fsk, ephrinA5-Fc, or both (Fig. 3F and G). The treatment of IMBX/Fsk, which elevates cAMP in α-cells, stimulates glucagon release at 11 mmol/L glucose. The treatment of ephrinA5-Fc, which does not increase F-actin density in α-cells, can further inhibit glucagon release at 1 mmol/L glucose. In combination, IMBX/Fsk fails to overcome ephrinA5-Fc inhibition of glucagon secretion.
Pseudoislets From Specific Cell Types
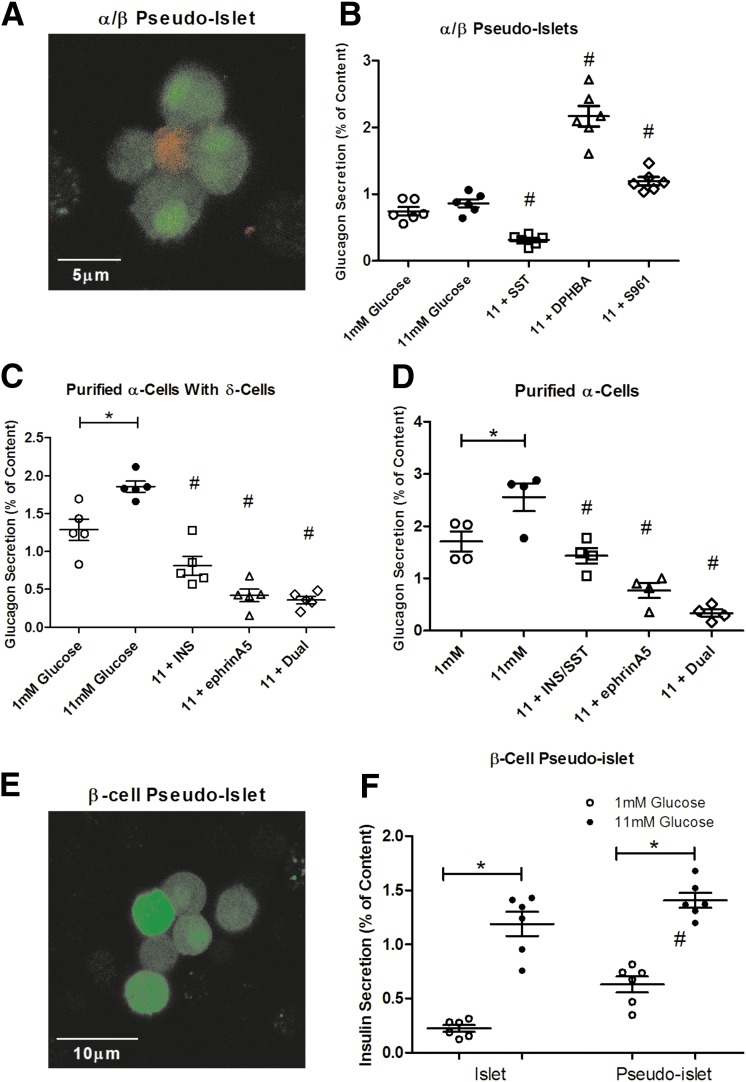
Using transgenic animals that express endogenous tags, we were able to isolate pure populations of α-, β-, and δ-cells and create pseudoislets independent of other islet cell types. With this approach, we know treatments will only affect the present cell types, minimizing complex indirect effects that could modulate glucagon secretion. We created pseudoislets composed of just α- and β-cells (Fig. 4A) and measured glucagon secretion in response to proposed regulators at 72 h (Fig. 4B). Without the presence of δ-cells and somatostatin, α-/β-cell pseudoislets fail to suppress glucagon release in response to high glucose. The addition of exogenous somatostatin potently inhibits glucagon secretion, similar to isletlike levels. We also blocked proposed glucagon modulators originating from the β-cell. Treatment with the selective EphA2/4 inhibitor DPHBA disrupts the reformed EphA4 forward signaling and results in glucagon secretion similar to dispersed α-cells. Antagonism of the insulin receptor with S961 enhances glucagon secretion, supporting the model in which insulin secreted by neighboring β-cells contributes to in the inhibition of glucagon secretion.
Figure 4.
Selective pseudoislets recapitulate aspects of the islet. A: Representative image of an α-/β-cell pseudoislet at 72 h. α-Cells (red) were identified by transgenic RFP expression, whereas β-cells (green) were identified by GFP expression. B: Glucagon secretion from α-/β-cell pseudoislets treated with 1 or 11 mmol/L glucose with or without 100 nmol/L somatostatin (SST), 12.5 μmol/L DPHBA, or 1 μmol/L S961 (n = 6 mice). C: Glucagon secretion from α-cells in coculture with δ-cells treated with 1 or 11 mmol/L glucose with or without 1 μmol/L insulin (INS), 4 µg/mL ephrinA5-Fc, or both (Dual; n = 5 mice). D: Glucagon secretion from α-cells treated with 1 or 11 mmol/L glucose with or without 1 μmol/L insulin and 100 nmol/L somatostatin, 4 µg/mL ephrinA5-Fc, or both (Dual) (n = 4 mice). E: Representative image of β-cell pseudoislets at 72 h. F: Insulin secretion from β-cell pseudoislets treated with 1 or 11 mmol/L glucose (n = 6 mice). Error bars represent SEM. *Differences between 1 and 11 mmol/L glucose. #Differences between control and conditions at the same glucose concentration (P < 0.05 by one-way ANOVA).
Because of the heterogeneity reported for α- and δ-cell arrangement in islets, it is unclear if α-cells form functional cell-to-cell contacts with δ-cells or other α-cells in an intact islet (32,33). To examine this further, we attempted to create pseudoislets from purified α-cells with or without δ-cells. Unfortunately, α-cells fail to aggregate with themselves or δ-cells, even after >72 h. Both purified α-cells with δ- and α-cells alone secrete glucagon similar to dispersed cells (Fig. 4C and D). The addition of the paracrine factors insulin and somatostatin and the EphA4/7 stimulator ephrinA5-Fc all inhibit glucagon secretion, which is maximally inhibited by combination treatment.
The inability of purified α-cells and δ-cells to form aggregates may be because of the lack of supporting β-cells. Data suggest β-cells are the core cell type for the formation of islets (32). To determine if pure β-cell populations form functional pseudoislets, we created β-cell pseudoislets (Fig. 4E) and measured insulin secretion (Fig. 4F). β-Cell pseudoislets exhibit near-normal GSIS and secrete insulin at isletlike levels at 72 h.
Discussion
Both β-cells and α-cells recover near-normal regulated hormone secretion upon the formation of small pseudoislets from the dispersed state (Fig. 1). Our data show that pseudoislet formation occurs more slowly for human cells than for mouse, but in both cases, insulin and glucagon recover to near-normal behaviors in parallel. In human pseudoislets, the response of glucagon secretion between low and high glucose moves from positive immediately after dispersion to negative (as it is in islets) after pseudoislet formation. This trend of recovery strongly follows the behavior observed in mouse pseudoislets, but the sample-to-sample variability of human islet preparations lowered the statistical significance of these differences. Taken together, these data highlight the importance of pseudoislet culture time with regard to function. Pseudoislets, prior to full recovery, may remain in a dysfunctional dispersed state rather than an isletlike state (19,25).
β-Cells recover regulated calcium oscillations and electrical coupling that potentiate GSIS, whereas glucose concentrations or cell clustering have little to no effect on the number of active α-cells or their calcium dynamics (Fig. 2). These effects are similar to what is seen in islets and is consistent with previous reports (1,4,27). The recovery of regulated glucagon secretion appears to depend on the restoration of GSIS from β-cells, as well as cell-to-cell contacts between α- and β-cells. As insulin becomes maximally secreted from β-cells, pseudoislet α-cells recover glucose-dependent cAMP levels. Concurrently, the contact of β- to α-cells appears to provide the necessary ephrin stimulation to activate EphA4/7 forward signaling and stimulate the formation of F-actin (Fig. 3). In β-cells, F-actin plays a critical glucose-sensing role by providing a barrier at low glucose to prevent aberrant insulin release (34). In α-cells, the role of F-actin appears to act differently as a barrier to tonically inhibit maximal glucagon secretion (5). When this barrier is lost, as seen in the dispersed state, glucagon secretion increases significantly. Recovery of this barrier by pseudoislet formation or EphA4/7 activation by ephrinA5-Fc potently inhibits glucagon secretion. Although ephrinA5-Fc only minimally increases F-actin density in islets and pseudoislets, it still inhibits glucagon secretion. This may indicate a change in actin matrix stability or remodeling, rather than an increase in the F-actin density. In neurons, changes in intracellular calcium and cAMP have been shown to alter F-actin density through downstream regulators (35,36). This is not the case in α-cells, in which the forced formation of cAMP does not alter the levels of F-actin, even in the dispersed state when EphA4/7 stimulation is absent (Supplementary Fig. 3C). Further investigation is required to determine if this pathway provides an additional fine-tuning mechanism for glucagon secretion independent of cAMP signaling.
Although flow-sorted populations of individual islet cells have been successfully isolated and interrogated previously (1,20,37), the creation of pseudoislets from specific fluorescent protein–labeled cell types provides additional insights into islet signaling and glucagon regulation (Fig. 4). The β-cells appear to drive pseudoislet formation and provide both cell-to-cell contacts and paracrine signals to regulate glucagon secretion. However, pseudoislets composed of only α- and β-cells do not recapitulate normal glucose inhibition of glucagon secretion. In this case, though, the addition of somatostatin, independent of any other δ-cell signal, leads to proper inhibition of glucagon secretion, and this inhibition is consistent with the lowering of cAMP levels by insulin and somatostatin as previously described (4). This observation is consistent with our hypothesis that the ephrinA ligand signal comes from the β-cells and not the δ-cells. Cultures consisting of only δ- and α-cells fail to form pseudoislets, which suggests that the β-cells may provide the cell-to-cell contacts required for islet integrity, and offers further evidence against α-cell EphA4/7 interactions with δ-cell ephrins. Despite the absence of pseudoislet formation, basal glucagon secretion is lowered when α-cells are cocultured δ-cells, putatively because of the basal release of somatostatin (Fig. 4C vs. Fig. 4D).
Altogether, our data indicate that glucagon secretion is regulated by multiple independent mechanisms. EphA4/7 signaling in α-cells appears to require ephrinA ligands from β-cells, and upon stimulation, these receptors regulate F-actin density to provide a secretion barrier for glucagon secretion. However, this barrier appears permissive and independent of glucose, which is consistent with glucagon secretion during glucose stimulation falling only to ∼50% of its maximal level. EphA4/7 signaling does not appear to directly affect α-cell cAMP levels, which instead are lowered by paracrine signals, including insulin and somatostatin. Thus, regulation of cAMP acts as an independent mechanism of indirect glucose regulation on glucagon secretion. Finally, our data support a mechanism in which the independent paracrine and juxtacrine pathways, which fail to fully suppress glucagon release alone, act additively to maximally inhibit glucagon secretion in response to glucose. Additional investigation is required to better understand the collaborative effect and relative contribution of both pathways on regulated glucagon secretion.
The complexity of regulated hormone secretion within the islet presents a continuing challenge in the pursuit of new therapies for diabetes. Potential reorganization of islet architecture after isolation complicates comparisons between in vivo and in vitro data. Although islet vasculature has been proposed to play an important role in in vivo communication among islet cells, the contribution of blood flow is clearly lost upon islet isolation. Still, pseudoislets provide an easily manipulated, reductionist model to study specific paracrine and cell-to-cell interactions, and despite the lack of blood flow, the resulting insulin and glucagon responses to glucose and other stimuli in pseudoislets are remarkably similar to what is measured in vivo. Our data support a model in which the paracrine interactions are not strictly dependent on blood flow. Regardless of the preparations, though, it is difficult to predict the overall concentrations and temporal exposure of the α-cell to paracrine factors in vivo, in ex vivo islets, or in pseudoislets of various sizes.
Although the importance of the size component of pseudoislets needs further exploration, it is clear that small pseudoislets can also recapitulate the islet state like larger pseudoislets from other species. This is of particular relevance because it has long been thought that size is a critical component to islet function, and pseudoislet dimensions must be comparable to intact islets for proper hormone secretion (14,16–18,22,25,38). Although large pseudoislets have been used in transplantation and β-cell mass replacement studies, this model can also be used to interrogate the function of the other islet cell types. With pseudoislets, the differences in glucagon secretion observed between islet and dispersed α-cells can be reconciled, affirming that α-cell function is under multiple levels of regulation that depend on other islet cells. These data argue that correction of dysfunctional glucagon secretion in patients with diabetes will require combination therapies that modulate different pathways simultaneously present in α-cells.
Supplementary Material
Article Information
Funding. This work was supported by National Institutes of Health grants R01-DK-098659, R01-DK-085064, S10-RR-25649, and S10-OD-10681 (to D.W.P.). Some experiments were supported by core laboratories provided by the Vanderbilt Diabetes Research and Training Center (DK-020593), the Diabetes Research Center (DK-20579), and Siteman Cancer Center (CA-091842) at Washington University in St. Louis. C.A.R. was supported in part by the Vanderbilt Multidisciplinary Training in Molecular Endocrinology program (DK-007563).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.A.R. and D.W.P. conceptualized the study. C.A.R. performed experiments and completed data analysis. C.A.R. and D.W.P. interpreted results and wrote and edited the manuscript. D.W.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1291/-/DC1.
References
- 1.Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem 2010;285:14389–14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 2006;55:2318–2323 [DOI] [PubMed] [Google Scholar]
- 4.Elliott AD, Ustione A, Piston DW. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Am J Physiol Endocrinol Metab 2015;308:E130–E143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchens T, Piston DW. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes 2015;64:3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007;28:84–116 [DOI] [PubMed] [Google Scholar]
- 7.Gylfe E. Glucose control of glucagon secretion-‘There’s a brand-new gimmick every year’. Ups J Med Sci 2016;121:120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briant L, Salehi A, Vergari E, Zhang Q, Rorsman P. Glucagon secretion from pancreatic α-cells. Ups J Med Sci 2016;121:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barg S, Galvanovskis J, Göpel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 2000;49:1500–1510 [DOI] [PubMed] [Google Scholar]
- 10.Huang Y-C, Rupnik MS, Karimian N, et al. In situ electrophysiological examination of pancreatic α cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion. Diabetes 2013;62:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 2000;141:111–117 [DOI] [PubMed] [Google Scholar]
- 12.Tian G, Sandler S, Gylfe E, Tengholm A. Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes 2011;60:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 14.Ju MK, Jeong JH, Lee JI, Kim YS, Kim MS. Proliferation and functional assessment of pseudo-islets with the use of pancreatic endocrine cells. Transplant Proc 2013;45:1885–1888 [DOI] [PubMed] [Google Scholar]
- 15.Ichihara Y, Utoh R, Yamada M, Shimizu T, Uchigata Y. Size effect of engineered islets prepared using microfabricated wells on islet cell function and arrangement. Heliyon 2016;2:e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Sun G, Wang S, et al. Engineering islet for improved performance by optimized reaggregation in alginate gel beads. Biotechnol Appl Biochem 3 March 2016 [Epub ahead of print]. DOI: 10.1002/bab.1489 [DOI] [PubMed] [Google Scholar]
- 17.Halban PA, Powers SL, George KL, Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes 1987;36:783–790 [DOI] [PubMed] [Google Scholar]
- 18.Hopcroft DW, Mason DR, Scott RS. Insulin secretion from perifused rat pancreatic pseudoislets. In Vitro Cell Dev Biol 1985;21:421–427 [DOI] [PubMed] [Google Scholar]
- 19.Dorrell C, Schug J, Canaday PS, et al. Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipeleers DG, in’t Veld PA, Van de Winkel M, Maes E, Schuit FC, Gepts W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology 1985;117:806–816 [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi H, He M, Wu P, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex [published correction appears in Neuron 2011;72:1091]. Neuron 2011;71:995–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima N. In vitro reconstitution of pancreatic islets. Organogenesis 2014;10:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosco D, Armanet M, Morel P, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010;59:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlén P. Spectral analysis of calcium oscillations. Sci STKE 2004;2004:pl15. [DOI] [PubMed] [Google Scholar]
- 25.Hilderink J, Spijker S, Carlotti F, et al. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J Cell Mol Med 2015;19:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benninger RKP, Remedi MS, Head WS, Ustione A, Piston DW, Nichols CG. Defects in beta cell Ca2+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia 2011;54:1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Marchand SJ, Piston DW. Glucose decouples intracellular Ca2+ activity from glucagon secretion in mouse pancreatic islet alpha-cells. PLoS One 2012;7:e47084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwetz TA, Reissaus CA, Piston DW. Differential stimulation of insulin secretion by GLP-1 and Kisspeptin-10. PLoS One 2014;9:e113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göpel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse -cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol 2000;528:509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Yu Q, Ahooghalandari P, et al. Submembrane ATP and Ca2+ kinetics in α-cells: unexpected signaling for glucagon secretion. FASEB J 2015;29:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavagnino Z, Dwight J, Ustione A, Nguyen T-U, Tkaczyk TS, Piston DW. Snapshot hyperspectral light-sheet imaging of signal transduction in live pancreatic islets. Biophys J 2016;111:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2010;2:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets 2009;1:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalwat MA, Thurmond DC. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp Mol Med 2013;45:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366 [DOI] [PubMed] [Google Scholar]
- 36.Dong J-M, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem 1998;273:22554–22562 [DOI] [PubMed] [Google Scholar]
- 37.Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types [published correction appears in Diabetologia 2013;56:1192]. Diabetologia 2011;54:2832–2844 [DOI] [PMC free article] [PubMed]
- 38.O’Sullivan ES, Johnson AS, Omer A, et al. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia 2010;53:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.