Abstract
Dok1 is an abundant Ras-GTPase-activating protein-associated tyrosine kinase substrate that negatively regulates cell growth and promotes migration. We now find that IκB kinase β (IKKβ) associated with and phosphorylated Dok1 in human epithelial cells and B lymphocytes. IKKβ phosphorylation of Dok1 depended on Dok1 S439, S443, S446, and S450. Recombinant IKKβ also phosphorylated Dok1 or Dok1 amino acids 430–481 in vitro. TNF-α, IL-1, γ radiation, or IKKβ overexpression phosphorylated Dok1 S443, S446, and S450 in vivo, as detected with Dok1 phospho-S site-specific antisera. Moreover, Dok1 with S439, S443, S446, and S450 mutated to A was not phosphorylated by IKKβ in vivo. Surprisingly, mutant Dok1 A439, A443, A446, and A450 differed from wild-type Dok1 in not inhibiting platelet-derived growth factor-induced extracellular signal-regulated kinase 1/2 phosphorylation or cell growth. Mutant Dok1 A439, A443, A446, and A450 also did not promote cell motility, whereas wild-type Dok1 promoted cell motility, and Dok1 E439, E443, E446, and E450 further enhanced cell motility. These data indicate that IKKβ phosphorylates Dok1 S439S443 and S446S450 after TNF-α, IL-1, or γ-radiation and implicate the critical Dok1 serines in Dok1 effects after tyrosine kinase activation.
Keywords: NF-κB, serine phosphorylation, cell migration
Dok1 or p62 dok is an abundant Ras-GTPase-activating protein-associated adaptor protein that is downstream of growth factor receptor and nonreceptor tyrosine k inases (1, 2). Related proteins include Dok2 (also known as FRIP or Dok-R), Dok3 (also known as Dok-L), Dok4, Dok5, and insulin receptor substrates (3–8). DOK proteins have an N-terminal pleckstrin homology domain, a phosphotyrosine-binding (PTB) domain, and a C terminus rich in proline, serine, and tyrosine (1, 2). The pleckstrin homology domain mediates association with membrane phospholipids, whereas the PTB domain mediates homodimerization and association with phosphotyrosine signaling molecules (9, 10). When it is tyrosine-phosphorylated, Dok1 interacts with other signaling molecules containing Src homology 2 domains, such as Ras-GTPase-activating protein, SHIP1, Nck, Csk, and SH2D1A (11–15).
DOK proteins down-modulate tyrosine kinase signaling effects. Dok1 can inhibit mitogen-activated protein kinase activation, cell proliferation, cell transformation, and leukemogenesis (9, 10, 16–18). Dok1 can also affect cell adhesion, spreading, migration, and apoptosis (13, 19, 20), and modulate T or B cell receptor signaling (18, 21–24). Pleckstrin homology domain-mediated plasma membrane translocation and tyrosine phosphorylation are critical for these Dok1 effects (10, 25).
After TNF-α, IL-1, or Toll receptor signaling, γ radiation, or tyrosine kinase signaling, IκB kinase β (IKKβ) phosphorylates IκBα S32S36, resulting in IκBα degradation, NF-κB activation, and increased transcription of genes important for costimulatory and survival effects (for reviews see refs. 15 and 26–37). Dok1 could have a role in the physiologic integration of tyrosine kinase with TNF, IL-1, and Toll receptor signaling (38–41). In this report, we investigate whether Dok1 S439S443 and S446S450, which are in a context similar to IκBα S32S36, can be phosphorylated by IKKβ and potentially modify Dok1 effects downstream of tyrosine kinases.
Materials and Methods
Plasmids. pcDNA3-Flag-Dok1, pcDNA3-Myc-Dok1, and pcDNA3-Flag-IKKβΔ9 have been described (15, 42). Human (Hu)Dok1 variants bearing specific mutations or deletions were obtained by PCR mutagenesis and cloned into expression vectors (15). Dok1-SSSS is wild-type Dok1. Dok1-AAAA has S439, S443, S446, and S450 replaced by A. Mutants were cloned into expression plasmids and sequence-confirmed. pRK5-Flag-IKKβ (WT), pRK5-Myc-IKKα (WT), and kinase-dead (KD) mutants, pRK5-Flag-IKKβ (KD) and pRK5-Myc-IKKα (KD) were obtained from D. Goeddel (Tularik, South San Francisco, CA). The expression plasmids for ataxia-telangiectasia-mutated (ATM) were obtained from Y. Shiloh (Tel Aviv University, Tel Aviv) and M. Kastan (St. Jude Children's Research Hospital, Memphis, TN). Glycogen synthetase kinase 3β expression plasmids were obtained from J. Woodgett through G. Johnson (Ontario Cancer Institute, Toronto). GFP-Dok1-SSSS was created by inserting the coding sequences of the full-length cDNA of Dok1 in-frame with GFP into pEGFP-C1 (Clontech).
Reagents and Antibodies. TNF-α, IL-1, platelet-derived growth factor (PDGF), anti-Flag M2, M5, and M2 affinity gels were obtained from Sigma and R & D Systems. His-tagged recombinant IKK2 (IKKβ) was provided by H. Allen (Abbott Bioresearch Center, Worcester, MA). λ-Phosphatase and leukocyte antigen-related-tyrosine-phosphatase, monoclonal antibodies against phospho-extracellular signal-regulated kinase (Erk)1/2 and phospho-IκBα were from New England Biolabs. IKKα- and IKKβ-specific, monoclonal phosphotyrosine, goat Erk1/2, mouse phospho-IκBα, and rabbit IκBα antibodies were obtained from Santa Cruz Biotechnology. Polyclonal antibodies against phosphoserine (pS) 15 (S15) of p53 was obtained from Cell Signaling Technology (Beverly, MA). Polyclonal rabbit anti-Dok1 was described (1) and obtained from J. Cambier (St. Jude Children's Research Hospital). Rabbit polyclonal anti-phospho-Dok1-Ser 439, -Ser 443, -Ser 446, and -Ser 450 antibodies were raised against keyhole limpet hemocyanin-coupled peptides: Dok1-pS439-SSS, H2N-CTG-pS-GIKSHNSA LYSQVQK-COOH; Dok1-S-pS443-SS, H2N-CTGSGIK-pS-HNSALYS-QVQK-COOH; Dok1-SS-pS446-S, H2N-CTGSGIKSHN-pS-ALYSQVQK-COOH; and Dok1-SSS-pS450, H2N-CTGSGIK-SHNSALY-pS-QVQK-COOH by Covalab (Lyon, France).
Cell Culture and Transfections. Human embryonic kidney (HEK) 293, HEK 293 stably transfected with empty vector pcDNA3, pcDNA3-Flag-Dok1-SSSS, pcDNA3-Flag-Dok1-AAAA, pEGFP-C1, or pEGFP-Dok1-SSSS, and 293T cells were maintained in DMEM containing 10% FBS (D10). Clones expressing Dok1 or containing empty vector, and polyclonal 293/GFP- or 293/GFP-Dok1-expressing cells were isolated after selection in D10 containing 800 μg/ml G418. Epstein–Barr virus immortalization lymphoblastoid cell lines from a healthy individual (lymphoblastoid cell line 2145) or from ataxia-telangiectasia (AT) patients expressing a truncated ATM protein (AT-11 and AT-14) were obtained from G. Lenoir (International Agency for Research on Cancer) (43). These cell lines, as well as BJAB (a Burkitt's lymphoma cell line) and BJAB, stably transfected with DNA3 or pcDNA3-Flag-IKKβΔ9, were maintained in RPMI medium 1640 supplemented with 10% FBS. Cells were transfected by electroporation or by Superfect (Qiagen, Valencia, CA) (15). To determine growth curves, cells were seeded in six-well plates at a density of 1 × 104 and cultured in D10. The medium was changed every 48 h and the number of cells was counted every 24 h. For cytokine stimulation and ionizing radiation, cells were treated with TNF-α (20 ng/ml), IL-1 (20 ng/ml), or PDGF (20 ng/ml), or were exposed to γ radiation (20 Gy) by using a 137Ce IBL-437c irradiator (CIS Biointernational, Gif-sur-Yvette, France), and harvested at the indicated time points. Equal amounts of protein from the total cell extracts were analyzed by Western blot.
Cell Spreading and Wound Healing. For cell spreading assays, 293 cells stably transfected were seeded at a density of 105 cells per ml in 60-mm-diameter culture dishes coated with collagen type I (10 μg/ml, Sigma). After incubation at 37°C, the cells were examined under a light microscope equipped with phase-contrast optics (model IX 70, Olympus) and photographed. For the wounding test, cells were seeded on 35-mm-diameter dishes precoated with collagen type I and grown to confluence in the presence of serum. The medium was changed every 24 h. The wound was made by scraping across the center of the dish with 0.1- to 2-μl tips. The medium was changed after scraping to eliminate nonadherent cells. The cells were then incubated at 37°C and examined at different times by using a Leica (Bannockburn, IL) DM IRB microscope (objective N plan ×10). The area of the wound was measured in pixels by using metamorph software (Universal Imaging, West Chester, PA).
Immunoprecipitation, GST Pull-Down Assays, and Western Blotting. Immunoprecipitation, GST pull-down assays, and Western blot analyses were described (15).
Assay of Erk Activation and Kinase Assays. Cells were deprived of serum for 24 h and then incubated with PDGF (20 ng/ml, Calbiochem) for indicated times. Antibodies specific for activated Erks and total Erks were used for immunoblotting. The in vitro kinase assays were described (44).
Confocal Microscopy and Immunofluorescence. Cells were seeded in 35-mm glass-bottom Petri dishes coated with collagen I, and immunofluorescence was as described (45). F-actin distribution was observed after incubation of cells with rhodamine B isothiocyanate-conjugated phalloidin (Molecular Probes) at 0.4 units/ml for 20 min at room temperature. Samples were imaged with a Zeiss LSM 510 microscope by using a ×63 (numerical aperture of 1.4) Plan Neo Fluor objective.
Results
IKKβ Associates with and Phosphorylates Dok1. To test whether Dok1 associates with IKKβ, Myc-tagged Dok1 and Flag-tagged IKKβ (F-IKKβ) were expressed in 293T cells (Fig. 1A). Approximately 0.5–1% of Dok1 in the cell lysates coprecipitated with ≈10–20% of F-IKKβ. The coimmunoprecipitation was specific because Dok1 was not precipitated from cells in which F-IKKβ was not expressed (Fig. 1A). Furthermore, ≈0.1% of endogenous Dok1 specifically associated with ≈20% of IKKβ in F-IKKβ immunoprecipitates from lysates of BJAB cells constitutively expressing F-IKKβ (Fig. 1B) or with ≈4% of endogenous IKKβ in nontransfected BJAB cells (Fig. 1C). Thus, corrected for the efficiency of IKKβ immunoprecipitation, 0.5–5% of Dok1 is stably associated with IKKβ in 293T epithelial cells or BJAB B lymphoblasts. By using polyclonal Dok1 antibody to immunoprecipitate Dok1 from BJAB B lymphoblasts, ≈0.5% of IKKβ appeared to be stably Dok1-associated, after correction for the ≈20% efficiency of Dok1 precipitation (Fig. 1C); polyclonal antibody could interfere with or inhibit intermolecular associations. Moreover, pervanadate inhibition of phosphatases substantially increased Dok1 tyrosine phosphorylation and resulted in almost 2-fold more Dok1 or phosphotyrosine-Dok1 association with overexpressed F-IKKβ in 293T cells (Fig. 1A). Interestingly, IKKβ overexpression decreased Dok1 tyrosine phosphorylation (Fig. 1A), which is consistent with the possibility that IKKβ may down-modulate Dok1 effects downstream of tyrosine kinases.
Fig. 1.
IKKβ associates with Dok1, inducing a mobility shift. (A) HEK 293T cells were transfected with expression plasmids for Myc-Dok1 (Dok1) and Flag-IKKβΔ9 (F-IKKβ). After 48 h, half of the cultures were treated with pervanadate (PV). Immunoprecipitates were analyzed by immunoblotting. L, 1% lysate; F, 30% of immunoprecipitate; IgG, Ig heavy chain. (B) Protein extracts from BJAB cells stably expressing Flag-IKKβΔ9 were immunoprecipitated and analyzed as in A. (C) Protein extracts from BJAB cells were immunoprecipitated with mouse anti-IKKβ or rabbit anti-Dok1. Immunoblotting was performed by using mouse anti-IKKβ, rabbit anti-IKKα, or rabbit anti-Dok. A faint band above IKKα may represent IKKβ due to crossreactivity. (D) HEK 293Tcells were cotransfected with vectors expressing Myc-Dok1 and Flag-IKKβ. After 24 h, cells were fixed. IKKβ was visualized with mouse anti-IKKβ and Dok1 with rabbit anti-Dok1. F-actin was visualized with rhodamine isothiocyanate-conjugated phalloidin. Colors are confocal interface false colors with F-actin-labeled phalloidin excited at 546 nm and IKKβ-labeled cyanin 5 excited at 633 nm. Arrowheads in the merge image indicate Dok1 and IKKβ colocalization. Data are representative of at least three independent experiments.
In transfected HEK 293T cells, overexpressed Dok1 and IKKβ were distributed in a reticular pattern throughout the cytoplasm, with enhancement near the plasma membrane and in cytoplasmic extensions, near sites of F-actin accumulation (Fig. 1D and data not shown).
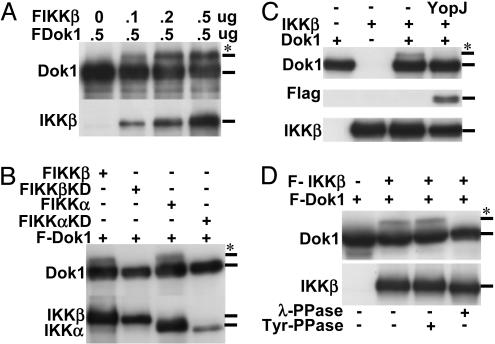
IKKβ overexpression with Dok1 resulted in a slower migrating Dok1 fraction (Fig. 2A). The mobility shift occurred with native, Flag-, or Myc-tagged Dok1 (data not shown). The fraction of Dok1 shift varied directly with the amount of IKKβ expression vector and the amount of IKKβ expressed (Fig. 2 A), implicating IKKβ in Dok1 modification. IκB kinase α (IKKα) overexpression also induced similar Dok1 modification, whereas KD IKKβ(KD) or IKKα(KD) did not induce Dok1 modification (Fig. 2B). Moreover, F-IKKβ(KD) or M-IKKα(KD) had dominant negative effects on wild-type F-IKKβ-induced Dok1 modification (data not shown). Furthermore, Yersinia YopJ, an inhibitor of IKKβ and other mitogen-activated protein kinases (46), prevented IKKβ-induced Dok1 modification (Fig. 2C). The Dok1 mobility shift disappeared with λ-Ser/Thr phosphatase treatment, but not with leukocyte antigen-related tyrosine phosphatase treatment (Fig. 2D). Thus, increased IKKβ or IKKα activity causes Dok1 Ser/Thr phosphorylation.
Fig. 2.
IKKβ is a Dok1 kinase. (A) HEK 293T cells were transfected with F-Dok1 and increasing amounts of F-IKKβ expression vector DNAs. Cell lysates were analyzed by Western blotting. *, IKKβ-modified Dok1. (B) HEK 293T cells were transfected with F-Dok1 together with F-IKKβ, F-IKKβKD, F-IKKα, or F-IKKα KD expression vector DNAs. (C) F-Dok1, F-IKKβ, and F-YopJ expression vector DNAs were transfected into 293T cells and cell lysates were analyzed by Western blot. (D) HEK 293T Cells were transfected with F-Dok1 with or without F-IKKβ expression vector DNA. Aliquots of whole-cell lysates were treated with Ser/Thr phosphatase (λ-PPase) or tyrosine phosphatase (leukocyte antigen-related-Tyr-PPase), and proteins were analyzed by Western blot. Similar results were obtained in three independent experiments.
To assess whether Dok1 is a direct IKKβ substrate, IKKβ was expressed and purified from HEK 293 cells or from Sf9 insect cells, and incubated in an in vitro kinase reaction with Escherichia coli-expressed putative substrates, GST-Dok1, GST-Dok1 amino acids 430–481, GST-IκBα positive control, and GST-IκBaA32A36 negative control proteins. IKKβ from 293 or Sf9 cells specifically phosphorylated GST-IκBα and, to a lesser extent, GST-Dok1, GST-Dok1 430–481, and IKKβ, whereas IKKβ failed to phosphorylate GST-IκBaA32A36 (Fig. 8, which is published as supporting information on the PNAS web site). Overexpression of mitogen-activated protein kinase/Erk kinase kinase 1, an upstream activator of IKK (47) also induced Dok1 mobility shift in 293T cells (data not shown). These data indicate that IKKβ can directly phosphorylate Dok1 or Dok1 amino acids 430 and 481 and that Dok1 amino acids 430–481 are a sufficient substrate for IKKβ phosphorylation.
IKKβ Phosphorylation Sites in Dok1. HuDok1 S439S443 and S446S450 and the homologous sites in murine (Mu)Dok1 and HuDok2 are separated by three amino acids, as are the critical serines for IKKβ phosphorylation sites in IκBα, β, ε, and Cactus (Fig. 3A). HuDok1 lacks a D before S439, S443, S446, or S450, and this fact may be the basis for less efficient phosphorylation, relative to IκBα, β, ε, and Cactus (Fig. 8). However, Hu and MuDok1 have a Y before S450, and phosphorylation of that tyrosine may mimic D or E in IκBα and IκBε, respectively, and account for the increased association of tyrosine-phosphorylated Dok1 with IKKβ in Fig. 1 A. The increased association could also be due to Dok1 dimerization after tyrosine phosphorylation.
Fig. 3.
Dok1 is an IKKβ substrate. (A)IκBα, IκBβ, IκBε, and Drosophila Cactus serines that are IKKβ-phosphorylated are compared with HuDok1 S439, S443, S446, and S450, with MuDok1, and with HuDok2. (B) HEK 293T cells were transfected with wild-type Dok1 (F-Dok1-SSSS) or various alanine substitution mutants, with or without F-IKKβ. Total cell lysates were analyzed by Western blot using anti-Dok1 or anti-IKKβ antibodies. Data are representative of two or more independent experiments.
The role of Dok1 S439S443S446S450 in IKKβ phosphorylation was further evaluated by determining whether IKKβ overexpression could induce the phosphor ylation of Dok1 A439A443A446A450 (Fig. 3B). IKKβ overexpression resulted in substantial Dok1 phosphorylation and slower mobility, whereas Dok1 A439A443A446A450 was not detectably phosphorylated, and Dok1 with A substituted for any three of the four Ss was not extensively phosphorylated (Fig. 3B). Thus, Dok1 S439S443S446, and S450 are critical for IKKβ phosphorylation. However, Dok1 with As substituted for the first or last two Ss was intermediate in the intensity of highly phosphorylated Dok1, indicating that S439S443 and S446S450 can independently enable extensive phosphorylation with reduced efficiency (Fig. 3B). These data are most consistent with the possibility that IKKβ-induced Dok1 S/T phosphorylation extends sequentially beyond S439–S450.
Dok1 pS443- or pS450-specific antibodies were raised in rabbits purified on phosphopeptide affinity columns and readily detected phosphorylated Dok1 in extracts from 293T cells after overexpression of IKKβ and Dok1 (Fig. 4). As expected, phosphospecific antibody reactivity was not detected with overexpression of IKKβ and Dok1 A439A443A446A450 (Fig. 4). Dok1 antibody detected Dok1 and Dok1 A439A443A446A450 equally (Fig. 4). Antibodies to pS443 and pS450 reacted at low levels with wild-type Dok1, but not with Dok1 A439A443A446A450 in the absence of cotransfected IKKβ (Fig. 4). Some of this low-level reactivity is likely due to phosphorylation by endogenous IKK, given the high antibody specificity for Dok1 over Dok1 A439A443A446A450. However, pS450 antibody did have low nonphosphospecific reactivity with Dok1 A450, whereas antibody to pS443 appeared to lack reactivity with Dok1 A443 (Fig. 4). Overall, these data indicate that antibody to pS443 and pS450 have substantial specificity and that IKKβ overexpression causes Dok1 phosphorylation at these sites. Similar specificity was obtained with antibody to peptide pS446, but not with antibody to peptide pS439 (data not shown).
Fig. 4.
Dok1 pS443- and pS450-specific antibodies. HEK 293T cells were transfected with an expression vector for F-Dok1-SSSS, AAAA, ASSS, SASS, SSAS, or SSSA with or without F-IKKβ. Dok1 phosphorylation was evaluated by using anti-pS443 or anti-pS450 affinity-purified antibody. Dok1 and IKKβ levels were monitored by Western blot. These data are representative of at least three independent experiments.
TNF-α, IL-1, and γ Irradiation Induce IKKβ-Mediated Dok1 Phosphorylation. To determine whether IKKβ-mediated Dok1 phosphorylation occurs after physiologic IKKβ activation, Flag-Dok1 or Flag-Dok1 AAAA stably expressing 293 cells were stimulated by TNF-α or IL-1, potent IKKβ activators. Whereas total Dok1 levels were similar in most clones and did not change after TNF-α or IL-1 treatment, TNF-α or IL-1 consistently induced pS443 and pS450 reactivity within 10 min. Phosphorylation was maximal at 10–20 min and persisted at nearly maximal levels for at least 30 min (Fig. 5A and data not shown for pS450). As expected, TNF-α or IL-1 did not cause Dok1-AAAA phosphorylation, as was evident by the absence of change in Dok1 pS443 or pS450 antibody reactivity in Western blots of 293 cell extracts (data not shown). TNF-α also induced endogenous Dok1 S443 and S450 phosphorylation by 10 min in HeLa cells (Fig. 5B). Moreover, overexpression of dominant-negative IKKβ(KD) inhibited TNF-α induced Dok1 phosphorylation in 293 cells expressing F-Dok1 (data not shown).
Fig. 5.
TNF-α, IL-1, or γ radiation induces Dok1 pS443 and pS450. (A) HEK 293 cells stably transfected with F-Dok1-SSSS were treated with TNF-α (20 ng/ml) or IL-1 (20 ng/ml), and protein extracts were analyzed by immunoblot using Dok1 and Dok1 pS443. (B) HeLa cells were treated with TNF-α (20 ng/ml) for 10 min. Protein extracts were immunoprecipitated with rabbit antibody to Dok1 and analyzed by immunoblot with Dok1 and Dok1 pS443 or pS450 antibody. (C and D) BJAB or Epstein–Barr virus-transformed lymphoblasts from a normal control (lymphoblastoid cell line 2145) or from two people with AT (AT11 and AT14) were exposed to γ radiation (20 Gy) and equal protein extracts were immunoblotted with Dok1 and Dok1 pS450, or p53 pS15. These data are representative of two or more independent experiments.
Ionizing radiation also activated IKKβ (48, 49) and induced Dok1 pS450 reactivity, which reached its maximum at 1–2 h and persisted for at least 6 h (Fig. 5D). Radiation-induced phosphorylated Dok1 had an electrophoretic mobility similar to that observed with IKKβ overexpression (data not shown). Phosphorylation of S443 was also induced, but to lesser extent (data not shown). Ionizing radiation-induced IKKβ-mediated NF-κB activation depends on the kinase ATM that is mutated in AT (48, 49). As expected, ATM was required for Dok1 phosphorylation, and Dok1 S450 was not phosphorylated in lymphoblastoid cell lines from two AT patients (Fig. 5D; AT-11 and AT-14) harboring a nonfunctional truncated ATM protein (43). Thus, ionizing radiation-induced Dok1 phosphorylation is ATM-dependent. Phosphorylation of p53 at S15 was observed in AT mutant cell lines at 20 Gy because other ATM family members such as ATR can phosphorylate p53 (50). These data indicate that ATM is the principal kinase for the initiation of Dok1 phosphorylation after ionizing radiation exposure. We cannot exclude the possibility that ATM also directly phosphorylates Dok1 because Dok1 S310 and S450 are potential SQ/TQ ATM phosphorylation motif sites (1, 50). In fact, ATM overexpression induced Dok1 pS450 reactivity, but the extent of phosphorylation was much less than that induced by IKKβ or ionizing radiation (data not shown). Taken together, these data indicate that TNF-, IL-1-, or γ radiation-induced IKKβ activation results in Dok1 S443, S450 phosphorylation.
Dok1 S439, S443, S446, and S450 Are Required for Dok1 Effects on Cell Proliferation, on PDGF-Induced Erk1/2 Activation, and on Cell Motility. Wild-type Dok1 overexpression down-modulates cell proliferation and mitogen-activated protein kinase activation (16). The role of the Dok1 IKKβ phosphorylation sites in Dok1 effects on cell proliferation and Erk1/2 kinase activity in 293 cells was evaluated by comparing the effects of stable Dok1 or Dok1-AAAA overexpression on cell proliferation and Erk1/2 kinase activity. As expected (16), cells overexpressing Dok1 grew more slowly than control cells (Fig. 6A). In contrast, cells expressing similar amounts of Dok1-AAAA grew at a rate that was indistinguishable from control cells (Fig. 6A). PDGF also induced less Erk1/2 phosphorylation in cells that overexpress wild-type Dok1, whereas PDGF-induced Erk1/2 phosphorylation was normal or elevated in cells that overexpress Dok1-AAAA (Fig. 6B). Thus, the Dok1 serines that are phosphorylated by IKKβ are required for Dok1 overexpression-mediated inhibition of Erk1/2 phosphorylation and cell proliferation, which is consistent with a role for IKKβ-mediated phosphorylation in Dok1 effects downstream of tyrosine kinases.
Fig. 6.
Dok1 inhibits cell proliferation and Erk1/2 phosphorylation, whereas Dok1-AAAA does not affect cell proliferation and slightly increases Erk1/2 phosphorylation. (A) HEK 293 cells stably transfected with Dok1, Dok1-AAAA, or empty pcDNA3 expression vectors were monitored for growth. Data are mean ± SE of three independent experiments carried out in triplicate. Similar results were also obtained from two other independent sets of expressing clones. (B) Equal protein extracts from PDGF-treated pcDNA3-, Dok1-, or Dok1-AAAA-transfected cells lines were analyzed by immunoblotting for pERK1/2, Erk1/2, and Dok1. Data are representative of at least three independent experiments.
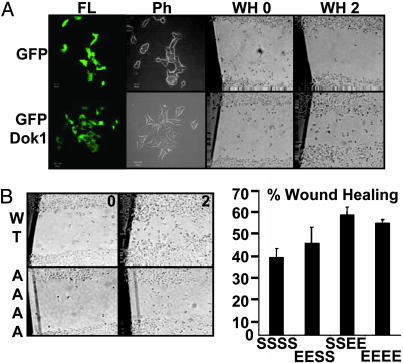
As described (13, 18, 19), Dok1 overexpression increased 293 cell spreading and migration on coverslips coated with collagen I (Fig. 7A). Although polyclonal cells converted to GFP expression were indistinguishable from polyclonal cells expressing GFP-Dok1, in their growth on plastic (except for GFP-Dok1 localization to the cytoplasm and GFP distribution throughout the cell), cells expressing GFP-Dok1 became flatter and more spread out than GFP-expressing cells within hours of plating on a collagen-I substrate in complete medium with serum (Fig. 7A Left). Furthermore, GFP-Dok1-expressing cells migrated more rapidly into a wound in the cell layer than did GFP control cells (Fig. 7B Right). In contrast, Flag-Dok1-AAAA-converted cells were significantly slower in migration than Flag-Dok1 cells (Fig. 7B Left). Moreover, cells expressing Dok1-SSEE or Dok1-EEEE migrated significantly faster than did wild-type Dok1-expressing cells (Fig. 7B Right). These data implicate Dok1 S439, S443, S446, and S450 in Dok1 effects on cell spreading and migration, which is consistent with previously observed TNF-α- or IL-1-induced increase in cell spreading; such effects have also been attributed to other mitogen-activated protein kinase pathways (51–53).
Fig. 7.
Dok1, Dok1-SSEE, and Dok1-EEEE increase cell migration, whereas Dok1-AAAA inhibits cell migration. (A) Polyclonal selected 293/GFP-or 293/GFP-Dok1-expressing cells were plated in serum on coverslips coated with collagen I. Cells were observed by fluorescence (FL) and phase contrast (Ph) for wound healing (WH) 2 h later. Healing (WH) in the cell layer was photographed for the indicated cell lines at 0 (WH 0) and 2 h (WH 2). Cell migration was evaluated based on cell filling of the wound area by using metamorph software. (B) Wound healing test was performed as in A for cell lines expressing Flag-tagged Dok1 wild-type (WT), Dok1-AAAA mutant (AAAA), Dok1-EESS, Dok1-SSEE, or Dok1-EEEE. The percentage filling at 2 h for each cell line is plotted. Data are means ± SE of three independent experiments carried out in triplicate.
Importantly, Dok1 SSSS or AAAA overexpression did not affect IKKβ-mediated IκBα phosphorylation or subsequent degradation after TNF-α treatment (data not shown), indicating that Dok1 does not compete with IκBα for IKKβ, even when Dok1 is overexpressed.
Discussion
These experiments indicate that physiologic IKKβ activation results in Dok1 S443, S446, and S450 phosphorylation. IKKβ (and α) associated with and phosphorylated Dok1 on S439S443 and S446S450. Extensive IKKβ-mediated Dok1 phosphorylation, in vivo, depended on S439, S443, S446, and S450, and Dok1 amino acids 430–481 were phosphorylated by recombinant IKKβ, in vitro. Furthermore, TNF-α, IL-1, or ionizing radiation induced IKKβ activation and Dok1 S443, S446, and S450 phosphorylation, in vivo, as assayed with Dok1 pS site-specific antisera. Moreover, Dok1 phosphorylation after ionizing radiation was ATM-dependent, which was consistent with the expected ATM role in activating IKKβ. Dok1 S443, S446, and S450 phosphorylations were down-stream of TNF-α, IL-1, or ionizing radiation-induced IKKβ activation in human epithelial and B cells.
Dok1 was a less efficient than IκBα as an in vitro substrate for activated IKKβ. Furthermore, Dok1 overexpression did not affect IKKβ-mediated NF-κB activation. Dok1 therefore appears to be an alternative IKKβ substrate that does not inhibit IKKβ phosphorylation of IκBα.
The data presented here support the hypothesis that Dok1 S439,S443,S446, and S450, which are phosphorylated by IKKβ, are critical for previously characterized Dok1 effects downstream of tyrosine kinase signaling. Activated IKKβ phosphorylation of these Dok1 serines may augment Dok1 effects. Indeed, mutation of Dok1 S439, S443, S446, and S450 to A resulted in Dok1 being unable to inhibit PDGF-mediated Erk1/2 activation or cell proliferation.
Most relevant to a role for Dok1 serine phosphorylation were motility studies in which wild-type Dok1 overexpression increased cell motility, Dok1 with S439, S443, S446, and S450 mutated to A439, A443, A446, and A450 was unable to increase cell motility, and Dok1 with S439, S443, S446, and S450 mutated to E439, E443, E446, and E450 was slightly superior to wild-type Dok1 in increasing cell motility. Moreover, as shown in Fig. 1D, Dok1 and IKKβ had a diffuse reticular distribution, with accentuation at sites of F-actin, which is consistent with a role for IKKβ in filopodia formation, possibly related to cell motility. Altogether, these biochemical and reverse genetic data are consistent with the hypothesis that IKKβ phosphorylation of Dok1 is important for Dok1 effects on cell motility.
Other evidence supports a role for Dok1 in cell motility. Mu-Dok1 Y361, which is equivalent to Hu-Dok1 Y362, is also a specific c-Abl substrate in fibroblasts and mediates Nck recruitment followed by Pak1 or mitogen-activated protein kinase Erk kinase kinase 1 activation (13, 54–57). Mouse fibroblasts lacking c-Abl, Dok1, or Nck have fewer filopodia than cells expressing the disrupted gene (57).
Dok1 is now among a growing list of “nonclassical” IKKβ substrates. Dok1 S439S443 and S446S450 are similar in amino acid sequence to IκBα S32S36. Furthermore, Dok1 Y449 is an important Csk tyrosine kinase site and Y449 phosphorylation next to S450 may mimic the D or E that precedes S32 and S36 in IκBα. Y449 phosphorylation would enhance Dok1 similarity to classical IKKβ substrates. Y449 phosphorylation also increases Dok1, SH2D1A, SHP-2, or SHIP binding (11–15). Whereas most IKKβ substrates, including IκBα, p65, or p105 are important for NF-κB activation (26), β-catenin, SRC-3, and insulin receptor substrate 1, another Dok family member, have also been identified to be nonclassical IKKβ substrates (58–60). These other IKKβ substrates are also phosphorylated by other kinases, such as glycogen synthetase kinase 3β (61) and Dok1 may also be a more promiscuous Ser/Thr kinase substrate. However, we have recently found that glycogen synthetase kinase 3β overexpression in 293T cells had less effect than IKKβ on Dok1 phosphorylation, by using Dok1 pS450-specific antibody (data not shown).
Further evaluation of the role of IKKβ in mediating TNF-α, IL-1, or γ radiation effects through phosphorylation of Dok1 S439–450 should include studies in IKKβ knockout, RNAi knocked down, or IKKβ chemically inhibited cells. Investigations with murine IKKβ knock out fibroblasts were hindered by poor reactivity of HuDok1 pS-specific sera with MuDok1. We also note that IKKα was present in Dok1 immunoprecipitations and could also have a role in Dok1 S phosphorylation.
Supplementary Material
Acknowledgments
We thank T. Gilmore (Boston University, Boston) for GST IκBα plasmids; G. Mosialos, T. Gilmore, E. Cahir-McFarland, J. Hall, M. Tommasino, F. Roy and Z.-Q. Wang for contributing advice; J. Cheney for editing; G. Mollon (International Agency for Research on Cancer) for illustrations; D. Goeddel, H. Allen, J. Dixon, Y. Shiloh, M. Kastan, G. Johnson, J. Woodgett, J. Cambier, and G. Lenoir for reagents; I. Verma (The Salk Institute, La Jolla, CA) for IKK null mouse embryonic fibroblasts; and B. Chapot for technical assistance. This work was supported by National Institutes of Health Grants CA47006 and CA85180 (to E.D.K.), by a fellowship from the Association pour la Recherche sur le Cancer (to B.S.), and by Ministère de l'Education Nationale de la Recherche et de la Technologie fellowships (to F.S. and O.D.).
Author contributions: S.L., C.A., F.S., O.D., P.J., E.D.K., and B.S.S. designed research; S.L., C.A., F.S., O.D., J.A., V.P., J.M., B.S., and B.S.S. performed research; S.L., C.A., F.S., O.D., J.A., V.P., J.M., B.S., R.K., P.J., E.D.K., and B.S.S. contributed new reagents/analytic tools; S.L., C.A., F.S., O.D., J.A., V.P., J.M., B.S., R.K., P.J., E.D.K., and B.S.S. analyzed data; and S.L., C.A., F.S., O.D., P.J., E.D.K., and B.S.S. wrote the paper.
Abbreviations: IKKβ, IκB kinase β; IKKα, IκB kinase α; Erk, extracellular signal-regulated kinase; pS, phosphoserine; HuDok, human Dok; MuDok, murine Dok; KD, kinase-dead; AT, ataxia-telangiectasia; ATM, AT-mutated; PDGF, platelet-derived growth factor; HEK, human embryonic kidney; F-IKKβ, Flag-tagged IKKβ.
References
- 1.Carpino, N., Wisniewski, D., Strife, A., Marshak, D., Kobayashi, R., Stillman, B. & Clarkson, B. (1997) Cell 88, 197–204. [DOI] [PubMed] [Google Scholar]
- 2.Yamanashi, Y. & Baltimore, D. (1997) Cell 88, 205–211. [DOI] [PubMed] [Google Scholar]
- 3.Cong, F., Yuan, B. & Goff, S. P. (1999) Mol. Cell. Biol. 19, 8314–8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Cristofano, A., Carpino, N., Dunant, N., Friedland, G., Kobayashi, R., Strife, A., Wisniewski, D., Clarkson, B., Pandolfi, P. P. & Resh, M. D. (1998) J. Biol. Chem. 273, 4827–4830. [DOI] [PubMed] [Google Scholar]
- 5.Grimm, J., Sachs, M., Britsch, S., Di Cesare, S., Schwarz-Romond, T., Alitalo, K. & Birchmeier, W. (2001) J. Cell Biol. 154, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemay, S., Davidson, D., Latour, S. & Veillette, A. (2000) Mol. Cell. Biol. 20, 2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelms, K., Snow, A. J., Hu-Li, J. & Paul, W. E. (1998) Immunity 9, 13–24. [DOI] [PubMed] [Google Scholar]
- 8.White, M. F. (1998) Mol. Cell. Biochem. 182, 3–11. [PubMed] [Google Scholar]
- 9.Songyang, Z., Yamanashi, Y., Liu, D. & Baltimore, D. (2001) J. Biol. Chem. 276, 2459–2465. [DOI] [PubMed] [Google Scholar]
- 10.Zhao, M., Schmitz, A. A., Qin, Y., Di Cristofano, A., Pandolfi, P. P. & Van Aelst, L. (2001) J. Exp. Med. 194, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat, A., Johnson, K. J., Oda, T., Corbin, A. S. & Druker, B. J. (1998) J. Biol. Chem. 273, 32360–32368. [DOI] [PubMed] [Google Scholar]
- 12.Neet, K. & Hunter, T. (1995) Mol. Cell. Biol. 15, 4908–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi, T., Matozaki, T., Inagaki, K., Tsuda, M., Fukunaga, K., Kitamura, Y., Kitamura, T., Shii, K., Yamanashi, Y. & Kasuga, M. (1999) EMBO J. 18, 1748–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattler, M., Verma, S., Pride, Y. B., Salgia, R., Rohrschneider, L. R. & Griffin, J. D. (2001) J. Biol. Chem. 276, 2451–2458. [DOI] [PubMed] [Google Scholar]
- 15.Sylla, B. S., Murphy, K., Cahir-McFarland, E., Lane, W. S., Mosialos, G. & Kieff, E. (2000) Proc. Natl. Acad. Sci. USA 97, 7470–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cristofano, A., Niki, M., Zhao, M., Karnell, F. G., Clarkson, B., Pear, W. S., Van Aelst, L. & Pandolfi, P. P. (2001) J. Exp. Med. 194, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick, M. J., Dong, L. Q., Hu, D., Langlais, P. & Liu, F. (2001) J. Biol. Chem. 276, 42843–42850. [DOI] [PubMed] [Google Scholar]
- 18.Yamanashi, Y., Tamura, T., Kanamori, T., Yamane, H., Nariuchi, H., Yamamoto, T. & Baltimore, D. (2000) Genes Dev. 14, 11–16. [PMC free article] [PubMed] [Google Scholar]
- 19.Hosooka, T., Noguchi, T., Nagai, H., Horikawa, T., Matozaki, T., Ichihashi, M. & Kasuga, M. (2001) Mol. Cell. Biol. 21, 5437–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamakawa, N., Tsuchida, K. & Sugino, H. (2002) EMBO J. 21, 1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato, I., Takai, T. & Kudo, A. (2002) J. Immunol. 168, 629–634. [DOI] [PubMed] [Google Scholar]
- 22.Martelli, M. P., Boomer, J., Bu, M. & Bierer, B. E. (2001) J. Biol. Chem. 276, 45654–45661. [DOI] [PubMed] [Google Scholar]
- 23.Nemorin, J. G., Laporte, P., Berube, G. & Duplay, P. (2001) J. Immunol. 166, 4408–4415. [DOI] [PubMed] [Google Scholar]
- 24.Tamir, I., Stolpa, J. C., Helgason, C. D., Nakamura, K., Bruhns, P., Daeron, M. & Cambier, J. C. (2000) Immunity 12, 347–358. [DOI] [PubMed] [Google Scholar]
- 25.Liang, X., Wisniewski, D., Strife, A., Clarkson, B. & Resh, M. D. (2002) J. Biol. Chem. 277, 13732–13738. [DOI] [PubMed] [Google Scholar]
- 26.Karin, M. & Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18, 621–663. [DOI] [PubMed] [Google Scholar]
- 27.Karin, M. & Lin, A. (2002) Nat. Immunol. 3, 221–227. [DOI] [PubMed] [Google Scholar]
- 28.Silverman, N. & Maniatis, T. (2001) Genes Dev. 15, 2321–2342. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin, A. S. J. (1996) Annu. Rev. Immunol. 14, 649–683. [DOI] [PubMed] [Google Scholar]
- 30.Arsura, M., Mercurio, F., Oliver, A. L., Thorgeirsson, S. S. & Sonenshein, G. E. (2000) Mol. Cell. Biol. 20, 5381–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann, B., Weber, C. K., Troppmair, J., Whiteside, S., Israel, A., Rapp, U. R. & Wirth, T. (2000) Proc. Natl. Acad. Sci. USA 97, 4615–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane, L. P., Mollenauer, M. N., Xu, Z., Turck, C. W. & Weiss, A. (2002) Mol. Cell. Biol. 22, 5962–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petro, J. B., Rahman, S. M., Ballard, D. W. & Khan, W. N. (2000) J. Exp. Med. 191, 1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saijo, K., Mecklenbrauker, I., Santana, A., Leitger, M., Schmedt, C. & Tarakhovsky, A. (2002) J. Exp. Med. 195, 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su, T. T., Guo, B., Kawakami, Y., Sommer, K., Chae, K., Humphries, L. A., Kato, R. M., Kang, S., Patrone, L., Wall, R., et al. (2002) Nat. Immunol. 3, 780–786. [DOI] [PubMed] [Google Scholar]
- 36.Wooten, M. W., Seibenhener, M. L., Neidigh, K. B. & Vandenplas, M. L. (2000) Mol. Cell. Biol. 20, 4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung, K. C., Rose, D. W., Dhillon, A. S., Yaros, D., Gustafsson, M., Chatterjee, D., McFerran, B., Wyche, J., Kolch, W. & Sedivy, J. M. (2001) Mol. Cell. Biol. 21, 7207–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herndon, T. M., Shan, X. C., Tsokos, G. C. & Wange, R. L. (2001) J. Immunol. 166, 5654–5664. [DOI] [PubMed] [Google Scholar]
- 39.Khan, W. N. (2001) Immunol. Res. 23, 147–156. [DOI] [PubMed] [Google Scholar]
- 40.Weil, R., Schwamborn, K., Alcover, A., Bessia, C., Di Bartolo, V. & Israel, A. (2003) Immunity 18, 13–26. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, K., Yamashita, Y., Miyazato, A., Ohya, K., Kitanaka, A., Ikeda, U., Shimada, K., Yamanaka, T., Ozawa, K. & Mano, H. (2000) J. Biol. Chem. 275, 24945–24952. [DOI] [PubMed] [Google Scholar]
- 42.Sylla, B. S., Hung, S. C., Davidson, D. M., Hatzivassiliou, E., Malinin, N. L., Wallach, D., Gilmore, T. D., Kieff, E. & Mosialos, G. (1998) Proc. Natl. Acad. Sci. USA 95, 10106–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angèle, S., Laugé, A., Fernet, M., Moullan, N., Beauvais, P., Couturier, P., Stoppa-Lyonnet, D. & Hall, J. (2003) Hum. Mutat. 21, 169–170. [DOI] [PubMed] [Google Scholar]
- 44.Li, N. & Karin, M. (2000) Methods Enzymol. 319, 273–279. [DOI] [PubMed] [Google Scholar]
- 45.Ory, S., Munari-Silem, Y., Fort, P. & Jurdic, P. (2000) J. Cell Sci. 113, 1177–1188. [DOI] [PubMed] [Google Scholar]
- 46.Orth, K., Palmer, L. E., Bao, Z. Q., Stewart, S., Rudolph, A. E., Bliska, J. B. & Dixon, J. E. (1999) Science 285, 1920–1922. [DOI] [PubMed] [Google Scholar]
- 47.Lee, F. S., Peters, L. C., Dang, L. C. & Maniatis, T. (1998) Proc. Natl. Acad. Sci. USA 95, 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, N., Banin, S., Ouyang, H., Li, G., Courtois, G., Shiloh, Y., Karin, M. & Rotman, G. (2001) J. Biol. Chem. 276, 8898–8903. [DOI] [PubMed] [Google Scholar]
- 49.Piret, B., Schoonbroodt, S. & Piette, J. (1998) Oncogene 18, 2261–2271. [DOI] [PubMed] [Google Scholar]
- 50.Kastan, M. B. & Lim, D. (2000) Nat. Rev. Mol. Cell Biol. 1, 179–186. [DOI] [PubMed] [Google Scholar]
- 51.Chen, Y., Ke, Q., Yang, Y., Rana, J. S., Tang, J., Morgan, J. P. & Xiao, Y. F. (2003) FASEB J. 17, 2231–2239. [DOI] [PubMed] [Google Scholar]
- 52.Corcione, A., Ottonello, L., Tortolina, G., Tasso, P., Ghiotto, F., Airoldi, I., Taborelli, G., Malavasi, F., Dallegri, F. & Pistoia, V. (1997) Blood 90, 4493–4501. [PubMed] [Google Scholar]
- 53.Miossec, P., Yu, C. L. & Ziff, M. (1984) J. Immunol. 133, 2007–2011. [PubMed] [Google Scholar]
- 54.Master, Z., Jones, N., Tran, J., Jones, J., Kerbel, R. S. & Dumont, D. J. (2001) EMBO J. 20, 5919–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami, H., Yamamura, Y., Shimono, Y., Kawai, K., Kurokawa, K. & Takahashi, M. (2002) J. Biol. Chem. 277, 32781–32790. [DOI] [PubMed] [Google Scholar]
- 56.Yujiri, T., Ware, M., Widmann, C., Oyer, R., Russell, D., Chan, E., Zaitsu, Y., Clarke, P., Tyler, K., Oka, Y., et al. (2000). Proc. Natl. Acad. Sci. USA 97, 7272–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodring, P. J., Meisenhelder, J., Johnson, S.A., Zhou, G. L., Field, J., Shah, K., Bladt, F., Pawson, T., Niki, M., Pandolfi, P. P., et al. 2004) J. Cell Biol. 165, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao, Z., Hwang, D., Bataille, F., Lefevre, M., York, D., Quon, M. J. & Ye, J. (2002) J. Biol. Chem. 277, 48115–48121. [DOI] [PubMed] [Google Scholar]
- 59.Lamberti, C., Lin, K. M., Yamamoto, Y., Verma, U., Verma, I. M., Byers, S. & Gaynor, R. B. (2001) J. Biol. Chem. 276, 42276–42286. [DOI] [PubMed] [Google Scholar]
- 60.Wu, R. C., Qin, J., Hashimoto, Y., Wong, J., Xu, J., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (2002) Mol. Cell. Biol. 22, 3549–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harwood, A. J. (2001) Cell 105, 821–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.