Abstract
Background
Randomized controlled trials evaluate the effectiveness of interventions for central venous access devices, however, high complication rates remain. Scoping reviews map the available evidence and demonstrate evidence deficiencies to focus ongoing research priorities.
Method
A scoping review (January 2006–December 2015) of randomized controlled trials evaluating the effectiveness of interventions to improve central venous access device outcomes; including peripherally inserted central catheters, non-tunneled, tunneled and totally implanted venous access catheters. MeSH terms were used to undertake a systematic search with data extracted by two independent researchers, using a standardized data extraction form.
Results
In total, 178 trials were included (78 non-tunneled [44%]; 40 peripherally inserted central catheters [22%]; 20 totally implanted [11%]; 12 tunneled [6%]; 6 non-specified [3%]; and 22 combined device trials [12%]). There were 119 trials (68%) involving adult participants only, with 18 (9%) pediatric and 20 (11%) neonatal trials. Insertion-related themes existed in 38% of trials (67 RCTs), 35 RCTs (20%) related to post-insertion patency, with fewer trials on infection prevention (15 RCTs, 8%), education (14RCTs, 8%), and dressing and securement (12 RCTs, 7%). There were 46 different study outcomes reported, with the most common being infection outcomes (161 outcomes; 37%), with divergent definitions used for catheter-related bloodstream and other infections.
Conclusion
More high quality randomized trials across central venous access device management are necessary, especially in dressing and securement and patency. These can be encouraged by having more studies with multidisciplinary team involvement and consumer engagement. Additionally, there were extensive gaps within population sub-groups, particularly in tunneled devices, and in pediatrics and neonates. Finally, outcome definitions need to be unified for results to be meaningful and comparable across studies.
Introduction
Central venous access devices (CVADs) provide access to the greater vascular system to administer therapy contraindicated to be given peripherally, for longer term treatment, and for venous monitoring and blood sampling [1]. Patients requiring CVADs are heterogeneous, with varying ages, acute and chronic illnesses, across hospitals and in community care. There are many different CVAD types inserted for different treatment requirements; for example, long or short term duration, and continuous or intermittent therapy. CVAD care is complex and multi-faceted; clinicians from diverse clinical specialties are involved in their insertion and management [1–4].
Despite the prevalence of CVADs in acute and chronic care, serious insertion and management complications associated with CVADs continue to be prevalent [5–10]. Many complications, including bloodstream infection, are considered a preventable source of patient harm and have a significant negative impact on patients and healthcare costs [11]. Developing, testing and implementing effective interventions to prevent CVAD-associated harm are important considerations for healthcare researchers, clinicians, and patients. In order to improve the quality and safety of CVADs, many randomized controlled trials (RCTs) have been conducted to evaluate the effectiveness of healthcare interventions.
The RCT is considered to be the “gold standard” for evaluating the effectiveness of an intervention, as it provides reliable evidence with minimal risk of bias compared to other study designs [12]. Clinicians refer to results of RCTs and systematic reviews to guide clinical decision-making, however, this may be challenged if results cannot be generalized to their population because of too few RCTs, small sample sizes, poor reporting, and a lack of clear effect [13]. Therefore, it is important for researchers to develop their research agenda based upon the identified priority areas and clinical needs, while minimizing unnecessary duplication of research and associated costs.
Scoping reviews are used to map the existing research in a given field and to highlight the gaps in evidence [14–17]. They examine the breadth of literature published, with the aim of providing insights and guidance for clinicians and researchers on where to focus their research [17]. The aim of this scoping review was to reveal the RCTs conducted in relation to CVADs in the past decade and to synthesize the patient populations and clinical settings studied, interventional themes addressed, and the outcome measures used.
Methods
Review framework
This scoping review was conducted using the framework outlined by Arksey and O’Malley [14] and modified by the Cochrane Public Health Group [14, 16], which is used extensively [18–20]. The framework consists of the following steps: 1. Identify the research question; 2. Identify relevant studies; 3. Select studies for inclusion; 4. Sort, collate, and analyze data; and 5. Summarize and report results [14]. Consensus on methodology and inclusion/exclusion criteria were established during the first phase of the project to ensure consistency in decision making.
Identify the research questions
The objectives of the review were to answer the following questions:
What RCTs have been conducted on the effectiveness of interventions to improve CVAD outcomes within contemporary literature (< 10 years), and what were the study characteristics?
What CVAD types, population demographics, and clinical settings were included?
What were the interventional themes studied?
What outcome measures were reported?
Identify relevant studies
A systematic search was undertaken. Studies were eligible for inclusion if they met predefined inclusion criteria: (1) employed an RCT design; (2) evaluated interventions to improve outcomes associated with CVADs; and (3) were published between 1 January 2006 and 31 December 2015. CVADs were limited to peripherally inserted central catheter (PICC), non-tunneled CVAD (NTCVAD), tunneled CVAD, and totally implantable venous access device (TIVAD) used in any patient population. Umbilical catheters and dialysis catheters were excluded from the review as they are used in special population groups only. Studies comprising more than one CVAD type were included in the scoping review if the article specified the results for each device type, allowing the results of the target devices to be extracted. All participant ages and settings (inpatient and ambulatory) were included. We excluded non-RCT designs (including cross-over design), secondary analysis of RCTs, non-English studies without an English abstract, and non-human studies.
Search strategy
The search strategy was developed with the assistance of a university health sciences librarian. Databases were systematically and independently searched on the 19th February, 2016. Databases included PubMed (US National Library of Medicine), Cochrane Central Register of Controlled Trials (the Cochrane Library), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Medline (US National Library of Medicine). (See S1 File for search terms). Reference lists of retrieved systematic reviews were reviewed to ensure all potential RCTs were included.
Study selection and data extraction
References were imported into EndNote™ (Clarivate Analytics) and then sorted and examined throughout the inclusion/exclusion process [14]. Each reference, title and abstract was initially dual-screened by a pair of two independent investigators (MT, GB, AU, SK). Two independent reviewers then reviewed the full text of selected studies for inclusion/exclusion criteria, and, if eligible, extracted the data using a standardized data extraction form. If the group was unclear of the inclusion, all four reviewers met until an agreement was reached.
Data sorting, collating, and analysis
Data were extracted into a Microsoft Excel file to organize the data under the following headings: first author; year of publication; country; first author profession; title of journal; method of randomization; study population (inpatient/ambulant; neonates/pediatrics/adults; clinical specialities); sample size; discipline of CVAD inserter; interventional theme; outcome measures; key findings; and grant funding. The risk of bias of the individual studies was not formally assessed in the same way as a systematic review [21]. This is because of the differing goals of scoping reviews, which are designed to illuminate the breadth rather than the depth of available research [14, 15]. However, this review incorporates the appraisal of the methodological quality by including assessment of randomization methods and outcome definitions [22]. Authors were not contacted for further information or to obtain full-text.
Summarizing and reporting results
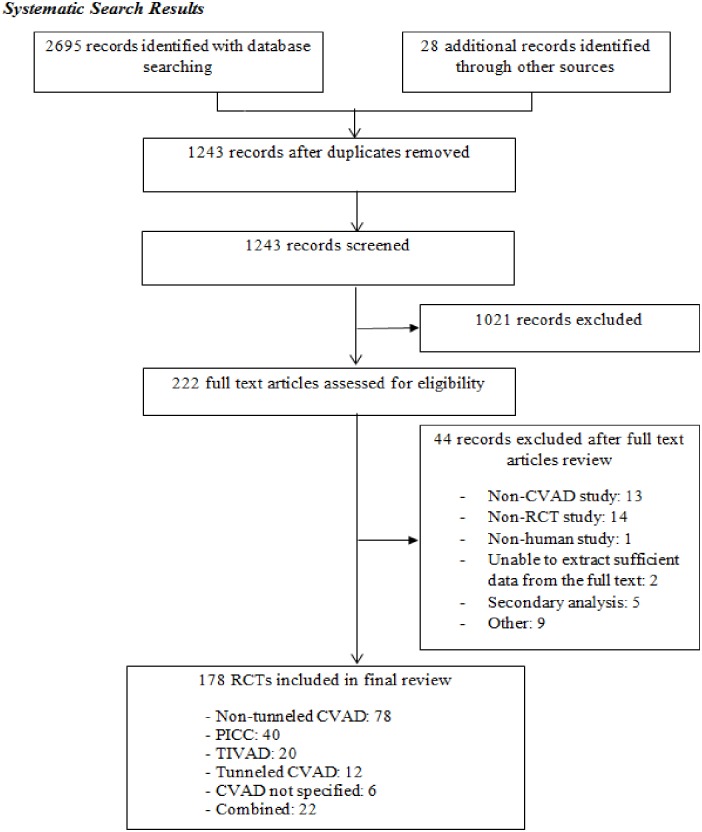
Preliminary tables were constructed to identify topics that had been the focus of many RCTs and topics that were lacking in evidence. Microsoft Excel was used to create graphs and tables. Fig 1 describes the flow of inclusion and exclusion for the study selection, in accordance with the referred Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. Database searching identified 2,695 RCTs and an additional 28 RCTs were added after reviewing the reference lists of 77 identified systematic reviews. After duplicates and studies with non-relevant abstracts were removed, 222 RCTs were reviewed. Twenty-nine RCTs provided abstract information only.
Fig 1. Flowchart of articles screened for inclusion in the scoping review.
RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device.
Results
Characteristics of included RCTs
The final review included 178 RCTs (78 non-tunneled [24–101]; 40 PICC [102–141]; 20 totally implanted [142–161]; 12 tunneled [162–173]; 6 non-specified [174–179]; 22 combined [180–201]; See S1 Table for included studies). The non-specified category was created to classify studies that did not specify the type of CVAD. There were nine pilot studies (6%) and 28 multi-center studies (16%). The largest number of RCTs were published in the USA (32 RCTs; 18%), followed by Italy (12 RCTs; 7%), and Brazil, Netherlands, France, and Germany (10 RCTs each; 6%).
First authors were mainly medical doctors (96 RCTs; 54%), followed by departmental information only (48 RCTs; 27%), and nurses (13 RCTs; 7%). Information regarding grant monies was listed as public/departmental source (43 RCTs; 24%), followed by missing/not stated (27 RCTs; 15%), or missing/abstract information only (25 RCTs; 14%). Industry grants accounted for 19 RCTs (11%), and 13 RCTs received both public and private funding (7%). Forty percent (n = 70) of RCTs used computer-generated allocation, which is the standard method of randomization [202]. However, 42 RCTs (24%) did not state the randomization method (excluding those that provided abstract information only), and some studies had questionable randomization methods, such as alternate allocation, random admission to ward allocation, and lottery method, which can potentially be vulnerable to subversion [202].
Population demographics and clinical settings of included RCTs
Patient populations studied were mostly adults (119 RCTs: 68%) (See Table 1). In younger patients, neonates were the target population in 20 RCTs (11%) and pediatrics in 18 RCTs (10%). There was a difference in frequency of use of CVAD types in different populations. Non-tunneled CVADs were the most popular catheter type studied in adults (n = 23,435, 60% of all adult RCTs), compared to 24% of all pediatric RCTs (n = 431) and 4% of all neonate RCTs (n = 87). Most studied CVAD types for pediatrics and neonates were tunneled CVADs (n = 744, 41% of all pediatric RCTs) and PICCs respectively (n = 1572, 78% of neonate RCTs). Pediatrics comprised 4% (n = 1816) of the total sample size compared to adults (n = 38,979, 84%) and neonates (n = 2009, 4%).
Table 1. Population table of included RCTs (N = 178 studies, 46,258 participants).
| NTCVAD | PICC | TIVAD | Tunneled | Combined | CVAD-NS | Total | |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Population (number of participants) | |||||||
| Adult | 23,435 (60%) | 2,742 (7%) | 4,322 (11%) | 917 (2%) | 7,222 (19%) | 341 (1%) | 38,979 (100%) |
| Pediatrics | 431 (24%) | 240 (13%) | 151 (8%) | 744 (41%) | 250 (14%) | 0 | 1,816 (100%) |
| Neonates | 87 (4%) | 1,572 (78%) | 0 | 0 | 350 (18%) | 0 | 2,009 (100%) |
| Combined | 240 (18%) | 0 | 0 | 0 | 1089 (82%) | 0 | 1,329 (100%) |
| Staff | 724 (88%) | 32 (4%) | 0 | 0 | 0 | 65 (8%) | 821 (100%) |
| Unknown | 394 (30%) | 250 (19%) | 0 | 0 | 0 | 660 (51%) | 1,304 (100%) |
| Total | 25,311 (55%) | 4,836 (10%) | 4,473 (10%) | 1,661 (4%) | 8,911 (19%) | 1,066 (2%) | 46,258 (100%) |
| Setting (number of studies) | |||||||
| Inpatient | 65 (47%) | 27 (19%) | 11 (8%) | 12 (9%) | 20 (14%) | 3 (2%) | 138 (100%) |
| Outpatient | 0 | 3 (38%) | 4 (50%) | 0 | 1 (13%) | 0 | 8 (100%) |
| Both | 0 | 2 (33%) | 4 (67%) | 0 | 0 | 0 | 6 (100%) |
| Staff | 11 (85%) | 1 (8%) | 0 | 0 | 0 | 1 (8%) | 13 (100%) |
| Not stated | 2 (15%) | 7 (54%) | 1 (8%) | 0 | 1 (8%) | 2 (15%) | 13 (100%) |
| Total | 78 (44%) | 40 (22%) | 20 (11%) | 12 (7%) | 22 (12%) | 6 (3%) | 178 (100%) |
| Clinical setting (number of studies) | |||||||
| Intensive Care Unit (ICU) | 37 (61%) | 19 (30%) | 0 | 0 | 4 (7%) | 1 (2%) | 61 (100%) |
| Adult ICU | (35) | (2) | (0) | (0) | (1) | (1) | (39) |
| Pediatric ICU | (1) | (1) | (0) | (0) | (0) | (0) | (2) |
| Neonatal ICU | (1) | (16) | (0) | (0) | (3) | (0) | (20) |
| Hematology/Oncology | 5 (10%) | 4 (8%) | 16 (33%) | 10 (20%) | 11 (22%) | 3 (7%) | 49 (100%) |
| Operating room | 18 (90%) | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 20 (100%) |
| All patients requiring vascular access/outpatients/not stated | 2 (10%) | 10 (50%) | 4 (20%) | 1 (5%) | 3 (15%) | 0 | 20 (100%) |
| Education/Training facility | 10 (91%) | 0 | 0 | 0 | 0 | 1 (9%) | 11 (100%) |
| Medical/Surgical | 2 (25%) | 3 (38%) | 0 | 1 (12%) | 2 (25%) | 0 | 8 (100%) |
| Combined | 4 (66%) | 1 (17%) | 0 | 0 | 1 (17%) | 0 | 6 (100%) |
| Radiology department | 0 | 3 (100%) | 0 | 0 | 0 | 0 | 3 (100%) |
| Emergency department* | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 78 (45%) | 40 (22%) | 20 (11%) | 12 (7%) | 22 (12%) | 6 (3%) | 178 (100%) |
*Two studies were undertaken in ED in conjunction with other clinical areas, but no study was undertaken in ED alone.
RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; NTCVAD: Non-tunneled Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device; CVAD NS: Central Venous Access Device Not Specified
The most prevalent clinical specialties for CVAD RCTs were intensive care units (ICU) (61 RCTs; 34%), with a further 4 RCTs including some ICU patients, followed by hematology/oncology settings (49 RCTs; 27%). Most non-tunneled CVAD studies (78 RCTs) took place in ICUs (37 RCTs; 47%) or operating rooms (18 RCTs, 23%). PICCs were studied predominantly in ICUs (19 RCTs), of which the majority were neonatal ICU (16 RCTs; 84%). Hematology/oncology was the most prevalent clinical setting for TIVAD (16 out of 20 RCTs; 80%), tunneled CVAD (10 out of 12 RCTs; 83%), and combined catheter (11 out of 22 RCTs, 50%) studies. No studies were conducted solely in the emergency department (ED), but two RCTs included some ED devices. Staff education was the focus of 13 RCTs (7%); these were primarily simulation studies and only two detailed nursing education. The majority of CVADs were inserted by doctors or medical interns (75 RCTs; 42%). Vascular access nurses or vascular access teams including nurses inserted CVADs in 17 RCTs (9%), and radiologists inserted CVADS in 13 RCTs (7%).
Interventional themes of included RCTs
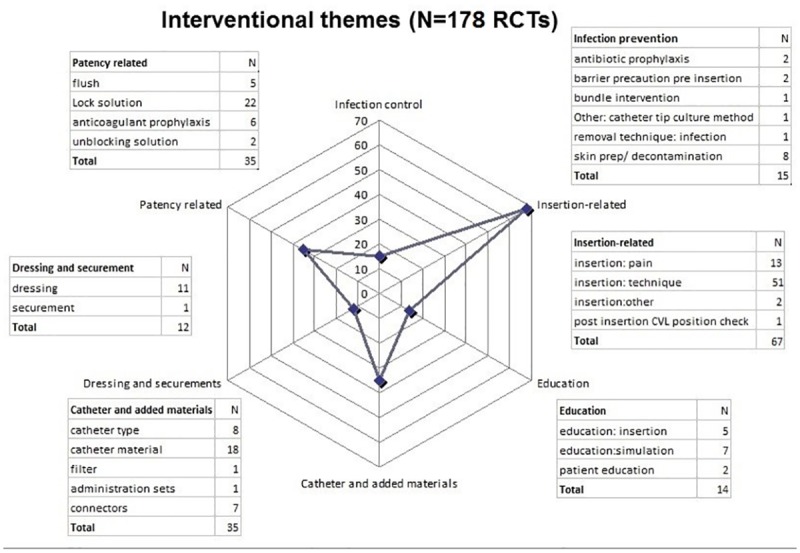
Among all CVAD types, insertion technique was the most common topic (51 RCTs, 29% of all studies), followed by lock solutions (22 RCTs, 12%) (See Fig 2, S2 and S3 Tables for detailed per CVAD type table). Only five studies examined flushing technique, in contrast to more common lock solution studies (22 RCTs). There were only one or two studies on each of the following topics: bundle interventions, securement, in-line filters, administration sets, unblocking solutions, and patient education.
Fig 2. Interventional themes of included RCTs (N = 178 RCTs).
There were 433 individual outcomes in this review, which were categorized into seven major themes (See Table 2). The most prevalent outcomes studied were: infective (n = 161, 37%), followed by catheter complications (n = 99, 23%), and catheter insertion outcomes (n = 101, 23%). These major categories were further subdivided into sub-categories (See S4 Table to see the variety of definitions of outcomes across the studies).
Table 2. Study outcomes (N = 433) of included RCTs (Detailed table per CVAD type in S4 Table).
| Reported Study Outcomes | Totals | |
|---|---|---|
| Catheter complications, N (%) | 99 (22.9%) | |
| Thrombosis | 29 (6.7%) | |
| Occlusion | 23 (5.3%) | |
| Mechanical failure a | 15 (3.4%) | |
| Early removal | 14 (3.3%) | |
| Dwell time | 12 (2.8%) | |
| Complication rate (not specified) | 5 (1.2%) | |
| Local edema/ inflammation | 1 (0.2%) | |
| Patient outcomes, N (%) | 29 (6.6%) | |
| Pain scores | 14 (3.2%) | |
| Patient satisfaction/ Quality of life | 6 (1.4%) | |
| Vital signs | 3 (0.7%) | |
| Mortality rate | 3 (0.7%) | |
| Psychological distress | 1 (0.2%) | |
| Patient comprehension | 1 (0.2%) | |
| Self-management ability | 1 (0.2%) | |
| Catheter insertion outcomes, N (%) | 101 (23.3%) | |
| Successful placement measures | 47 (10.9%) | |
| Insertion-related complications | 39 (9.0%) | |
| Insertion success: performance scores | 13 (3.0%) | |
| Use of ultrasound | 1 (0.2%) | |
| Requirement for repositioning | 1 (0.2%) | |
| Infective outcomes, N (%) | 161 (37.2%) | |
| Catheter-related blood stream infection | 47 (10.9%) | |
| Catheter-related infection | 29 (6.7%) | |
| CVAD tip colonization | 29 (6.7%) | |
| Contamination/ colonization of non-catheter materials including skin and hub | 20 (4.6%) | |
| Systemic infection/sepsis/ fever | 18 (4.2%) | |
| Local infection/ exit-site infection/phlebitis | 17 (3.9%) | |
| Microbial biofilm | 1 (0.2%) | |
| Patency-related outcomes, N (%) | 10 (2.3) | |
| Patency | 5 (1.2%) | |
| Thrombolytic/ fibrinolysis injection | 4 (0.9%) | |
| Anticoagulant treatment | 1 (0.2%) | |
| Intervention-related outcomes, N (%) | 21 (4.8%) | |
| Side effects/ tolerability | 16 (3.7%) | |
| Bleeding | 4 (0.9%) | |
| Skin necrosis | 1 (0.2%) | |
| Health service-related outcomes, N (%) | 12 (2.8%) | |
| Health economics/cost | 12 (2.8%) | |
| TOTAL | 433 (100%) | |
a Mechanical failures include migration, catheter defects, malfunction, infiltration, skin fixation failure, dislocation, fracture and other.
Discussion
This scoping review is the first to systematically identify the gaps in the recent RCT evidence for CVAD use. Many studies included in the review (67 RCTs, 38%) focused on effective CVAD insertion practices, however, comparatively few evaluated maintenance strategies, such as infection prevention (15 RCTs, 8%) and dressing and securement (12 RCTs, 7%). Important areas such as securement, patient education, and bundled interventions lack RCT data.
The findings of this review accord with a recent scoping review of RCTs in peripheral vascular access catheters, which found catheter insertion strategies were extensively studied, but there was a lack of robust evidence to support post-insertion care and maintenance, including dressings and securement, flushing practices, and infection prevention strategies [203]. CVAD insertion complications occur in 0.4–4.5% of procedures, in comparison to post-insertion complications, which can occur in up to 25% of the device life [5, 204–206]. CVADs are designed for prolonged use compared to peripheral intravenous catheters, and more evidence for post-insertion care is needed to avoid unnecessary complications and the need for catheter replacement.
It is important that the same RCT questions and outcome variables are replicated in several RCTs in different clinical settings before a precise and reliable assessment of effect can be determined, preferably in a systematic review and meta-analysis. Our review highlighted that this depth of RCT testing is not yet present in the literature for all areas of CVAD management, with many single RCTs focusing on an intervention in one study only [25, 47, 58, 67, 70, 72, 91, 107, 108, 122, 156, 163, 168, 177, 181, 185, 187, 194, 195, 199]. The potential barriers to undertaking and publishing RCTs in vascular access are likely to be due to a lack of research knowledge, skills, and funding. However, many such issues could be resolved if vascular access teams and other health professionals affiliate with local academics and incorporate research and publishing within their service roles [207].
In addition, the scoping review revealed the variety of outcome definitions used, particularly for infection outcomes. For example, CRBSI as per the Centers for Disease Control and Prevention (CDC) definition; CRBSI defined by other references [208–214], catheter-related infection defined by the authors, catheter-related infection not specified, and catheter-related sepsis. Half of the CRBSI outcomes included within the scoping review were defined by references other than the CDC, with some references dating from the 1990s despite the included RCTs being published from 2006 onwards. Furthermore, many catheter-related infections were not defined. Such heterogeneous reporting of infective outcomes makes the comparison of studies problematic and, with the weak effect, cannot be extrapolated to clinical practice.
CVAD-associated thrombosis outcomes were also diagnosed differently across studies. In the review thrombosis was categorized into three categories: 1. Diagnosed and screened by instruments, such as venography, ultrasound, MRI or CT scans; 2. Diagnosed clinically and then scanned by instruments; and 3. Diagnosed clinically only. Around 33% of the thrombosis outcome studies did not screen patients with instruments. This could potentially lessen the effect when compared to the studies that screened for thrombosis for all patients. With variable definitions, it is difficult to relate the evidence to clinically important outcomes. There is a need for consensus on definition for catheter complications including mechanical and infective outcomes in order to have meaningful results that are comparable across studies.
Pediatrics and neonates have been understudied across all CVAD types. There were only 18 RCTs in pediatrics, 20 RCTs in neonates, and 3 in combined adults and pediatrics, compared to 119 adult RCTs. Previous systematic reviews have concluded that more evidence from this population group is required to reach significant results in meta-analysis [215, 216]. Neonatal and pediatric populations have different underlying anatomy and physiology and indications for CVAD insertion and use. It is not recommended to extrapolate results from adult RCTs to neonatal and pediatric populations, where physiological and technical reasons may modify the effect [217]. Therefore, pediatric and neonatal clinicians have inadequate evidence on which to base their vascular access practice, which could contribute to poorer outcomes for children, families and the healthcare system. More RCTs to provide evidence to support CVAD insertion and management decisions are urgently needed in this vulnerable population [217].
This scoping review has several limitations. Primarily, it did not examine the risk of bias as assessed in systematic reviews. However, the aim was to capture the current CVAD themes being studied, which this review successfully captured. Secondly, some references were analyzed from abstracts without full text available. This could have potentially misclassified some variables due to a lack of complete information. Thirdly, research abstracts published in a language other than English were excluded due to cost and time involved in translating material, so these results reflect only the English literature. However, this is the first scoping review published on RCTs on CVADs, and it has made a unique and significant contribution to the body of knowledge in this area. It provides a platform for prioritizing rigorous research in areas such as CVAD maintenance to provide high level evidence to inform guidelines and practice. Additionally, it has given future recommendations on methods and reporting of the randomization process and unification of outcome definitions for RCT findings to be significant and useful for systematic reviews.
Conclusion
This scoping review has identified RCTs in a range of CVAD types to detect the gaps in evidence and highlight areas needing further research. Many RCTs focus on insertion-related themes, but there is a scarcity of RCTs in post-insertion care. There is also a need for consensus on outcome definitions to avoid heterogeneous outcomes, such as CRBSI, catheter-related infections, and thrombosis. Almost a quarter of RCTs did not state the randomization method, which may degrade the quality of the study. Researchers should report their results in accordance with the relevant CONSORT reporting guidelines including randomization method to ensure reliability [218].
More RCTs in post-insertion care are necessary, and these can be encouraged by having more interdisciplinary collaboration for CVAD research, including doctors, nurses, allied health professionals and patients. There are over 5 million CVADs inserted every year in the United States [219]. A recent systematic review of CVAD complications in pediatrics found that 25% of CVADs failed before completion of therapy [5]. The estimated proportion of catheter failure from all CVAD complications for adults is still unknown. In 2009, an estimated 18,000 central line-associated bloodstream infections occurred in ICU patients in the United States, with a single episode costing up to US$22,939 [220, 221]. These potentially preventable injuries cause direct harm to patients and place an enormous financial strain on healthcare institutions. This study has identified that more high quality evidence is necessary to inform CVAD maintenance practices to avoid such complications to reduce unnecessary CVAD replacements and associated cost.
Supporting information
(DOCX)
(DOCX)
(DOCX)
*One catheter material study had also a theme of heparin flush. One removal technique study included insertion technique, but this is only included in one category. RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; NTCVAD: Non-tunneled Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device; CVAD NS: Central Venous Access Device Not Specified; PICU: Pediatric Intensive Care Unit; NICU: Neonatal Intensive Care Unit.
(DOCX)
RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; NTCVAD: Non-tunneled Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device; CVAD NS: Central Venous Access Device Not Specified; PICU: Pediatric Intensive Care Unit; NICU: Neonatal Intensive Care Unit; CDC: Centers of Disease Control; MRI: Magnetic Resonance Imaging; USS: Ultra sound sonography.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Loveday H, Wilson J, Pratt R, Golsorkhi M, Tingle A, Bak A, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:S1–S70. 10.1016/S0195-6701(13)60012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor JE, McDonald SJ, Tan K. Prevention of central venous catheter-related infection in the neonatal unit: a literature review. J Matern Fetal Neonatal Med. 2015;28(10):1224–30. 10.3109/14767058.2014.949663 [DOI] [PubMed] [Google Scholar]
- 3.Davis MH. Pediatric central venous catheter management: A review of current practice. JAVA. 2013;18(2):93–8. [Google Scholar]
- 4.McMullan C, Propper G, Schuhmacher C, Sokoloff L, Harris D, Murphy P, & Greene WH. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61–9. [DOI] [PubMed] [Google Scholar]
- 5.Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5). [DOI] [PubMed] [Google Scholar]
- 6.Eisen LA, Narasimhan M, Berger JS, Mayo PH, Rosen MJ, Schneider RF. Mechanical complications of central venous catheters. Journal of Intensive Care Medicine. 2006;21:40+. 10.1177/0885066605280884 [DOI] [PubMed] [Google Scholar]
- 7.Webster CS, Merry AF, Emmens DJ, van Cotthem IC, Holland RL. A prospective clinical audit of central venous catheter use and complications in 1000 consecutive patients. Anaesth Intensive Care. 2003;31(1):80–6. [DOI] [PubMed] [Google Scholar]
- 8.Wilson TJ, Stetler WR, Fletcher JJ. Comparison of catheter-related large vein thrombosis in centrally inserted versus peripherally inserted central venous lines in the neurological intensive care unit. Clin Neurol Neurosurg. 2013;115(7):879–82. 10.1016/j.clineuro.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 9.Scott WL. Complications associated with central venous catheters. a survey. Chest. 1988;94(6):1221–4. [DOI] [PubMed] [Google Scholar]
- 10.Bozzetti F, Mariani L, Bertinet DB, Chiavenna G, Crose N, De Cicco M, et al. Central venous catheter complications in 447 patients on home parenteral nutrition: an analysis of over 100.000 catheter days. Clin Nutr. 2002;21(6):475–85. [DOI] [PubMed] [Google Scholar]
- 11.Schwebel C, Lucet JC, Vesin A, Arrault X, Calvino-Gunther S, Bouadma L, et al. Economic evaluation of chlorhexidine-impregnated sponges for preventing catheter-related infections in critically ill adults in the dressing study. Crit Care Med. 2012;40(1):11–7. 10.1097/CCM.0b013e31822f0604 [DOI] [PubMed] [Google Scholar]
- 12.Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005;90(8):840–4. 10.1136/adc.2004.058222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg AX, Hackam D, Tonelli M. Systematic review and meta-analysis: when one study is just not enough. Clin J Am Soc Nephrol. 2008;3(1):253–60. 10.2215/CJN.01430307 [DOI] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 15.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong R, Hall BJ, Doyle J, Waters E. ‘Scoping the scope’ of a cochrane review. Journal of Public Health. 2011;33(1):147–50. 10.1093/pubmed/fdr015 [DOI] [PubMed] [Google Scholar]
- 17.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 18.Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Medicine. 2015;13:224 10.1186/s12916-015-0465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galipeau J, Barbour V, Baskin P, Bell-Syer S, Cobey K, Cumpston M, et al. A scoping review of competencies for scientific editors of biomedical journals. BMC Medicine. 2016;14:16 10.1186/s12916-016-0561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunn F, Burn A-M, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Medicine. 2014;12:192 10.1186/s12916-014-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Altman DG, Stern JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. 2011. In: Cochrane Handbook for Systematic Reviews of Interventions [Internet]. The Cochrane Collaboration.
- 22.Daudt HM, Van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England). 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 24.Bracho-Blanchet E, Cortes-Sauza J, Davila-Perez R, Lezama-Del Valle P, Villalobos-Alfaro C, Nieto-Zermeno J. Usefulness of intravenous heparin to prevent thrombosis of central venous catheter in children. Cir Cir. 2010;78(5):423–9. [PubMed] [Google Scholar]
- 25.Boersma RS, Jie KS, Voogd AC, Hamulyak K, Verbon A, Schouten HC. Concentrated citrate locking in order to reduce the long-term complications of central venous catheters: a randomized controlled trial in patients with hematological malignancies. Support Care Cancer 2015;23(1):37–45. 10.1007/s00520-014-2320-2 [DOI] [PubMed] [Google Scholar]
- 26.Ball RD, Scouras NE, Orebaugh S, Wilde J, Sakai T. Randomized, prospective, observational simulation study comparing residents' needle-guided vs free-hand ultrasound techniques for central venous catheter access. Br J Anaesth. 2012;108(1):72–9. 10.1093/bja/aer329 [DOI] [PubMed] [Google Scholar]
- 27.Aziz N, Khan A, Iqbal J. Subclavian vein catheterization: Supraclavicular versus infraclavicular approach. Journal of Medical Sciences (Peshawar). 2013;21(4):187–9. [Google Scholar]
- 28.Atahan K, Cokmez A, Bekoglu M, Durak E, Tavusbay C, Tarcan E. The effect of antiseptic solution in central venous catheter care. Bratisl Lek Listy. 2012;113(9):548–51. [DOI] [PubMed] [Google Scholar]
- 29.Arvaniti K, Lathyris D, Clouva-Molyvdas P, Haidich AB, Mouloudi E, Synnefaki E, et al. Comparison of Oligon catheters and chlorhexidine-impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: a multicenter, randomized, controlled study. Crit Care Med 2012;40(2):420–9 p. 10.1097/CCM.0b013e31822f0d4b [DOI] [PubMed] [Google Scholar]
- 30.Apiliogullari B, Kara I, Apiliogullari S, Arun O, Saltali A, Celik JB. Is a neutral head position as effective as head rotation during landmark-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. J Cardiothorac Vasc Anesth. 2012;26(6):985–8. 10.1053/j.jvca.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 31.Aouad MT, Kanazi GE, Abdallah FW, Moukaddem FH, Turbay MJ, Obeid MY, et al. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg. 2010;111(3):724–8. 10.1213/ANE.0b013e3181e9c475 [DOI] [PubMed] [Google Scholar]
- 32.Antonelli M, Pascale G, Ranieri VM, Pelaia P, Tufano R, Piazza O, et al. Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs conventional catheters in intensive care unit patients. J Hosp Infect. 2012;82(2):101–7. 10.1016/j.jhin.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 33.Anton N, Cox PN, Massicotte MP, Chait P, Yasui Y, Dinyari PM, et al. Heparin-bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: a blinded randomized, controlled trial. Pediatrics. 2009;123(3):453–8 [DOI] [PubMed] [Google Scholar]
- 34.Alic Y, Torgay A, Pirat A. Ultrasound-guided catheterization of the subclavian vein: a prospective comparison with the landmark technique in ICU patients. Crit Care. 2009;13(Suppl 1):1-. [Google Scholar]
- 35.Alba GA, Kelmenson DA, Noble VE, Murray AF, Currier PF. Faculty staff-guided versus self-guided ultrasound training for internal medicine residents. Medical Education. 2013;47(11):1099–108 p. 10.1111/medu.12259 [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Singh D, Singh A. Ultrasonography: A novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213–6. 10.4103/0972-5229.60174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abualenain J, Calabrese K, Tansek R, Ranniger C. Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4 suppl. 1):S113. [Google Scholar]
- 38.Abdelkefi A, Achour W, Ben Othman T, Ladeb S, Torjman L, Lakhal A, et al. Use of heparin-coated central venous lines to prevent catheter-related bloodstream infection. J Support Oncol. 2007;5(6):273–8. [PubMed] [Google Scholar]
- 39.Gebhard RE, Szmuk P, Pivalizza EG, Melnikov V, Vogt C, Warters RD. The accuracy of electrocardiogram-controlled central line placement. Anesth Analg. 2007;104(1):65–70. 10.1213/01.ane.0000250224.02440.fe [DOI] [PubMed] [Google Scholar]
- 40.Fuentes Pumarola IC, Casademont Mercader R, Colomer Plana M, Cordon Bueno C, Sabench Casellas S, Felez Vidal M, et al. Comparative study of maintenance of patency of triple lumen central venous catheter. Enferm Intensiva. 2007;18(1):25–35. [DOI] [PubMed] [Google Scholar]
- 41.Fragou M, Gravvanis A, Dimitriou V, Papalois A, Kouraklis G, Karabinis A, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med 2011;39(7):1607–12 p. 10.1097/CCM.0b013e318218a1ae [DOI] [PubMed] [Google Scholar]
- 42.Fraenkel D, Rickard C, Thomas P, Faoagali J, George N, Ware R. A prospective, randomized trial of rifampicin-minocycline-coated and silver-platinum-carbon-impregnated central venous catheters. Crit Care Med. 2006;34(3):668–75. 10.1097/01.CCM.0000201404.05523.34 [DOI] [PubMed] [Google Scholar]
- 43.Fenik Y, Celebi N, Wagner R, Nikendei C, Lund F, Zipfel S, et al. Prepackaged central line kits reduce procedural mistakes during central line insertion: a randomized controlled prospective trial. BMC Med Educ. 2013;13:60 10.1186/1472-6920-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ezri T, Weisenberg M, Sessler DI, Berkenstadt H, Elias S, Szmuk P, et al. Correct depth of insertion of right internal jugular central venous catheters based on external landmarks: avoiding the right atrium. J Cardiothorac Vasc Anesth. 2007;21(4):497–501. 10.1053/j.jvca.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 45.Evans LV, Dodge KL, Shah TD, Kaplan LJ, Siegel MD, Moore CL, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462–9. 10.1097/ACM.0b013e3181eac9a3 [DOI] [PubMed] [Google Scholar]
- 46.Esteve F, Pujol M, Limón E, Saballs M, Argerich MJ, Verdaguer R, et al. Bloodstream infection related to catheter connections: a prospective trial of two connection systems. J Hosp Infect. 2007;67(1):30–4. 10.1016/j.jhin.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 47.Dettenkofer M, Wilson C, Gratwohl A, Schmoor C, Bertz H, Frei R, et al. Skin disinfection with octenidine dihydrochloride for central venous catheter site care: a double-blind, randomized, controlled trial. Clin Microbiol Infect. 2010;16(6):600–6. 10.1111/j.1469-0691.2009.02917.x [DOI] [PubMed] [Google Scholar]
- 48.Casey AL, Burnell S, Whinn H, Worthington T, Faroqui MH, Elliott TS. A prospective clinical trial to evaluate the microbial barrier of a needleless connector. J Hosp Infect. 2007;65(3):212–8. 10.1016/j.jhin.2006.09.029 [DOI] [PubMed] [Google Scholar]
- 49.Campos F, Jose Pereira A, Correa T, Biasi Cavalcanti A, Hora Passos R, Beller Ferri M, et al. Central venous catheterization: A randomized comparison between external and internal jugular access. Critical Care Conference: 29th International Symposium on Intensive Care and Emergency Medicine Brussels Belgium Conference Start: 20090324 Conference End: 20090327 Conference Publication: (varpagings). 2009;13:S79-s80.
- 50.Camargo LF, Marra AR, Buchele GL, Sogayar AM, Cal RG, de Sousa JM, et al. Double-lumen central venous catheters impregnated with chlorhexidine and silver sulfadiazine to prevent catheter colonisation in the intensive care unit setting: a prospective randomised study. J Hosp Infect. 2009;72(3):227–33. 10.1016/j.jhin.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 51.Byon HJ, Lee GW, Lee JH, Park YH, Kim HS, Kim CS, et al. Comparison between ultrasound-guided supraclavicular and infraclavicular approaches for subclavian venous catheterization in children—a randomized trial. Br J Anaesth. 2013;111(5):788–92. 10.1093/bja/aet202 [DOI] [PubMed] [Google Scholar]
- 52.Britt RC, Novosel T, Britt L, Sullivan M. The impact of central line simulation before the ICU experience. Am J Surg 2009;197(4):533–6. 10.1016/j.amjsurg.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 53.Lee YH, Kim TK, Jung YS, Cho YJ, Yoon S, Seo JH, et al. Comparison of needle insertion and guidewire placement techniques during internal jugular vein catheterization: The thin-wall introducer needle technique versus the cannula-over-needle technique. Crit Care Med 2015;43(10):2112–6. 10.1097/CCM.0000000000001167 [DOI] [PubMed] [Google Scholar]
- 54.Lee JB, Lee YM. Pre-measured length using landmarks on posteroanterior chest radiographs for placement of the tip of a central venous catheter in the superior vena cava. J Int Med Res. 2010;38(1):134–41. 10.1177/147323001003800115 [DOI] [PubMed] [Google Scholar]
- 55.Latif RK, Bautista AF, Memon SB, Smith EA, Wang C, Wadhwa A, et al. Teaching aseptic technique for central venous access under ultrasound guidance: a randomized trial comparing didactic training alone to didactic plus simulation-based training. Anesth Analg. 2012;114(3):626–33. 10.1213/ANE.0b013e3182405eb3 [DOI] [PubMed] [Google Scholar]
- 56.Lamkinsi T, Kettani A, Belkhadir Z, Tadili J, Benjelloun MY, Mosadik A, et al. Internal jugular venous cannulation: what is the best approach? Ann Fr Anesth Reanim. 2012;31(6):512–6. [DOI] [PubMed] [Google Scholar]
- 57.Laiq N, Majid A, Nawab J, Malik A. Central venous catheterization and cardiac surgeries. Journal of Medical Sciences (Peshawar). 2015;23(3):137–40. [Google Scholar]
- 58.Kwakman PH, Muller MC, Binnekade JM, van den Akker JP, de Borgie CA, Schultz MJ, et al. Medical-grade honey does not reduce skin colonization at central venous catheter-insertion sites of critically ill patients: a randomized controlled trial. Crit Care. 2012;16(5):R214 10.1186/cc11849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krikava I, Kolar M, Garajova B, Balik T, Sevcikova A, Pachl J, et al. Polyhexanide anti-infective coating of central venous catheters in prevention of catheter colonization and bloodstream infection: Study HC-G-H-0507. Crit Care. 2011;15:S80–s1. [Google Scholar]
- 60.Kocum A, Sener M, Calıskan E, Bozdogan N, Atalay H, Aribogan A. An alternative central venous route for cardiac surgery: supraclavicular subclavian vein catheterization. J Cardiothorac Vasc Anesth. 2011;25(6):1018–23. 10.1053/j.jvca.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 61.Khouli H, Jahnes K, Shapiro J, Rose K, Mathew J, Gohil A, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80–7 p. 10.1378/chest.10-0979 [DOI] [PubMed] [Google Scholar]
- 62.Kaye AD, Fox CJ, Hymel BJ, Gayle JA, Hawney HA, Bawcom BA, et al. The importance of training for ultrasound guidance in central vein catheterization. Middle East J Anaesthesiol. 2011;21(1):61–6 [PubMed] [Google Scholar]
- 63.Karakitsos D, Saranteas T, Patrianakos AP, Labropoulos N, Karabinis A. Ultrasound-guided "low approach" femoral vein catheterization in critical care patients results in high incidence of deep vein thrombosis. Anesthesiology. 2007;107(1):181–2. [DOI] [PubMed] [Google Scholar]
- 64.Karakitsos D, Labropoulos N, Groot E, Patrianakos AP, Kouraklis G, Poularas J, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162 10.1186/cc5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang M, Ryu HG, Son IS, Bahk JH. Influence of shoulder position on central venous catheter tip location during infraclavicular subclavian approach. Br J Anaesth. 2011;106(3):344–7 p. 10.1093/bja/aeq340 [DOI] [PubMed] [Google Scholar]
- 66.Kalfon P, de Vaumas C, Samba D, Boulet E, Lefrant JY, Eyraud D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032–9. 10.1097/01.CCM.0000259378.53166.1B [DOI] [PubMed] [Google Scholar]
- 67.Izquierdo Fuentes MT, Justel Garcia R, Moral Quintana C, Garcia Pastor E, Mora Muniz V, Martinez Estalella G. Sterile film as a barrier method in central venous catheter placement. Enferm Intensiva. 2008;19(1):35–41. [DOI] [PubMed] [Google Scholar]
- 68.Islam MT, Rahman Z, Rahman MS. Comparative study of stress response to central venous cannulation under local anesthesia and general anesthesia in patients undergoing open heart surgery. Mymensingh Med J. 2009;18(1): S82–92. [PubMed] [Google Scholar]
- 69.Ishizuka M, Nagata H, Takagi K, Kubota K. Dressing change reduces the central venous catheter-related bloodstream infection. Hepato-gastroenterology. 2011;58(112):1882–6. [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa Y, Kiyama T, Haga Y, Ishikawa M, Takeuchi H, Kimura O, et al. Maximal sterile barrier precautions do not reduce catheter-related bloodstream infections in general surgery units: a multi-institutional randomized controlled trial. Ann Surg 2010;251(4):620–3. 10.1097/SLA.0b013e3181d48a6a [DOI] [PubMed] [Google Scholar]
- 71.Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752–8. 10.1097/EJA.0b013e32832a3a84 [DOI] [PubMed] [Google Scholar]
- 72.Pontes-Arruda A, Dos Santos MC, Martins LF, González ER, Kliger RG, Maia M, et al. Influence of parenteral nutrition delivery system on the development of bloodstream infections in critically ill patients: an international, multicenter, prospective, open-label, controlled study—EPICOS study. JPEN J Parenter Enteral Nutr. 2012;36(5):574–86. 10.1177/0148607111427040 [DOI] [PubMed] [Google Scholar]
- 73.Poletti N. Central venous catheter in the short term and local infections: surgical anchor versus 2-octylcyano acrylate. SCENARIO: Official Italian Journal of ANIARTI. 2012;29(2):19–26 8p. [Google Scholar]
- 74.Pedrolo E, Santos MC, de Oliveira GLR, Mingorance P, Reichembach Danski MT, Boostel R. Chlorhexidine-impregnated dressing for central venous catheter: pilot clinical trial. Revista Enfermagem UERJ. 2014;22(6):760–4 p. [Google Scholar]
- 75.Pedrolo E, Danski MTR, De Lazzari PMLSM, Johann DA. Clinical controlled trial on central venous catheter dressings. Acta Paulista de Enfermagem. 2011;24(2):278–83 p. [Google Scholar]
- 76.Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Radiol Imaging. 2009;19(3):191 10.4103/0971-3026.54877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ovezov A, Zakirov I, Vishnyakova M, editors. Effectiveness and safety of the internal jugular vein catheterization in pediatrics: Ultrasound navigation vs anatomical landmarks (A prospective, randomized, double-blind study) Intensive Care Med; 2010: SPRINGER; 233 SPRING ST, NEW YORK, NY 10013 USA. [Google Scholar]
- 78.Osma S, Kahveci ŞF, Kaya FN, Akalın H, Özakın C, Yılmaz E, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156–62. 10.1016/j.jhin.2005.06.030 [DOI] [PubMed] [Google Scholar]
- 79.Oh AY, Jeon YT, Choi EJ, Ryu JH, Hwang JW, Park HP, et al. The influence of the direction of J-tip on the placement of a subclavian catheter: Real time ultrasound-guided cannulation versus landmark method, a randomized controlled trial. BMC Anesthesiol. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muller MC, Arbous MS, Spoelstra-de Man AM, Vink R, Karakus A, Straat M, et al. Transfusion of fresh-frozen plasma in critically ill patients with a coagulopathy before invasive procedures: a randomized clinical trial (CME). Transfusion. 2015;55(1):26–35; quiz 25. 10.1111/trf.12750 [DOI] [PubMed] [Google Scholar]
- 81.Mitre CI, Golea A, Acalovschi I, Mocan T, Caea AM, Ru C, et al. Ultrasound-guided external jugular vein cannulation for central venous access by inexperienced trainees. Eur J Anaesthesiol. 2010;27(3):300–3. 10.1097/EJA.0b013e328333c2d6 [DOI] [PubMed] [Google Scholar]
- 82.Mimoz O, Villeminey S, Ragot S, Dahyot-Fizelier C, Laksiri L, Petitpas F, et al. Chlorhexidine-based antiseptic solution vs alcohol-based povidone-iodine for central venous catheter care. Arch Intern Med. 2007;167(19):2066–72. 10.1001/archinte.167.19.2066 [DOI] [PubMed] [Google Scholar]
- 83.Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386(10008):2069–77. 10.1016/S0140-6736(15)00244-5 [DOI] [PubMed] [Google Scholar]
- 84.Milling T, Holden C, Melniker L, Briggs WM, Birkhahn R, Gaeta T. Randomized controlled trial of single-operator vs. two-operator ultrasound guidance for internal jugular central venous cannulation. Academic Emergency Medicine. 2006;13(3):245–7 p. 10.1197/j.aem.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 85.Mer M, Duse AG, Galpin JS, Richards GA. Central venous catheterization: A prospective, randomized, double-blind study. Clin Appl Thromb Hemost. 2008. [DOI] [PubMed] [Google Scholar]
- 86.Matzie KA, Philbrook L, Mitani A, Lipsitz S, Gerhard-Herman M, Pozner C, et al. Comparison of web-based and classroom-based training programs for point-of care, real-time ultrasound-guided central venous catheter placement. Am J Respir Crit Care Med. 2010;181(1 MeetingAbstracts). [Google Scholar]
- 87.Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242–9. 10.1111/anae.13187 [DOI] [PubMed] [Google Scholar]
- 88.Lim T, Ryu HG, Jung CW, Jeon Y, Bahk JH. Effect of the bevel direction of puncture needle on success rate and complications during internal jugular vein catheterization*. Critical Care Medicine. 2012;40(2):491–4 p. 10.1097/CCM.0b013e318232da48 [DOI] [PubMed] [Google Scholar]
- 89.Lee J-H, Bahk J-H, Ryu H-G, Jung C-W, Jeon Y. Comparison of the bedside central venous catheter placement techniques: landmark vs electrocardiogram guidance†. Br J Anaesth. 2009:aep046. [DOI] [PubMed] [Google Scholar]
- 90.Xiao Y, Seagull FJ, Bochicchio GV, Guzzo JL, Dutton RP, Sisley A, et al. Video-based training increases sterile-technique compliance during central venous catheter insertion. Crit Care Med. 2007;35(5):1302–6. 10.1097/01.CCM.0000263457.81998.27 [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Cui Z, Wang J, Hu Z, Kang H, Ji J, et al. A prospective randomized controlled trial on effect of norvancomycin tube sealing for prevention of central venous catheter-related infection in critical patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(7):468–72. 10.3760/cma.j.issn.2095-4352.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 92.Walz JM, Avelar RL, Longtine KJ, Carter KL, Mermel LA, Heard SO. Anti-infective external coating of central venous catheters: a randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Crit Care Med 2010;38(11):2095–102. 10.1097/CCM.0b013e3181f265ba [DOI] [PubMed] [Google Scholar]
- 93.Vokurka S, Bystricka E, Visokaiova M, Scudlova J. Once- versus twice-weekly changing of central venous catheter occlusive dressing in intensive chemotherapy patients: results of a randomized multicenter study. Med Sci Monit. 2009;15(3):Cr107–10. [PubMed] [Google Scholar]
- 94.Valles J, Fernandez I, Alcaraz D, Chacon E, Cazorla A, Canals M, et al. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infect Control Hosp Epidemiol 2008;29(09):847–53. [DOI] [PubMed] [Google Scholar]
- 95.Timsit J, Schwebel C, Bouadma L, Geoffroy A, Garrouste-Orgeas M, Pease S, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: A randomized controlled trial. JAMA. 2009;301(12):1231–41. 10.1001/jama.2009.376 [DOI] [PubMed] [Google Scholar]
- 96.Smith CC, Huang GC, Newman LR, Clardy PF, Feller-Kopman D, Cho M, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc. 2010;5(3):146–51. 10.1097/SIH.0b013e3181dd9672 [DOI] [PubMed] [Google Scholar]
- 97.Schallom ME, Prentice D, Sona C, Micek ST, Skrupky LP. Heparin or 0.9% sodium chloride to maintain central venous catheter patency: a randomized trial. Critical care medicine. 2012;40(6):1820–6. 10.1097/CCM.0b013e31824e11b4 [DOI] [PubMed] [Google Scholar]
- 98.Samantaray A, Rao MH. Effects of fentanyl on procedural pain and discomfort associated with central venous catheter insertion: A prospective, randomized, double-blind, placebo controlled trial. Indian J Crit Care Med. 2014;18(7):421–6 p. 10.4103/0972-5229.136069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruschulte H, Franke M, Gastmeier P, Zenz S, Mahr KH, Buchholz S, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Ann Hematol. 2009;88(3):267–72. 10.1007/s00277-008-0568-7 [DOI] [PubMed] [Google Scholar]
- 100.Rando K, Castelli J, Pratt JP, Scavino M, Rey G, Rocca ME, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessel. 2014;6(1):13–23 p. [PMC free article] [PubMed] [Google Scholar]
- 101.Pedrolo E, Reichembach Danski MT, Adami Vayego S. Chlorhexidine and gauze and tape dressings for central venous catheters: a randomized clinical trial. Revista Latino-Americana de Enfermagem (RLAE). 2014;22(5):764–71 p. Language: English. Entry Date: 20150101. Revision Date: 20150712. Publication Type: Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jun L, Yanqun H, Jiexian Y. Time limit research on guide wire retrieve in PICC insertion. Journal of Nursing Science. 2011;26:7–8. [Google Scholar]
- 103.Johnston AJ, Streater CT, Noorani R, Crofts JL, Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the 'ELeCTRiC' study. J Vasc Access. 2012;13(4):421–5. [DOI] [PubMed] [Google Scholar]
- 104.Itkin M, Mondshein JI, Stavropoulos SW, Shlansky-Goldberg RD, Soulen MC, Trerotola SO. Peripherally inserted central catheter thrombosis—Reverse tapered versus nontapered catheters: A randomized controlled study. J Vasc Interv Radiol. 2014;25(1):85–91. 10.1016/j.jvir.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 105.Hosseini MB, Jodeiri B, Mahallei M, Abdoli-Oskooi S, Safari A, Salimi Z. Early outcome of peripherally inserted central catheter versus peripheral IV line in very low birth weight neonates. Feyz Journal of Kashan University of Medical Sciences. 2014;17(6):561–7. [Google Scholar]
- 106.Hockley SJ, Hamilton V, Young RJ, Chapman MJ, Taylor J, Creed S, et al. Efficacy of the CathRite system to guide bedside placement of peripherally inserted central venous catheters in critically ill patients: a pilot study. Crit Care Resusc. 2007;9(3):251–5. [PubMed] [Google Scholar]
- 107.Hill ML, Baldwin L, Slaughter JC, Walsh WF, Weitkamp JH. A silver-alginate-coated dressing to reduce peripherally inserted central catheter (PICC) infections in NICU patients: a pilot randomized controlled trial. J Perinatol 2010;30(7):469–73. 10.1038/jp.2009.190 [DOI] [PubMed] [Google Scholar]
- 108.Hemels MA, van den Hoogen A, Verboon-Maciolek MA, Fleer A, Krediet TG. Prevention of neonatal late-onset sepsis associated with the removal of percutaneously inserted central venous catheters in preterm infants. Pediatr Crit Care Med. 2011;12(4):445–8. 10.1097/PCC.0b013e3182070f5d [DOI] [PubMed] [Google Scholar]
- 109.Graf JM, Newman CD, McPherson ML. Sutured securement of peripherally inserted central catheters yields fewer complications in pediatric patients. JPEN J Parenter Enteral Nutr. 2006;30(6):532–5. 10.1177/0148607106030006532 [DOI] [PubMed] [Google Scholar]
- 110.Glauser F, Breault S, Babaker M, Jouannic AM, Qanadli SD. Bed-side versus fluoroscopically guided insertion of PICCS: Prospective randomized trial. J Vasc Access. 2014; 15(3):221. [Google Scholar]
- 111.Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone-iodine antisepsis for central venous catheter placement in neonates. J Perinatol. 2009;29(12):808–13. 10.1038/jp.2009.161 [DOI] [PubMed] [Google Scholar]
- 112.Feng BL, Tan XH, Tong L, Wang B, Zhou SJ. Comparison of the outcomes of central venous catheters inserted from the left side and right side: A prospective randomized controlled study. Chinese Journal of Clinical Nutrition. 2010;18(2):87–90. [Google Scholar]
- 113.Fan YY, Qin HY, Li J, Xin MZ. Randomized comparison of cancer chemotherapy patient's degree of comfort of two methods of peripherally inserted central venous catheters placement. Chinese Journal of Cancer Prevention and Treatment. 2013;20(21):1679–85. [Google Scholar]
- 114.Carvalho Onofre PS, Gonçalves Pedreira ML, Peterlini MA. Placement of peripherally inserted central catheters in children guided by ultrasound: a prospective randomized, and controlled trial. Pediatr Crit Care Med. 2012;13(5):e282–7. 10.1097/PCC.0b013e318245597c [DOI] [PubMed] [Google Scholar]
- 115.Caparas J, Hu JP, Hung HS. Does a novel method of PICC insertion improve safety? Nursing. 2014;44(5):65–7. 10.1097/01.NURSE.0000444725.83265.1d [DOI] [PubMed] [Google Scholar]
- 116.Broadhurst D. PICC catheter securement: A randomized controlled trial in the home care setting. J Vasc Access. 2014;15(3):218–9. [Google Scholar]
- 117.Bowers L, Speroni KG, Jones L, Atherton M. Comparison of occlusion rates by flushing solutions for peripherally inserted central catheters with positive pressure Luer-activated devices. J Infus Nurs. 2008;31(1):22–7. 10.1097/01.NAN.0000308542.90615.c2 [DOI] [PubMed] [Google Scholar]
- 118.Birch P, Ogden S, Hewson M. A randomised, controlled trial of heparin in total parenteral nutrition to prevent sepsis associated with neonatal long lines: the Heparin in Long Line Total Parenteral Nutrition (HILLTOP) trial. Arch Dis Child Fetal Neonatal Ed. 2010:fetalneonatal167403. [DOI] [PubMed] [Google Scholar]
- 119.Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer 2011;19(4):539–43. 10.1007/s00520-010-0849-2 [DOI] [PubMed] [Google Scholar]
- 120.Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A tale of two PICCs. Can Assoc Radiol J. 2012;63(4):323–8. 10.1016/j.carj.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 121.Xia C, Lu A, Sun J. Role of modified Seldinger technique combined with vascular ultrasonography in the placement of peripherally inserted central catheters in patients with breast cancer undergoing postoperative chemotherapy. Chinese Journal of Clinical Nutrition. 2014;22(3):187–90. [Google Scholar]
- 122.Wang YH. Influence of self-management education on patients with peripherally inserted central catheters. Chinese Journal of Clinical Nutrition. 2011;19(2):124–8. [Google Scholar]
- 123.Uslu S, Ozdemir H, Comert S, Bolat F, Nuhoglu A. The effect of low-dose heparin on maintaining peripherally inserted percutaneous central venous catheters in neonates. J Perinatol 2010;30(12):794–9. 10.1038/jp.2010.46 [DOI] [PubMed] [Google Scholar]
- 124.Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing central line placement. JAMA. 2006;295(7):793–800. 10.1001/jama.295.7.793 [DOI] [PubMed] [Google Scholar]
- 125.Shin SH, Kim HS, Lee J, Choi KY, Lee JH, Kim EK, et al. A comparative study of two remifentanil doses for procedural pain in ventilated preterm infants: a randomized, controlled study. Pediatr Crit Care Med. 2014;15(5):451–5. 10.1097/PCC.0000000000000123 [DOI] [PubMed] [Google Scholar]
- 126.Shah PS, Kalyn A, Satodia P, Dunn MS, Parvez B, Daneman A, et al. A randomized, controlled trial of heparin versus placebo infusion to prolong the usability of peripherally placed percutaneous central venous catheters (PCVCs) in neonates: the HIP (Heparin Infusion for PCVC) study. Pediatrics. 2007;119(1):e284–91. 10.1542/peds.2006-0529 [DOI] [PubMed] [Google Scholar]
- 127.Schweickert WD, Herlitz J, Pohlman AS, Gehlbach BK, Hall JB, Kress JP. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med 2009;37(4):1217–21. 10.1097/CCM.0b013e31819cee7f [DOI] [PubMed] [Google Scholar]
- 128.Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519–23. 10.5301/jva.5000280 [DOI] [PubMed] [Google Scholar]
- 129.Phipps K, Modic A, O'Riordan MA, Walsh M. A randomized trial of the Vein Viewer versus standard technique for placement of peripherally inserted central catheters (PICCs) in neonates. J Perinatol 2012;32(7):498–501. 10.1038/jp.2011.129 [DOI] [PubMed] [Google Scholar]
- 130.Periard D, Monney P, Waeber G, Zurkinden C, Mazzolai L, Hayoz D, et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost. 2008;6(8):1281–8. 10.1111/j.1538-7836.2008.03053.x [DOI] [PubMed] [Google Scholar]
- 131.Panagiotounakou P, Antonogeorgos G, Gounari E, Papadakis S, Labadaridis J, Gounaris AK. Peripherally inserted central venous catheters: frequency of complications in premature newborn depends on the insertion site. J Perinatol 2014;34(6):461–3. 10.1038/jp.2014.36 [DOI] [PubMed] [Google Scholar]
- 132.Ong CK, Venkatesh SK, Lau GB, Wang SC. Prospective randomized comparative evaluation of proximal valve polyurethane and distal valve silicone peripherally inserted central catheters. J Vasc Interv Radiol. 2010;21(8):1191–6. 10.1016/j.jvir.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 133.Miyagaki H, Nakajima K, Hara J, Yamasaki M, Kurokawa Y, Miyata H, et al. Performance comparison of peripherally inserted central venous catheters in gastrointestinal surgery: a randomized controlled trial. Clin Nutr. 2012;31(1):48–52. 10.1016/j.clnu.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 134.Michel F, Vialet R, Hassid S, Nicaise C, Garbi A, Thomachot L, et al. Sevoflurane for central catheter placement in neonatal intensive care: a randomized trial. Paediatr Anaesth. 2010;20(8):712–9. 10.1111/j.1460-9592.2010.03334.x [DOI] [PubMed] [Google Scholar]
- 135.Marcatto JO, Vasconcelos PC, Araujo CM, Tavares EC, Pereira e Silva Y. EMLA versus glucose for PICC insertion: a randomised triple-masked controlled study. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F467–8. Epub 2011/05/31. 10.1136/adc.2011.215152 [DOI] [PubMed] [Google Scholar]
- 136.Lyons MG, Phalen AG. A randomized controlled comparison of flushing protocols in home care patients with peripherally inserted central catheters. J Infus Nurs. 2014;37(4):270–81. 10.1097/NAN.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 137.Li J, Fan YY, Xin MZ, Yan J, Hu W, Huang WH, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103. 10.1016/j.ejon.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 138.Lemyre B, Sherlock R, Hogan D, Gaboury I, Blanchard C, Moher D. How effective is tetracaine 4% gel, before a peripherally inserted central catheter, in reducing procedural pain in infants: a randomized double-blind placebo controlled trial [ISRCTN75884221]. BMC medicine. 2006;4:11 10.1186/1741-7015-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lago P, Tiozzo C, Boccuzzo G, Allegro A, Zacchello F. Remifentanil for percutaneous intravenous central catheter placement in preterm infant: a randomized controlled trial. Paediatr Anaesth. 2008;18(8):736–44. 10.1111/j.1460-9592.2008.02636.x [DOI] [PubMed] [Google Scholar]
- 140.Khalidi N, Kovacevich DS, Papke-O'Donnell LF, Btaiche I. Impact of the positive pressure valve on vascular access device occlusions and bloodstream infections. JAVA 2009;14(2):84–91. [Google Scholar]
- 141.Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol 2013;33(10):791–4. 10.1038/jp.2013.58 [DOI] [PubMed] [Google Scholar]
- 142.Teichgraber UK, Streitparth F, Cho CH, Benter T, Gebauer B. A comparison of clinical outcomes with regular- and low-profile totally implanted central venous port systems. Cardiovasc Intervent Radiol. 2009;32(5):975–9. 10.1007/s00270-008-9477-3 [DOI] [PubMed] [Google Scholar]
- 143.Rosen J, Lawrence R, Bouchard M, Doros G, Gardiner P, Saper R. Massage for perioperative pain and anxiety in placement of vascular access devices. Adv Mind Body Med. 2013;27(1):12–23. [PubMed] [Google Scholar]
- 144.Nocito A, Wildi S, Rufibach K, Clavien PA, Weber M. Randomized clinical trial comparing venous cutdown with the Seldinger technique for placement of implantable venous access ports. Br J Surg. 2009;96(10):1129–34. 10.1002/bjs.6730 [DOI] [PubMed] [Google Scholar]
- 145.Miao J, Ji L, Lu J, Chen J. Randomized clinical trial comparing ultrasound-guided procedure with the Seldinger's technique for placement of implantable venous ports. Cell Biochem Biophys. 2014;70(1):559–63. 10.1007/s12013-014-9956-x [DOI] [PubMed] [Google Scholar]
- 146.Marcy PY, Largillier R, Poissonet G, Dassonville O, Machiavello JC, Poudenx M, et al. Prospective randomized trial comparing distal (arm port) to central (chest port) technique in ambulatory cancer patients. J Vasc Access. 2014;15(3):206–7. [Google Scholar]
- 147.Lavau-Denes S, Lacroix P, Maubon A, Preux PM, Genet D, Vénat-Bouvet L, et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol 2013;72(1):65–73. 10.1007/s00280-013-2169-y [DOI] [PubMed] [Google Scholar]
- 148.Knebel P, Lopez-Benitez R, Fischer L, Radeleff BA, Stampfl U, Bruckner T, et al. Insertion of totally implantable venous access devices: an expertise-based, randomized, controlled trial (NCT00600444). Ann Surg 2011;253(6):1111–7. 10.1097/SLA.0b013e318214ba21 [DOI] [PubMed] [Google Scholar]
- 149.Knebel P, Fischer L, Huesing J, Hennes R, Büchler M, Seiler C. Randomized clinical trial of a modified Seldinger technique for open central venous cannulation for implantable access devices. Br J Surg. 2009;96(2):159–65. 10.1002/bjs.6457 [DOI] [PubMed] [Google Scholar]
- 150.Karanlik H, Kurul S, Saip P, Unal ES, Sen F, Disci R, et al. The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg 2011;202(1):10–5 p. 10.1016/j.amjsurg.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 151.Hitz F, Klingbiel D, Omlin A, Riniker S, Zerz A, Cerny T. Athrombogenic coating of long-term venous catheter for cancer patients: a prospective, randomised, double-blind trial. Ann Hematol 2012;91(4):613–20 p. 10.1007/s00277-011-1343-8 [DOI] [PubMed] [Google Scholar]
- 152.Heden LE, von Essen L, Ljungman G. Effect of morphine in needle procedures in children with cancer. Eur J Pain. 2011;15(10):1056–60. 10.1016/j.ejpain.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 153.Hedén L, Essen L, Ljungman G. Effect of high-dose paracetamol on needle procedures in children with cancer—an RCT. Acta Paediatr 2014;103(3):314–9. 10.1111/apa.12509 [DOI] [PubMed] [Google Scholar]
- 154.Goossens GA, Verbeeck G, Moons P, Sermeus W, Wever I, Stas M. Functional evaluation of conventional 'Celsite' venous ports versus 'Vortex' ports with a tangential outlet: a prospective randomised pilot study. Support Care Cancer 2008;16(12):1367–74. 10.1007/s00520-008-0436-y [DOI] [PubMed] [Google Scholar]
- 155.Goossens GA, Jerome M, Janssens C, Peetermans WE, Fieuws S, Moons P, et al. Comparing normal saline versus diluted heparin to lock non-valved totally implantable venous access devices in cancer patients: a randomised, non-inferiority, open trial. Ann Oncol 2013;24(7):1892–9. 10.1093/annonc/mdt114 [DOI] [PubMed] [Google Scholar]
- 156.Ferreira Chacon JM, Hato de Almeida E, de Lourdes Simoes R, Lazzarin COV, Alves BC, Mello de Andrea ML, et al. Randomized study of minocycline and edetic acid as a locking solution for central line (port-a-cath) in children with cancer. Chemotherapy. 2011;57(4):285–91. 10.1159/000328976 [DOI] [PubMed] [Google Scholar]
- 157.Di Carlo I, Toro A, Pulvirenti E, Palermo F, Scibilia G, Cordio S. Could antibiotic prophylaxis be not necessary to implant totally implantable venous access devices? Randomized prospective study. Surg Oncol. 2011;20(1):20–5. 10.1016/j.suronc.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 158.Biffi R, Pozzi S, Bonomo G, Della Vigna P, Monfardini L, Radice D, et al. Cost effectiveness of different central venous approaches for port placement and use in adult oncology patients: evidence from a randomized three-arm trial. Ann Surg Oncol 2014;21(12):3725–31. 10.1245/s10434-014-3784-5 [DOI] [PubMed] [Google Scholar]
- 159.Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol 2009;20(5):935–40. 10.1093/annonc/mdn701 [DOI] [PubMed] [Google Scholar]
- 160.Biffi R, Orsi F, Pozzi S, Maldifassi A, Radice D, Rotmensz N, et al. No impact of central venous insertion site on oncology patients' quality of life and psychological distress. A randomized three-arm trial. Support Care Cancer 2011;19(10):1573–80 p. 10.1007/s00520-010-0984-9 [DOI] [PubMed] [Google Scholar]
- 161.Vandoni RE, Guerra A, Sanna P, Bogen M, Cavalli F, Gertsch P. Randomised comparison of complications from three different permanent central venous access systems. Swiss Med Wkly. 2009;139(21–22):313–6. [DOI] [PubMed] [Google Scholar]
- 162.Worth LJ, Slavin MA, Heath S, Szer J, Grigg AP. Ethanol versus heparin locks for the prevention of central venous catheter-associated bloodstream infections: a randomized trial in adult haematology patients with Hickman devices. J Hosp Infect 2014;88(1):48–51. 10.1016/j.jhin.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 163.van Rooden CJ, Schippers EF, Guiot HF, Barge RM, Hovens MM, van der Meer FJ, et al. Prevention of coagulase-negative staphylococcal central venous catheter-related infection using urokinase rinses: a randomized double-blind controlled trial in patients with hematologic malignancies. J Clin Oncol. 2008;26(3):428–33. 10.1200/JCO.2007.11.7754 [DOI] [PubMed] [Google Scholar]
- 164.Slobbe L, Doorduijn JK, Lugtenburg PJ, El Barzouhi A, Boersma E, van Leeuwen WB, et al. Prevention of catheter-related bacteremia with a daily ethanol lock in patients with tunnelled catheters: a randomized, placebo-controlled trial. PLoS One. 2010;5(5):e10840 10.1371/journal.pone.0010840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schoot RA, Ommen CH, Stijnen T, Tissing WJ, Michiels E, Abbink FC, et al. Prevention of central venous catheter-associated bloodstream infections in paediatric oncology patients using 70% ethanol locks: A randomised controlled multi-centre trial. European journal of cancer 2015;51(14):2031–8. 10.1016/j.ejca.2015.06.126 [DOI] [PubMed] [Google Scholar]
- 166.Sanders J, Pithie A, Ganly P, Surgenor L, Wilson R, Merriman E, et al. A prospective double-blind randomized trial comparing intraluminal ethanol with heparinized saline for the prevention of catheter-associated bloodstream infection in immunosuppressed haematology patients. J Antimicrob Chemother. 2008;62(4):809–15. 10.1093/jac/dkn284 [DOI] [PubMed] [Google Scholar]
- 167.Klek S, Szczepanek K, Hermanowicz A, Galas A. Taurolidine lock in home parenteral nutrition in adults: results from an open-label randomized controlled clinical trial. JPEN J Parenter Enteral Nutr. 2015;39(3):331–5. 10.1177/0148607114525804 [DOI] [PubMed] [Google Scholar]
- 168.Horne MK, McCloskey DJ, Calis K, Wesley R, Childs R, Kasten-Sportes C. Use of Heparin versus Lepirudin Flushes to Prevent Withdrawal Occlusion of Central Venous Access Devices. Pharmacotherapy. 2006;26(9):1262–7. 10.1592/phco.26.9.1262 [DOI] [PubMed] [Google Scholar]
- 169.Dümichen MJ, Seeger K, Lode HN, Kühl JS, Ebell W, Degenhardt P, et al. Randomized controlled trial of taurolidine citrate versus heparin as catheter lock solution in paediatric patients with haematological malignancies. J Hosp Infect. 2012;80(4):304–9 p. 10.1016/j.jhin.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 170.Douard MC, di Palma M, d'Agostino P, Chevret S, Kriegel I, Falissard B, et al. Prospective, double-blind, randomized trial of equimolar mixture of nitrous oxide/oxygen to prevent pain induced by insertion of venous access ports in cancer patients. Support Care Cancer. 2006;14(2):161–6. 10.1007/s00520-005-0852-1 [DOI] [PubMed] [Google Scholar]
- 171.Decembrino N, Brandolini M, Pagani M, Bottazzi A, Rubert L, Calafiore L, et al. Lock-therapy with ethanol for the salvage of colonized long-term central venous catheter: Experience in oncohematological pediatric patients. Bone Marrow Transplant 2014;49:S467. [Google Scholar]
- 172.Cesaro S, Tridello G, Cavaliere M, Magagna L, Gavin P, Cusinato R, et al. Prospective, randomized trial of two different modalities of flushing central venous catheters in pediatric patients with cancer. J Clin Oncol 2009;27(12):2059–65. 10.1200/JCO.2008.19.4860 [DOI] [PubMed] [Google Scholar]
- 173.Bruzoni M, Slater BJ, Wall J, Peter SD, Dutta S. A prospective randomized trial of ultrasound- vs landmark-guided central venous access in the pediatric population. J Am Coll Surg. 2013;216(5):939–43. 10.1016/j.jamcollsurg.2013.01.054 [DOI] [PubMed] [Google Scholar]
- 174.Yamamoto N, Kimura H, Misao H, Matsumoto H, Imafuku Y, Watanabe A, et al. Efficacy of 1.0% chlorhexidine-gluconate ethanol compared with 10% povidone-iodine for long-term central venous catheter care in hematology departments: a prospective study. American journal of infection control. 2014;42(5):574–6. 10.1016/j.ajic.2013.12.023 [DOI] [PubMed] [Google Scholar]
- 175.Pawlik MT, Lemberger P, Hansen E. Evaluation of CVC-set. Anasthesiol Intensivmed Notfallmed Schmerzther. 2006;41(2):79–85. 10.1055/s-2005-870451 [DOI] [PubMed] [Google Scholar]
- 176.Niers TM, Nisio M, Klerk CP, Baarslag HJ, Büller HR, Biemond BJ. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: a randomized, placebo-controlled study. J Thromb Haemost. 2007;5(9):1878–82. 10.1111/j.1538-7836.2007.02660.x [DOI] [PubMed] [Google Scholar]
- 177.Marsteller JA, Sexton JB, Hsu YJ, Hsiao CJ, Holzmueller CG, Pronovost PJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med 2012;40(11):2933–9 p. 10.1097/CCM.0b013e31825fd4d8 [DOI] [PubMed] [Google Scholar]
- 178.Marmol MT, Martins Braga FTM, Garbin LM, Moreli L, Benedita dos Santos C, Campos de Carvalho E. Central catheter dressing in a simulator: the effects of tutor's assistance or self-learning tutorial. Rev Lat Am Enfermagem. 2012;20(6):1134–41 p. [DOI] [PubMed] [Google Scholar]
- 179.Karpanen TJ, Casey AL, Nightingale P, Cook M, Elliott TS. Do silver-coated needleless intravascular catheter connectors reduce microbial contamination? Clin Microbiol Infect. 2011;17:S326. [Google Scholar]
- 180.Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009;373(9663):567–74. 10.1016/S0140-6736(09)60205-1 [DOI] [PubMed] [Google Scholar]
- 181.van den Hoogen A, Krediet TG, Uiterwaal CS, Bolenius JF, Gerards LJ, Fleer A. In-line filters in central venous catheters in a neonatal intensive care unit. J Perinat Med 2006;34(1):71–4. 10.1515/JPM.2006.009 [DOI] [PubMed] [Google Scholar]
- 182.Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med 2012;186(12):1272–8. 10.1164/rccm.201206-1038OC [DOI] [PubMed] [Google Scholar]
- 183.Schroeder AR, Axelrod DM, Silverman NH, Rubesova E, Merkel E, Roth SJ. A continuous heparin infusion does not prevent catheter-related thrombosis in infants after cardiac surgery. Pediatric Critical Care Medicine. 2010;11(4):489–95. [DOI] [PubMed] [Google Scholar]
- 184.Ruud E. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies—a randomized, controlled study. Acta Paediatr 2006;95(9):1053–9. 10.1080/08035250600729092 [DOI] [PubMed] [Google Scholar]
- 185.Perez-Parra A, Guembe M, Martin-Rabadan P, Munoz P, Fernandez-Cruz A, Bouza E. Prospective, randomised study of selective versus routine culture of vascular catheter tips: Patient outcome, antibiotic use and laboratory workload. J Hosp Infect. 2011;77(4):309–15. 10.1016/j.jhin.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 186.Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 2014;22(1):121–8. 10.1007/s00520-013-1941-1 [DOI] [PubMed] [Google Scholar]
- 187.Moll S, Kenyon P, Bertoli L, De Maio J, Homesley H, Deitcher SR. Phase II trial of alfimeprase, a novel-acting fibrin degradation agent, for occluded central venous access devices. J Clin Oncol 2006;24(19):3056–60. 10.1200/JCO.2006.05.8438 [DOI] [PubMed] [Google Scholar]
- 188.Konstantinou EA, Karampinis DF, Mitsos AP, Konstantinou MI, Mariolis-Sapsakos T, Kapritsou M, et al. Central vascular catheters versus peripherally inserted central catheters in nurse anesthesia. A perspective within the Greek health system. J Vasc Access. 2013;14(4):373–8. 10.5301/jva.5000160 [DOI] [PubMed] [Google Scholar]
- 189.Karthaus M, Kretzschmar A, Kröning H, Biakhov M, Irwin D, Marschner N, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol 2006;17(2):289–96. 10.1093/annonc/mdj059 [DOI] [PubMed] [Google Scholar]
- 190.Johansson E, Engervall P, Bjorvell H, Hast R, Bjorkholm M. Patients' perceptions of having a central venous catheter or a totally implantable subcutaneous port system-results from a randomised study in acute leukaemia. Support Care Cancer. 2009;17(2):137–43. 10.1007/s00520-008-0449-6 [DOI] [PubMed] [Google Scholar]
- 191.Jain K, Pellegrini L, Slavotinek J, Eaton M, McLeay W, Price TJ, et al. A randomised trial comparing two types of chronic indwelling central venous catheter (CVC) devices used for the delivery of chemotherapy to patients with non-haematological malignancy-peripherally inserted central venous catheters (PICC) vs subcutaneous. Asia Pac J Clin Oncol. 2010;6(194). [Google Scholar]
- 192.Handrup MM, Møller JK, Schrøder H. Central venous catheters and catheter locks in children with cancer: a prospective randomized trial of taurolidine versus heparin. Pediatr Blood Cancer. 2013;60(8):1292–8. 10.1002/pbc.24482 [DOI] [PubMed] [Google Scholar]
- 193.Handrup MM, Fuursted K, Funch P, Møller JK, Schrøder H. Biofilm formation in long-term central venous catheters in children with cancer: a randomized controlled open-labelled trial of taurolidine versus heparin. APMIS. 2012;120(10):794–801. 10.1111/j.1600-0463.2012.02910.x [DOI] [PubMed] [Google Scholar]
- 194.Gabrail N, Sandler E, Charu V, Anas N, Lim E, Blaney M. TROPICS 1: a phase III, randomized, double-blind, placebo-controlled study of tenecteplase for restoration of function in dysfunctional central venous catheters. J Vasc Interv Radiol. 2010;21(12):1852–8. 10.1016/j.jvir.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 195.Filippi LL. Fusidic acid and heparin lock solution for the prevention of catheter-related bloodstream infections in critically ill neonates: a retrospective study and a prospective, randomized trial. Pediatr Crit Care Med. 2007;8(6):556–62. 10.1097/01.PCC.0000288711.46009.58 [DOI] [PubMed] [Google Scholar]
- 196.De Cicco M, Matovic M, Balestreri L, Steffan A, Pacenzia R, Malafronte M, et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: a randomized controlled study based on serial venographies. Ann Oncol 2009;20(12):1936–42. 10.1093/annonc/mdp235 [DOI] [PubMed] [Google Scholar]
- 197.Casey AL, Karpanen TJ, Nightingale P, Cook M, Elliott TS. Microbiological comparison of a silver-coated and a non-coated needleless intravascular connector in clinical use. J Hosp Infect. 2012;80(4):299–303. 10.1016/j.jhin.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 198.Burlacu CL, McKeating K, McShane AJ. Remifentanil for the insertion and removal of long-term central venous access during monitored anesthesia care. J Clin Anesth 2011;23(4):286–91 p. 10.1016/j.jclinane.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 199.Bowers N, Eisenberg N, Jaskolka J, Roche-Nagle G. Using a multimedia presentation to improve patient understanding and satisfaction with informed consent for minimally invasive vascular procedures: A pilot study. J Vasc Surg 2013;57(5 suppl. 1):59s–60s. [DOI] [PubMed] [Google Scholar]
- 200.Bisseling TM, Willems MC, Versleijen MW, Hendriks JC, Vissers RK, Wanten GJ. Taurolidine lock is highly effective in preventing catheter-related bloodstream infections in patients on home parenteral nutrition: a heparin-controlled prospective trial. Clin Nutr. 2010;29(4):464–8. 10.1016/j.clnu.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 201.Barreras F, Cabeza M, Collantes de Terán L. Clinical efficacy and safety of Securflux®, an anti-reflux device for intravenous infusion. J Vasc Access. 2013;14(1):77–82. 10.5301/jva.5000095 [DOI] [PubMed] [Google Scholar]
- 202.Vickers AJ. How to randomize. J Soc Integr Oncol. 2006;4(4):194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takashima M, Ray-Barruel G, Keogh S, Rickard C. Randomised controlled trials in peripheral vascular access catheters: a scoping review. Vascular Access. 2015;1(2):10–37. [Google Scholar]
- 204.Alexandrou E, Spencer TR, Frost SA, Mifflin N, Davidson PM, Hillman KM. Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates—a report from 13 years of service. Crit Care Med 2014;42(3):536–43. 10.1097/CCM.0b013e3182a667f0 [DOI] [PubMed] [Google Scholar]
- 205.Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311–25. 10.1016/S0140-6736(13)60592-9 [DOI] [PubMed] [Google Scholar]
- 206.Ezaru CS, Mangione MP, Oravitz TM, Ibinson JW, Bjerke RJ. Eliminating arterial injury during central venous catheterization using manometry. Anesth Analg. 2009;109(1):130–4. 10.1213/ane.0b013e31818f87e9 [DOI] [PubMed] [Google Scholar]
- 207.Ullman AJ, Kleidon T, Rickard CM. The role of the vascular access nurse practitioner in developing evidence, promoting evidence-based vascular access practice and improving health services. Vascular Access. 2015;1(1):10–20. [Google Scholar]
- 208.Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Infect Control Hosp Epidemiol 2001;22(04):222–42. [DOI] [PubMed] [Google Scholar]
- 209.Timsit J, editor Updating of the 12th consensus conference of the Societe de Reanimation de langue francaise (SRLF): Catheter related infections in the intensive care unit. Ann Fr Anesth Reanim; 2005. [DOI] [PubMed]
- 210.Fallat ME, Gallinaro RN, Stover BH, Wilkerson S, Goldsmith LJ. Central venous catheter bloodstream infections in the neonatal intensive care unit. J Pediatr Surg 1998;33(9):1383–7. [DOI] [PubMed] [Google Scholar]
- 211.Mimoz O, Pieroni L, Lawrence C, Edouard A, Costa Y, Samii K, et al. Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients. Crit Care Med 1996;24(11):1818–23. [DOI] [PubMed] [Google Scholar]
- 212.Tablan O, Anderson L, Besser R, Bridges C, Hajjeh R. CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 213.Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis. 2007;7(10):645–57. 10.1016/S1473-3099(07)70235-9 [DOI] [PubMed] [Google Scholar]
- 214.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36(5):309–32. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 215.Ainsworth S, McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst Rev. 2015;(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brandao LR, Shah N, Shah PS. Low molecular weight heparin for prevention of central venous catheterization-related thrombosis in children. Cochrane Database Syst Rev. 2014;3:Cd005982. [DOI] [PubMed] [Google Scholar]
- 217.Harron K, Ramachandra G, Mok Q, Gilbert R, on behalf of the CATCH team. Consistency between guidelines and reported practice for reducing the risk of catheter-related infection in British paediatric intensive care units. Intensive Care Med. 2011;37:1641–7. 10.1007/s00134-011-2343-9 [DOI] [PubMed] [Google Scholar]
- 218.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123–33. 10.1056/NEJMra011883 [DOI] [PubMed] [Google Scholar]
- 220.Hu KK, Veenstra DL, Lipsky BA, Saint S. Use of Maximal Sterile Barriers during Central Venous Catheter Insertion: Clinical and Economic Outcomes. Clin Infect Dis 2004;39(10):1441–5. 10.1086/425309 [DOI] [PubMed] [Google Scholar]
- 221.Liang SY, Marschall J. Update on emerging infections: news from the Centers for Disease Control and Prevention. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med. 2011;58(5):447–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
*One catheter material study had also a theme of heparin flush. One removal technique study included insertion technique, but this is only included in one category. RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; NTCVAD: Non-tunneled Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device; CVAD NS: Central Venous Access Device Not Specified; PICU: Pediatric Intensive Care Unit; NICU: Neonatal Intensive Care Unit.
(DOCX)
RCT: Randomized Controlled Trial; CVAD: Central Venous Access Device; NTCVAD: Non-tunneled Central Venous Access Device; PICC: Peripherally Inserted Central Catheter; TIVAD: Totally Implantable Vascular Access Device; CVAD NS: Central Venous Access Device Not Specified; PICU: Pediatric Intensive Care Unit; NICU: Neonatal Intensive Care Unit; CDC: Centers of Disease Control; MRI: Magnetic Resonance Imaging; USS: Ultra sound sonography.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.