Abstract
In this study, we investigated the association between the renin-angiotensin system (RAS), endoplasmic reticulum (ER) stress and atherosclerosis (AS) in uremic apolipo-protein E knockout (apoE−/−) mice. Mild uremia was induced by a 5/6 nephrectomy (5/6 Nx) in 10-week-old apoE−/− mice. Four weeks after nephrectomy, the mice received losartan or no treatment for 16 weeks. Sham-operated mice served as the controls. We found that uremia accelerated AS at the aortic root. The activation of ER stress and the significant upregulation of pro-inflammatory cytokines and chemokines were observed in the uremic mice. Phosphorylated inositol-requiring 1α (p-IRE1α), an ER stress marker protein, was mainly expressed in macrophages in the atherosclerotic lesions. Treatment with losartan significantly attenuated aortic AS, inhibited ER stress and reduced aortic inflammation. In in vitro experiments, angiotensin II (Ang II) increased the levels of the common ER stress maker, glucose-regulated protein 78 (GRP78) and the phosphorylation of IRE1α in RAW264.7 macrophages. Treatment with losartan inhibited the activation of ER stress and the upregulation of GRP78, and enhanced the expression of nuclear factor-κB (NF-κB) inhibitor (IκB) in Ang II-stimulated RAW264.7 macrophages. IRE1α-siRNA suppressed inflammation and downregulated IκB expression and IκB kinase (IKK) phosphorylation, which inhibited IκB degradation and NF-κB p65 nuclear translocation in Ang II-treated RAW264.7 macrophages. These findings suggest that RAS activation accelerates AS by promoting ER stress-related inflammation in uremic mice.
Keywords: uremia, atherosclerosis, angiotensin II, endoplasmic reticulum stress, inflammation
Introduction
Patients with chronic renal failure (CRF) have a greater risk of developing cardiovascular disease (CVD) than subjects with normal renal function. Atherosclerosis (AS) is an important risk factor for the development of CVD in patients with CRF (1–4). Previous studies have reported that inflammation plays a key role in the development of AS in patients with CRF (5–7).
The endoplasmic reticulum (ER) is a multifunctional organelle that performs protein synthesis, folding and maturation. An accumulation of unfolded or misfolded proteins in the ER lumen leads to ER stress, which restores homeostasis in the ER. The release of ER stress sensors, including inositol-requiring 1α (IRE1α), double-stranded RNA-dependent protein kinase (PKR)-like ER kinase (PERK) and activating transcription factor 6 (ATF6) stimulates three distinct downstream signaling pathways (8,9). Severe and prolonged ER stress disrupts homeostasis and causes tissue injury and organ dysfunction. ER stress has been implicated in various types of CVD. Increasing evidence indicates that ER stress is the upstream signal for the inflammatory reaction, and is thus a key player in AS initiation and progression (10–13).
As regards other diseases, the suppression of the renin-angiotensin system (RAS) can also inhibit ER stress to decrease angiotensin II (Ang II)-induced inflammation (14,15). Thus, RAS activation promotes ER stress-related inflammation and may aggravate AS and other diseases. According to previous studies, RAS inhibition effectively abolishes the pro-atherogenic effects of uremia, and these effect are mainly dependent on an anti-inflammatory mechanism (16,17). However, the association between RAS-mediated ER stress and uremia-associated AS has not yet been examined to date, at least to the best of our knowledge. In this study, we investigated the effects of RAS on ER stress-induced inflammation in uremic apolipoprotein E knockout (apoE−/−) mice by blocking the Ang II receptor to further clarify the mechanisms of action of RAS in experimental uremia-associated AS.
Materials and methods
Serum biochemistry
Serum levels of urea nitrogen (BUN) and creatinine (CRE) were measured enzymatically with reagents from Nanjing Jiancheng Bioengineer Institute (Nanjing, China). The serum level of Ang II was measured by ELISA according to the instructions provided by the manufacturer (Jianglai Biotechnology Co., Shanghai, China).
Animal models
Ten-week-old male apoE−/− mice (homozygous apoE-deficient male mice, back-crossed 20 times from the C57BL/6 strain; Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were housed in the animal facility of Chongqing Medical University, Chongqing, China. The animals were maintained in an air-conditioned, pathogen-free and light-controlled environment and were fed standard mouse chow (2018S; Harlan Teklad, Madison, WI, USA) and sterile water. Experimental mild uremia was induced by 5/6 nephrectomy (5/6 Nx, termed SNx). In total, 31 apoE−/− mice were randomly allocated to undergo SNx (n=21) or sham operation (n=10). At 10 weeks of age, both the upper poles of the right kidney were resected, and electrocoagulation of the incision was performed to stop bleeding. After 2 weeks, the whole left kidney was removed. The control animals underwent a sham operation (the bilateral kidneys were only exposed, without any resection of the kidneys). Anesthesia was achieved by an intraperitoneal injection of sodium pentobarbital (Sigma Chemical Co., St. Louis, MO, USA) at a dose of 40 mg/kg.
Four weeks following nephrectomy, the uremic mice were randomly allocated into 2 subgroups as follows: the mice treated with the Ang II type 1 (AT1) receptor antagonist, losartan (Merck Sharp & Dohme Pty. Ltd., Macquarie Park, NSW, Australia) at a dose of 30 mg/kg in drinking water (n=10) or no medicine (n=11) for 12 weeks. The sham-operated mice received no medicine (n=10).
After the 12-week-intervention, each mouse was anesthetized, and blood was collected from the retro-orbital venous plexus. The blood was centrifuged at 3,000 rpm, at 4°C, and the plasma was stored at −80°C for analysis. For each mouse, following perfusion, the heart and aorta were separated from the iliac arteries. The heart and aortic root were dissected from the distal aorta and stored in 4% paraformaldehyde solution for histological analyses. The remaining aorta was stored at −80°C for gene and protein evaluations of the atherosclerotic lesions. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Chongqing Medical University. The protocols were approved by the Institutional Ethics Committee for Animal Experiments of Chongqing Medical University.
Cell culture and treatment
RAW264.7 murine macrophages were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA). The cells were plated on tissue culture dishes before the experiment and were cultured at 37°C under 5% CO2 in a humidified incubator. In all the experiments, the RAW264.7 macrophages were grown to 90% confluence and were serum-starved for 12 h prior to exposure to to various concentrations of Ang II (0.01, 0,1, 1, or 10 µg/ml). Prior to exposure to 1 μg/ml Ang II for 8 h, the cells were treated with losartan (10 μmol/l) 1 h before.
Infection with lentivirus
To verify whether Ang II-induced inflammation is associated with ER stress, the RAW264.7 macrophages were transfected with a specific siRNA-carrying lentiviral vector directed against IRE1α (forward, 5′-GGAAUUACUGGCUUCUCAUdTdT-3′ and reverse, 5′-AUGAGAAGCCAGUAAUUCCdTdT-3′) (18). A nonsense sequence served as the negative control. Following transfection for 48 h, the cells were exposed to Ang II for 8 h. Target gene silencing was validated by reverse transcription-quantitative PCR (PCR).
Histopathological analysis
Following fixation, the hearts and aortic roots were paraffin-embedded and sectioned. The sections were stained with hematoxylin-eosin or special antibodies, respectively. For immunohistochemistry, paraffinized sections were deparaffinized. Endogenous peroxidase activity was blocked with 3% H2O2 for 20 min, and non-specific staining was blocked by incubation with goat serum for 15 min. The samples were stained with anti-CD68 (BA3638; 1:100; Boster, Wuhan, China) or anti-p-IRE1α (Ser724; ab48187; 1:300; Abcam, Cambridge, UK) antibodies overnight at 4°C, followed by HRP-conjugated secondary antibodies (SP-9001; ZSGB-Bio, Beijing, China) at a 1:200 dilution for 30 min at 37°C. Immunohistochemical staining was performed by exposure to 3,3′-diaminobenzidine, and counterstaining was developed with hematoxylin. The sections were examined under a light microscope (Leica, Wetzlar, Germany) and quantified using Image-Pro Plus 6.0 software.
RT-qPCR
Total RNA was extracted from the rat aorta tissues or RAW264.7 macrophages using TRIzol reagent according to the manufacturer's instructions (Takara Bio, Inc., Otsu, Japan). RNA was reverse transcribed using the PrimeScript™ RT reagent kit (Takara Bio, Inc.). qPCR was performed using an Applied Biosystems StepOnePlus Real-Time PCR system and SYBR®-Green PCR master mix (both from Applied Biosystems, Foster City, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as an internal quantitative control. All cDNA were run twice in separate runs. The relative mRNA expression of each molecule was calculated using the 2−ΔΔCt method and normalized to GADPH. The primer sequences are listed in Table I.
Table I.
Sequences of primers for the detected genes.
| Gene | Primer sequences (5′→3′) |
|---|---|
| IL-6 | F: GAGGATACCACTCCCAACAGACC |
| R: AAGTGCATCATCGTTGTTCATACA | |
| TNF-α | F: CATGAGCACAGAAAGCATGATCCG |
| R: AAGCAGGAATGAGAAGAGGCTGAG | |
| CCL2/MCP-1 | F: CTTCCTCCACCACCATGCA |
| R: CCAGCCGGCAACTGTGA | |
| CX3CL1 | F: GTGCTGACCCGAAGGAGAAA |
| R: CACCCGCTTCTCAAACTTGC | |
| GADPH | F: TGCTGAGTATGTCGTGGAGTCTA |
| R: AGTGGGAGTTGCTGTTGAAATC |
F, forward; R, reverse; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CCL2, chemokine (C-C motif) ligand 2; MCP-1, monocyte chemoattractant protein-1; CX3CL1, chemokine (C-X3-C motif) ligand 1; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Western blot analysis
The aorta tissue or RAW264.7 macrophages were lysed for 30 min at 4°C in lysis buffer. Total protein concentrations were determined using bicinchoninic acid reagent. Total protein (50–100 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride membranes. After blocking, the membranes were incubated with anti-IRE1α (ab37073; 1:800; Abcam), anti-glucose-regulated protein 78 (GRP78; 11587-1-AP; 1:500; ProteinTech, Wuhan, China), anti-p-IRE1α (Ser724; ab48187; 1:800; Abcam), IκB kinase α (IKKα; 2682S; Cell Signaling Technology, Danvers, MA, USA), IκB kinase β (IKKβ; 2684S; Cell Signaling Technology), anti-p-IκB kinase α/β (p-IKK; Ser176/180; 2697T; 1:800; Cell Signaling Technology), IκB (YM3718; 1:500; ImmunoWay, Newark, DE, USA), anti-NF-κB p65 (YT5339; 1:500; ImmunoWay), anti-β-actin (6008-1-Ig; 1:750; ProteinTech) and anti-LaminB (sc-6216; 1:500; ZSGB-Bio) primary antibodies overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies (ZB-2301; ZSGB-Bio) were applied the following day for 2 h at 37°C. The bands were visualized using chemiluminescence (Beyotime, Shanghai, China). Band density was analyzed using fusion software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and normalized to β-actin or Lamin B density.
Statistical analyses
Statistical analyses were performed using SPSS software version 19.0. The data are presented as the means ± SEM for in vivo experiments and as the means ± SD for in vitro experiments. Analyses of variance (one-way ANOVA) followed by Bonferroni's or Dunnett's tests and the Kruskal-Wallis test followed by post-hoc tests were performed to evaluate group differences.
Results
Effect of losartan treatment on serum biochemistry and the development of AS in uremic mice
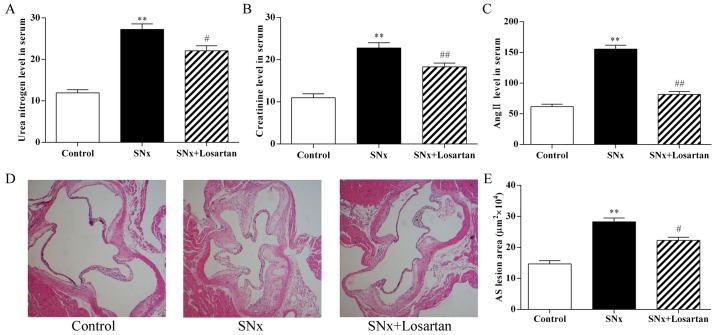
As shown in Fig. 1, 16 weeks after nephrectomy, the serum BUN and CRE concentrations were markedly increased in the uremic mice compared to the control mice (P<0.01; Fig. 1A and B). These increases indicated that the model was successfully developed. In addition, the serum Ang II levels was significantly increased in the uremic mice compared to the controls (P<0.01; Fig. 1C). Treatment with losartan treatment improved renal function in the uremic mice (BUN, P<0.05; CRE, P<0.01; Fig. 1A and B). The level of Ang II was also decreased in the losartan treatment group (P<0.01; Fig. 1C). At 16 weeks after nephrectomy, the uremic mice displayed a significant increase in the aortic root plaque area compared with the control mice (282,293±12,375 vs. 146,627±10,411 μm2, P<0.01) (Fig. 1D and E). Treatment with losartan treatment markedly decreased the lesion area in the uremic mice (222,703±9,857, P<0.05) (Fig. 1D and E). These results indicate RAS inhibition ameliorated the development of accelerated AS in uremia mice.
Figure 1.
Effects of losartan treatment on serum biochemistry and the development of atherosclerosis in uremic mice. (A–C) Quantitative analysis of the level of urea nitrogen, creatinine and angiotensin II (Ang II) in serum. n=10, 11, 10 for the control, SNx and SNx + losartan groups, respectively. (D and E) Quantitative analysis of aortic root AS lesion area. Hematoxylin and eosin staining; original magnification, ×40. n=10, 11, 10 for the control, SNx and SNx + losartan groups, respectively. SNx, subtotal nephrectomized apolipoprotein E knockout (apoE−/−) mice. Losartan was administered at the doses of 30 mg/kg for 12 weeks from 4 weeks after SNx. Data are the means ± SEM. **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. SNx group.
Effect of losartan treatment on vascular inflammation and ER stress in uremic mice
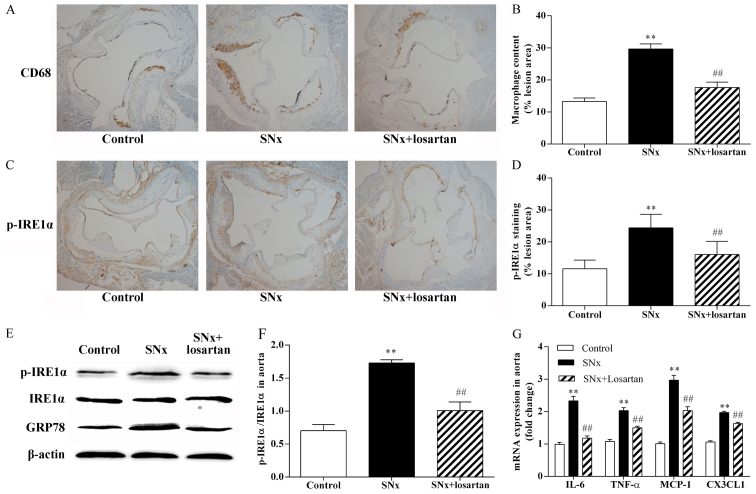
As shown in Fig. 2, macrophage inflammation plays an important role in the development of AS and ER stress is the upstream signal for the inflammatory reaction. Using immunohistochemistry on adjacent sections, we stained macrophages with anti-CD68 antibodies and using p-IRE1α, a typical ER stress sensor, to evaluate the state of ER stress in the aortic root sections. The uremic mice had a significantly increased macrophage content within the aortic root plaques compared with the control mice (29.64±1.59 vs. 13.27±1.11%, P<0.01) (Fig. 2A and B). The uremic mice also exhibited a significant increase in p-IRE1α expression (24.40±1.28 vs. 11.58±0.86%, P<0.01) (Fig. 2C and D). The co-localization of CD68 and p-IRE1α on adjacent sections indicated that p-IRE1α was mainly expressed in macrophages within the plaques. The results of western blot analysis revealed that the phosphorylation of IRE1α was significantly increased in the aortas of the uremic mice (P<0.01) (Fig. 2E and F). Treatment with losartan significantly reduced the atherosclerotic plaque macrophage content in the uremic mice (17.62±1.71%, P<0.01) (Fig. 2A and B). Treatment with losartan also markedly decreased p-IRE1α expression (16.05±1.29, P<0.01) (Fig. 2C and D) and markedly inhibited IRE1α phosphorylation in the uremic mice (P<0.01; Fig. 2E and F). We also observed the upregulation of the common ER stress maker, GRP78, in the aortas of the uremic mice (P<0.01; Fig. 2E). The upregulation of GRP78 was significantly inhibited in the aortas of the losartan-treated uremic mice (P<0.01; Fig. 2E). In addition, we compared the mRNA expression levels of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and chemokines, such as chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemoattractant protein-1 (MCP-1) and chemokine (C-X3-C motif) ligand 1 (CX3CL1), in the mouse aortas. The mRNA expression levels of the pro-inflammatory cytokines and chemokines were higher in the uremic mice than in the controls (P<0.01; Fig. 2G). Treatment with losartan markedly decreased the mRNA expression levels of the pro-inflammatory cytokines and chemokines in the uremic mice (P<0.05; Fig. 2G).
Figure 2.
Effects of losartan treatment on vascular inflammation and ER stress in uremic mice. The co-localization of CD68 and p-inositol-requiring 1α (IRE1α) was examined by immunohistochemistry using adjacent sections. (A and B) Quantitative analysis of macrophages content in lesion plaques. Immunohistochemistry with CD68, original magnification, ×40. n=10, 11, 10 for the control, SNx and SNx + losartan groups, respectively. (C and D) Quantitative analysis of p-IRE1α in lesion plaques in mice. Immunohistochemistry with p-IRE1α; original magnification, ×40. (E and F) Western blot analysis of the activation of IRE1α and the expression of glucose-regulated protein 78 (GRP78) in aortas of mice. n=5, 6, 5 for the control, SNx and SNx + losartan groups, respectively. (G) RT-qPCR analysis of pro-inflammatory cytokine and chemokine gene expression in aortas. n=5, 5, 5 for the control, SNx and SNx + losartan groups, respectively. Data are the means ± SEM. **P<0.01 vs. control group; ##P<0.01 vs. SNx group.
Effect of losartan on the expression of ER stress marker proteins and the NF-κB inhibitor protein IκB in Ang II-stimulated RAW264.7 macrophages
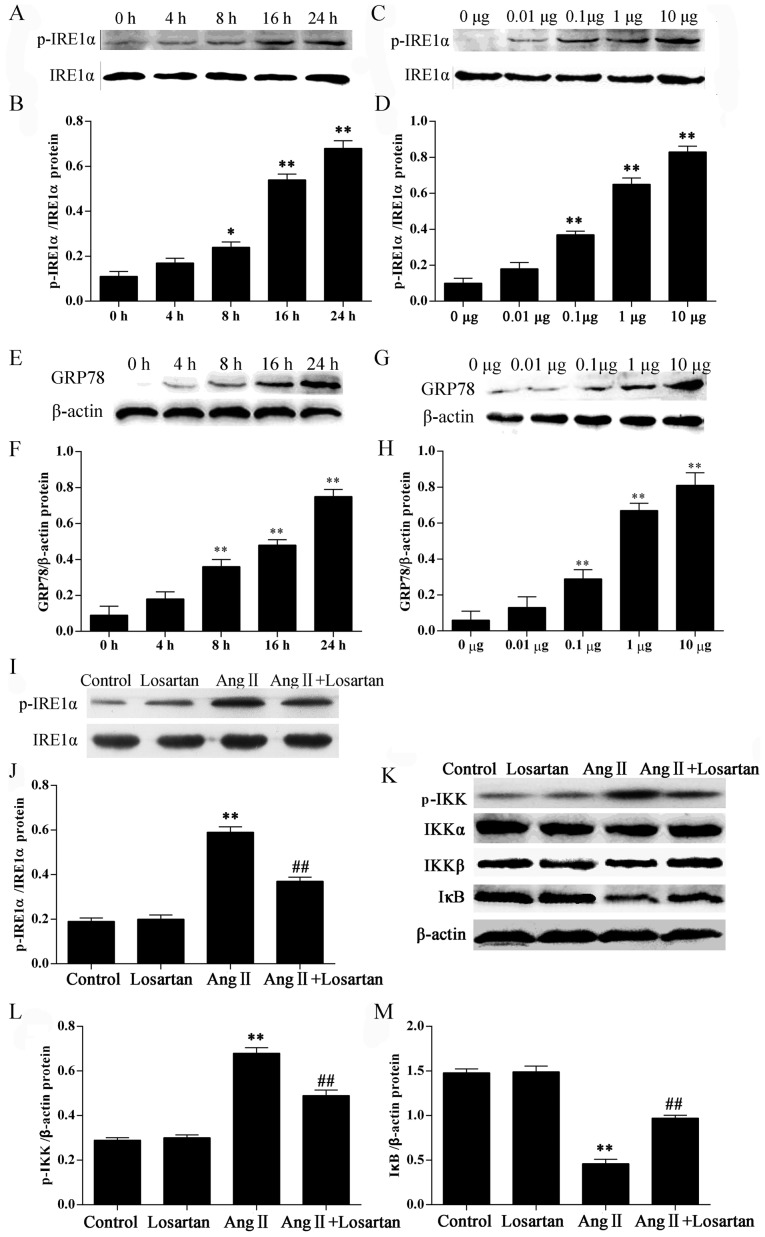
In this study, we examined the ER stress response in RAW264.7 macrophages following treatment with Ang II by measuring the protein levels of ER stress markers. Stimulation of the RAW264.7 macrophages with Ang II significantly increased the phosphorylation of IRE1α in a time− and dose-dependent manner (P<0.05; Fig. 3A–D). Stimulation of the RAW264.7 macrophages with Ang II also significantly increased the GRP78 levels in a time- and dose-dependent manner (P<0.05; Fig. 3E–H). Stimulation with Ang II also increased the phosphorylation of IKK (P<0.05; Fig. 3K and L) in the RAW264.7 macrophages. Losartan is a common AT1 receptor antagonist. Losartan significantly inhibited the phosphorylation of IRE1α and IKK (P<0.01; Fig. 3I–L) compared with the Ang II-stimulated RAW264.7 macrophages not treated with losartan. IκB is a regulatory protein binding to the transcription factor, NF-κB, retaining it in the cytoplasm. Losartan significantly enhanced the expression of IκB in the Ang II-stimulated RAW264.7 macrophages (P<0.01; Fig. 3K and M), suggesting the inhibition of NF-κB by losartan in the Ang II-stimulated RAW264.7 macrophages.
Figure 3.
Effects of losartan on the expression of ER stress marker proteins and nuclear factor-κB (NF-κB) inhibitor protein IκB in angiotensin II (Ang II)-stimulated RAW264.7 macrophages. RAW264.7 macrophages were stimulated with 1 μg/ml Ang II for 4, 8, 16 and 24 h [for inositol-requiring 1α (IRE1α) activation (A and B); for glucose-regulated protein 78 (GRP78) (E and F)] or with 0.01, 0.1, 1, or 10 μg/ml Ang II for 24 h [for IRE1α activation (C and D); for GRP78 (G and H)] and examined by western blot analysis. Culture medium was used as a blank control. (I–M) Western blot analysis of the activation of IRE1α, IKKα/β and IκB. The results were expressed as the means ± SD. n=3 independent experiments; *P<0.05 and **P<0.05 vs. control; ##P<0.05 vs. Ang II.
Effect of IRE1α-siRNA on the Ang II-induced inflammatory response in RAW264.7 macrophages
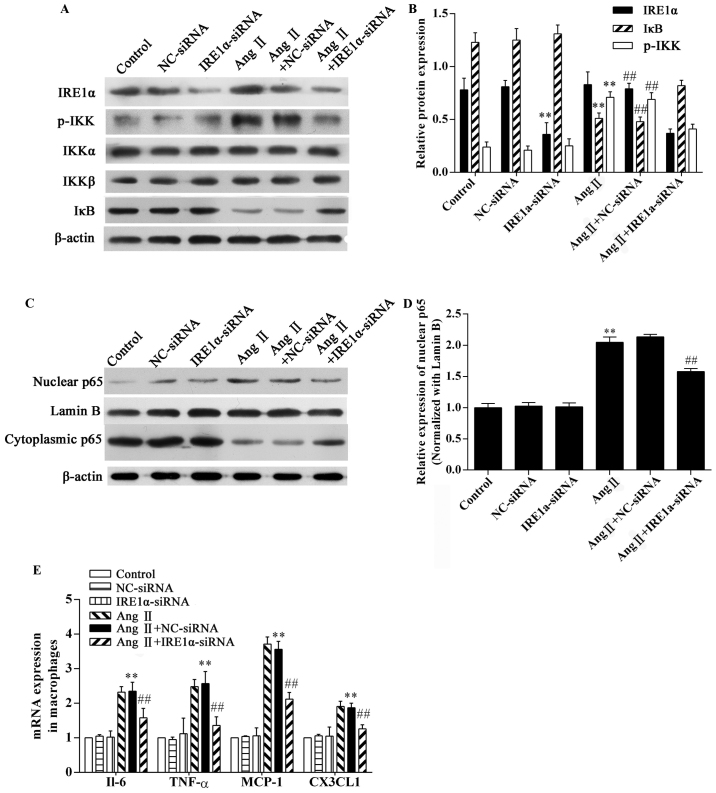
To confirm the role of ER stress in Ang II-induced inflammation, RAW264.7 macrophages were transfected with IRE1α-specific siRNA or control-siRNA 48 h prior to exposure to Ang II. The expression of the ER stress sensor, IRE1α, was downregulated in the IRE1α-siRNA group (Fig. 4A and B). The results of western blot analysis demonstrated that stimulation with Ang II significantly increased IKK phosphorylation (P<0.01; Fig. 4A and B) and caused a marked degradation of IκB in the RAW264.7 macrophages (P<0.01; Fig. 4A and B). Ang II stimulation significantly increased NF-κB p65 in nuclear extracts from macrophages, and the cytoplasmic NF-κB p65 content decreased (Fig. 4C). Compared with the Ang II-exposed group, IRE1α-siRNA decreased IKK phosphorylation and inhibited IκB degradation (P<0.01; Fig. 4A and B) and inhibited NF-κB p65 nuclear translocation (P<0.01; Fig. 4C and D). In addition, Ang II stimulation significantly upregulated the mRNA expression of pro-inflammatory cytokines and chemokines in the RAW264.7 macrophages (P<0.01; Fig. 4E). The depletion of IRE1α expression markedly downregulated the mRNA expression of pro-inflammatory cytokines and chemokines (Fig. 4E). Without the presence of Ang II, the IRE1α-specific siRNA did not affect IKK phosphorylation, IκB degradation, or NF-κB p65 nuclear translocation (Fig. 4). The negative control (NC-siRNA) did not influence the effects of Ang II (Fig. 4). Thus, Ang II-mediated inflammation in RAW264.7 macrophages is partially dependent on the activation of ER stress.
Figure 4.
Effects of inositol-requiring 1α (IRE1α) siRNA on the angiotensin II (Ang II)-induced inflammatory response in RAW264.7 macrophages. The RAW264.7 macrophages transfected with or without IRE1α siRNA were stimulated with 1 μg/ml Ang II for 8 h and then subjected to western blot analysis and gene expression analysis. Western blot analysis of the expression of (A and B) IRE1α, p-IKK, IKKα/β, IκB, and (C and D) NF-κB p65. (E) RT-qPCR analysis of the gene expression of the indicated cytokines. The results were expressed as the mean ± SD. n=3 independent experiments; **P<0.05 vs. control; ##P<0.05 vs. Ang II.
Discussion
A number of experimental and clinical studies have demonstrated that RAS activation occurs during uremia, and RAS plays a key role in the development and progression of uremic AS (16,17,19). Similar to these previous findings (16), our study found that the Ang II serum concentration was significantly increased in uremic mice, and that RAS blockade with losartan significantly abolished the pro-atherogenic effects in apoE−/− mice subjected to SNx. However, the exact mechanisms through which RAS promotes large vessel disease in patients with CRF were not fully explained.
Atherosclerotic plaques, particularly advanced lesions, contain abundant pro-inflammatory cytokines and chemokines (20,21). Pedersen et al reported that the pro-inflammatory response overrules the pro-atherogenic potential of traditional factors during uremia-induced AS (6). In the present study, we induced experimental mild uremia by subjecting apoE−/− mice to SNx. The levels of Ang II were found to increase after SNx. It has been demonstrated that the anti-atherogenic effects of losartan are due to the direct inhibition of Ang II activity (22). Other events that occur due to Ang II, such as hypertension (15), may also be regulated by losartan. Furthermore, treatment with losartan has been shown to prevent both coronary vascular injury and myocyte damage induced by continuous Ang II infusion in rats (23). We aim to investigate the signaling pathways mediating the effects of losartan in the future.
Chronic inflammation is an important risk factor for the progression of AS in patients with CRF. According to our study, macrophage accumulation was increased, and pro-inflammatory cytokine and chemokine gene expression was upregulated in the aortas from uremic mice. Treatment with losartan, an AT1 receptor blocker, reduced macrophage accumulation and inhibited aortic inflammation in the uremic mice. These molecular and cellular changes were associated with accelerated AS. These findings are consistent with those of previous studies (16,17). RAS inhibition abolished the pro-atherogenic effecta of uremia, which was mainly dependent on its anti-inflammatory mechanisms (16).
A recent study demonstrated that ER stress is a key player during the development of AS. Prolonged ER stress triggers macrophage and endothelial cell apoptosis, which in turn leads to plaque necrosis. Another important pro-atherogenic effect of prolonged ER stress is the activation of inflammatory pathways in cells within the plaques, especially macrophages (11). To determine the association between RAS and ER stress in uremic mice, we assessed the expression and activation of the ER stress response signaling proteins. GRP78 is a prominent ER-resident chaperone, which binds to the three ER stress sensors. During ER stress, GRP78 stably interacts with misfolded or unfolded proteins, and the three previously mentioned GRP78-bound proteins, PERK, IRE1α and ATF6 are free. The upregulation of GRP78 is a common ER stress marker (10). In this study, we observed that GRP78 upregulation was significantly inhibited in the aortas of losartan-treated uremic mice. The three free proteins can activate their respective downstream signaling pathways, and prolong the activation of ER stress, causing disease. In this study, among the three branches of ER stress signaling, we focused on IRE1α to address the inflammation caused by uremia. In addition, the effects of Ang II and losartan upon endothelial and vascular smooth cells in the pathogenesis of AS cannot be neglected. A previous in vivo study demonstrated that IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor (VEGF)-A expression and contributes to angiogenesis (24). Exogenous Ang II can activate IRE1 protein expression in a dose-dependent manner in endothelial cells, and the activation of IRE1 is necessary for both VEGF production and capillary tube formation from endothelial cells induced by Ang II (25).
IRE1α activates both the NF-κB and AP-1 transcriptional pathways, which are crucial regulators of the inflammatory response (26–29). It is widely accepted that IRE1 is involved in the activation of NF-κB induced by ER stress. In a previous study, the activation of NF-κB by the ER stress-inducing agents, thapsigargin and tunicamycin, was inhibited by a dominant-negative IRE1 (27). Our results also suggested that the NF-κB activation from the ER is transduced by IRE1. Moreover, the study by Miyazaki-Anzai et al demonstrated that CHOP inhibition alone was not sufficient to inhibit CKD-dependent AS. The IRE1α-NF-κB axis of the ER stress signal may be more important in the development of CKD-dependent AS (30). In this study, losartan treatment decreased IRE1α activation in atherosclerotic plaques. From this perspective, we suggest that RAS activation may be associated with ER stress-induced inflammation during uremic AS.
To further explore the association between RAS activation and ER stress-related inflammation, we performed in vitro experiments. In the in vivo experiments, we discovered the co-localization of CD68 and p-IRE1α by immunohistochemistry using adjacent sections, showing that p-IRE1α was mainly expressed in macrophages within the plaques. ER stress-induced inflammation in macrophages is an important contributor to AS (11). During early development of AS, monocytes migrate into the arterial intima by chemotaxis and then mature into macrophages. Macrophage infiltration into the arteries is a key factor for atherosclerotic plaque initiation. Macrophages in plaques produce pro-inflammatory cytokines to promote AS progression (31,32). In our in vitro experiment, Ang II was used to stimulate RAW264.7 macrophages as Ang II is the primary effector of RAS. The results demonstrated that ER stress was induced by Ang II stimulation in RAW264.7 macrophages. GRP78 expression was significantly upregulated, and IRE1α phosphorylation was increased in the Ang II-stimulated macrophages. During ER stress, IRE1α is activated by auto-phosphorylation at S724, and IRE1α autophosphorylation induces a conformational change in its cytosolic domain, which can subsequently bind to the adaptor protein TNF-α-receptor associated factor 2 (TRAF2). The IRE1α-TRAF2 complex can phosphorylate IKK, which leads to IκB degradation and the nuclear translocation of NF-κB (26–28). In this study, we determined that stimulation with Ang II significantly upregulated IKK phosphorylation, IκB degradation and NF-κB nuclear translocation. More importantly, IRE1α depletion effectively downregulated IKK phosphorylation and inhibited IκB degradation and NF-κB nuclear translocation.
In previous studies, Ang II stimulation was shown to effectively activate an inflammatory response. Guo et al indicated that NF-κB is a critical inflammatory transcription factor for Ang II-induced inflammation in RAW264.7 macrophages (33). NF-κB is a pro-atherogenic factor, mainly due to its regulation of many pro-inflammatory genes that are linked to AS (34,35). This study indicated that ER stress is an upstream signal of the NF-κB pathway. Ang II stimulation significantly elevated pro-inflammatory cytokine and chemokine gene expression in RAW264.7 macrophages, which was mostly dependent on the activation of the IRE1α/IKK/NF-κB pathway. In this study, losartan significantly enhanced the expression of IκB in Ang II-stimulated RAW264.7 macrophages, suggesting the inhibition of NF-κB by losartan in Ang II-stimulated RAW264.7 macrophages. This further confirmed the effect of losartan on the IRE1α/IKK/NF-κB pathway in vitro. In addition, it has been demonstrated that phosphorylated forms of IRE1α could be detected by slower migration upon stimulation with lipopolysaccharide (36). The knockdown of IRE1α in J774 macrophages resulted in reduced IL-6 induction by tunicamycin and lipopolysaccharide co-treatment (36). Similarly, the effect of IRE1α-siRNA on the Ang II-induced inflammatory response is not only due to the reduced macrophage number.
Furthermore, MCP-1 has been detected in atherosclerotic lesions (37,38). Blocking the expression of MCP-1 or its receptor CCR2 decreases atheroma formation in hypercholesterolemic mice (39,40). In future studies we aim to explore the influence of MCP-1 and its receptor, CCR2, on ER stress-induced AS.
In conclusion, our findings demonstrated that the blockade of RAS by losartan almost completely prevented the acceleration of atherosclerotic lesion formation in uremic apoE−/− mice. This effect was associated with the modulation of chronic inflammation via ER stress-dependent mechanisms. This study further clarified the mechanism through which RAS aggravates and accelerates atherogenesis in uremic mice.
Acknowledgments
We are grateful to Mr. Zhengyi Wang, Ms. Jingmei Xie and Ms. Yao Xiao for providing experiment technical support.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Amann K, Gross ML, Ritz E. Pathophysiology underlying accelerated atherogenesis in renal disease: Closing in on the target. J Am Soc Nephrol. 2004;15:1664–1666. doi: 10.1097/01.ASN.0000128365.76153.5B. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 4.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO. Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623–2631. doi: 10.1097/01.ASN.0000088722.56342.A8. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee D, Recio-Mayoral A, Chitalia N, Kaski JC. Insulin resistance, inflammation, and vascular disease in nondiabetic predialysis chronic kidney disease patients. Clin Cardiol. 2011;34:360–365. doi: 10.1002/clc.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen TX, Binder CJ, Fredrikson GN, Nilsson J, Bro S, Nielsen LB. The pro-inflammatory effect of uraemia overrules the anti-atherogenic potential of immunization with oxidized LDL in apoE−/− mice. Nephrol Dial Transplant. 2010;25:2486–2491. doi: 10.1093/ndt/gfq059. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26:3537–3543. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 8.Pluquet O, Pourtier A, Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol. 2015;308:C415–C425. doi: 10.1152/ajpcell.00334.2014. [DOI] [PubMed] [Google Scholar]
- 9.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 10.Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic Biol Med. 2015;78:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh T, Endo M, Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int J Inflamm. 2011;2011:259462. doi: 10.4061/2011/259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Lhoták S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL, Liu BC. Involvement of endoplasmic reticulum stress in angiotensin II-induced LRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin. 2015;36:821–830. doi: 10.1038/aps.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young CN, Li A, Dong FN, Horwath JA, Clark CG, Davisson RL. Endoplasmic reticulum and oxidant stress mediate nuclear factor-κB activation in the subfornical organ during angiotensin II hypertension. Am J Physiol Cell Physiol. 2015;308:C803–C812. doi: 10.1152/ajpcell.00223.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardi S, Candido R, Toffoli B, Carretta R, Fabris B. Prevention of accelerated atherosclerosis by AT1 receptor blockade in experimental renal failure. Nephrol Dial Transplant. 2011;26:832–838. doi: 10.1093/ndt/gfq524. [DOI] [PubMed] [Google Scholar]
- 17.Bro S, Binder CJ, Witztum JL, Olgaard K, Nielsen LB. Inhibition of the renin-angiotensin system abolishes the proatherogenic effect of uremia in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1080–1086. doi: 10.1161/ATVBAHA.107.139634. [DOI] [PubMed] [Google Scholar]
- 18.Yao S, Miao C, Tian H, Sang H, Yang N, Jiao P, Han J, Zong C, Qin S. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J Biol Chen. 2014;289:4032–4042. doi: 10.1074/jbc.M113.524512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/S0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 20.Mach F. The role of chemokines in atherosclerosis. Curr Atheroscler Rep. 2001;3:243–251. doi: 10.1007/s11883-001-0067-y. [DOI] [PubMed] [Google Scholar]
- 21.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7(Suppl 1):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang C, Wu ZG, Ding J, Jiang JF, Huang GZ, Du RZ, Ge JB. Losartan inhibited expression of matrix metalloproteinases in rat atherosclerotic lesions and angiotensin II-stimulated macrophages. Acta Pharmacol Sin. 2004;25:1426–1432. [PubMed] [Google Scholar]
- 23.Kabour A, Henegar JR, Devineni VR, Janicki JS. Prevention of angiotensin II induced myocyte necrosis and coronary vascular damage by lisinopril and losartan in the rat. Cardiovasc Res. 1995;29:543–548. doi: 10.1016/S0008-6363(96)88532-4. [DOI] [PubMed] [Google Scholar]
- 24.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, Kaufman RJ, Chevet E, Bikfalvi A, Moenner M. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67:6700–6707. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Bai YP, Hong D, Gao HC, Li LF, Li CC, Zhu LP, Sun Q, Zhang GG. Ang II induces capillary formation from endothelial cells via the AT1R-dependent inositol requiring enzyme 1 pathway. Biochem Biophys Res Commun. 2013;434:552–558. doi: 10.1016/j.bbrc.2013.03.113. [DOI] [PubMed] [Google Scholar]
- 26.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and downregulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Wang D, Xiao Y, Zhou X, Wang L, Chen B, Li Q, Guo X, Huang Q. Endoplasmic reticulum stress plays a role in the advanced glycation end product-induced inflammatory response in endothelial cells. Life Sci. 2014;110:44–51. doi: 10.1016/j.lfs.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki-Anzai S, Masuda M, Demos-Davies KM, Keenan AL, Saunders SJ, Masuda R, Jablonski K, Cavasin MA, Kendrick J, Chonchol M, et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein (CHOP) regulates chronic kidney disease-induced vascular calcification. J Am Heart Assoc. 2014;3:e000949. doi: 10.1161/JAHA.114.000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Guo F, Chen XL, Wang F, Liang X, Sun YX, Wang YJ. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J Interferon Cytokine Res. 2011;31:351–361. doi: 10.1089/jir.2010.0073. [DOI] [PubMed] [Google Scholar]
- 34.Xanthoulea S, Curfs DM, Hofker MH, de Winther MP. Nuclear factor kappa B signaling in macrophage function and atherogenesis. Curr Opin Lipidol. 2005;16:536–542. doi: 10.1097/01.mol.0000180167.15820.ae. [DOI] [PubMed] [Google Scholar]
- 35.de Winther MPJ, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 36.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeya M, Yoshimura T, Leonard EJ, Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–539. doi: 10.1016/0046-8177(93)90166-E. [DOI] [PubMed] [Google Scholar]
- 38.Ylä-Herttuala S, Lipton BA, Rosenfeld ME, Särkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 40.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/S1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]