Abstract
Neural stem cells with the characteristics of astrocytes persist in the subventricular zone (SVZ) of the juvenile and adult brain. These cells generate large numbers of new neurons that migrate through the rostral migratory stream to the olfactory bulb. The developmental origin of adult neural stem cells is not known. Here, we describe a lox–Cre-based technique to specifically and permanently label a restricted population of striatal radial glia in newborn mice. Within the first few days after labeling, these radial glial cells gave rise to neurons, oligodendrocytes, and astrocytes, including astrocytes in the SVZ. Remarkably, the rostral migratory stream contained labeled migratory neuroblasts at all ages examined, including 150-day-old mice. Labeling dividing cells with the S-phase marker BrdUrd showed that new neurons continue to be produced in the adult by precursors ultimately derived from radial glia. Furthermore, both radial glia in neonates and radial glia-derived cells in the adult lateral ventricular wall generated self-renewing, multipotent neurospheres. These results demonstrate that radial glial cells not only serve as progenitors for many neurons and glial cells soon after birth but also give rise to adult SVZ stem cells that continue to produce neurons throughout adult life. This study identifies and provides a method to genetically modify the lineage that links neonatal and adult neural stem cells.
Keywords: neurogenesis, oligodendrocytes, subependyma
The mammalian brain retains neural stem cells in the subventricular zone (SVZ) of the lateral ventricular wall. These cells generate new neurons (1–4) that migrate along the rostral migratory stream (RMS) to the olfactory bulb, where they differentiate into granular and periglomerular interneurons (5, 6). Although they are actively neurogenic, adult SVZ stem cells express glial fibrillary acidic protein (GFAP) and have the morphology and ultrastructure of astrocytes (7–10). These findings challenge the classical view that all astrocytes are terminally differentiated glial cells belonging to a lineage separate from that of neurons (7, 11). However, the developmental origin of these neurogenic astrocytes remains elusive and controversial.
Here, we tested the hypothesis that adult SVZ neural stem cells are derived from radial glia. Radial glia have a long, RC2-positive (12) basal process that extends from their cell body in the ventricular zone (VZ) through the parenchyma toward the brain surface (13). Radial glia have anatomical features of cells in the astroglial lineage, including endfeet on blood vessels, intermediate filaments, and glycogen granules. At the end of histogenesis, many radial glia differentiate into parenchymal astrocytes (14, 15). For these reasons, radial glia classically were viewed as immature glial cells that guide neuronal migration and function as scaffolding for brain development (16, 17). However, studies in songbirds (18), where radial glia persist into adult life, and work in the developing rodent brain (19–25) indicate that radial glia also function as neural progenitors. We (11) and others (26–28) have hypothesized that neural stem cells may be contained within what was classically considered the macroglial lineage (i.e., neuroepithelial cells → radial glia → astrocytes).
In the present study, we genetically tagged a restricted population of radial glia that persist in the lateral wall of the lateral ventricle of postnatal day (P) 0 mice (29). We show that these radial glial cells give rise to neurons, astrocytes, ependymal cells, and oligodendrocytes. More importantly, we show that these neonatal radial glial cells give rise to the SVZ astrocytes that maintain neurogenesis in the adult mammalian brain. This work identifies the neonatal origin of adult SVZ neural stem cells.
Materials and Methods
Labeling of P0 Radial Glia by Striatal Adenovirus (Ad) Injection. All protocols followed the guidelines of the Laboratory Animal Resource Center at the University of California, San Francisco. Newborn mice were anesthetized by hypothermia and placed onto the platform of a stereotaxic injection rig where their heads were stabilized by a customized head mold. Striatal injections (20 nl) of Ad were targeted unilaterally as shown in Fig. 1 G and H. We avoided the ventricle, VZ, and SVZ by angling the injection needle at 45° and following a lateral approach. After injection, animals were returned to their mother and monitored every 10 min until they resumed nursing.
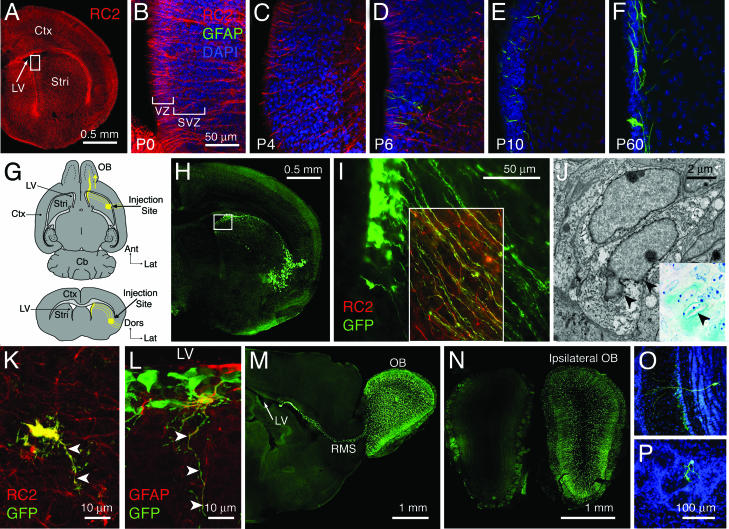
Fig. 1.
Radial glia are labeled specifically in the neonatal mouse brain. (A) RC2+ radial glial processes extend from the VZ to the brain surface. (B–F) In the region boxed in A, the disappearance of RC2+ radial glia correlates with the appearance of GFAP+ SVZ astrocytes. (G) Radial glial processes can be infected by an Ad injected into the ventrolateral striatum. (H) This injection results in the labeling of cells at the injection site and in the dorsolateral VZ. (I) VZ cells (box in H) have long radial processes that express RC2 (boxed region) and extend from the VZ through the injection site. (J) These cells have the ultrastructure of radial glia. (J Inset) In the same cells (from P2 R26R mice), X-Gal precipitate (arrowheads) is shown at the light microscope. (K and L) In the early postnatal brain, radial glia retract their processes (arrowheads) as they transform into astrocytes in the striatum (K) and in the SVZ (L). (M–P) Their progeny, however, continue to generate large numbers of neuroblasts in the P90 brain (M) that migrate along the RMS to the olfactory bulb (N) and differentiate into granular (O) and periglomerular (P) cells. Cb, cerebellum; Ctx, cortex; LV, lateral ventricle; Stri, striatum.
Immunohistochemistry. Animals were deeply anesthetized and killed by transcardial perfusion of saline followed by 4% paraformaldehyde. After 24 h in 4% paraformaldehyde, 50-μm vibratome sections were cut and immunostained with antibodies directed against the RC2 antigen (mouse monoclonal IgM, Developmental Studies Hybridoma Bank, Iowa City, IA), GFAP (mouse monoclonal IgG1, Chemicon; rabbit polyclonal IgG, DAKO), EGFP (rabbit polyclonal IgG, Novus Biologicals, Littleton, CO; sheep polyclonal Ig, Biogenesis, Bournemouth, U.K.), doublecortin (guinea pig polyclonal IgG, Chemicon), antineuronal nuclei (NeuN; mouse monoclonal IgG1, Chemicon), heat stable antigen (CD24; rat monoclonal IgG, Pharmingen), olig2 [mouse monoclonal IgG, a generous gift from David Rowitch (Harvard Medical School, Boston)], β-tubulin type III (Tuj1; mouse monoclonal IgG2a, Covance, Princeton), and O4 (mouse monoclonal IgG, Chemicon) following standard protocols. Staining was visualized with secondary antibodies conjugated to 7-amino-4-methylcoumarin-3-acetic acid, Cy3, or Alexa Fluor 488, 568, 594, or 647 fluorophores or to biotin followed by streptavidin and diaminobenzidine. Omission of the primary antibodies eliminated staining at all developmental ages examined. Sections were counterstained with DAPI.
Electron Microscopy. Tissue processing for electron microscopy after β-galactosidase histochemistry was performed as described in ref. 30. Cell counting in the neonatal VZ by using electron microscopy was performed as described in ref. 31. The ultra-structural criteria to identify radial glia and adult SVZ cell types are described in refs. 31 and 32.
Viral Diffusion Assay. To determine whether Ad injected in the ventrolateral striatum could be diffusing through the striatum and infecting cells at or near the VZ, we injected Ad expressing Cre recombinase (Ad:Cre) into the lateral striatum of wild-type (CD1) P0 pups along with bromophenol blue to visualize the injection site. At various time points after injection (5 and 20 min and 1, 4, 8, and 24 h), we killed the animals and microdissected equally sized explants of the injection site, the ipsilateral ventricular wall, and the striatum halfway between these two sites (see Fig. 4A, which is published as supporting information on the PNAS web site). We then cultured these explants for 3 days on top of monolayers of EGFP Ad:Cre-reporting (Z/EG strain) SVZ astrocytes (n = 3 per condition). We found EGFP+ cells in monolayers cultured with the injection site explant (Fig. 4B) but never from monolayers cultured with the ventricular wall explant. Furthermore, the number of cells infected by each explant rapidly decreased over time; after 4 h we could not detect any infectious particles.
Grafting of Radial Glial Cell Bodies. To obtain labeled radial glia, we injected heterozygous Z/EG pups at P0, killed the animals at P3, and exposed the lateral wall of the lateral ventricle under a fluorescent dissecting scope. This procedure allowed us to visualize the injection site and a well defined patch of EGFP+ radial glial cell bodies in the VZ (Fig. 2K). We microdissected the labeled cells in the VZ (n = 6 grafts) and the injection site (n = 4 grafts) and stereotactically grafted them into the VZ/SVZ of their wild-type littermates, which we then killed at P30. In a separate experiment, we also grafted labeled cells from the injection site (n = 6) into the ventrolateral striatum. Radial glial grafts, but not grafts from cells labeled locally at the injection site, produced all of the cell types seen from the injection alone.
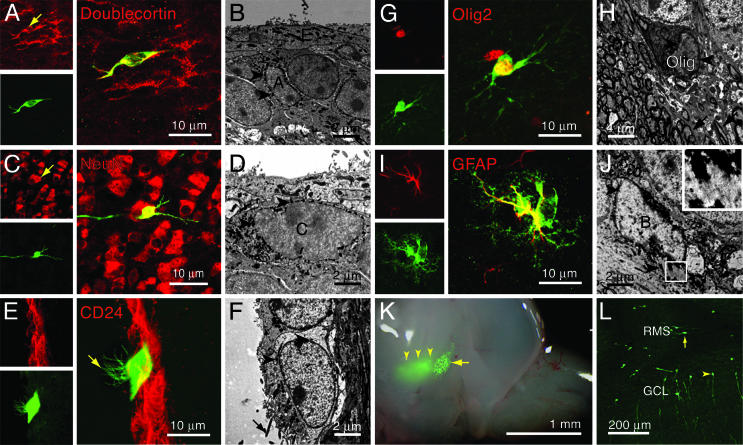
Fig. 2.
Radial glia produce all classes of brain cells. (A–J) Radial glial progeny were identified with the confocal microscope by EGFP expression in Z/EG mice and with the electron microscope by dark, perinuclear grains of X-Gal precipitate in R26R mice (black arrowheads). The progeny include doublecortin+ neuroblasts (type A cells) (A and B), NeuN+ neurons (C), ciliated (arrows) CD24+ ependymal cells (E and F), and Olig2+ oligodendrocytes whose processes (arrows) intercalate between myelinated axons (G and H). (D, I, and J) Radial glia also produce GFAP+ SVZ astrocytes or type B cells (I and J; intermediate filaments shown in Inset) and neurogenic transit-amplifying type C cells (D). (K) A whole mount of the lateral ventricular wall reveals the injection site deep in the tissue at a deeper focal plane (yellow arrowheads) and a well defined patch of labeled radial glial cell bodies (arrow). (L) When grafted into a wild-type mouse, radial glial cell bodies (but not cell bodies from the injection site) produce all cell types obtained by viral injection alone, including neuroblasts (arrow) and olfactory bulb neurons (yellow arrowhead) in the adult brain. GCL, granule cell layer.
BrdUrd Treatment and Immunohistochemistry. To demonstrate the continual production of neurons in the adult, newborn Z/EG mice were injected unilaterally with Ad:Cre into the ventrolateral striatum. Thirty days later, BrdUrd was added to their drinking water (1 mg/ml) for 3 days, following a standard protocol (6). Immediately thereafter or 15 days later, animals were perfused with 4% paraformaldehyde as described above. Vibratome sections of 50 μm were cut, treated for BrdUrd staining by using a protocol described in ref. 33, and immunostained for EGFP (rabbit polyclonal IgG, Abcam, Cambridge, U.K.), BrdUrd (rat monoclonal IgG, Oxford Biotechnology, Oxfordshire, U.K.), and GFAP (mouse monoclonal IgG, Chemicon). Staining was revealed by secondary antibodies conjugated to Alexa Fluor 488, 594, or 647 fluorophores, respectively. Omission of the primary antibodies eliminated staining. Double-labeled cells were identified at the light microscope and confirmed by confocal analysis.
Clonal Generation of Neurospheres. To determine whether radial glia or their adult progeny could form neurospheres, Z/EG animals were injected with Ad:Cre at P0, and the lateral ventricular wall of P2, P10, and P90 animals was dissected, dissociated, and placed in neurosphere medium as described in ref. 6. After amplification in culture, cells were sorted for EGFP by FACS on a MoFlo Cytomation (Freiburg, Germany) cell sorter at ≈100 cells per sec. Upon sorting, we obtained a bimodal distribution, with EGFP+ cell peak ≈2 orders of magnitude greater than the peak for EGFP– cells. Visual inspection confirmed strong EGFP fluorescence in >98% of sorted cells. We sorted the cells a second time by using the same parameters to achieve an ≈99.9% pure population of EGFP+ cells. Visual inspection confirmed that all neurospheres formed by these cells were strongly green fluorescent. Upon plating at clonal density (20 cells per μl), these cells generated neurospheres that were individually differentiated and stained for EGFP, O4, Tuj1, and GFAP as described in ref. 6.
Results
Maturation of the SVZ. We first studied the composition of the lateral ventricular wall at different ages. At P0, the striatal VZ contains primarily radial glial cell bodies and a few immature ependymal cells but no astrocytes (29). The cell bodies of subcortical (striatal) radial glia line the lateral wall of the lateral ventricle, and their long RC2+ basal processes curve through the striatum to contact the brain surface (Fig. 1 A). To determine the time course of astrocyte formation in the SVZ, we immunostained for GFAP (31) and RC2 to label astrocytes and radial glia, respectively, at P0, P2, P4, P6, P8, P10, and P60 (n = 4 per age group). The disappearance of radial glia from the lateral ventricular wall correlates with the appearance of GFAP+ astrocytes (at P6) directly below the VZ in the developing SVZ (Fig. 1 B–F). We hypothesized that striatal radial glia in the neonatal brain give rise to the neurogenic astrocytes found in the adult SVZ.
Radial Glial Targeting and Lineage Tracing. To test this hypothesis, we developed a technique to trace the progeny of a restricted population of radial glia. We found that radial glia in P0 mice can be labeled by a replication incompetent, EGFP-expressing Ad (Ad:GFP). To ensure that we were specifically labeling radial glia and not other cells in the VZ or its vicinity, we infected radial glia at their distal basal processes by stereotactically injecting Ad:GFP (20 nl) into ventrolateral striatum of P0 mice near the border to the piriform cortex (Fig. 1G). Two days after infection, we observed labeling at the injection site and in a restricted region of the dorsolateral striatal VZ, 1.10 ± 0.13 mm (mean ± SEM) away from the injection site (Figs. 1H and 2K). Labeled cells in the VZ had the distinctive morphology of radial glia and had RC2+ processes that traversed the injection site (see below). Thus, radial glial cell bodies can be labeled retrogradely with Ad. The rapid infection, short half-life, and limited diffusion of Ad through brain parenchyma (34) result in rapid, localized labeling. However, the adenoviral genome is not incorporated into cellular DNA and becomes diluted with cell division. Therefore, we used a Cre–lox-based strategy to trace the long-term progeny of radial glia. We used the same targeting strategy to infect the distal processes of radial glia but injected P0 Z/AP (35), Z/EG (36), or R26R (37) mice with Ad:Cre (38). Z/AP mice express alkaline phosphatase (AP), Z/EG mice express EGFP, and R26R mice express LacZ upon Cre-mediated recombination, so infected radial glia and their progeny are labeled permanently.
As with Ad:GFP-injected mice, Ad:Cre-injected Z/AP, Z/EG, or R26R mice had AP+, EGFP+, or LacZ+ cells, respectively, at the injection site and in the VZ. We confirmed that all labeled cells in the VZ were radial glia by immunostaining for RC2 (Fig. 1I) and with electron microscopy (Fig. 1J; 527 cells analyzed) 2 days after injection. We never detected labeled cells in the medial ventricular wall or on the contralateral side, indicating that the Ad:Cre did not leak into the ventricular lumen. Furthermore, cells were not nonspecifically labeled at or near the VZ by viral diffusion through the parenchyma, because infective Ad:Cre particles completely disappeared from the injection site after 4 h and were never detected near the VZ. Therefore, all labeled cells in the VZ correspond to radial glia infected at their distal processes.
Radial Glia Produce All Four Major Classes of Brain Cells. We then examined the progeny of radial glia in Z/AP, Z/EG, and R26R mice that survived 4, 7, 10, 15, 30, 45, 90, or 150 days (n ≥ 4 per age group) after Ad:Cre injection. At all time points, we observed labeled cells in the VZ and SVZ, at the injection site in the ventrolateral striatum, and scattered stellate astrocytes in the striatum. We identified radial glial progeny by using Golgi-like AP staining for morphology (see Fig. 5 C–L, which is published as supporting information on the PNAS web site), immunofluorescence for cell-type specific markers, and electron microscopy. At short survivals (P4–P10), we observed radial glia retracting their basal process from the injection site and transforming into striatal (Fig. 1K) and SVZ (Fig. 1L) astrocytes. From P4 on, the SVZ and RMS contained many migratory neuroblasts (type A cells) (Figs. 1M and 2 A and B), and from P10 on, the ipsilateral olfactory bulb contained large numbers of labeled granular (Figs. 1 M–O and 2C) and periglomerular (Fig. 1 M, N, and P) neurons. In addition, we identified labeled ependymal cells lining the lateral ventricular wall (Fig. 2 E and F) and oligodendrocytes in the corpus callosum and striatum (Fig. 2 G and H). We provide a more detailed description of the generation of ependymal cells from radial glia in a separate study (39). Importantly, we also observed labeled type B cells, the astrocytic stem cells of the adult SVZ (7–10) (Fig. 2 I and J), and type C cells, the neurogenic transit-amplifying cells that are produced by SVZ astrocytes (7, 31, 40) (Fig. 2D), strongly suggesting that radial glia give rise to adult neural stem cells.
Radial Glia, Not Striatal Cells, Act as Progenitors. To demonstrate that these cell types are derived exclusively from radial glia, we microdissected either labeled radial glia from the VZ (Fig. 2K, arrow) or labeled cells from the injection site (Fig. 2K, arrowheads) and stereotactically grafted them into the VZ/SVZ of wild-type mice. The pure population of labeled radial glia produced all cell types we observed in Ad:Cre-injected animals, including SVZ astrocytes, neuroblasts, and olfactory bulb interneurons (Fig. 2L). However, cells grafted from the injection site into the VZ/SVZ or into the ventrolateral striatum never produced ependymal cells, oligodendrocytes, neuroblasts, or olfactory bulb neurons. In a separate experiment, we found that radial glia could be labeled by adenoviral injection into the lateral ventricle of P0 Z/AP mice, demonstrating that they contact the ventricular surface. These labeled cells gave rise to all progeny we observed with injection into the ventrolateral striatum, including types B, C, and A cells and olfactory bulb interneurons (Fig. 5 J–L). Thus, the progenitor cells in this system are those that have the defining characteristics of striatal radial glia: cell bodies in the VZ that contact the ventricular surface and RC2+ processes that project through the striatum.
Radial Glia Produce Adult Neural Stem Cells. Types B, C, and A cells compose the neurogenic lineage of the adult brain (7, 30). Types C and A cells compose transient populations that disappear when neurogenesis is eliminated by treatment with antimitotic agents (7, 41, 42). However, we observe large numbers of these labeled cells in adult (up to P150) mice, long after radial glia have disappeared. This observation suggests that labeled radial glia generate the actively neurogenic adult stem cell lineage. To test this inference, we injected Z/EG mice with Ad:Cre in the ventrolateral striatum at P0 and allowed them to survive for 30 days. At P30, we added 1 mg/ml of the S-phase marker BrdUrd to their drinking water for 3 days, as described in ref. 7. We then killed these animals at P33 (n = 8) or P45 (n = 9) and immunostained for EGFP and BrdUrd. In the brains of the P33 mice, we found BrdUrd and EGFP double-labeled astrocytes in the SVZ (Fig. 3 A and B) and neuroblasts throughout the RMS (Fig. 3C). In P45 mice, most of these double-labeled cells had migrated to the olfactory bulb and matured into interneurons (Fig. 3D), but some remained in the SVZ and RMS. Thus, radial glia generate a population of cells that continue to produce neurons in the adult brain long after radial glia have disappeared. But are these cells truly stem cells?
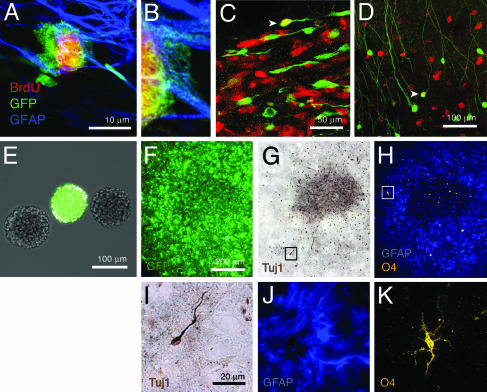
Fig. 3.
Radial glia-derived adult stem cells remain actively neurogenic in vivo. (A) Radial glia-derived (EGFP+) GFAP+ astrocytes in the adult (P33) SVZ are labeled by the S-phase marker BrdUrd. (B) GFAP EGFP double-labeling shown at higher magnification. (C) We also found many double-labeled migratory neuroblasts. (D) These adult-born neuroblasts migrate to the olfactory bulb and differentiate into interneurons. Radial glia and their adult progeny are self-renewing and multipotent in vitro. (E) EGFP+ cells isolated from the ventricular wall of P2, P10, and P90 Z/EG mice injected with Ad:Cre in the ventrolateral striatum at P0 clonally produce primary neurospheres. These EGFP+ neurospheres were sorted by FACS and grown again at clonal density for several passages to ensure clonality and self-renewal. (F–H) All cells in clonally grown neurospheres are EGFP+ (F) and differentiate into Tuj1+ neurons (visualized by diaminobenzidine, dark brown spots in G), GFAP+ astrocytes, and O4+ oligodendrocytes (H). (I) A higher magnification of a Tuj1+ neuron (box in G). (J and K) GFAP+ processes of astrocytes (J) and an O4+ oligodendrocyte (K) (box in H). The differentiated neurosphere shown in F–K was isolated from a P90 mouse, but we obtained similar results from all ages we examined, demonstrating that both radial glia and their progeny are multipotent, self-renewing stem cells. (F–H and I–K share the same scale bar.)
To address this question, we generated neurospheres from the lateral wall of the lateral ventricle of P2, P10, and P90 Z/EG animals injected with Ad:Cre in the ventrolateral striatum at P0. A neurosphere is a floating cell mass that results from the expansion of a single stem cell grown in dissociated culture (2, 43–45). Neurospheres are thought to be formed by stem cells because they are self-renewing (neurospheres form from dissociated neurospheres) and multipotent (neurospheres have the ability to differentiate into multiple cell types) (46–49). Immediately after isolation from the ventricular wall, all age groups we examined generated primary EGFP+ neurospheres (Fig. 3E) that differentiated into neurons, oligodendrocytes, and astrocytes. This result demonstrates that radial glia and their progeny are multipotent in vitro. To demonstrate self-renewal and to ensure a pure population of EGFP+ cells, we passaged primary neurospheres three or more times and then purified EGFP+ cells by FACS. We then passaged these cells at least three more times, sorted them again by FACS, and grew them at clonal density (20 cells per μl) for three passages. At each passage, we ensured a single-cell suspension by visual inspection after passage through a cell strainer. After these passages, we hand-picked individual neurospheres and transferred them to separate wells for differentiation. These clonally generated, radial glia-derived neurospheres from P2, P10, and P90 animals differentiated into neurons, astrocytes, and oligodendrocytes (Fig. 3 F–K). These experiments demonstrate that individual radial glia and their progeny in the adult brain act as stem cells.
Discussion
We demonstrate that radial glia in the neonatal ventricular wall produce multiple classes of brain cells: astrocytes, oligodendrocytes, ependymal cells, and neurons. Importantly, we also show that these radial glia give rise to adult SVZ stem cells that maintain the neurogenic lineage in the adult brain. This conclusion is based on four separate observations. First, infected radial glia produced GFAP+ SVZ astrocytes, which have been identified as the neurogenic stem cells in the SVZ (7–10). Second, the adult RMS was full of labeled migratory neuroblasts that only could have been produced in the adult brain from neurogenic adult stem cells, because radial glia disappear from the brain soon after birth. We confirmed this result by double-labeling SVZ astrocytes, neuroblasts, and olfactory bulb neurons with BrdUrd. Finally, we demonstrated that radial glia and their progeny isolated from the P2, P10, and P90 brain clonally generate self-renewing, multipotent neurospheres. We conclude that striatal radial glia give rise to SVZ astrocytes that continue to generate neurons in the adult brain. This finding extends recent evidence that radial glia can generate neurons (18–25) and supports the hypothesis (11, 26–28) that the stem cells of the adult mammalian brain are derived from radial glia, which are ultimately derived from neuroepithelial cells.
Fundamental questions about neural stem cells could be addressed by studying their lineage in greater detail. We have demonstrated that individual radial glia and their progeny act as multipotent stem cells in vitro, but are they multipotent in vivo as individual cells or as a population? To what extent does the potential of radial glia change when they transform into astrocytes? Is this process permanent or reversible? What distinguishes multipotent radial glia from radial glia that produce only neurons and astrocytes? To address these questions, the genetic recombination technique we developed to label radial glia could be adapted to introduce or eliminate a gene of interest and then trace a subset of embryonic or adult stem cells. Such work would shed light on brain development and maintenance and advance efforts to manipulate neural stem cells for therapeutic purposes.
Supplementary Material
Acknowledgments
We thank Gail Martin (University of California, San Francisco) for providing Cre reporter mice and Carlos Lois (Massachusetts Institute of Technology, Cambridge) for providing Ad:Cre; Nuria Flames for help with Ad injections and tissue processing; and Cynthia Yaschine and Matthew G. H. Chun for valuable comments that improved the manuscript. This work was supported by grants from the National Institutes of Health and a generous gift from Frances and John Bowes. F.T.M. was supported by Aging and Neurodegenerative Diseases Training Grant AG00278-01 from the National Institutes of Health/National Institute on Aging. A.D.T. was supported by Damon Runyon Cancer Research Fund Grant DRG-1601.
Author contributions: F.T.M., A.D.T., and A.A.-B. designed research; F.T.M., A.D.T., and J.M.G.-V. performed research; F.T.M., A.D.T., J.M.G.-V., and A.A.-B. analyzed data; and F.T.M., A.D.T., and A.A.-B. wrote the paper.
Abbreviations: Ad, adenovirus; Ad:Cre, Ad expressing Cre recombinase; Ad:GFP, EGFP-expressing Ad; GFAP, glial fibrillary acidic protein; Pn; postnatal day n; SVZ, subventricular zone; VZ, ventricular zone.
References
- 1.Lois, C. & Alvarez-Buylla, A. (1993) Proc. Natl. Acad. Sci. USA 90, 2074–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morshead, C. M., Reynolds, B. A., Craig, C. G., McBurney, M. W., Staines, W. A., Morassutti, D., Weiss, S. & van der Kooy, D. (1994) Neuron 13, 1071–1082. [DOI] [PubMed] [Google Scholar]
- 3.Gage, F. H. (2000) Science 287, 1433–1438. [DOI] [PubMed] [Google Scholar]
- 4.Temple, S. (2001) Nature 414, 112–117. [DOI] [PubMed] [Google Scholar]
- 5.Luskin, M. B. (1998) J. Neurobiol. 36, 221–233. [PubMed] [Google Scholar]
- 6.Alvarez-Buylla, A. & García-Verdugo, J. M. (2002) J. Neurosci. 22, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doetsch, F., Caille, I., Lim, D. A., García-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Cell 97, 703–716. [DOI] [PubMed] [Google Scholar]
- 8.Laywell, E. D., Rakic, P., Kukekov, V. G., Holland, E. C. & Steindler, D. A. (2000) Proc. Natl. Acad. Sci. USA 97, 13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skogh, C., Eriksson, C., Kokaia, M., Meijer, X. C., Wahlberg, L. U., Wictorin, K. & Campbell, K. (2001) Mol. Cell. Neurosci. 17, 811–820. [DOI] [PubMed] [Google Scholar]
- 10.Imura, T., Kornblum, H. I. & Sofroniew, M. V. (2003) J. Neurosci. 23, 2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla, A., García-Verdugo, J. M. & Tramontin, A. D. (2001) Nat. Rev. Neurosci. 2, 287–293. [DOI] [PubMed] [Google Scholar]
- 12.Misson, J. P., Edwards, M. A., Yamamoto, M. & Caviness, V. S., Jr. (1988) Brain Res. Dev. Brain. Res. 44, 95–108. [DOI] [PubMed] [Google Scholar]
- 13.Noctor, S. C., Flint, A. C., Weissman, T. A., Wong, W. S., Clinton, B. K. & Kriegstein, A. R. (2002) J. Neurosci. 22, 3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmechel, D. E. & Rakic, P. (1979) Anat. Embryol. 156, 115–152. [DOI] [PubMed] [Google Scholar]
- 15.Voigt, T. (1989) J. Comp. Neurol. 289, 74–88. [DOI] [PubMed] [Google Scholar]
- 16.Rakic, P. (1972) J. Comp. Neurol. 145, 61–84. [DOI] [PubMed] [Google Scholar]
- 17.Misson, J. P., Austin, C. P., Takahashi, T., Cepko, C. L. & Caviness, V. S., Jr. (1991) Cereb. Cortex 1, 221–229. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Buylla, A., Theelen, M. & Nottebohm, F. (1990) Neuron 5, 101–109. [DOI] [PubMed] [Google Scholar]
- 19.Tamamaki, N., Nakamura, K., Okamoto, K. & Kaneko, T. (2001) Neurosci. Res. 41, 51–60. [DOI] [PubMed] [Google Scholar]
- 20.Malatesta, P., Hartfuss, E. & Gotz, M. (2000) Development (Cambridge, U.K.) 127, 5253–5263. [DOI] [PubMed] [Google Scholar]
- 21.Miyata, T., Kawaguchi, A., Okano, H. & Ogawa, M. (2001) Neuron 31, 727–741. [DOI] [PubMed] [Google Scholar]
- 22.Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. (2001) Nature 409, 714–720. [DOI] [PubMed] [Google Scholar]
- 23.Gotz, M., Hartfuss, E. & Malatesta, P. (2002) Brain Res. Bull. 57, 777–788. [DOI] [PubMed] [Google Scholar]
- 24.Anthony, T. E., Klein, C., Fishell, G. & Heintz, N. (2004) Neuron 41, 881–890. [DOI] [PubMed] [Google Scholar]
- 25.Li, H., Babiarz, J., Woodbury, J., Kane-Goldsmith, N. & Grumet, M. (2004) Dev. Biol. 271, 225–238. [DOI] [PubMed] [Google Scholar]
- 26.Campbell, K. & Gotz, M. (2002) Trends Neurosci. 25, 235–238. [DOI] [PubMed] [Google Scholar]
- 27.Doetsch, F. (2003) Nat. Neurosci. 6, 1127–1134. [DOI] [PubMed] [Google Scholar]
- 28.Kriegstein, A. R. & Gotz, M. (2003) Glia 43, 37–43. [DOI] [PubMed] [Google Scholar]
- 29.Tramontin, A. D., García-Verdugo, J. M., Lim, D. A. & Alvarez-Buylla, A. (2003) Cereb. Cortex 13, 580–587. [DOI] [PubMed] [Google Scholar]
- 30.Herrera, D. G., García-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Ann. Neurol. 46, 867–877. [DOI] [PubMed] [Google Scholar]
- 31.Doetsch, F., García-Verdugo, J. M. & Alvarez-Buylla, A. (1997) J. Neurosci. 17, 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinds, J. W. & Ruffett, T. L. (1971) Z. Zellforsch. Mikrosk. Anat. 115, 226–264. [DOI] [PubMed] [Google Scholar]
- 33.Seri, B., García-Verdugo, J. M., McEwen, B. S. & Alvarez-Buylla, A. (2001) J. Neurosci. 21, 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peltekian, E., Parrish, E., Bouchard, C., Peschanski, M. & Lisovoski, F. (1997) J. Neurosci. Methods 71, 77–84. [DOI] [PubMed] [Google Scholar]
- 35.Lobe, C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. & Nagy, A. (1999) Dev. Biol. 208, 281–292. [DOI] [PubMed] [Google Scholar]
- 36.Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C. G. (2000) Genesis 28, 147–155. [PubMed] [Google Scholar]
- 37.Soriano, P. (1999) Nat. Genet. 21, 70–71. [DOI] [PubMed] [Google Scholar]
- 38.Anton, M. & Graham, F. L. (1995) J. Virol. 69, 4600–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spassky, N., Merkle, F. T., Flames, N., Tramontin, A. D., García-Verdugo, J. M. & Alvarez-Buylla, A., J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 40.Doetsch, F., Petreanu, L., Caille, I., García-Verdugo, J. M. & Alvarez-Buylla, A. (2002) Neuron 36, 1021–1034. [DOI] [PubMed] [Google Scholar]
- 41.Doetsch, F., García-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Proc. Natl. Acad. Sci. USA 96, 11619–11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lois, C. & Alvarez-Buylla, A. (1994) Science 264, 1145–1148. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds, B. A. & Weiss, S. (1992) Science 255, 1707–1710. [DOI] [PubMed] [Google Scholar]
- 44.Gage, F. H., Ray, J. & Fisher, L. J. (1995) Annu. Rev. Neurosci. 18, 159–192. [DOI] [PubMed] [Google Scholar]
- 45.Weiss, S., Reynolds, B. A., Vescovi, A. L., Morshead, C., Craig, C. G. & van der Kooy, D. (1996) Trends Neurosci. 19, 387–393. [DOI] [PubMed] [Google Scholar]
- 46.McKay, R. (1997) Science 276, 66–71. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, S. J., Shah, N. M. & Anderson, D. J. (1997) Cell 88, 287–298. [DOI] [PubMed] [Google Scholar]
- 48.Gage, F. H. (1998) Curr. Opin. Neurobiol. 8, 671–675. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Buylla, A. & Temple, S. (1998) J. Neurobiol. 36, 105–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.