Abstract
Upon exposure to invading microorganisms, neutrophils undergo NETosis, a recently identified type of programmed cell death, and release neutrophil extracellular traps (NETs). NETs are described as an antimicrobial mechanism, based on the fact that NETs can trap microorganisms and exhibit bactericidal activity through the action of NET-associated components. In contrast, the components of NETs have been recognized as damage-associated molecular pattern molecules (DAMPs), which trigger inflammatory signals to induce cell death, inflammation and organ failure. In the present study, to clarify the effect of NETs on cytokine production by macrophages, mouse macrophage-like J774 cells were treated with NETs in combination with lipopolysaccharide (LPS) as a constituent of pathogen-associated molecular patterns. The results revealed that NETs significantly induced the production of interleukin (IL)-1β by J774 cells in the presence of LPS. Notably, the NET/LPS-induced IL-1β production was inhibited by both caspase-1 and caspase-8 inhibitors. Furthermore, nucleases and serine protease inhibitors but not anti-histone antibodies significantly inhibited the NET/LPS-induced IL-1β production. Moreover, we confirmed that caspase-1 and caspase-8 were activated by NETs/LPS, and the combination of LPS, DNA and neutrophil elastase induced IL-1β production in reconstitution experiments. These observations indicate that NETs induce the production of IL-1β by J774 macrophages in combination with LPS via the caspase-1 and caspase-8 pathways, and NET-associated DNA and serine proteases are involved in NET/LPS-induced IL-1β production as essential components.
Keywords: macrophage, neutrophil extracellular traps, IL-1β, lipopolysaccharide, NETosis, damage-associated molecular pattern molecules
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). In response to invading microorganisms and their products [e.g., lipopolysaccharide (LPS)], immune cells produce a broad array of inflammatory mediators, including cytokines (2). Cytokines mediate a variety of responses; for example, interleukin (IL)-1β induces the expression of adhesion molecules on endothelial cells to promote the recruitment of inflammatory cells at the site of inflammation (3). Notably, inflammatory cytokines are overwhelmingly produced during sepsis, and the dysregulated production of cytokines can lead to hypotension, multiple organ failure and ultimately death (2,4). In recent years, it has been reported that cell death contributes to the production of cytokines in sepsis (5,6), and that inhibition of cell death (e.g., pyroptosis) reduces the production of cytokines and improves the survival of septic models (5–8). Thus, much attention has been focused on the relationship between the production of cytokines and host cell death during sepsis.
Neutrophils, the most abundant leukocytes in humans, defend against invading microorganisms and are considered as an essential part of the innate immune system (9,10). Neutrophils exhibit antimicrobial potential by the ability to phagocytose microorganisms and release antimicrobial agents (e.g., antimicrobial peptides and lytic enzymes) (9). Moreover, upon exposure to bacteria, neutrophils undergo NETosis (a type of programmed cell death) and release neutrophil extracellular traps (NETs) (11). Notably, NETs are described as an antimicrobial mechanism, based on the fact that NETs can trap microorganisms and exert bactericidal activity by the action of NET-associated components [e.g., antimicrobial peptides and neutrophil elastase (NE)] (12,13). In contrast, the major components of NETs (histone, DNA and granule proteins) are recognized as damage-associated molecular pattern molecules (DAMPs), which trigger inflammatory signals to induce cell death, inflammation and organ failure (14–18); for example, extracellular histones were found to mediate mortality in mouse fatal liver injury (17). Importantly, NETs, recognized as a complex of DAMPs, were found to induce the cell death of epithelial and endothelial cells (15), and provide a scaffold for platelet binding and aggregation to form the thrombosis in sepsis (19). Moreover, NETs activate macrophages to induce cytokine production in atherosclerosis (20).
However, it has not been clarified how NETs affect and induce the production of cytokines by macrophages in sepsis. Therefore, in the present study, we utilized LPS (the component of Gram-negative bacteria) as a stimulus to mimic the septic environment, and treated mouse macrophage-like J774 cells with NETs in combination with LPS. The results indicated that NETs significantly induced the production of IL-1β by J774 cells in the presence of LPS. Notably, the NET/LPS-induced IL-1β production was inhibited by both caspase-1 and caspase-8 inhibitors. Moreover, nucleases and serine protease inhibitors significantly inhibited the NET/LPS-induced IL-1β production. These observations suggest that NETs induce the production of IL-1β from LPS-stimulated macrophages via the caspase-1 and caspase-8 pathways, and NET-associated DNA and serine proteases are likely involved in the NET/LPS-induced IL-1β production as essential components.
Materials and methods
Reagents
Ac-YVAD (Tyr-Val-Ala-Asp)-CHO was purchased from Peptide Institute (Osaka, Japan). Ac-IETD (Ile-Glu-Thr-Asp)-CHO was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Z-VAD (Val-Ala-Asp)-FMK was purchased from InvivoGen (San Diego, CA, USA). Deoxyribonuclease I (DNase I) and micrococcal nuclease (MNase) were purchased from Takara Bio, Inc. (Shiga, Japan). LPS (Escherichia coli B55:05), 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (ABESF), α1-anti-trypsin, calf thymus (CT)-histone, CT-DNA, N-acetyl-L-cysteine (NAC) [a reactive oxygen species (ROS) scavenger] and neutrophil elastase (elastase from human leukocytes) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse histone H2AX monoclonal antibody (MAB3406) and mouse histone H4 polyclonal antibody (AF5215) were purchased from R&D Systems, Inc. (Minneapolis, MN, USA), and used for the neutralization of the NET-associated histones.
Mice
Male BALB/c mice (15 weeks old; Sankyo Labo Service, Tokyo, Japan) were used in the experiments. Mice were bred under specific pathogen-free (SPF) conditions, and housed in temperature-controlled, air-conditioned facilities with a 12/12-h light/dark cycle, and food and water ad libitum. All experiments were approved by the Ethics Committee for the Use of Laboratory Animals of Juntendo University, Graduate School of Medicine (Tokyo, Japan; permit no. 270193).
Isolation of neutrophils
Murine bone marrow-derived neutrophils were harvested from BALB/c mice. Briefly, the tibia and femur were isolated and flushed with RPMI-1640 medium (Sigma-Aldrich). The red blood cells were then removed from bone marrow cells by hypotonic lysis (0.2% NaCl), and the resultant cell suspension was centrifuged at 600 × g for 5 min. The cell pellet was resuspended in PBS, and further centrifuged (1,600 × g, 30 min) over 62% Percoll (GE Healthcare Life Sciences, Marlborough, MA, USA). Murine neutrophils at the bottom of a 62% layer were collected and washed in PBS (21). Neutrophils were resuspended in RMPI-1640 medium at a cell density of 1.7×106 cells/ml.
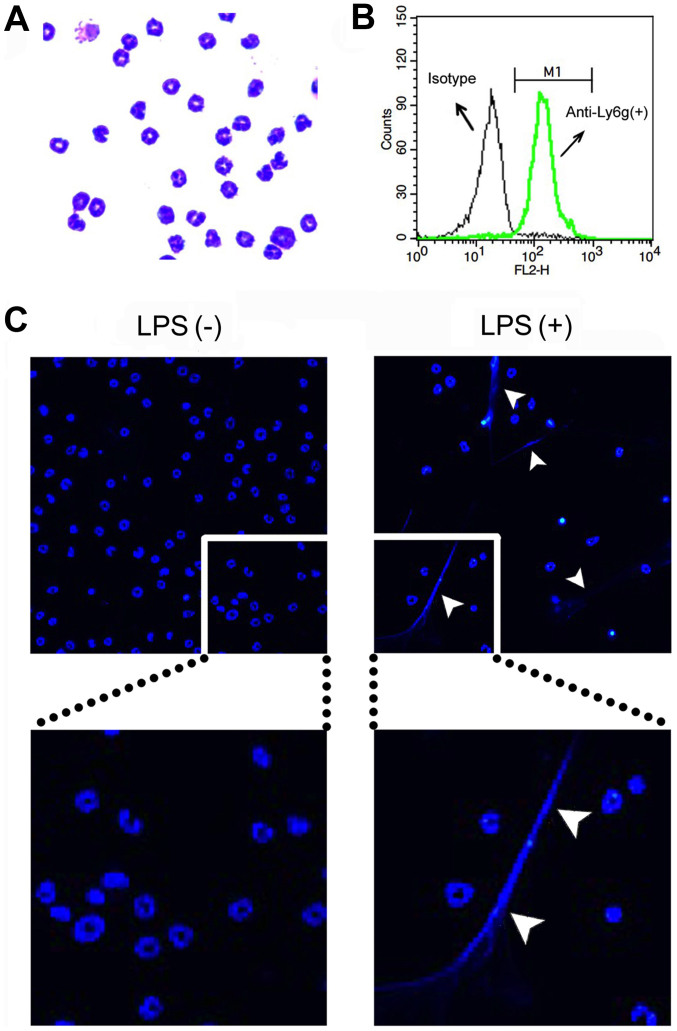
Purities of the neutrophils were determined by both cytospin counts with Giemsa stain (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and forward scatter/side scatter gating of cells stained with PE/Cy5-conjugated anti-Ly6g (a neutrophil-specific marker) monoclonal antibody (RB6-8C5; Abcam, Cambridge, MA, USA) [isotype control, a PE/Cy5-conjugated rat IgG2b monoclonal antibody (eB149/10H5), eBioscience, Inc., San Diego, CA, USA] using a flow cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). The neutrophil fraction contained >90% neutrophils as determined by Giemsa staining (Fig. 1A) and flow cytometry (Ly6g staining) (Fig. 1B).
Figure 1.
Preparation of neutrophil extracellular traps (NETs). (A) Purities of bone marrow-derived neutrophils were determined by both cytospin counts with Giemsa staining and (B) flow cytometry of forward scatter/side scatter gating of cells stained with PE/Cy5-conjugated anti-Ly6g (a neutrophil-specific marker) monoclonal antibody (isotype control, a PE/Cy5 conjugated rat IgG2b mAb). For evaluating the formation of NETs, neutrophils were seeded over a 12-mm glass coverslip and treated without (−) or with (+) 200 ng/ml lipopolysaccharide (LPS) for 4 h to induce NETs. (C) Then, the cells were stained with Hoechst 33342 and photographed with a fluorescence microscope system Axioplan 2. The areas of the respective images were enlarged and are shown below. Arrowheads indicate the formation of NETs. Images are representative of 3 separate experiments.
Formation and preparation of NETs
To evaluate NET formation by microscopy, neutrophils (1.3×106 cells/well) were seeded over a 12-mm glass coverslip (Thermo Fisher Scientific, Pittsburg, PA, USA) in 24-well plates (Iwaki Brand; Asahi Techno Glass Corporation, Tokyo, Japan) for 30 min. After adhesion, the cells were treated with 200 ng/ml LPS for 4 h to induce NETs. Then, the cells were washed with RPMI-1640 medium and stained with Hoechst 33342 (0.8 µg/ml). Thereafter, the coverslips were mounted on a slide glass by using aqueous medium Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA), and the cells were photographed using a fluorescence microscope system Axioplan 2 (Carl Zeiss, Jena, Germany) (Fig. 1C).
To prepare NETs as a stimulant, neutrophils (1.7×106 cells/well) were seeded in 6-well plates for 30 min in a total volume of 1 ml RPMI-1640 medium, and then were treated with 200 ng/ml LPS for 4 h to induce NETs. Then, the cells were washed twice with fresh PRMI-1640 medium, and the NETs were collected by extensively pipetting with 1 ml RPMI-1640 medium (22). Thereafter, the NETs were recovered as a supernatant at a concentration of 1.7×106 cell equivalents/ml by centrifugation at 400 × g and 10,000 × g. Alternatively, the neutrophils were incubated without LPS for 4 h, and the supernatants were recovered and used as the control.
Measurement of NET-associated protein
The concentration of the NET-associated protein (µg/ml) was quantified using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's instructions using samples prepared with (+) or without (−) LPS stimulation of neutrophils.
Measurement of NET-associated DNA
The concentration of NET-associated DNA was quantified by adding 5 µg/ml Hoechst 33342 (FLICA™ caspase-1 assay) to samples (100 µl) in a 96-well plate (Nunc™ MicroWell™ 96-well microplates; Thermo Fisher Scientific). The emission (E) at 460 nm (excitation at 355 nm) was measured using samples prepared with (+) or without (−) LPS stimulation of neutrophils, and RPMI-1640 medium as a background. The relative fold change was calculated as follows: Relative fold change = (Ewith LPS − ERPMI-1640)/(Ewithout LPS − ERPMI-1640).
Measurement of NET-associated NE
The NET-associated NE was detected by western blotting. Briefly, samples prepared with (+) or without (−) LPS stimulation of neutrophils were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore Corp., Billerica, MA, USA). The membranes were blocked with BlockOne (Nakara, Tokyo, Japan), probed with rat anti-mouse NE mAb (R&D Systems, Inc.), followed by horseradish peroxidase-conjugated anti-rat IgG (Santa Cruz Biotechnology, Inc.). Signals were detected with SuperSignal West Dura chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, IL, USA), and quantified as grey-scale using LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan) and MultiGauge software (Fujifilm). Relative fold change was calculated as follows; Relative fold change = grey-scalewith LPS/grey-scalewithout LPS.
Measurement of NET-associated myeloperoxidase (MPO)-DNA complex
The NET-associated MPO-DNA complex was detected by MPO-DNA ELISA. Briefly, a 96-well plate (Thermo Fisher Scientific) was coated with 5 µg/ml anti-mouse MPO monoclonal antibody (AF3667; R&D Systems, Inc.) overnight at 4°C. After washing 3 times, 20 µl of the samples prepared with (+) or without (−) LPS stimulation of neutrophils and RPMI-1640 medium (as a background) were added to the wells with 80 µl of incubation buffer containing a peroxidase-labeled anti-DNA monoclonal antibody (MCA-33; Cell Death ELISAPlus; Roche Diagnostics, Indianapolis, IN, USA). The plate was incubated for 2 h with shaking at 300 rpm at room temperature. After 3 washes, 100 µl peroxidase substrate (ABTS; Cell Death ELISAPlus) was added, and absorbance (A) at 405 nm was measured after a 20-min incubation at room temperature in the dark. Relative fold change was calculated as follows: Relative fold change = (Awith LPS − ARPMI-1640)/(Awithout LPS − ARPMI-1640).
Measurement of NET-associated histone-DNA complex
The NET-associated histone-DNA complex was detected using Cell Death ELISAPlus (Roche Diagnostics) according to the manufacturer's instructions. Briefly, 20 µl of the samples prepared with (+) or without (−) LPS stimulation of neutrophils and RPMI-1640 medium (as a background) were added to a streptavidin-coated plate with 80 µl of incubation buffer containing a peroxidase-labeled anti-DNA mAb. The plate was incubated for 2 h with shaking at 300 rpm at room temperature. After 3 washes, 100 µl peroxidase substrate (ABTS) was added, and absorbance at 405 nm was measured after a 20-min incubation at room temperature in the dark. Relative fold change was calculated as follows: Relative fold change = (Awith LPS − ARPMI-1640)/(Awithout LPS − ARPMI-1640).
Cell culture
A murine macrophage-like cell line J774 was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Sanko Junyaku, Tokyo, Japan) and 100 U/ml penicillin/100 µg/ml streptomycin (Nacalai Tesque, Kyoto, Japan) at 37°C in 5% CO2. J774 cells (2×104 cells/well) were seeded in 96-well culture plates (Iwaki Brand) overnight, and then treated with NETs (1.7×105 cell equivalents) for 24 h in the absence or presence of 10 ng/ml LPS at 37°C in 5% CO2. Thereafter, the supernatants were recovered for the IL-1β and lactate dehydrogenase (LDH) assays.
Assays for caspase-1 and caspase-8 activation
J774 cells (2×104 cells/well) were seeded in CC2 Chamber Slide system 8-wells (Thermo Fisher Scientific) overnight, and then treated with NETs (1.7×105 cell equivalents) for 24 h in the absence or presence of 10 ng/ml LPS at 37°C in 5% CO2. Thereafter, the activation of caspase-1 and caspase-8 was assayed using the FLICA™ caspase-1 assay kit (ImmunoChemistry Technologies, LLC, Bloomington, MN, USA) and FLICA™ caspase-8 assay kit (ImmunoChemistry Technologies, LLC), respectively. Briefly, after treatment with LPS and NETs, the cells were washed with fresh RPMI-1640 medium, and then the cells were labeled with FAM-YVAD-FMK or FAM-LETD-FMK (fluorescent-labeled caspase-1 or caspase-8 inhibitor that binds with activated caspase-1 or caspase-8, respectively) by incubation for 1 h at 37°C. After washing, the cells were labeled with Hoechst 33342 (0.8 µg/ml) for 10 min. Finally, the slide was mounted with a cover glass (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) using aqueous medium Vectashield, and the cells were photo-graphed using a fluorescence microscope system Axioplan 2.
Quantification of IL-1β and LDH
IL-1β 1evels in the supernatants were determined using a commercially available mouse IL-1β ELISA kit (eBioscience, Inc.), according to the manufacturer's instructions. LDH activity in the supernatants was determined for the evaluation of cell death; LDH activity in the supernatants and 1% Triton X-100-lysed cells (as a total activity of 100%) was determined using a commercially available LDH assay kit (Takara Bio, Inc.), according to the manufacturer's instructions.
Statistical analysis
Data are shown as the means ± standard deviation. A statistical test was performed using one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test (GraphPad Prism; GraphPad Software, Inc., San Diego, CA, USA). Otherwise, a statistical test was performed using the Student's t-test for experiments with two groups (GraphPad Prism). P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of the NETs
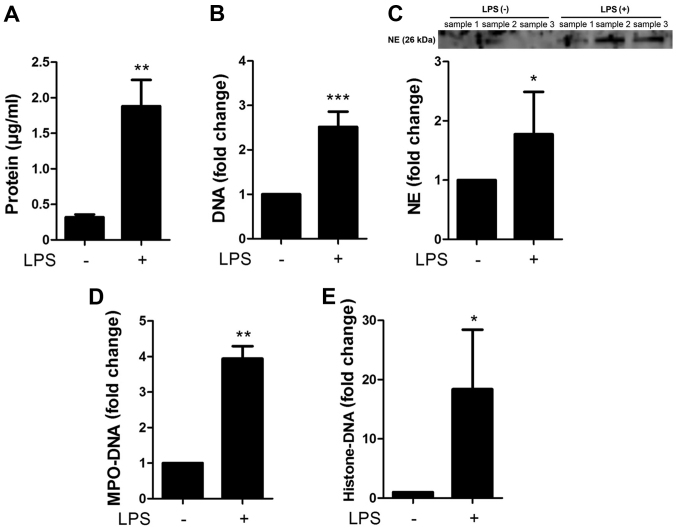
The NETs were characterized by measuring the concentrations of proteins, DNA, NE, MPO-DNA complex and histone-DNA complex contained in the preparations of NETs (with LPS stimulation) and the controls (without LPS stimulation) (Fig. 2A). The preparation of NETs contained 1.88±0.37 µg/ml protein, whereas the controls contained 0.32±0.04 µg/ml protein. Moreover, the NETs contained 2.52±0.34-fold higher DNA, 1.77±0.72-fold higher NE, 3.94±0.35-fold higher MPO-DNA complex and 18.4±10.0-fold higher histone-DNA complex than the controls (Fig. 2B–E), confirming that NETs contain the complexes of DNA, histone and granule proteins.
Figure 2.
Characterization of the neutrophil extracellular traps (NETs). NETs were characterized by measuring the concentrations of (A) proteins, (B) DNA, (C) neutrophil elastase (NE), (D) myeloperoxidase (MPO)-DNA complex and (E) histone-DNA complex contained in the preparations of NETs [+, with lipopolysaccharide (LPS) stimulation] and control (−, without LPS stimulation). Data show the means ± standard deviation of 3–6 separate experiments. *P<0.05, **P<0.01, ***P<0.001. Images are representative of 3 separate experiments.
Treatment with NETS and LPS induces IL-1β production by macrophages via the caspase-1 and caspase-8 pathways
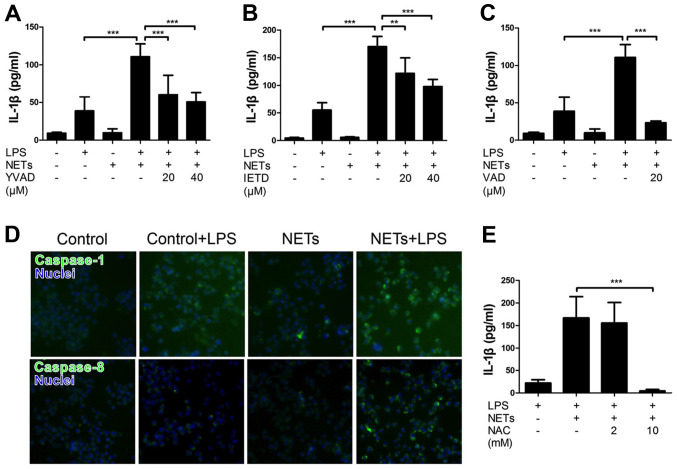
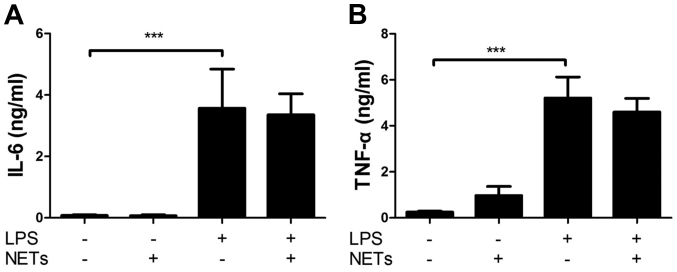
We first examined the effect of NETs and LPS treatment on the cytokine production by macrophages by measuring the release of IL-1β and LDH. Mouse macrophage-like J774 cells were treated with NETs and LPS for 24 h. The results indicated that LPS or NET treatment alone did not essentially induce the production of IL-1β (Fig. 3A) and LDH (cell death, data not shown). Notably, the treatment of J774 cells with both NETs and LPS significantly induced the release of IL-1β (Fig. 3A), but not LDH (data not shown). Notably, Ac-YVAD-CHO (a caspase-1-specific inhibitor), Ac-IETD-CHO (a caspase-8-specific inhibitor) and Z-VAD-FMK (a pan caspase inhibitor) inhibited the NET/LPS-induced production of IL-1β (Fig. 3A–C), suggesting that the NET/LPS-induced IL-1β production was dependent on the activation of caspase-1 and caspase-8. In fact, we confirmed the activation of caspase-1 and caspase-8 by staining the activated caspase with FAM-YVAD-FMK (a fluorescence-labeled caspase-1 inhibitor) and FAM-LETD-FMK (a fluorescence-labeled caspase-8 inhibitor), respectively. As shown in Fig. 3D, LPS or NET treatment alone did not significantly induce either caspase-1 or the caspase-8 activation; however, the NET/LPS treatment considerably increased the activation of both caspase-1 and caspase-8. These observations indicate that treatment with NETs and LPS induced the production of IL-1β by macrophages via caspase-1- and caspase-8-dependent pathways. Furthermore, NAC (a ROS scavenger) inhibited the NET/LPS-induced IL-1β production by J774 cells (Fig. 3E), suggesting the involvement of ROS in the NET/LPS-induced IL-1β production by macrophages. Separately, we confirmed that LPS alone significantly increased the levels of IL-6 and tumor necrosis factor (TNF)-α compared with the NETs alone (Fig. 4A and B). Importantly, NET/LPS treatment did not further increase the levels of IL-6 and TNF-α production compared with LPS alone. Moreover, NETs alone did not essentially induce the production of IL-6 and TNF-α compared with LPS, suggesting that NETs, used in this study, if any, contained little amount of LPS for activating J774 cells.
Figure 3.
Effect of lipopolysaccharide (LPS) and neutrophil extracellular trap (NET) treatment on the interleukin (IL)-1β production by J774 cells. Macrophage-like J774 cells (2×104 cells) were treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h in 100 µl RPMI-1640 medium in the absence or presence of (A) Ac-YVAD-CHO (a caspase-1-specific inhibitor), (B) Ac-IETD-CHO (a caspase-8-specific inhibitor) or (C) Z-VAD-FMK (a pan-caspase inhibitor) at the indicated concentrations (µM). Thereafter, the supernatants were recovered for the assays of IL-1β. Data show the means ± standard deviation (SD) of 3-5 separate experiments. Values are compared between NET/LPS treatment and LPS alone or in the presence of caspase inhibitors. (D) J774 cells were treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h. Thereafter, the cells were stained with FAM-YVAD-FMK or FAM-LETD-FMK (fluorescent-labeled caspase-1 or caspase-8 inhibitor that binds with activated caspase-1 or caspase-8, respectively) and Hoechst 33342 (for nuclear staining, blue) and photographed with a fluorescence microscope system. Images of cells are representative of 4 separate experiments. (E) J774 cells (2×104 cells) were treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h in 100 µl RPMI-1640 medium in the absence or presence of NAC (a reactive oxygen species scavenger). Thereafter, the supernatants were recovered for the assays of IL-1β. Data show the means ± SD of 3 separate experiments. Values are compared between the NET/LPS treatment in the absence and presence of NAC. **P<0.01, ***P< 0.001.
Figure 4.
Effect of lipopolysaccharide (LPS) and neutrophil extracellular trap (NET) treatment on the production of interleukin (IL)-6 and tumor necrosis factor (TNF)-α by J774 cells. J774 cells (2×104 cells) were treated with LPS (10 ng/ml), NETs (1.7×105 cell equivalents) or their combinations in 100 µl RPMI-1640 medium for 24 h. Thereafter, the supernatants were recovered for the assays of (A) IL-6 and (B) TNF-α. Data show the means ± standard deviation of 3 separate experiments. Values are compared between without and with LPS. ***P<0.001.
NET-associated DNA is an important component of the NET/LPS-induced IL-1β production
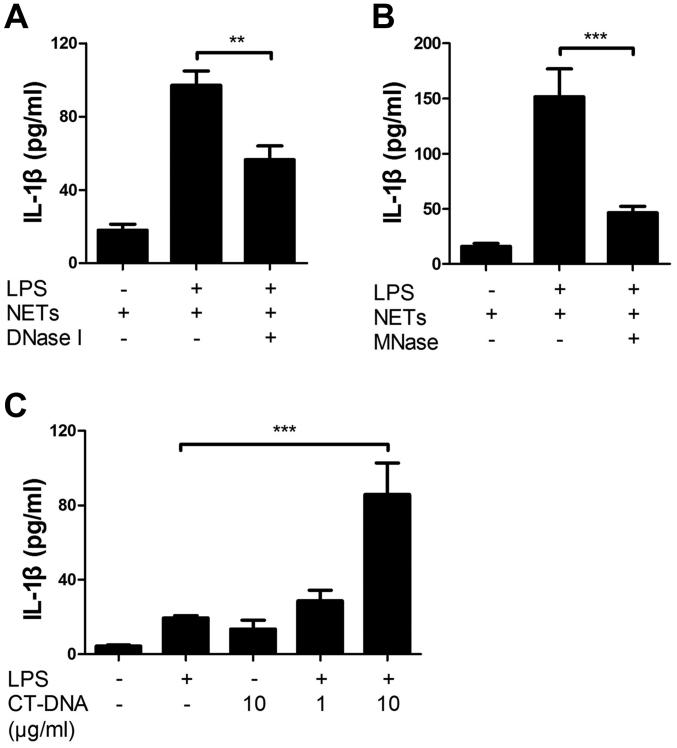
Furthermore, we investigated the involvement of NET-associated DNA in the NET/LPS-induced IL-1β production by using nucleases. Thus, J774 cells were treated with NETs/LPS in the presence of nucleases (DNase I or MNase) for 24 h. Preliminarily, we confirmed that DNase I and MNase totally degraded DNA (as evidence by agarose gel electrophoresis) and did not show cytotoxicity (as evidenced by LDH assay) (data not shown). Notably, both DNase I and MNase significantly inhibited the NET/LPS-induced IL-1β production by J774 cells (Fig. 5A and B). Next, we examined the effect of DNA on IL-1β production by J774 cells using CT-DNA to mimic the NET-associated DNA. CT-DNA alone did not induce the production of IL-1β; however, CT-DNA and LPS significantly induced IL-1β production (Fig. 5C). These observations indicate that NET-associated DNA is an important component of NET/LPS-induced IL-1β production by macrophages.
Figure 5.
Involvement of neutrophil extracellular trap (NET)-associated DNA in the NET/lipopolysaccharide (LPS)-induced interleukin (IL)-1β production. J774 cells (2×104 cells) were treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h in 100 µl RPMI-1640 medium in the absence or presence of nucleases [(A) deoxyribonuclease I (DNase I); (B) micrococcal nuclease (MNase)]. Thereafter, the supernatants were recovered for the assays of IL-1 β. (C) Furthermore, J774 cells were treated with LPS (10 ng/ml) and calf thymus (CT)-DNA (1 and 10 µg/ml) for 24 h, and then the supernatants were recovered for the assays of IL-1β. Data shows the means ± standard deviation of 3-6 separate experiments. Values are compared between the NET/LPS treatment in the absence and presence of (A and B) nucleases, or LPS alone and (C) CT-DNA/LPS treatment. **P<0.01, ***P<0.001.
NET-associated serine proteases are important components of NET/LPS-induced IL-1β production
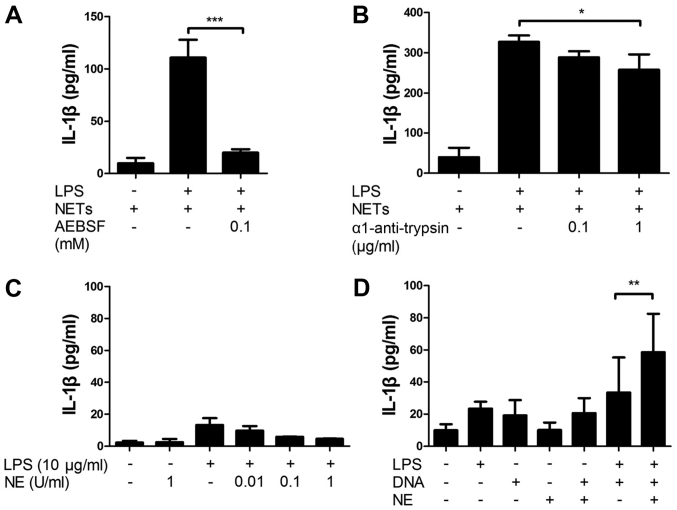
Next, we investigated the involvement of NET-associated serine proteases in the NET/LPS-induced IL-1β production using serine protease inhibitors. Thus, the J774 cells were treated with NETs/LPS in the presence of serine protease inhibitors (AEBSF, a permeable serine inhibitor or α1-anti-trypsin, an impermeable serine protease inhibitor) for 24 h. Importantly, both AEBSF and α1-anti-trypsin significantly inhibited the NET/LPS-induced IL-1β production by J774 cells (Fig. 6A and B). These results indicate that NET-associated serine proteases are important components of the NET/LPS-induced IL-1β production by macrophages. To confirm the involvement of serine proteases in IL-1β production, the J774 cells were incubated with NE. Unexpectedly, neither NE alone nor the NE/LPS treatment essentially induced the production of IL-1β (Fig. 6C). Notably, however, the NE/DNA treatment significantly induced the IL-1β production by the J774 cells in the presence of LPS (Fig. 6D), suggesting that NE induces IL-1β production from macrophages in combination with DNA.
Figure 6.
Involvement of neutrophil extracellular trap (NET)-associated serine proteases in the NET/lipopolysaccharide (LPS)-induced interleukin (IL)-1β production. J774 cells were (2×104 cells) treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h in 100 µl RPMI-1640 medium in the absence or presence of serine protease inhibitors: (A) AEBSF or (B) α1-anti-trypsin. Thereafter, the supernatants were recovered for the assays of IL-1β. Data show the means ± standard deviation (SD) of 3-5 separate experiments. Values are compared between the NET/LPS treatment in the absence and presence of serine protease inhibitors. J774 cells (2×104 cells) were treated with (C) LPS (10 ng/ml) and neutrophil elastase (NE) (0.01 and 0.1 U/ml) in 100 µl RPMI-1640 medium for 24 h. Otherwise, J774 cells were treated with (D) LPS (10 ng/ml), NE (0.1 U/ml), CT-DNA (1 µg/ml) or their combinations for 24 h. Thereafter, the supernatants were recovered for the assays of IL-1β. Data show the means ± SD of 3–6 separate experiments. Values are compared between the LPS/DNA and LPS/DNA/NE. *P<0.05, **P<0.01, ***P<0.001.
NET-associated histones are unlikely involved in NET/LPS-induced IL-1β production
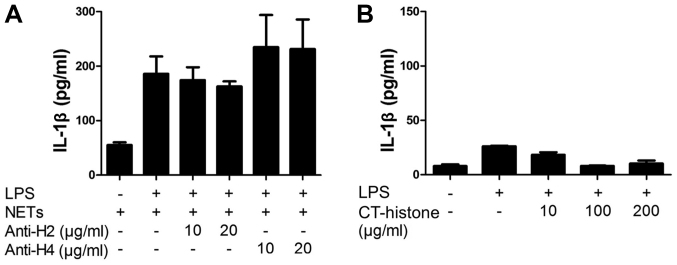
Finally, we investigated the involvement of NET-associated histones in the NET/LPS-induced IL-1β production by macrophages using histone antibodies. Thus, the J774 cells were treated with NETs/LPS in the presence of histone antibodies (anti-histone H2 mAb or anti-histone H4 pAb) for 24 h. Notably, neither anti-histone H2 mAb nor anti-histone H4 pAb inhibited the NET/LPS-induced IL-1β production by J774 cells (Fig. 7A). Further, we examined the direct effect of histones on the IL-1β production by J774 cells using CT-histones. As a result, CT-histones did not induce IL-1β production by the J774 cells in the presence of LPS (Fig. 7B). These observations indicate that NET-associated histones were unlikely involved in the NET/LPS-induced IL-1β production by macrophages in our experiments.
Figure 7.
Involvement of neutrophil extracellular trap (NET)-associated histones in NET/lipopolysaccharide (LPS)-induced interleukin (IL)-1β production. J774 cells (2×104 cells) were treated with LPS (10 ng/ml) and NETs (1.7×105 cell equivalents) for 24 h in 100 µl RPMI-1640 medium in the absence or presence of (A) histone antibodies (anti-histone H2 and anti-histone H4). Thereafter, the supernatants were recovered for the assays of IL-1β. (B) Furthermore, J774 cells were treated with LPS (10 ng/ml) and calf thymus (CT)-histones (10, 100 and 200 µg/ml) for 24 h, and then the supernatants were recovered for the assays of IL-1β. Data shows the means ± standard deviation of 4 separate experiments.
Discussion
Neutrophils play an important role as effectors of inflammation, tissue injury and host defense against microbial infection (9,10). Besides the well-known antimicrobial potential, neutrophils trap and kill invading microorganisms by extracellularly releasing NETs via NETosis, a recently identified type of programmed cell death (11–13). Importantly, the components of NETs (such as DNA and NE) are demonstrated to be potential mediators for cytokine production in sepsis (11). For example, degradation of DNA (by administration of recombinant DNase I) was found to inhibit over-produced cytokines in septic mice (23–25); and knockout of NE significantly reduced the production of IL-1β in response to Pseudomonas aeruginosa infection in mice (26). In the present study, we revealed that NETs, as a complex of DAMPs (containing DNA, histone and serine proteases) induced the production of IL-1β by macrophage-like J774 cells in the presence of LPS via the action of caspase-1 and caspase-8, and that the NET-associated DNA and serine proteases were involved in the production of IL-1β by the cells.
IL-1β is a prototypical inflammatory cytokine, which stimulates both local and systemic inflammatory responses (3), and acts synergistically with other cytokines to cause tissue injury in sepsis (27). The production of IL-1β is mediated mainly by the activation of caspase-1 (27–29), and requires two distinct stimuli, microbial pathogen-associated molecular patterns (PAMPs, e.g., lipoproteins and LPS) and endogenous DAMPs (e.g., DNA and ATP) (28,29). Stimulation by PAMPs initiates a signaling cascade that leads to cellular activation (including the upregulation of inflammatory cytokine genes) (30). In contrast, stimulation by DAMPs activates caspase-1, which is involved in the processing and release of IL-1β (30). Additionally, recent studies have revealed that caspase-8 acts as either a direct enzyme for the processing and release of IL-1β or as an initiator for the activation of caspase-1, in response to PAMPs and DAMPs (e.g., LPS and ATP) (31–34). In the present study, LPS and NETs were regarded as PAMPs and DAMPs, respectively. Importantly, LPS or NET treatment alone did not essentially elicit IL-1β production from J774 cells, and treatment with both LPS and NETs significantly induced IL-1β production (Fig. 3A). Importantly, the NET/LPS-induced IL-1β production was inhibited by not only Ac-YVAD-CHO (a caspase-1-specific inhibitor) but also Ac-IEAD-CHO (a caspase-8-specific inhibitor) (Fig. 3A and B). Moreover, we confirmed that the NET/LPS treatment activated both caspase-1 and caspase-8 (Fig. 3D). These observations suggest that the NET/LPS treatment induced the production of IL-1β via the action of caspase-1 and caspase-8 (Fig. 8). Furthermore, it has been recently reported that ROS can be common upstream activators of the caspase-1 and caspase-8 pathways (35,36). Thus, we investigated the effect of NAC (an ROS scavenger) on the NET/LPS-induced IL-1β production. Notably, NAC inhibited the NET/LPS-induced IL-1β production by J774 cells (Fig. 3E), supporting the involvement of ROS in the NET/LPS-induced IL-1β production by macrophages. Furthermore, it has been reported that LPS alone can efficiently induce the production of other cytokines (e.g., IL-6 and TNF-α), and the additional treatment of DAMPs (e.g., ATP) cannot augment the LPS-induced production of these cytokines (37,38). In the present study, we confirmed that LPS alone significantly increased the levels of IL-6 and TNF-α compared with the NETs alone (Fig. 4), and the NET/LPS treatment did not further increase the levels of IL-6 and TNF-α production compared with LPS alone, suggesting that NETs may not be important for the production of these cytokines in sepsis compared with PAMPs and other DAMPs.
Figure 8.
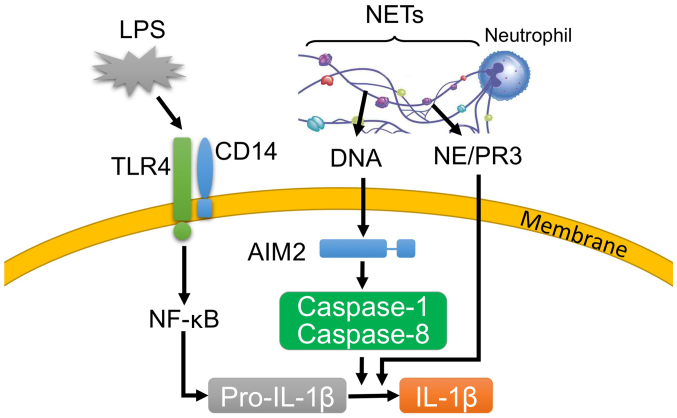
Postulated mechanism for the neutrophil extracellular trap (NET)/lipopolysaccharide (LPS)-induced production of interleukin (IL)-1β by macrophage-like J774 cells. LPS induces the expression of pro-IL-1β in the TLR4 pathway. Alternatively, intracellular DNA, which is derived from phagocytosed NETs, activates caspase-1 and caspase-8 via absent in melanoma 2 (AIM2). The activated caspase-1 and caspase-8 process and release IL-1β. Moreover, NET-associated serine proteases [e.g., proteinase 3 and neutrophil elastase (NE)] likely participate in the NET/LPS-induced IL-1β production by processing IL-1β. NF-κB, nuclear factor-κB.
Genomic DNA is the main component of NETs (14). In this study, nucleases (DNase I and MNase) inhibited the NET/LPS-induced production of IL-1β (Fig. 5A and B), suggesting that DNA is an important component of the NET/LPS-induced production of IL-1β. We also confirmed that extracellular DNA (CT-DNA) induced the production of IL-1β in combination with LPS (Fig. 5C). Previous studies demonstrated that cytosol absent in melanoma 2 (AIM2) is essential for the recognition of cytosol DNA to induce the activation of caspase-1/caspase-8 and production of IL-1β (39,40). In our system, we did not transfect DNA into the cytosol; however, extracellular DNA could be phagocytosed by macrophages in the presence of LPS, since LPS can potently augment the phagocytic ability of macrophages (41). Importantly, NETs are phagocytosed by macrophages either in the presence or absence of invading microorganisms (20,24). Thus, it is reasonable to speculate that the NETs were phagocytosed by macrophages, and then the NET-associated DNA induced the production of IL-1β via the recognition of DNA by AIM2 (Fig. 8).
Notably, genomic DNA is normally localized in the nuclei, and is extracellularly released during cell death (e.g., NETosis and necrosis) (42). Importantly, the serum levels of extracellular DNA in septic patients are significantly higher than that in healthy individuals (43). Notably, degradation of DNA by the administration of recombinant DNase I was found to inhibit the over-produced cytokines in septic mice (23–25). Importantly, NET-associated DNA (characterized by DNA-histone, DNA-MPO or DNA-NE complex) is detected in blood, and its level is correlated with that of extracellular DNA in blood in sepsis (44,45). These observations suggest that the NET-associated DNA may contribute to the cytokine production in sepsis.
Neutrophil serine proteases (including proteinase 3, NE and cathepsin G) are important regulators of inflammation (14,46). Importantly, NET-associated serine proteases mediate the production of cytokines and chemokines [e.g., IL-1β and monocyte chemotactic protein-1 (MCP-1)] by inflammatory cells (47). In the present study, both AEBSF (a cell permeable serine protease inhibitor) and α1-anti-trypsin (a cell impermeable serine protease inhibitor) significantly inhibited the NET/LPS-induced production of IL-1β (Fig. 6A and B), suggesting that NET-associated serine proteases are important for the production of IL-1β. Notably, the inhibitory effect of α1-anti-trypsin was significantly lower than that of AEBSF (Fig. 6A and B), suggesting a possibility that intracellular NET-associated serine proteases are more important for the production of IL-1β. Notably, the possibility may be supported by the findings that NET-associated serine proteases are phagocytosed by macrophages accompanied with NETs (20,24), as discussed above, and that neutrophil serine proteases (e.g., proteinase 3 and NE) can process IL-1β (48). Furthermore, we aimed to evaluate the effect of serine proteases on the production of IL-1β using NE. Unexpectedly, NE (0.01 U and 0.1 U) did not essentially induce the production of IL-1β by J774 cells in the absence or presence of LPS (Fig. 6C). Thus, based on the high affinity of NE for DNA (516–712 nM) (49), we evaluated the combined effect of NE (0.1 U/ml) and CT-DNA (1 µg/ml) on IL-1β production. The results revealed that NE and CT-DNA induced the production of IL-1β in the presence of LPS (Fig. 6D), suggesting that neutrophil serine proteases such as NE can be an important component of NETs for the production of IL-1β by macrophages in combination with DNA (Fig. 8).
NETs are described as a complex of antimicrobial components with the ability to trap and kill bacteria (11); however, NETs exacerbate the pathophysiology of sepsis via the actions of inducing cell death and thrombus formation (15,19). Furthermore, we revealed that NETs, as a complex of DAMPs, induce the production of IL-1β by macrophages in combination with LPS via the action of caspase-1 and caspase-8, and that DNA and serine proteases are important components of NETs for IL-1β production. Thus, our findings suggest a possibility that NETs play a role in cytokine production and have an adverse impact on sepsis, and provide a novel insight that the degradation and/or inhibition of NET-associated effective components may be a therapeutic target of sepsis.
In conclusion, the present results demonstrated that NETs significantly induced the IL-1β production by mouse macrophage-like J774 cells in combination with LPS. Importantly, the NET/LPS-induced IL-1β production was inhibited by both caspase-1 and caspase-8 inhibitors. Moreover, nucleases and serine protease inhibitors significantly inhibited the NET/LPS-induced IL-1β production. These observations suggest that NETs induce the production of IL-1β from macrophages in combination with LPS via the caspase-1 and caspase-8 pathways, and NET-associated DNA and serine proteases are likely involved in the NET/LPS-induced IL-1β production as essential components (Fig. 8).
Acknowledgments
We are grateful to Dr Kaori Suzuki (Department of Host Defense and Biochemical Research, Juntendo University, Graduate School of Medicine) for the helpful discussion. This study was supported by a Grant-in-Aid (grant no. 26460538) for Scientific Research from the Japan Society for the Promotion of Science, a Grant-in-Aid (grant no. S0991013) and a Grant-in-Aid (grant no. S1311011) from the Ministry of Education, Culture, Sport, Science, and Technology, Japan (MEXT) for the Foundation of Strategic Research Projects in Private Universities.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien JM, Jr, Ali NA, Aberegg SK, Abraham E. Sepsis. Am J Med. 2007;120:1012–1022. doi: 10.1016/j.amjmed.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 5.Pinheiro da Silva F, Nizet V. Cell death during sepsis: Integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis. 2009;14:509–521. doi: 10.1007/s10495-009-0320-3. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Murakami T, Suzuki K, Tamura H, Reich J, Kuwahara-Arai K, Iba T, Nagaoka I. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int Immunol. 2016;28:245–253. doi: 10.1093/intimm/dxv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 10.Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 11.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 12.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfaidi M, Wilson H, Daigneault M, Burnett A, Ridger V, Chamberlain J, Francis S. Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium. J Biol Chem. 2015;290:24067–24078. doi: 10.1074/jbc.M115.659029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhang X, Monestier M, Esmon L, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon L, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 20.Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis-induced neutrophil extracellular traps activate human macrophages. J Innate Immun. 2013;5:591–602. doi: 10.1159/000348676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, Thorlacius H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L586–L596. doi: 10.1152/ajplung.00365.2013. [DOI] [PubMed] [Google Scholar]
- 24.Meng W, Paunel-Görgülü A, Flohé S, Hoffmann A, Witte I, MacKenzie C, Baldus SE, Windolf J, Lögters TT. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai SH, Khan M, Dwivedi DJ, Ross CA, Zhou J, Gould TJ, Gross PL, Weitz JI, Fox-Robichaud AE, Liaw PC, Canadian Critical Care Translational Biology Group Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock. 2015;44:166–172. doi: 10.1097/SHK.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 26.Karmakar M, Sun Y, Hise AG, Rietsch A, Pearlman E. Cutting edge: IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J Immunol. 2012;189:4231–4235. doi: 10.4049/jimmunol.1201447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ, Mocarski ES, Dubyak GR. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem. 2015;290:20167–20184. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Kebir D, Filep JG. Targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells. 2013;2:330–348. doi: 10.3390/cells2020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Z, Murakami T, Suzuki K, Tamura H, Kuwahara-Arai K, Iba T, Nagaoka I. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS One. 2014;9:e85765. doi: 10.1371/journal.pone.0085765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23:201–212. doi: 10.1038/cr.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 40.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand RJ, Kohler JW, Cavallo JA, Li J, Dubowski T, Hackam DJ. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J Pediatr Surg. 2007;42:927–932. doi: 10.1016/j.jpedsurg.2007.01.023. discussion 933. [DOI] [PubMed] [Google Scholar]
- 42.Beyer C, Pisetsky DS. Modeling nuclear molecule release during in vitro cell death. Autoimmunity. 2013;46:298–301. doi: 10.3109/08916934.2012.750297. [DOI] [PubMed] [Google Scholar]
- 43.Meng W, Paunel-Görgülü A, Flohé S, Witte I, Schädel-Höpfner M, Windolf J, Lögters TT. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm. 2012;2012:149560. doi: 10.1155/2012/149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, Scortegagna GT, Silva RL, Barroso-Sousa R, Souto FO, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka K, Koike Y, Shimura T, Okigami M, Ide S, Toiyama Y, Okugawa Y, Inoue Y, Araki T, Uchida K, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One. 2014;9:e11–1888. doi: 10.1371/journal.pone.0111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham CT. Neutrophil serine proteases: Specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 47.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 48.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1β processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas MP, Whangbo J, McCrossan G, Deutsch AJ, Martinod K, Walch M, Lieberman J. Leukocyte protease binding to nucleic acids promotes nuclear localization and cleavage of nucleic acid binding proteins. J Immunol. 2014;192:5390–5397. doi: 10.4049/jimmunol.1303296. [DOI] [PMC free article] [PubMed] [Google Scholar]