Abstract
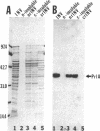
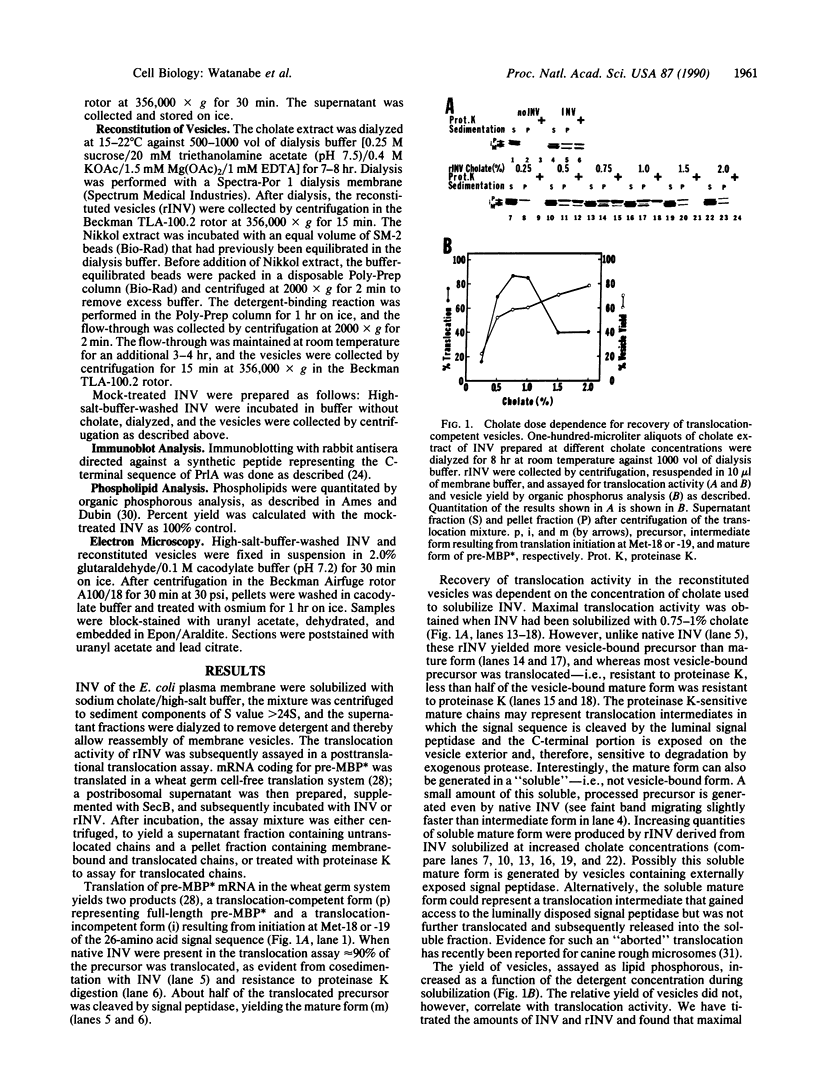
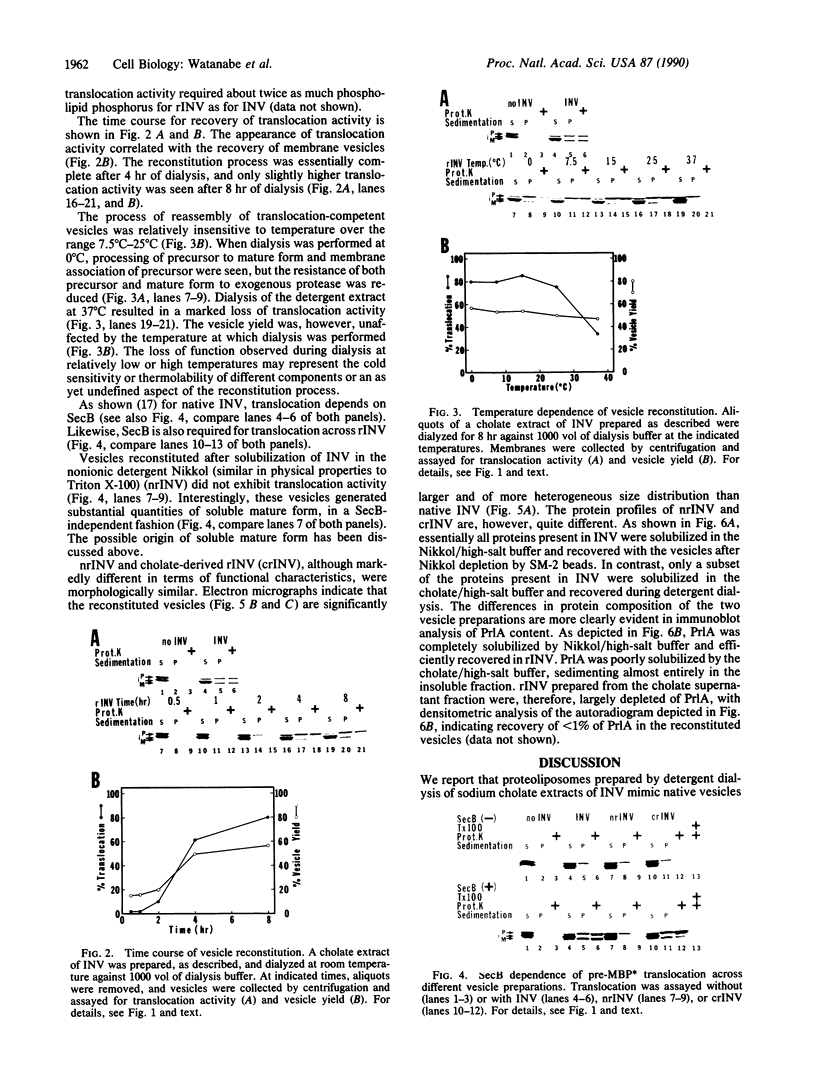
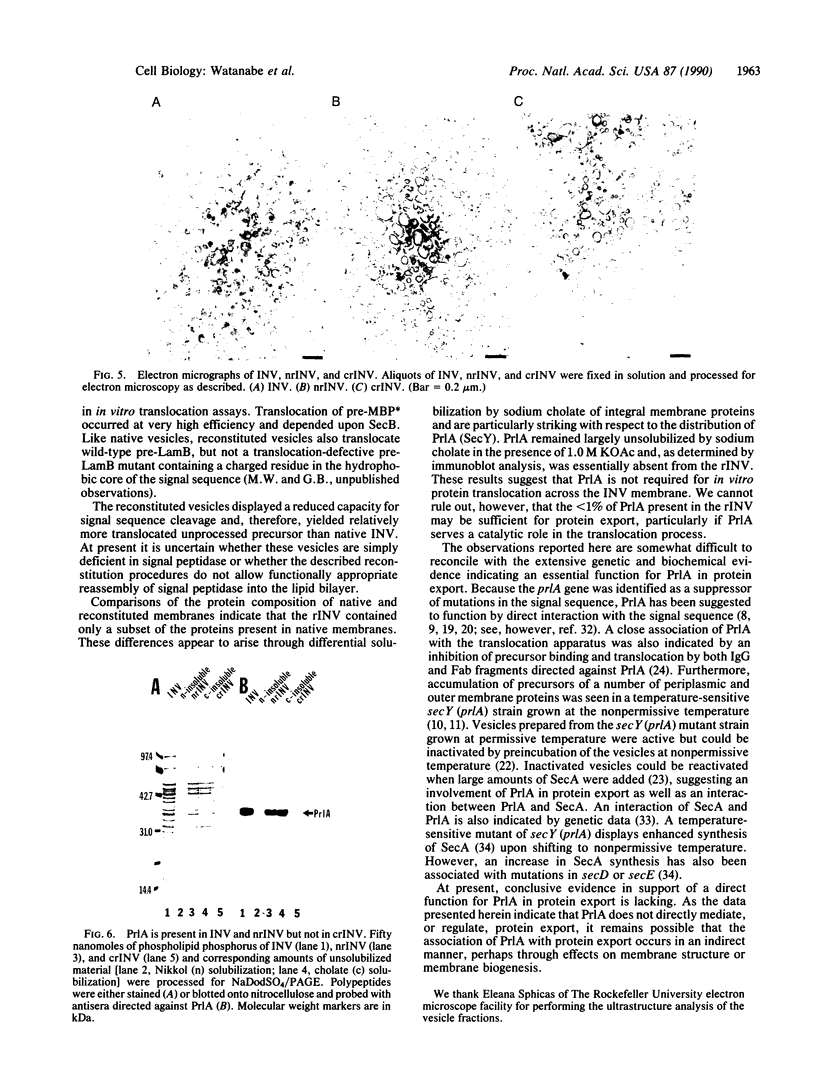
Proteoliposomes were reconstituted by detergent dialysis of a sodium cholate extract of inverted vesicles derived from Escherichia coli plasma membrane. The translocation of precursor proteins into reconstituted vesicles occurred at high efficiency and was SecB dependent. The protein composition of the reconstituted vesicles differed markedly from that of native vesicles. Immunoblot analysis of the sodium cholate extract and of the reconstituted vesicles indicated that PrlA (SecY) protein remained largely unsolubilized under the described conditions and was virtually absent from the reconstituted vesicles, suggesting that PrlA may not be required for in vitro translocation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Brickman E. R., Oliver D. B., Garwin J. L., Kumamoto C., Beckwith J. The use of extragenic suppressors to define genes involved in protein export in Escherichia coli. Mol Gen Genet. 1984;196(1):24–27. doi: 10.1007/BF00334087. [DOI] [PubMed] [Google Scholar]
- Cabelli R. J., Chen L., Tai P. C., Oliver D. B. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988 Nov 18;55(4):683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Cabelli R., Oliver D., Tai P. C. SecA suppresses the temperature-sensitive SecY24 defect in protein translocation in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8953–8957. doi: 10.1073/pnas.85.23.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P. M., Li P., Kumamoto C. A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989 Feb;171(2):813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Sako T. Inhibition and resumption of processing of the staphylokinase in some Escherichia coli prlA suppressor mutants. J Biol Chem. 1988 Dec 15;263(35):19077–19082. [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Chen L., Fandl J., Tai P. C. Purification of the Escherichia coli secB gene product and demonstration of its activity in an in vitro protein translocation system. J Biol Chem. 1989 Feb 5;264(4):2242–2249. [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo E. E., Oliver D. B. Regulation of the Escherichia coli secA gene by protein secretion defects: analysis of secA, secB, secD, and secY mutants. J Bacteriol. 1988 Jul;170(7):3281–3282. doi: 10.1128/jb.170.7.3281-3282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako T., Iino T. Distinct mutation sites in prlA suppressor mutant strains of Escherichia coli respond either to suppression of signal peptide mutations or to blockage of staphylokinase processing. J Bacteriol. 1988 Nov;170(11):5389–5391. doi: 10.1128/jb.170.11.5389-5391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Riggs P. D., Jacq A., Fath M. J., Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989 Jul;3(7):1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- Schmidt M. G., Oliver D. B. SecA protein autogenously represses its own translation during normal protein secretion in Escherichia coli. J Bacteriol. 1989 Feb;171(2):643–649. doi: 10.1128/jb.171.2.643-649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Gansheroff L. J., Silhavy T. J. New suppressors of signal-sequence mutations, prlG, are linked tightly to the secE gene of Escherichia coli. Genes Dev. 1989 Jul;3(7):1045–1052. doi: 10.1101/gad.3.7.1045. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. Binding of a soluble factor of Escherichia coli to preproteins does not require ATP and appears to be the first step in protein export. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2248–2252. doi: 10.1073/pnas.86.7.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. Cytosolic factor purified from Escherichia coli is necessary and sufficient for the export of a preprotein and is a homotetramer of SecB. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2728–2732. doi: 10.1073/pnas.86.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989 Aug 25;58(4):695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. Site-specific antibodies against the PrlA (secY) protein of Escherichia coli inhibit protein export by interfering with plasma membrane binding of preproteins. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1895–1899. doi: 10.1073/pnas.86.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., Ray P. H., Bassford P. J., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]