Abstract
Ex vivo perfusion systems offer a reliable, reproducible method for studying acute physiological responses of an organ to various environmental manipulations. unlike in vitro culture systems, the cellular organization, compartmentalization and three-dimensional structure of ex vivo–perfused organs are maintained. these particular parameters are crucial for the normal physiological function of the placenta, which supports fetal growth through transplacental exchange, nutritional synthesis and metabolism, growth factor promotion and regulation of both maternally and fetally derived molecules. the perfusion system described here, which can be completed in 4–5 h, allows for integrated, physiological studies of de novo synthesis and metabolism and transport of materials across the live mouse placenta, not only throughout a normal gestation period but also following a variety of individual or combined genetic and environmental perturbations compromising placental function.
INTRODUCTION
Organ perfusion systems can simulate physiological conditions, providing a framework for the study of numerous biochemical functions, including metabolism, membrane transfer, drug transport and delivery, toxicological studies and drug efficacy studies1–28. Ex vivo perfusion of isolated organs in particular allows for precise control of the physiological environment and can provide a unique opportunity for studying organ-specific physiological responses to environmental factors with a resolution and reproducibility that may not be possible in intact animals1–3,8–12,18,20,22,24–26,29,30. In this article, we describe the procedure used in our laboratory for the dissection and perfusion of mid-to-late-gestation mouse placentas.
Development and applications of the protocol
To investigate directly the impact of placental function on fetal brain development9,31,32, we sought to devise a mouse placenta perfusion system allowing for transport and metabolic studies throughout gestation. This model can be used in a wide array of integrated physiological studies, such as assessing changes in placental de novo synthesis, molecular transport and metabolism of various compounds throughout normal gestation and amidst manipulations of genetic and environmental conditions. We recently used this method to demonstrate that a fraction of the maternal delivery of the essential amino acid tryptophan is enzymatically converted to serotonin within the placenta; the neo-synthesized placental serotonin is then released into the fetal blood stream through the umbilical vein, from where it can reach the fetal forebrain9.
The method described here allows for perfusions of live mouse placentas in early (embryonic day (E)12–14) and late pregnancy (E16–18), providing a framework for directly comparing physiology at different stages of pregnancy. Furthermore, the use of the mouse placenta as a model allows for direct investigation of the effect of a variety of perturbations, individually or in combination, such as genetic deletions, inflammation or maternal drug exposure on placental physiology. For instance, the ex vivo placenta perfusion system, combined with the application of transgenic mouse models such as trophoblastic or syncytiotrophoblastic-specific gene deletions33–36, allows for the unique opportunity to explore cellular and biochemical processes underlying the impact of environmental or maternal perturbations on placental function throughout gestation. Another potential application of the ex vivo perfusion system is the real-time, simultaneous visualization of blood flow and transport or metabolism of fluorescently labeled molecules across the live placenta. Our preliminary studies indicate that the ex vivo perfusion apparatus, coupled with a two-photon imaging system, can be used to monitor maternal and fetal blood flows simultaneously by infusing fluorescently labeled dextran molecules through the uterine and/or umbilical arteries (Supplementary Fig. 1 and Supplementary Video 1; N.G., J. Burford, J. Peti-Peterdi and A.B., unpublished data). This approach can also be used for in vivo visualization of cell dynamics in the mouse placenta37. Although this system has been used primarily for placental perfusions in our laboratory, the application of this method to other organs or tissue systems can be readily accomplished, pending that target organs have a simple path of blood irrigation (e.g., simple arterial-venous input/output).
Comparison with other methods
Ever since Panigel et al.4,13 demonstrated the first dual recirculation placental perfusion model in 1967 (ref. 14), human term placental perfusion studies have been preferred for pathophysiology, metabolism and developmental pharmacology studies. There are several benefits to conducting postpartum placental studies in humans. Perhaps most notably, the use of postpartum tissue precludes ethical concerns, as both the isolation and perfusion of placental tissue are noninvasive with regard to both the mother and the newborn. Furthermore, in vivo transplacental transfer data obtained by comparing maternal blood concentrations with fetal cord blood concentrations of a given compound at term make postpartum placental perfusion studies directly comparable to in vivo measures.
However, the drawback of using term placenta is that mid-gestation and term placentas are very different in terms of transport and metabolic capacities, as suggested by reports of differential gene expression38–40. The transfer and regulation of compounds that enter the fetal blood supply are controlled by at least four distinct mechanisms, ranging from passive and active transmembranous transport to enzymatic metabolization and phagocytosis41–46. At the molecular level, changes in expression of transporter genes responsible for active transport of nutrients during pregnancy leads to a dynamic state of maternofetal and feto-maternal transfer throughout gestation47–49. There are also physical differences in the barrier structure that emerge during gestation; for instance, in the human placenta the syncytiotrophoblastic cell layer is ~20 μm thick during the first trimester, and it progressively thins during gestation down to ~5 μm in thickness at term50–53. This change in thickness, coupled with an increase in the number of fetal capillaries, may also contribute to the dynamic state of transplacental transfer that changes continuously throughout gestation54.
Thus, very little is known about the transplacental transfer and metabolism of molecules at earlier stages of human gestation. However, this knowledge is crucial if we are to better understand the mechanisms underlying fetal programming of adult diseases. Indeed, it is now becoming evident that several metabolic, cardiovascular and even psychiatric disorders that manifest in adulthood are linked to alterations of maternal-fetal interactions and fetal development that occur at specific periods of gestation31,55–63. The existence of time-sensitive windows during pregnancy for the development of specific mental disorders in the offspring suggests that alterations of maternal homeostatic conditions during early-to-mid gestation can have profound consequences on fetal brain development.
Another common method for studying placental physiology is in vitro culture of isolated placental cell types (e.g., trophoblasts). Although in vitro explant or dissociated cell culture systems allow for studies of transport and metabolic mechanisms at the molecular and cellular level, they lack the cellular organization, compartmentalization and three-dimensional structure of intact, physiologically active placentas. These parameters are particularly important for the normal function of the placenta39,54,64,65.
Our ex vivo perfusion system can provide a direct validation of the role of cellular and molecular pathways initially characterized in vitro. Therefore, we see the ex vivo perfusion method as a complement to current in vitro methods. The overall principle of the method described is comparable to human placental perfusion systems, adapted for an organ of much smaller size. Because of key interspecies differences66,67, results obtained in the mouse model must always be carefully extrapolated to human placental physiology. Initial studies in our laboratory were devoted to determining the effect of perfusion rate, incubation temperature, incubation medium composition and various surgical techniques on the viability of the perfused organ. Below we present the optimized protocol that we are now using for the perfusion of mid-to-late-gestation mouse placentas. Despite the straightforward appearance of the techniques, some hands-on experience and fine motor control are necessary to obtain accurate and reproducible results. The incubation and infusing media should be optimized for each individual experiment, as some compositions may not be suitable for certain metabolic studies.
Experimental design
In our experience and in that of others, it is essential to begin perfusing with fresh, viable tissue, as ischemic cell death progresses rapidly after mice are killed68–70. This implies that organs should be harvested as quickly as possible from the animal and transferred directly into a thermodynamically controlled incubation chamber that has been prefilled with oxygenated incubation medium. It should be noted that different tissues and organ systems require specific storage and incubation media71, and that each experiment should be optimized for the individual needs of the organ in question. In addition, organ viability should be monitored during and after perfusion to ensure that the specific medium and perfusion parameters meet the organ’s metabolic needs. Although the surgical and perfusion procedures are still being perfected, extra tissue to be used for cannulation can be kept on ice to limit the harmful effects of warm ischemia. However, in our experience, exposure to low temperatures increases vascular wall stiffness, which can impede the cannulation process. For this reason, we recommend the use of fresh tissue whenever possible.
Because of the small diameter of the vasculature of mid-to-late-gestation mouse placentas, the catheters used in this perfusion system are terminated with a polyimide-coated fused silica capillary tube, available in various sizes between 90 and 200 μm in external diameter. The optimal diameter of the catheter depends on the size of the vasculature in question, as well as on the operator preference. Although this perfusion system is optimized for use with E14 placenta, the system can be easily adapted to any mid-to-late-gestation mouse placenta simply by increasing or decreasing catheter size within this 90–200-μm range. Although smaller-diameter tubing may appear more straightforward for the cannulation of micro-vasculature, tubing diameter is directly proportional to rigidity, and overly pliable catheters can be more troublesome than their larger counterparts. It is therefore our opinion that a catheter of just slightly smaller diameter than the vasculature in question should be chosen, as it provides the best compromise between rigidity and size.
Although the choice of medium is not completely evidence based, most researchers use culture or incubation media such as Dulbecco’s modified Eagle’s medium (DMEM) or Krebs-Ringer saturated with 95% O2/5% CO2 (refs. 9, 12, 19 and 27). In our experience, this oxygen saturation is sufficient to maintain placental health for the duration of the experiment (ANTICIPATED RESULTS). Perfusion studies conducted under relative hypoxia should be possible by changing the oxygen saturation of perfusion and incubation media. Specific medium may not be suitable to every experiment, and any medium that is capable of sustaining a viable organ throughout the duration of the experiment is suitable.
MATERIALS
REAGENTS
Pregnant mouse with placental material of desired age ! CAUTION Animal tissue should be collected in compliance with legislative and institutional requirements.
Isoflurane (Western Medical Supply, cat. no. 7263) ! CAUTION Isoflurane is a powerful anesthetic and should always be used inside a laminar flow hood.
PBS (VWR, cat. no. 97064-158; see Reagent Setup)
L-Tryptophan (Sigma, cat. no. T0254)
Ethanol (95%, vol/vol; Millipore, cat. no. EX0280-3)
Ultrapure water
Distilled water
DMEM (Invitrogen, cat. no. 21063-045)
NaCl (VWR, cat. no. BDH8014-500G)
MgCl2 (VWR, cat. no. BDH0244-500G)
KCl (VWR, cat. no. BDH0258-500G)
Na2HPO4 (VWR, cat. no. JT3827-19)
CaCl2 (VWR, cat. no. BDH4525-500GP)
HEPES (VWR, cat. no. BDH4518-500GP)
D-Glucose (VWR, cat. no. 97061-168)
EQUIPMENT
BubbleStop syringe warmer (Automate Scientific, cat. no. 10-4-60-G)
ValveLink 8.2 (Automate Scientific, cat. no. 01-18)
Teflon valve box (Automate Scientific, cat. no. 02-01-02)
Vacuum bottle, 4 liters (VWR, cat. no. 16331-100)
Teflon tubing, 1/16th inch (Automate Scientific, cat. no. bi-20202275)
Syringes, 60 ml (Automate Scientific, cat. no. 06-60C)
Ultralow-flow peristaltic pump (×2) (Instech, cat. no. P720/10k)
Low-flow peristaltic pump (Instech, cat. no. P720)
USB interface for perfusion pumps (Bioptechs, cat. no. 13161603-13)
C-Flex tubing, 0.020-inch inner diameter (i.d.) (Instech, cat. no. P720/TS-BS020SBS)
C-Flex tubing, 0.031-inch i.d. (Instech, cat. no. P720/TS-BS031SBS)
Cyanoacrylate glue (Office Depot, cat. no. 903720)
Micro-Renathane tubing, 0.033 inch (Braintree Scientific, cat. no. MRE-033-CL-100)
Silica tubing, 10 m, 090 μm (Polymicro, cat. no. TSP020090)
Silica tubing, 10 m, 105 μm (Polymicro, cat. no. TSP040105)
Silica tubing, 10 m, 150 μm (Polymicro, cat. no. TSP075150)
Silica tubing, 10 m, 200 μm (Polymicro, cat. no. TSP100200)
Micro clamp (×5), 7 mm (Fine Science Tools, cat. no. 00396-01)
Micro clamp, 11 mm (Fine Science Tools, cat. no. 00398-02)
Incubation chamber (Warner Instruments, cat. no. QE-2)
In-line heater (Warner Instruments, cat. no. SH-27B)
Temperature controller (×2) (Warner Instruments, cat. no. TC324B)
Bin cable (Warner Instruments, cat. no. 64-0106)
Catheter holder (×3) (Bioscience Tools, cat. no. MH-2)
Tubing holder (×2) (Bioscience Tools, cat. no. MTH-S)
Medium-flow peristaltic pump (VWR, cat. no. 54856-075)
Dremel rotary tool (Amazon, cat. no. DR8000-03)
Eppendorf tubes, 1.5 ml
Hypodermic needles, 27 gauge (VWR, cat. no. 15141-192)
Hypodermic needles, 22 gauge (VWR, cat. no. MJ8881-250206)
Forceps
Surgical scissors
Vannas spring scissors, 4-mm cutting edge (Fine Science Tools, cat. no. 15018-10)
Vascular clamp forceps (Fine Science Tools, cat. no. 00071-14)
Surgical thread, 5/0 silk (VWR, cat. no. 100190-110)
Dissection microscope
95% O2/5% CO2 gas tank with a regulator
Polyvinyl chloride (PVC) tubing, 3/4 inch (VWR, cat. no. 89068-574)
Dewar flask (VWR, cat. no. 82021-112)
Liquid nitrogen
REAGENT SETUP
Preparation of buffer solutions
A variety of buffering solutions may be used to simulate maternal blood flow for the perfusion system. Freshly prepare buffer solutions and maternal and fetal input solutions on the day of the experiment using ultrapure water, and then store the solutions at 4 °C until use. Before perfusion, warm input solutions to above 40 °C to promote degassing (see PROCEDURE Step 4), and then oxygenate the incubation buffer using 95% O2/5% CO2. The buffers we use are as follows: PBS, composed of 10 mM Na2HPO4, 2.7 mM KCl and 137 mM NaCl; HEPES, composed of 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES and 10 mM glucose; and DMEM (see Reagents).
EQUIPMENT SETUP
Perfusion system
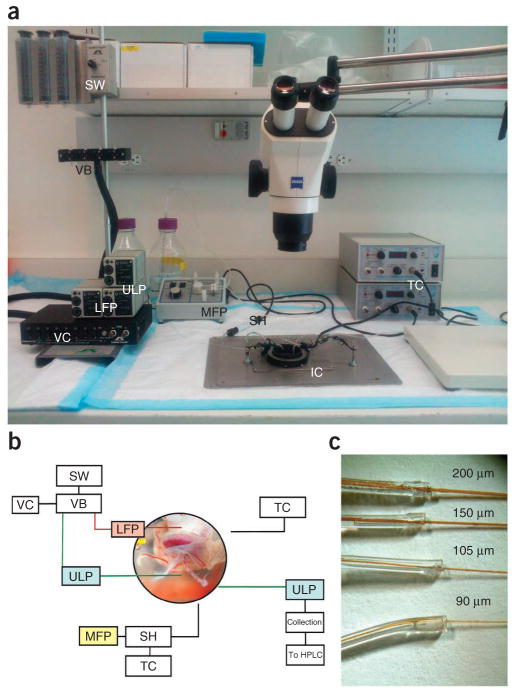
Load the BubbleStop syringe warmer with 60-ml syringes and connect it to the Teflon valve box with 1/16th-inch Teflon tubing (Fig. 1a,b). Connect the valve box to the ValveLink 8.2 software and ensure that it is controlled by the software. Connect the output of the Teflon valve box to 1/16-inch Teflon tubing, stepped down to 0.033-inch Micro-Renathane tubing, and then seal it with cyanoacrylate. Connect this Micro-Renathane tubing to the low-flow peristaltic pump, which delivers the simulated maternal input to the placenta. Bubble the incubation medium with 95% O2/5% CO2 through 3/4-inch PVC tubing connected to a bubbling stone, and then pump through the medium-flow peristaltic pump into the in-line solution heater and finally into the incubation chamber. Use the TC324B temperature controller to control the in-line solution heater and the incubation dish to maintain a steady state of 37 °C. Stimulate the fetal circulation by infusing input medium from the BubbleStop syringe warmer through the umbilical artery via an ultralow-flow pump. Collect the flow through the umbilical vein by a second ultralow-flow peristaltic pump and deliver it to a series of collection tubes. The low- and ultralow-flow peristaltic pumps may be controlled using a computer interface through the use of a USB controller.
Figure 1.
Overview of the ex vivo perfusion system. (a) BubbleStop syringe warmer (SW); Teflon valve box (VB); ValveLink controller (VC); low-flow peristaltic pump (LFP); medium-flow peristaltic pump (MFP); in-line solution heater (SH); incubation chamber (IC); temperature controllers (TC); ultralow-flow peristaltic pumps (ULP). (b) Layout and function of the components of the ex vivo perfusion system. (c) Polyamide-coated, fused silica capillaries of various diameters inserted into Micro-Renathane tubing of 0.033-inch i.d. and sealed with multiple applications of cyanoacrylate.
Peristaltic pumps and catheter design
Load the low-flow and ultralow- flow peristaltic pumps (Fig. 1c) with C-Flex tubing sets, 0.031-inch i.d. and 0.020-inch i.d., respectively. Connect Micro-Renathane tubing to the C-Flex tubing sets and seal them with cyanoacrylate. Terminate this tubing with one of four sizes of polyamide-coated fused silica capillaries and seal it with cyanoacrylate.
PROCEDURE
Preparation for the perfusion experiment ● TIMING 1.5 h
-
1|
Turn the solution heater on and wait until it reaches 45 °C.
-
2|
Prepare the desired incubation medium. To simulate physiological conditions, we use PBS, DMEM or HEPES buffer.
-
3|
Prepare maternal and fetal input solutions. We use PBS, DMEM or HEPES buffer to simulate maternal and fetal blood supplies, spiked with our particular compound of interest.
-
4|
Add the maternal and fetal solutions to 60-ml syringe tubes and place them in the syringe warmer to promote solution degassing and prevent air embolisms during perfusion (supplementary Video 2). Solutions should be near physiological temperatures as they enter the placenta.
▲ CRITICAL STEP The solutions should be warmed and agitated sufficiently to promote the release of gas at the new equilibrium temperature. Failure to sufficiently heat the solutions above the physiological temperature will result in the release of gas (in the form of air bubbles) upon entering the organ, causing embolism, poor perfusion and organ death. The temperature at which the perfusate leaves the catheter and enters the uterine artery is lower (at 37 °C), as the catheters are submerged in the thermostatted incubation chamber for a sufficient duration to reach equilibrium.
-
5|
Prewarm the incubation medium to 37 °C and oxygenate it by turning on the in-line solution heater and bubbling 95% O2/5% CO2 through the solution.
-
6|
Load the incubation chamber with a 60-mm tissue culture dish and secure it in place.
-
7|
Turn on the incubation chamber heater and begin circulation of the incubation medium at a rate of 7 ml min −1 to ensure that the incubation environment is stabilized.
-
8|
Turn on the vacuum system connected to the extraction tube to remove excess incubation medium.
-
9|
Once maternal inputs are sufficiently warmed and degassed, activate the infusing and extracting peristaltic pumps at the desired rate of flow (Fig. 2). Allow the medium to flow for at least 15 min before cannulating the placenta, ensuring that all air has been removed from the catheters.
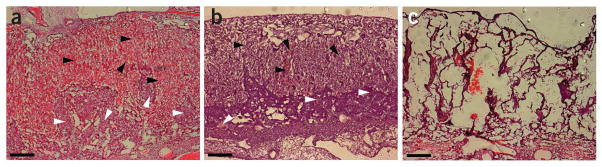
▲ CRITICAL STEP Peristaltic pumps should be calibrated and set to deliver and extract the medium at the known rate of flow for the organ in question. Because of substantial vascular remodeling, the physiological rate of flow for a mouse placenta is constantly increasing throughout development72–74. In accordance with published data75, we found a perfusion rate of 18 μl min −1 through the uterine artery and 5 μl min −1 through the umbilical vasculature to be close to physiological values and effective at clearing endogenous blood pools in an E14 placenta; at the same time it was not so forceful as to damage the overarching structure of the organ (Fig. 2).
? TROUBLESHOOTING
-
10|
Sterilize the surgical tools with 70% (vol/vol) ethanol, label the collection tubes for the fetal eluate and fill a Dewar flask with liquid nitrogen for snap-freezing samples after collection.
Figure 2.
Effect of perfusion rate on placental morphology. E14 mouse placenta sectioned at 10-μm thickness on a Reichert Jung 1800 cryostat and stained with H&E. (a) Maternal and fetal blood spaces of fresh unperfused placenta still contain large pools of maternal (white arrowheads) and fetal (black arrowheads) blood. (b) After perfusion through the umbilical artery with PBS at 5 μl min −1 and through the uterine artery at 16 μl min −1 for 80 min, the tissue is mostly devoid of blood, but the overreaching morphology is still intact. (c) After perfusion with PBS at 12 μl min −1 for 80 min through the umbilical artery, the organ is clearly distorted, blood spaces are compressed by the flow rate and membranous tissue is completely destroyed. All animal experiments have been conducted in accordance with the National Institutes of Health Animal Use Guidelines and approved by the Institutional Animal Care and Use Committee at The University of Southern California. Scale bars, 200 μm.
Collection and preparation of tissue ● TIMING 5–10 min
-
11|
Euthanize a mouse by placing it in a desiccator filled with isoflurane gas to induce loss of consciousness. Once loss of consciousness is observed, quickly perform a cervical dislocation and proceed with organ retrieval.
! CAUTION Euthanasia through the use of isoflurane should be performed in a laminar airflow cabinet. Mouse tissue should always be collected in compliance with all relevant institutional animal care and use committee requirements.
▲ CRITICAL STEP Mouse euthanasia and organ retrieval should be performed as quickly as possible, as organ viability declines rapidly postmortem.
-
12|
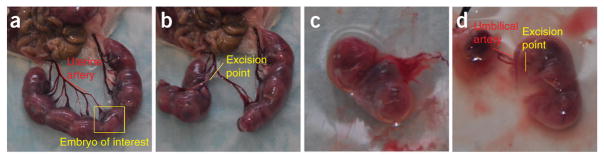
Open the abdominal cavity of the euthanized mouse and lay out the uterine horns (Fig. 3a).
-
13|
Select a placenta with a large and intact portion of the uterine artery, excising as close to neighboring branches as possible (Fig. 3b).
▲ CRITICAL STEP Maintaining as much intact uterine artery as possible will help with both cannulation and securing of the micro clamp. Well-sharpened spring scissors should always be used for severing vasculature, as a clean cut aids in visualization of the lumen during cannulation.
-
14|
After severing the uterine artery, separate the desired amniotic sac from the surrounding embryos and place it in a culture dish containing 37 °C incubation medium (Fig. 3c).
-
15|
Open the amniotic sac, separating the embryo from the placenta, and carefully sever the umbilical cord as close to the embryo as possible, preserving the umbilical vasculature for cannulation (Fig. 3d).
-
16|
Transfer the placenta to the incubation chamber.
Figure 3.
Extraction and isolation of an E18 mouse placenta. (a) Once the mouse has been euthanized and the peritoneal cavity opened, splay the uterine horns out to reveal the uterine artery for excision. (b) Remove the embryos surrounding the placenta of interest and preserve 4–5 mm of uterine artery for cannulation. (c) Once separated, immediately transfer the uterine segment containing the placenta of interest to the incubation chamber. (d) At this point, open the amniotic sac and separate the placenta from the embryo, severing the umbilical cord as near to the embryo as possible. All animal experiments have been conducted in accordance with the National Institutes of Health Animal Use Guidelines and approved by the Institutional Animal Care and Use Committee at The University of Southern California.
Cannulation of the placenta and flushing of maternal blood ● TIMING 20–25 min
-
17|
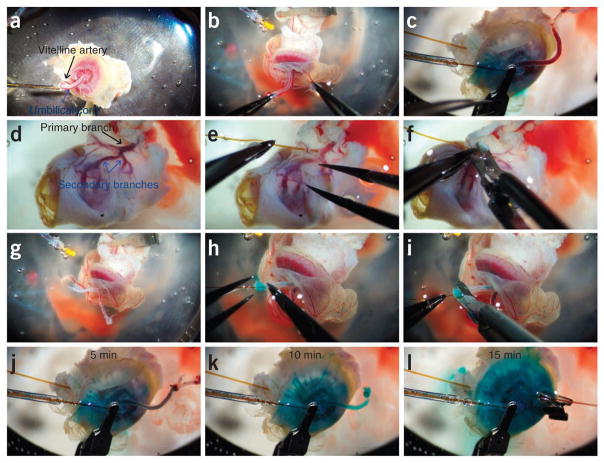
Before cannulation of the placenta, remove the vitelline artery from the umbilical cord (Fig. 4a) and separate the umbilical artery and vein (Fig. 4b,c and supplementary Video 3).
▲ CRITICAL STEP Exceptional care must be taken when grasping or manipulating fetal vasculature. Whenever possible, all forceps grasps should be placed parallel to the artery or vein, along the vascular wall. Perpendicular placement of the forceps will pinch the vasculature, thus obstructing blood flow and potentially piercing the vascular wall, causing solution to leak from the artery or vein (Fig. 4c; see also supplementary Fig. 2 and supplementary Video 2).
? TROUBLESHOOTING
-
18|
Invert the placenta, exposing the maternal artery for cannulation (Fig. 4d). Lightly grasp the uterine artery with one pair of forceps while carefully inserting the catheter ~2 mm into the artery, through to one of the secondary arterial branches (Fig. 4e and supplementary Video 4).
▲ CRITICAL STEP In vivo, the closed nature of the maternal circulation provides sufficient pressure to pump the maternal blood through the secondary arterial branches. In this ex vivo setup, owing to the open nature of the uterine artery, the maternal flow must be infused directly into the secondary branch. For a closed circuit perfusion, recirculation of the maternal solution can be achieved by cannulating the open end of the uterine artery and ligating the uterine vein.
-
19|
Reposition one pair of forceps to hold the catheter in place while the micro clamp is applied (Fig. 4f).
-
20|
Carefully invert the placenta to expose the umbilical vein and artery (Fig. 4g). Be careful not to disturb the cannulation of the uterine artery during this process.
-
21|
Lightly grasp the umbilical artery and insert the catheter into the lumen (Fig. 4h and supplementary Video 5).
▲ CRITICAL STEP The umbilical artery is frequently void of blood at the start of the cannulation process, thus inhibiting visualization of the lumen. Furthermore, the thick wall of the umbilical artery can hinder cannulation. It is often helpful to lightly poke around the opening of the umbilical artery to expand the lumen and provide contrast between the artery and the arterial wall. Care must be taken during this step not to puncture or pierce the arterial wall.
? TROUBLESHOOTING
-
22|
Carefully apply the micro clamp to the cannulated artery (Fig. 4i).
-
23|
Continue perfusing for 15 min to remove native fetal and maternal blood pools (Fig. 4j–l).
-
24|
Lightly grasp the umbilical vein and insert the catheter into the lumen (Fig. 4l).
▲ CRITICAL STEP The umbilical vein is greatly expanded when compared with the umbilical artery owing to the positive pressure from the infusing solutions. This facilitates cannulation of the umbilical vein and aids in visualization of the lumen. Placement of the micro clamp on the umbilical vein may not be necessary depending on your experiment. The use of an extracting flow rate slightly lower than the innate venous flow will help prevent collapse of the vascular wall, and the lack of a micro clamp will allow the excess eluate to flow directly into the bath.
? TROUBLESHOOTING
Figure 4.
Preparation and perfusion of mouse placental tissue. (a,b) After the placenta is removed from the peritoneal cavity, remove the vitelline artery (a) from the umbilical cord and separate the umbilical artery and vein (b). Be careful to avoid puncturing the vascular wall (c). (d,e) Invert the placenta, exposing the uterine artery (d) for cannulation with a 200-μm fused silica catheter (e). (f,g) Clamp the artery on to the body of the catheter using an 11-mm micro clamp (f) and revert the placenta to the original orientation, exposing the umbilical vasculature (g). (h,i) Cannulate the umbilical artery with a 105–150-μm fused silica catheter (h), and then clamp with a 7-mm micro clamp (i). (j–l) Perfuse the mouse placenta through both the uterine and umbilical arteries for an additional 15 min before collections begin to remove all innate maternal and fetal blood. All animal experiments have been conducted in accordance with the National Institutes of Health Animal Use Guidelines and approved by the Institutional Animal Care and Use Committee at The University of Southern California.
Perfusion and sample collection ● TIMING 2–3 h, depending on the number of samples
-
25|
Continue the placental perfusion for the duration of the experiment, changing collection tubes and switching maternal inputs at the desired time points. Viability measures should be performed throughout and immediately following perfusion to ensure placental health for the duration of the experiment (ANTICIPATED RESULTS). Sample tubes should be snap-frozen in liquid nitrogen immediately after collection and stored at - 80 °C.
? TROUBLESHOOTING
Troubleshooting advice can be found in table 1. In the case of an arterial leak, see also supplementary Video 2.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 9 | Air bubbles | Solution not prewarmed | Ensure solution is prewarmed to at least 40–50 °C |
| Air pockets in catheters | Agitate the catheters as they are flushed to ensure all air is purged. | ||
| 17 | Arterial leak | Pierced or punctured wall | An arterial leak can be fixed by suturing the vascular wall to the catheter downstream of the leak (Supplementary Fig. 2 and Supplementary Video 2) |
| 21 | Arterial rigor | Excessive delay | Surgical processes improve over time, decreasing the delay, but as a short-term solution the umbilical vein and artery can be separated after the uterine artery has been cannulated, reducing umbilical contraction and stiffening |
| 24 | Venous collapse | Elevated negative pressure | Lowering negative pressure and removing the complete vascular seal can improve perfusion efficiency, but it does lead to reduced collection rate |
| Vascular wall suction | Use of a larger-diameter catheter can prevent possible wall suction, as the larger diameter is more difficult to obstruct |
● TIMING
Steps 1–10, preparation for the perfusion experiment: 1.5 h
Steps 11–16, collection and preparation of tissue: 5–10 min
Steps 17–24, cannulation of the placenta and flushing of blood: 20–25 min
Step 25, perfusion and sample collection: 2–3 h
ANTICIPATED RESULTS
Typically, a mouse placenta at mid-to-late gestation is capable of sustaining flow in this ex vivo system for a minimum of 120 min before vascular collapse leads to reduced output from the umbilical vein. Several parameters are monitored to assay organ viability throughout perfusion; however, as noted earlier for human term placenta16, there is no standard set of parameters, and thus viability measures vary between laboratories. Common parameters include fetal circulation pressure, eluate pH, oxygen consumption, fetal oxygen transfer, glucose consumption, lactate production, the synthesis and secretion of proteins and cell death assays. Some indications point to fetal volume loss being the optimal measure of tissue viability and integrity20. However, there is no generally accepted maximum allowable volume loss and these measures vary across species. In our experience, fetal volume loss and vascular collapse precedes cell death, but additional viability measures should still be performed; therefore, we used a combination of placental lactate dehydrogenase (LDH) activity, fetal eluate loss and cell apoptosis assays as organ viability controls.
Fetal eluate volume should be monitored throughout the perfusion to confirm that no major volume loss is present. In our experience, an input of 5 μl min −1 through the umbilical artery of a healthy E14 placenta elicits a flow rate of 4.5 μl min −1 through the umbilical vein directly after euthanasia, with no more than an average fetal volume loss of 20% over a 90-min perfusion (supplementary Fig. 3a). Consequently, perfusions yielding fetal volume loss in excess of 20% should be discarded.
In normal tissues, hypoxia, tissue damage and cell toxicity have been shown to increase metabolic activities, such as glycolysis76–78. Hypoxic conditions in term human placenta, such as those caused by incomplete perfusion, have been shown to enhance glycolysis and increase the activity of LDH, which converts pyruvate to lactate76. Quantification of LDH activity can therefore be used as a reliable measure of cell toxicity throughout placental perfusions. LDH assays can be performed using a colorimetric assay kit (Sigma, cat. no. MAK066) that quantifies placental production of NADH, an index of glucose conversion to pyruvate and thus of placental LDH activity. Results show that in our conditions placental LDH activity remains low and stable throughout the perfusion (supplementary Fig. 3b), until 90 min after euthanasia. When we adjusted experimental parameters to allow for an increased perfusion time of 120 min, we observed a threefold increase in LDH activity, thus confirming that our experimental parameters are optimized for 90-min perfusions. Increased placental LDH activity indicates that cellular toxicity has commenced and the organ should be discarded.
Caspase-3, a member of the cysteine-aspartic acid protease (caspase) family, which is sequentially activated during the execution phase of cell apoptosis79–82, can be used after perfusion as a marker for cell apoptosis. We stained fresh placentas, as well as perfused and unperfused placentas at 40, 80 and 120 min after euthanasia, for activated caspase-3 (Millipore, cat. no. AB3623) as previously described83. We designated a region of interest within the decidua (0.6 mm2) across the different time points and conditions, and we performed cell counting across three sections for each time point under the criteria of colocalized activated caspase-3 and DAPI staining (supplementary Fig. 3c–l). In accordance with the lactate production assays, activated caspase-3 staining indicates that generalized cell apoptosis does not commence until well after completion of the perfusion.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (NICHD) (grant 5R21HD065287 to A.B.) and a NARSAD (National Alliance for Research on Schizophrenia and Depression; now the Brain and Behavior Research Foundation) Young Investigator award (to A.B.). We thank P. Levitt for his support during the initial development of this protocol. We acknowledge J. Burford and J. Peti-Peterdi for their valuable contributions in two-photon live imaging.
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS N.G. conducted the experiments. N.G. and A.B. conceived the protocol, interpreted the data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Stulc J, Stulcová B, Svihovec J. Transport of calcium across the dually perfused placenta of the rat. J Physiol. 1990;420:295–311. doi: 10.1113/jphysiol.1990.sp017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak-Wegzryan A. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and preterm deliveries perfused in vitro. Pediatrics. 2003;112:460. [Google Scholar]
- 3.Cygalova LH, Hofman J, Ceckova M, Staud F. Transplacental pharmacokinetics of glyburide, rhodamine 123, and BODIPY FL prazosin: effect of drug efflux transporters and lipid solubility. J Pharmacol Exp Ther. 2009;331:1118–1125. doi: 10.1124/jpet.109.160564. [DOI] [PubMed] [Google Scholar]
- 4.Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114:822–828. doi: 10.1016/0002-9378(72)90909-x. [DOI] [PubMed] [Google Scholar]
- 5.Myren M, Mose T, Mathiesen L, Knudsen LE. The human placenta—an alternative for studying foetal exposure. Toxicol In Vitro. 2007;21:1332–1340. doi: 10.1016/j.tiv.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Bond H, et al. Artificial perfusion of the fetal circulation of the in situ mouse placenta: methodology and validation. Placenta. 2006;27:S69–S75. doi: 10.1016/j.placenta.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Hauguel S, Challier JC, Cedard L, Olive G. Metabolism of the human placenta perfused in vitro: glucose transfer and utilization, O2 consumption, lactate and ammonia production. Pediatr Res. 1983;17:729–732. doi: 10.1203/00006450-198309000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ala-Kokko TI, Myllynen P, Vähäkangas K. Ex vivo perfusion of the human placental cotyledon: implications for anesthetic pharmacology. Int J Obstet Anesth. 2000;9:26–38. [Google Scholar]
- 9.Bonnin A, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loughhead AM, et al. Placental passage of tricyclic antidepressants. Biol Psychiatry. 2006;59:287–290. doi: 10.1016/j.biopsych.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler CP, Yudilevich DL. Transport and metabolism of adenosine in the perfused guinea-pig placenta. J Physiol. 1988;405:511–526. doi: 10.1113/jphysiol.1988.sp017345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polliotti BM, et al. Long-term dual perfusion of isolated human placental lobules with improved oxygenation for infectious diseases research. Placenta. 1996;17:57–68. doi: 10.1016/s0143-4004(05)80644-7. [DOI] [PubMed] [Google Scholar]
- 13.Panigel M. Radioangiographic study of circulation in the villi and intervillous space of isolated human placental cotyledon kept viable by perfusion. J Physiol (Paris) 1967;59:277. [PubMed] [Google Scholar]
- 14.Miller RK. Human placenta in vitro: characterization during 12 h of dual perfusion. Contrib Gynecol Obstet. 1985;13:77–84. [PubMed] [Google Scholar]
- 15.Sweiry JH, Yudilevich DL. Characterization of folate uptake in guinea pig placenta. Am J Physiol. 1988;254:C735–C743. doi: 10.1152/ajpcell.1988.254.6.C735. [DOI] [PubMed] [Google Scholar]
- 16.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- 17.Suzue T. Physiological activities of late-gestation rat fetuses in vitro. Neurosci Res. 1992;14:145–157. doi: 10.1016/0168-0102(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 18.Staud F, et al. Corticosterone transfer and metabolism in the dually perfused rat placenta: effect of 11β-hydroxysteroid dehydrogenase type 2. Placenta. 2006;27:171–180. doi: 10.1016/j.placenta.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Schröder H, Leichtweiss HP. Perfusion rates and the transfer of water across isolated guinea pig placenta. Am J Physiol. 1977;232:H666–H670. doi: 10.1152/ajpheart.1977.232.6.H666. [DOI] [PubMed] [Google Scholar]
- 20.Pienimäki P, et al. Pharmacokinetics of oxcarbazepine and carbamazepine in human placenta. Epilepsia. 1997;38:309–316. doi: 10.1111/j.1528-1157.1997.tb01122.x. [DOI] [PubMed] [Google Scholar]
- 21.Penfold P, Drury L, Simmonds R, Hytten FE. Studies of a single placental cotyledon in vitro: I. The preparation and its viability. Placenta. 1981;2:149–154. doi: 10.1016/s0143-4004(81)80018-5. [DOI] [PubMed] [Google Scholar]
- 22.Pollex EK, Feig DS, Lubetsky A, Yip PM, Koren G. Insulin glargine safety in pregnancy: a transplacental transfer study. Diabetes Care. 2010;33:29–33. doi: 10.2337/dc09-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halkoaho A, et al. Ethical aspects of human placental perfusion: interview of the mothers donating placenta. Placenta. 2010;31:686–690. doi: 10.1016/j.placenta.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Staud F, et al. Expression and transport activity of breast cancer resistance protein (Bcrp/Abcg2) in dually perfused rat placenta and HRP-1 cell line. J Pharmacol Exp Ther. 2006;319:53–62. doi: 10.1124/jpet.106.105023. [DOI] [PubMed] [Google Scholar]
- 25.Vähäkangas K, Myllynen P. Experimental methods to study human transplacental exposure to genotoxic agents. Mutat Res. 2006;608:129–135. doi: 10.1016/j.mrgentox.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Heikkine T, Ekblad U, Laine K. Transplacental transfer of citalopram, fluoxetine and their primary demethylated metabolites in isolated perfused human placenta. BJOG. 2002;109:1003–1008. doi: 10.1111/j.1471-0528.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Santo S. Trophoblast viability in perfused term placental tissue and explant cultures limited to 7–24 h. Placenta. 2003;24:882–894. doi: 10.1016/s0143-4004(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 28.Suzue T. Perfusion method and their physiological activities. Neurosci Res. 1994;21:173–176. doi: 10.1016/0168-0102(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 29.Bajoria R, Contractor SF. Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta. Pediatr Res. 1997;42:520–527. doi: 10.1203/00006450-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Tohyama K, Kusuhara H, Sugiyama Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004;145:4384–4391. doi: 10.1210/en.2004-0058. [DOI] [PubMed] [Google Scholar]
- 31.Bonnin A, Levitt P. Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 2012;37:299–300. doi: 10.1038/npp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada Y, et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007;25:233–237. doi: 10.1038/nbt1280. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel PL, Leone G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis. 2007;134:129–134. doi: 10.1002/dvg.20276. [DOI] [PubMed] [Google Scholar]
- 35.Renaud SJ, Karim Rumi MA, Soares MJ. Review: Genetic manipulation of the rodent placenta. Placenta. 2011;32(suppl 2):S130–S135. doi: 10.1016/j.placenta.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morioka Y, Isotani A, Oshima RG, Okabe M, Ikawa M. Placenta-specific gene activation and inactivation using integrase-defective lentiviral vectors with the Cre/loxP system. Genesis. 2009;47:793–798. doi: 10.1002/dvg.20563. [DOI] [PubMed] [Google Scholar]
- 37.Zenclussen AC, Olivieri DN, Dustin ML, Tadokoro CE. In vivo multiphoton microscopy technique to reveal the physiology of the mouse placenta. Am J Reprod Immunol. 2012;1600:1–8. doi: 10.1111/j.1600-0897.2012.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitras V, Fenton C, Paulssen R, Vårtun Å, Acharya G. Differences in gene expression between first and third trimester human placenta: a microarray study. PLoS ONE. 2012;7:e33294. doi: 10.1371/journal.pone.0033294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology. 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 40.Mikheev AM, et al. Profiling gene expression in human placentae of different gestational ages: an OPRU network and UW SCOR study. Reprod Sci. 2008;15:866–877. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battaglia FC, Regnault TR. Placental transport and metabolism of amino acids. Placenta. 2001;22:145–161. doi: 10.1053/plac.2000.0612. [DOI] [PubMed] [Google Scholar]
- 42.Pacifici GM. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- 43.Bell AW. Placental transport of nutrients and its implications for fetal growth. J Reprod Fertil Suppl. 1999;54:401–410. [PubMed] [Google Scholar]
- 44.Apgar V, Papper ME. Transmission of drugs across the placenta. Anesth Analg. 1951;31:309–320. [PubMed] [Google Scholar]
- 45.Hay WW. Placental transport of nutrients to the fetus. Horm Res. 1994;42:215–222. doi: 10.1159/000184196. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia FC. Placental transport: a function of permeability and perfusion. Am J Clin Nutr. 2007;85:591S–597S. doi: 10.1093/ajcn/85.2.591S. [DOI] [PubMed] [Google Scholar]
- 47.Coan PM, et al. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008;586:4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coan PM, et al. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588:527–538. doi: 10.1113/jphysiol.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devaskar SU. Expression of genes involved in placental glucose uptake and transport in the nonobese diabetic mouse pregnancy. Am J Obstet Gynecol. 1994;171:1316–1323. doi: 10.1016/0002-9378(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 50.Boyd JD, Hamilton WJ. Development and structure of the human placenta from the end of the 3rd month of gestation. J Obstet Gynaecol Br Commonw. 1967;74:161–226. doi: 10.1111/j.1471-0528.1967.tb14864.x. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton WJ. Trophoblast in human uterno-placental arteries. Nature. 1966;212:906–908. doi: 10.1038/212906a0. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann P, Stegner HE. Functional differentiation of the human placental syncytiotrophoblast. Z Zellforsch Mikrosk Anat. 1972;135:361–382. [PubMed] [Google Scholar]
- 53.Boyd JD. Observations on the vacuolar structure of the human syncytiotrophoblast. Z Zellforsch Mikrosk Anat. 1968;88:57–79. doi: 10.1007/BF00492234. [DOI] [PubMed] [Google Scholar]
- 54.Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- 55.Brown AS, et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 56.Enayati M, et al. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2012;87:295–302. doi: 10.1016/j.brainresbull.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Lau C. Fetal programming of adult disease: implications for prenatal care. Obstet Gynecol. 2011;117:978–985. doi: 10.1097/AOG.0b013e318212140e. [DOI] [PubMed] [Google Scholar]
- 58.Nathanielsz PW. Fetal programming: from gene to functional systems—an overview. J Physiol. 2003;547:3–4. [Google Scholar]
- 59.Stolp H, Neuhaus A, Sundramoorthi R, Molnár Z. The long and the short of it: gene and environment interactions during early cortical development and consequences for long-term neurological disease. Front Psychiatry. 2012;3:50. doi: 10.3389/fpsyt.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci. 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 63.Ponder KL, et al. Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: implications for fetal programming. Dev Psychol. 2011;53:711–723. doi: 10.1002/dev.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilligan J, Tong M, Longato L, de la Monte SM, Gundogan F. Precision-cut slice culture method for rat placenta. Placenta. 2012;33:67–72. doi: 10.1016/j.placenta.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rennie MY, Whiteley KJ, Kulandavelu S, Adamson SL, Sled JG. 3D visualisation and quantification by microcomputed tomography of late gestational changes in the arterial and venous feto-placental vasculature of the mouse. Placenta. 2007;28:833–840. doi: 10.1016/j.placenta.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Cox B, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 68.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 69.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 70.Jia Z, Chen Q, Qin H. Ischemia-induced apoptosis of intestinal epithelial cells correlates with altered integrin distribution and disassembly of f-actin triggered by calcium overload. J Biomed Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/617539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Killinger WAJ, Dorofi DB, Keagy BA, Johnson GJ. Endothelial cell preservation using organ storage solutions. Transplantation. 1992;53:979–982. doi: 10.1097/00007890-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Hilgers RHP, et al. Uterine artery structural and functional changes during pregnancy in tissue kallikrein-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1826–1832. doi: 10.1161/01.ATV.0000090672.07568.60. [DOI] [PubMed] [Google Scholar]
- 73.Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol. 2006;5:1421–1428. doi: 10.1152/ajpheart.00031.2006. [DOI] [PubMed] [Google Scholar]
- 74.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology. 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacLennan MJ, Keller BB. Umbilical arterial blood flow in the mouse embryo during development and following acutely increased heart rate. Ultrasound Med Biol. 1999;25:361–370. doi: 10.1016/s0301-5629(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 76.Kay HH, Zhu S, Tsoi S. Hypoxia and lactate production in trophoblast cells. Placenta. 2007;28:854–860. doi: 10.1016/j.placenta.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 78.Tissot van Patot MC, et al. Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am J Physiol Regul Integr Comp Physiol. 2010;298:166–172. doi: 10.1152/ajpregu.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hernandez LD, et al. Encyclopedia of Life Sciences. John Wiley & Sons; 2001. Caspases and cell death. [Google Scholar]
- 80.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 81.Edison N, et al. The IAP-antagonist ARTS initiates caspase activation upstream of cytochrome C and SMAC/Diablo. Cell Death Differ. 2012;19:356–368. doi: 10.1038/cdd.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz SM. Cell death and the caspase cascade. Circulation. 1998;97:227–229. doi: 10.1161/01.cir.97.3.227. [DOI] [PubMed] [Google Scholar]
- 83.Mu J, et al. Apoptosis and related proteins in placenta of intrauterine fetal death in prostaglandin F receptor-deficient mice. Biol Reprod. 2003;68:1968–1974. doi: 10.1095/biolreprod.102.008029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.