Abstract
Background
miR-181a is a small non-coding RNA known to be dysregulated in osteoarthritis (OA), but the role of miR-181a in human OA remains unclear. The aim of this study was to identify its function and molecular target in chondrocytes during OA pathogenesis.
Material/Methods
The function of miR-181a was assessed by gain-of-function studies in human OA chondrocytes. Potential targets of miR-181a were predicted using series of bioinformatics and intersection analysis, then confirmed by luciferase reporter assay. Gene expression was quantified using quantitative reverse transcription PCR (qRT-PCR) assays, and protein production was quantified by Western blot analysis.
Results
The FITC apoptosis assay results indicated that the upregulation of miR-181a led to an increase of apoptosis rate in chondrocytes. Then bioinformatic analysis identified potential target sites of the miR-181a located in the 3′ untranslated region of GPD1L. Dual-luciferase reporter assays results showed that GPD1L is a target gene of miR-181a. Furthermore, Western blot and qRT-PCR analysis demonstrated that miR-181a inhibited GPD1L gene expression. Increased GPD1L and decreased miRNA-181a were observed in tissues from osteoarthritis patients. Moreover, we found a highly negative correlation between miRNA-181a and GPD1L.
Conclusions
Our results demonstrated that miR-181a may play an important role in the pathogenesis of OA through targeting GPD1L and regulating chondrocyte apoptosis.
MeSH Keywords: Apoptosis, Chondrocytes, Down-Regulation, MicroRNAs, Osteoarthritis
Background
MicroRNAs (miRNAs), a class of short non-coding RNA molecules of 17–25 nucleotides, have been regarded as a class of crucial gene regulators that silence target mRNAs by binding to complementary sequences in 3′ untranslated regions (3′UTR) to induce target mRNA degradation or translational repression [1]. There is accumulating evidence that miRNAs play a vital role in cartilage differentiation and homeostasis, and in the pathobiology of osteoarthritis and therefore function as an integral part of the regulatory network in chondrocyte fate and cartilage function [2,3]. Growing evidence shows differential expression patterns of miRNAs between osteoarthritis (OA) and normal plasma [4], synovial fluid [5], and cartilage [6]. Moreover, several miRNAs have been identified to participate in the “tug-of-war” between tissue homeostasis and OA pathogenesis [7]. miR-181a was previously identified to be associated with OA. Expression of miR-181a can be detected in cells with chondrocytic phenotype [8]; its expression is downregulated in OA but upregulated in synovial fluid from OA patients treated with hyaluronic acid [5]. However, the potential function and mechanism of miR-181a in human OA pathogenesis remains unclear.
Apoptosis has been proved to be involved in maintaining the homeostasis of various tissues in the adult human body, and the role of apoptosis in the chondrocytes in the etiology and progression of OA has been well documented [9,10]. Apoptosis occurs in osteoarthritic cartilage; however, the relative contributor and regulator of chondrocyte apoptosis in the pathogenesis of OA has rarely been reported [11]. It has been confirmed that miRNA is involved in the disrupted balance of pro- and anti-apoptotic proteins, thus resulting in over-proliferation and/or dysfunctional removal of cells in a variety of diseases [12–14]. miRNAs may also be important mediators of chondrocyte apoptosis in OA.
In this present study, we show that miR-181a functions as an inducer of chondrocyte apoptosis in vitro, and that miR-181a can directly target the chondrocyte apoptosis suppressor GPD1L. Increased GPD1L and decreased miRNA-181a were observed in tissues from OA patients. Moreover, we found a highly negative correlation between miRNA-181a and GPD1L, and showed that knockdown of GPD1L induced chondrocyte apoptosis. Our findings indicated that dysregulation of miR-181a may modulate OA through targeting GPD1L and regulating chondrocyte apoptosis.
Material and Methods
Materials
Rabbit polyclonal antibodies specific to GPD1L and GAPDH was purchased from Abcam (Cambridge, MA, USA); miR-181a mimic and negative control, miR-181a inhibitor and negative control, Lipofectamine 3000, and TRIzol from Life Technologies (Carlsbad, CA). DMEM, fetal bovine serum (FBS), and all other cell culture reagents from Life Technologies (Grand Island, NY); wild-type 3′UTR of GPD1L mRNA (wt-GPD1L- 3′UTR) and vector containing mismatches in predicted miR-181a-binding site (full muntant-GPD1L-3′UTR) were from Addgene (Cambridge, MA); GPD1L siRNA (sc-78210) and the negative control siRNA (sc-37007) were obtained from Santa Cruz (Santa Cruz Biotechnology, CA).
Specimen selection and cell culture
Cartilage tissues were obtained at the time of knee replacement from patients diagnosed with OA according to the American College of Rheumatology criteria for this disease. The OA patients included 3 males and 2 females, with a mean age of 72.4 years (63–85 years). Normal human articular cartilage tissues from knee joints, including 3 males and 2 females, with a mean age of 42.6 years (30–50 years), were obtained from traumatic amputees in our hospital. Ethics approval was obtained from the Ethics Committee of our hospital and written consent was obtained from all of the patients.
Articular cartilages were cut into small fragments, firstly digested with trypsin (Invitrogen, CA, USA) for 30 min and then treated with 0.3% collagenase (Invitrogen) for 4 h at 37°C for dissociation. After that, cell suspension was filtered through 40 μm cell filter (BD Falcon, MA), and cells were collected. Chondrocytes were then resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS, HyClone Laboratories, Logan, UT), 100 units/ml penicillin, and 100 units/ml streptomycin. Chondrocytes were cultured at 37°C in humidified air with 5% CO2. Primary chondrocytes at 80% confluence were used for all the studies.
Transfection
Chondrocytes at approximately 60%–70% confluence were transfected using LipofectamineTM 3000 reagents (Invitrogen, CA). After 5 h of transfection, the medium was replaced with fresh DMEM medium with 10% FBS.
Annexin-V-fluorescein isothiocyanate (FITC) apoptosis assay
After transfection for 48 h, the chondrocytes (1×104) were harvested and the translocation of phosphatidylserine was detected by using the Annexin-V-FLUOS staining kit (Roche Applied Science, Mannheim, Germany). Briefly, the harvested chondrocytes were washed and incubated after being labeled with 5 ml of Annexin-V-fluorescein isothiocyanate (FITC) and 5 ml of PI. The labeled chondrocytes were then analyzed by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) flow cytometry
Target prediction
Putative targets of miR-181a were predicted bioinformatically by specific programs as follows: TargetScan (http://www.targetscan.org), miRanda database (http://www.microrna.org), and Pictar database (http://pictar.mdc-berlin.de/). Putative miRNA: mRNA interaction was based on the total context score and probability of conserved targeting (PCT). The more negative the context score and the higher the PCT, the higher the probability of miRNA: mRNA binding. Relevant targets predicted by all 3 databases were chosen for lab experimentation.
Quantitative reverse transcription PCR
Total RNA of tissues and cells were isolated using TRIzol (Invitrogen Life Technologies). Subsequently, cDNA was synthesized from 1 μg RNA using a specific primer for miRNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), or with a primer for message RNA using a reverse transcription kit (Takara, Dalian, China). After reverse transcriptase reaction, real-time PCR was performed on an ABI 7900 Real-Time PCR system (Applied Biosystems, Foster City, CA). For the quantification of miRNA expression, RT reaction was processed at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. Gene expression levels were quantified at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The primers used were as follows: GPD1L, 5′-ACGGTGGTTG ATGATGCAGACACT-3′ (forward), and 5′-CGGATGACGGCCGCT TTGGT-3′ (reverse); GPD1L, 5′-CGCTGGGAATCACCCTCATC-3′ (forward), and 5′-CATTACTTTGCTGCCGATGGT-3′ (reverse); β-actin, 5′-ATCGTCCACCGCAAATGCTTCTA-3′ (forward), and 5′-AGCCATGCCAATCTCATCTTGTT-3′(reverse). β-actin and small nuclear RNA U6 were used as internal normalized references for mRNA and miRNA, respectively.
Luciferase reporter assay
For the luciferase reporter assay, cells were co-transfected with mutant-type or wild-type pmirGLO-report-GPD1L vector, and co-transfected with miR-181a inhibitor/mimics or their negative control, respectively. Each sample was co-transfected with 50 ng of pRL-TK plasmid expressing Renilla luciferase (Promega, Madison, WI). Chondrocytes were collected 24 h after transfection and analyzed using the dual-luciferase reporter assay (Promega Corporation, Madison, WI). The Renilla luciferase activities were normalized to firefly luciferase.
Western blot analysis
Total protein was dissolved in radio-immunoprecipitation assay buffer (150 mm NaCl, 10 mm Tris, pH 7.2, 5 mm EDTA, 1% Triton X-100, 0.1% SDS, 1% deoxycholic acid). Protein samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. The membranes were blocked using 5% BSA for 30 min and incubated with diluted (1:1,000) polyclonal antibodies specific for GPD1L and GAPDH overnight at 4°C, followed by hybridized with secondary antibodies. After washing 3 times with TBST, the specific proteins were visualized using an enhanced chemiluminescence method kit (GE Healthcare Life Sciences, Piscataway, NJ).
Statistical analysis
Each experiment was repeated 3 times. Analyses were carried out using the Statistical Package for the Social Sciences (version 21.0; SPSS, Chicago, IL). Data are expressed as mean ± standard deviation (SD). The difference between groups was estimated by using Student’s t-test. Pearson correlation coefficient were used to assess the correlation between miR-181a and GPD1L expression levels. A P value low than 0.05 was considered to be a statistically significant difference.
Results
miR-181a induces chondrocyte apoptosis
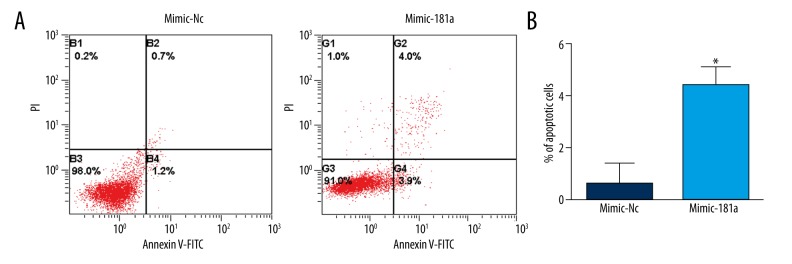
To investigate the effect of miR-181a on chondrocytes, we detected whether miR-181a overexpression affects the percentage of apoptosis chondrocytes. As shown in Figure 1, the ectopic expression of miR-181a chondrocytes showed a significant increase in the apoptotic rate of chondrocytes (P<0.01).
Figure 1.
Overexpression of miR-181a induced chondrocyte apoptosis. (A) After transfection for 48 h, Annexin-V-FITC and PI co-staining were used for flow cytometric analysis. FITC, fluorescein isothiocyanate; PI, propidium iodide. (B) Quantification of the percentage of apoptotic cells. * P<0.05.
miR-181a targets prediction
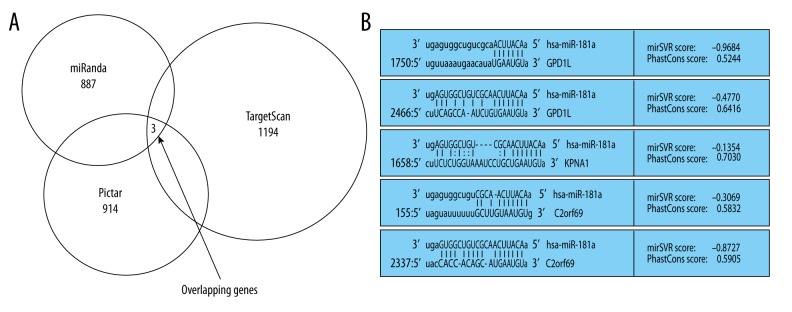
miRNA is an important post transcription negative regulator for protein coding genes. Therefore, we first predicted targets of miR-181a by using online bioinformatics tools TargetScan and miRanda, as well as the Pictar databases. Among predicted targets of miR-181a from these 3 databanks, 3 overlapping genes ranked as most probable targets (Figure 2). One of them, GPD1L, is implicated in hypoxia-inducible factor 1α (HIF-1α) expression and hydroxylation [15,16], and HIF-1α has been confirmed as an important regulator contributing to the synthesis of ECM and chondrocyte fate in OA [17]. Thus, we next determined whether GPD1L is a direct target of miR-181a in chondrocytes, and whether miR-181a regulates chondrocyte apoptosis by targeting GPD1L, thereby affecting the pathogenesis of OA.
Figure 2.
MiR-181a targets prediction. (A) Three genes were selected as potential targets of miR-181a using 3 classic prediction databases. (B) The specific binding sites of miR-18a within the 3′UTR of potential targets.
miR-181a directed targets GPD1L
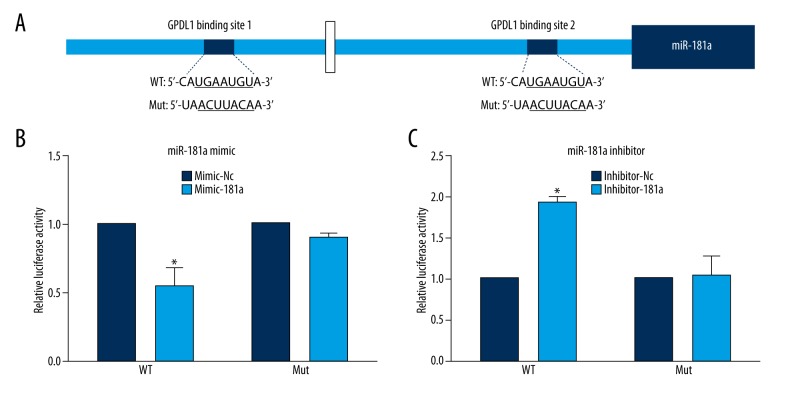
Next, we tested whether miR-181a targets GPD1L through a seed site in the 3′-UTR of GPD1L mRNA. We constructed pmirGLO-GPD1L-UTR, a dual-luciferase reporter plasmid containing 2 tandem miR-181a recognition sequences from the 3′-UTR of GPD1L mRNA, and pmirGLO-GPD1L-mut-UTR reporter, the mutated recognition sequences (Figure 3A). We performed dual-luciferase reporter assays by using these vectors. Our results showed that the reporter luciferase activity of the wild-type 3′-UTR was significantly decreased in the miR-181a mimic group (Figure 3B), while luciferase activity of the mutant 3′-UTR reporter was not significantly changed by the miR-181a mimics. The miR-181a inhibitor (Inhibitor-181a)-transfected cells showed the opposite results (Figure 3C). In summary, GPD1L is a direct target gene of miR-181a.
Figure 3.
GPD1L is a direct target of miR-181a. (A) Schematic representation of the sequence alignment of the wild-type GPD1L 3′-UTR indicating the binding sites of the miR-181a and the mutant GPD1L 3′-UTR for pmirGLO-report (WT, wild-type; MUT, mutant). (B) Compared with the negative control group (Mimic-Nc), miR-181a mimics significantly inhibited the reporter luciferase activity of the wild-type GPD1L 3′-UTR (* p<0.05) but not that of the mutant GPD1L 3′-UTR (p>0.05) (means ±SD; n=3). (C) Compared with the inhibitor negative control group, the reporter luciferase activity of the wild-type GPD1L 3′-UTR was significantly increased by miR-181a inhibitor (* p<0.05) compared to the mutant GPD1L 3′-UTR (* p>0.05) (means ±SD; n=3).
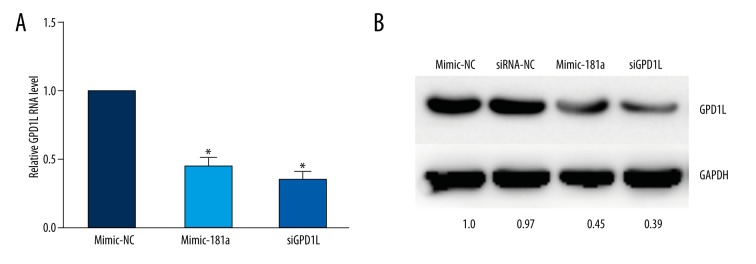
Using Western blot and RT-qPCR analysis, we then determined whether miR-181a downregulates GPD1L protein or mRNA in chondrocytes. Our results showed that miR-181a-overexpression resulted in a sharp decreased in GPD1L mRNA levels (Figure 4A) and reduced GPD1L protein level (Figure 4B), which is comparable to the effect of GPD1L siRNA in chondrocytes.
Figure 4.
GPD1L expression is regulated by miR-181a. (A) miR-181a mimic and GPD1L siRNA effectively reduced the expression of GPD1L mRNA. (* p<0.05). (B) miR-181a mimic and GPD1L siRNA effectively reduced the expression of GPD1L protein. (* p<0.05) (means ±SD; n=3).
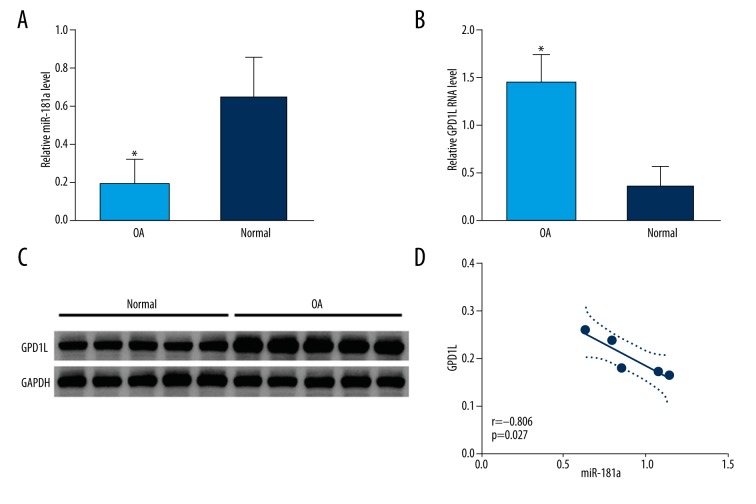
The expression of miRNA-181a and GPD1L in OA patients
We then assessed miRNA-181a and GPD1L expression levels in osteoarthritis patients. As shown by real-time PCR analysis, the expression level of miRNA-181a was significantly decreased, and the expression of GPD1L mRNA was markedly increased in tissues from osteoarthritis patients compared to tissues from traumatic amputees (Figure 5A, 5B). Figure 5C displays the Western blotting results, showing that GPD1L protein was downregulated in tissues from osteoarthritis patients compared to that of the control group (P<0.05). Interestingly, a highly negative correlation between miRNA-181a and GPD1L was found in tissues from osteoarthritis patients (Figure 5D).
Figure 5.
Expression of miR-181a and GPD1L in healthy and OA cartilage. (A) The qRT-PCR analysis of expression of miR-181a in OA cartilages compared to healthy cartilage from traumatic amputees (normal control). The expression of miR-181a is significantly lower in cartilage from OA patients compared to cartilage from traumatic amputees (P<0.01). (B) The levels of GPD1L mRNA in tissues from OA patients and from traumatic amputees (normal control) were analyzed by qRT-PCR. OA cartilage exhibited higher levels of GPD1L mRNA than did healthy cartilage (P<0.01). (C) The level of the GPD1L protein in tissues from OA patients and from traumatic amputees was analyzed by Western blot analysis. (D) Correlation analysis of the mRNA expression of GPD1L and miR-181a in OA and healthy tissue mentioned in Figures 1A and B.
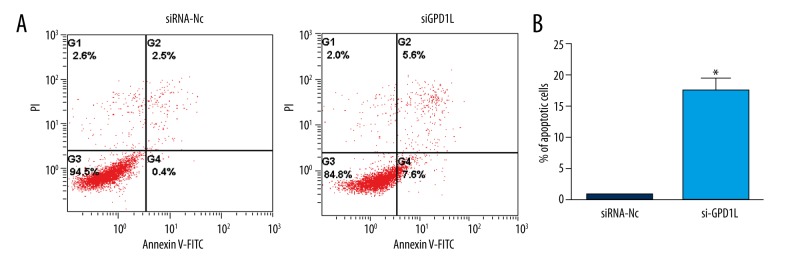
GPD1L inhibits chondrocyte apoptosis
To investigate the effect of GPD1L on chondrocytes, we examined whether the decrease of GPD1L affects the apoptosis rate of chondrocyte. As shown in Figure 6, GPD1L-knockdown chondrocytes showed a significantly increased apoptotic rate (P<0.01).
Figure 6.
Knockdown of GPD1L induced chondrocyte apoptosis. (A) After transfection for 48 h, Annexin-V-FITC and PI co-staining were used for flow cytometric analysis. (B) Quantification of the percentage of apoptotic cells. * P<0.05.
Discussion
Osteoarthritis is the most prevalent chronic joint disease in the elderly population [18]. Articular cartilage is composed of a small number of chondrocytes and a large amount of extracellular matrix (ECM). Chondrocytes, the unique cell type resident in cartilage, mediates the cartilage homeostasis by express various ECM molecules such as type II collagen and sulfated proteoglycans, which are essential components of chondrocyte anabolism [19]. Recently, numerous experiments have focused on the role of miRNAs in chondrocytes and cartilage [3]. The present study presents an important finding that the expression of miR-181a functions as an inducer of chondrocytes apoptosis via downregulating GPD1L, showing the critical function of miR-181a in the development of OA.
miRNAs are essential regulatory molecules and orchestrate gene expressions implicated in the regulation of articular cartilage homeostasis through translational inhibition and mRNA stability. It has been established that miRNAs are important regulators of synthesis and degradation of ECM components, and that they participate extensively in chondrogenesis, chondrocyte differentiation, proliferation, and apoptosis [2,7,20]. Among them, miR-181a has been demonstrated to be downregulated in OA [5], but its expression and role in chondrocyte has not been explored. In the present study, we investigated the effects of miR-181a on chondrocyte apoptosis, and found that overexpression of miR-181a induces chondrocyte apoptosis, suggesting that miR-181a is a pivotal regulator in OA pathogenesis.
To understand the role of miR-181a in the pathogenesis of OA, we used computational databanks to help identify the targets of miR-181a. Among the 3 overlapping targets predicted, we focused on the gene GPD1L. GPD1L is a master regulator for the hydroxylation of HIF-1α, and its expression leads to decrease of HIF-1α expression [16]. HIF-1α was reported to protect articular cartilage by promoting the chondrocyte phenotype, maintaining chondrocyte viability, and supporting metabolic adaptation to a hypoxic environment [17]. In addition, HIF-1α-induced HSP70 overexpression increased the expression levels of ECM genes and cell viability, and protected chondrocytes from apoptosis [21], indicating that GPD1L may also be involved in modulating of chondrocytes apoptosis. Kelly et al. reported that hypoxia-induced miR-210 represses GPD1L and contributes to increased HIF-1α protein levels [16], proposed a model in which GPD1L mediates the function of miRNAs in different physiologic or pathologic conditions. Our studies found that the mimic of miR-181a significantly downregulated the mRNA and protein expression of GPD1L and miR-181a by directly targeting GPD1L in chondrocytes, and the expression level of GPD1L was negative correlated with miRNA-181a in tissues from OA patients. Moreover, GPD1L-knockdown chondrocytes showed a significantly increased apoptotic rate, indicating that GPD1L is indeed involved in modulation of different cellular microenvironments by mediating miRNAs function.
In addition to chondrocyte apoptosis, it would be interesting to investigate the role of miR-181a in OA angiogenesis, as GPD1L signaling is also involved in endothelial progenitor cell tube formation and migration [16]. It has been reported that hypoxia-induced miR-181a enhances the expression of vascular endothelial growth factor [22]. Future experiments are necessary to address miR-181a function in depth.
Conclusions
The present study demonstrates that miR-181a facilitates chondrocyte apoptosis through directly targeting GPD1L, which ameliorates the pathological process of OA. AgomiR-based therapy targeted at miR-181a could potentially be effective in limiting OA progression.
Footnotes
Source of support: Departmental sources
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–33. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 2.Hong E, Reddi AH. MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: Implications for tissue engineering. Tissue Eng Part B Rev. 2012;18(6):445–53. doi: 10.1089/ten.TEB.2012.0116. [DOI] [PubMed] [Google Scholar]
- 3.Mirzamohammadi F, Papaioannou G, Kobayashi T. MicroRNAs in cartilage development, homeostasis, and disease. Curr Osteoporos Rep. 2014;12(4):410–19. doi: 10.1007/s11914-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgonio Cuadra VM, González-Huerta NC, Romero-Córdoba S, et al. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PLoS One. 2014;9(6):e97690. doi: 10.1371/journal.pone.0097690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu JF, Zhang SJ, Zhao C, et al. Altered microRNA expression profile in synovial fluid from patients with knee osteoarthritis with treatment of hyaluronic acid. Mol Diagn Ther. 2015;19(5):299–308. doi: 10.1007/s40291-015-0155-2. [DOI] [PubMed] [Google Scholar]
- 6.Díaz-Prado S, Cicione C, Muiños-López E, et al. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet Disord. 2012;13:144. doi: 10.1186/1471-2474-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldring MB, Marcu KB. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol Med. 2012;18(2):109–18. doi: 10.1016/j.molmed.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumiyoshi K, Kubota S, Ohgawara T, et al. Novel role of miR-181a in cartilage metabolism. J Cell Biochem. 2013;114(9):2094–100. doi: 10.1002/jcb.24556. [DOI] [PubMed] [Google Scholar]
- 9.Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59(12):959–65. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–54. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou Y, Tan C, An H, et al. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Monit. 2012;18(6):BR247–52. doi: 10.12659/MSM.882901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Li Y, Kong D, et al. Cross-talk between miRNA and Notch signaling pathways in tumor development and progression. Cancer Lett. 2010;292(2):141–48. doi: 10.1016/j.canlet.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan G, Mathur R, Hu X, et al. miRNA response to DNA damage. Trends Biochem Sci. 2011;36(9):478–84. doi: 10.1016/j.tibs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki H, Wadhwa R, Taira K. World of small RNAs: From ribozymes to siRNA and miRNA. Differentiation. 2004;72(2–3):58–64. doi: 10.1111/j.1432-0436.2004.07202006.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu SC, Chuang Sm, Hsu CJ, et al. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 2014;5:e1485. doi: 10.1038/cddis.2014.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. 2011;31(13):2696–706. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang FJ, Luo W, Lei GH. Role of HIF-1α and HIF-2α in osteoarthritis. Joint Bone Spine. 2015;82(3):144–47. doi: 10.1016/j.jbspin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Aigner T, Sachse A, Gebhard PM, Roach HI. Osteoarthritis: Pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58(2):128–49. doi: 10.1016/j.addr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Kang F, Cho Y, Kim JH. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Mol Cells. 2015;38(8):677–84. doi: 10.14348/molcells.2015.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang J, Liu H, Zhou Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage-related diseases. J Cell Mol Med. 2013;17(12):1515–24. doi: 10.1111/jcmm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchida S, Arai Y, Takahashi KA, et al. HIF-1α-induced HSP70 regulates anabolic responses in articular chondrocytes under hypoxic conditions. J Orthop Res. 2014;32(8):975–80. doi: 10.1002/jor.22623. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Wei L, Chen Q, Terek RM. MicroRNA regulates vascular endothelial growth factor expression in chondrosarcoma cells. Clin Orthop Relat Res. 2015;473(3):907–13. doi: 10.1007/s11999-014-3842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]