Abstract
Background
Pituitary adenomas are mostly benign tumors, although certain cases have invasiveness, which might be related with high expression of miR-106b. The PTEN-PI3K/AKT signal pathway is known to be related with cell migration and invasion. Among these, PTEN is the target gene for miR-106b. Whether miR-106b affects invasiveness of pituitary adenoma via PTEN-PI3K/AKT is unclear.
Material/Methods
Both invasive and non-invasive pituitary adenoma tissue samples were collected from our Neurosurgery Department, in parallel with brain tissues after head contusion surgery. Pituitary adenoma cell line HP75 was cultured in vitro and divided into NC and miR-106b inhibitor groups for measuring cell cycle/proliferation. Malignant growth of cells was measured by agarose gel clonal assay, while cell migration and invasion were reflected by starch assay and Transwell assay, respectively. The expression of PTEN, PI3K/AKT, and MMP-9 was measured.
Results
MiR-106b was significantly up-regulated in pituitary adenoma but PTEN was down-regulated, especially in invasive tumors. The inhibition of miR-106b remarkably suppressed proliferation and anchorage-independent growth of HP75 cells, with major arrest of cell cycles. The inhibition of miR-106b significantly depressed starch healing and invasive potency of cells. A negative targeted regulation existed between miR-106b and PTEN, as the inhibition of miR-106b significantly enhanced PTEN expression, affecting the activity of downstream PI3K/AKT signaling pathway, thus affecting migration and invasion of pituitary adenoma.
Conclusions
MiR-106b can affect migration and invasion of pituitary adenoma cells via regulating PTEN and further activity of the PI3K/AKT signaling pathway and MMP-9 expression.
MeSH Keywords: Growth Hormone-Secreting Pituitary Adenoma, Matrix Metalloproteinase 9, PTEN Phosphohydrolase
Background
Pituitary adenoma is a kind of endocrine tumor [1] and is a common intracranial tumor, as it accounts for about 10% of all primary intracranial tumors, making it the third-most common after glioma and meningioma [2]. Pituitary adenoma often occurs in young people, and presents as a series of endocrine symptoms, including acral growth and decreased sexual function. Although it is a benign tumor, pituitary adenoma can have infiltrative growth, which is one of the biological behaviors of malignant tumors [3]. It often invades adjacent tissues, including vascular wall, cavernous sinus, or even brain tissues, causing various complications. This aggressive invasion of pituitary adenoma is closely correlated with tumor recurrence and prognosis [4]. In pituitary tumors with invasive growth, the tumor size is normally large, or accompanied with focal infiltration into peripheral tissues, thus severely affecting neural functions. Such invasive growth also causes difficulty in complete surgical resection of tumors, as well as post-operative recurrence and worse prognosis. Some studies show that the occurrence of pituitary adenoma might be related with oncogene expression or inactivation of tumor suppressor genes, although the detailed mechanism is still unclear [5–7]. MicroRNA (miR) is a biological marker for human malignant tumors, and exerts critical roles in biological processes in cancer. The abnormal expression of miR functions as a tumor suppressor gene or an oncogene in malignant tumors. It is a member of the miR-106b~25 family, which consists of miR-106b, miR-93, and miR-25. MiR-106b is a known oncogenic miR [8]. Its abnormally high expression can be observed in various tumor tissues, and has multiple functions regulating tumor cell proliferation, migration, and invasion [9–12]. Previous studies have shown that high expression of miR-106b can facilitate growth of gastric carcinoma cells via accelerating the cell cycle [13], and can enhance metastasis of breast cancer via inducing the TGF-β signal pathway [14]. miR-106b can also affect invasion or metastasis of endometrial carcinoma [15], cervical cancer [16], hepatocellular carcinoma [17], renal carcinoma [18], and pulmonary carcinoma [19] via various mechanisms. A recent study also revealed that up-regulation of miR-106b can facilitate the epithelial-mesenchymal transition (EMT) of tumor cells, thus facilitating tumor invasion and metastasis [17]. A recent study showed the existence of miR-106 abnormal expression in pituitary adenoma, thus indicating its potential role in tumor pathogenesis [20]. However, the detailed mechanism of miR-106b in pituitary adenoma occurrence is still unknown, as is the relationship with tumor invasiveness. PI3K/AKT is a multiple signal pathway correlated with the occurrence of pituitary adenoma. As an important tumor suppressor gene, PTEN can negatively regulate the PI3K-AKT signal pathway to inhibit tumor occurrence and progression. PTEN is a lipid phosphatase, and can convert membrane PIP3 into PIP2 via de-phosphorylation, and can antagonize the phosphorylation of PI3K on PIP2, thus affecting PI3K-induced cell proliferation and survival signals. Moreover, the PTEN-PI3K/AKT signal pathway exerts critical functions in mediating tumor cell invasion/migration properties [21]. As a target gene for miR-106b, the expression of PTEN is negatively regulated by miR-106b, thus affecting multiple biological functions of tumor cells [22,23]. The correlation between the miR-106/PTEN-PI3K/AKT signal pathway and invasiveness of pituitary adenoma still requires further investigation.
Material and Methods
Reagents and materials
Human pituitary adenoma cell line HP75 was provided by ATCC (USA). Cell culture medium was purchased from Gibco (USA). RNA extraction reagent Trizol was purchased from Invitrogen (USA). Reverse transcription and fluorescent quantitative PCR kits were purchased from Takara (China). Oligonucleotides and PCR primers for transfection were designed and synthesized by Gimma (China). Rabbit anti-PTEN and mouse anti-MMP-9 antibody were purchased from Abcam (USA). Rabbit anti-p-AKT was purchased from Bioworld (USA).
Clinical information
A total of 50 pituitary adenoma patients in the Department of Neurosurgery in Shandong Provincial Hospital affiliated to Shandong University from June 2014 to August 2015 were recruited in this study. All patients had received definite diagnosis by imaging, surgery, and pathological examination. No treatment has given to any patient before the surgery. There were 23 males and 27 females in the patient cohort, ages 25–68 years (average age=37.2 years). There were 8, 7, 15, and 20 cases of ACTH adenoma, prolactin adenoma, GH adenoma, and nonfunctional adenoma, respectively. A total of 18 patients were classified into the non-invasive pituitary tumor group while the other 32 were classified into the invasive adenoma group. The criteria for tumor invasiveness were: (1) Grade III~V or phase C, D, or E tumors according to Hardy-Wilson guideline; (2) Destruction of cavernous sinus or parasellar tissues by pre-op CT/MRI imaging; (3) Infiltration into sellar diaphragm, or sellar bottom bone regions by tumor cells as shown by biopsy during the surgery; and (4) Perforation of inner wall of cavernous sinus under endoscopy. Non-invasive pituitary adenoma was defined as the limitation of tumor body within the sellar region, without any compression on peripheral structures. Exclusive criteria were: Post-operative pathological examination revealed other types of tumors, including cranial-pharyngeal carcinoma, meningioma of sellar nodules, and Lyske cyst, despite pre-operative diagnosis of pituitary adenoma. Another cohort of 10 normal brain tissues collected from head trauma surgery were recruited as the control group, in which there were 4 males and 6 females, ages 26–61 years (average age=36.5 years). We obtained consent from all patients for sample collection, along with approval by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University.
Cell culture and transfection
Human pituitary adenoma cell line HP75 was inoculated in DMEM containing 12.5% horse serum and 2.5% fetal bovine serum. We transfected 2×105 HP75 cells into 6-well plates. Until reaching 60%~80% confluence, lipofectamine 2000 was used in transfection in negative control, missense oligonucleotide (5′-CAGUA CUUUU GUGUA GUACA A-3′), and miR-106b inhibitor groups (5′-AUCUG CACUG UCAGC ACUUU A-3′). In brief, lipofectamine 2000 and oligonucleotides were cultured along with cells in serum-free Opti-MEMI medium after culturing at 37°C with 5% CO2 for 4~6 hours. Fresh normal culture medium was used for 48-h continuous incubation.
qRT-PCR for gene expression
Total RNA was extracted by Trizol reagent. Purity and quality of RNA were assayed by UV spectrometry and agarose gel electrophoresis. MiR-106a specific stem-loop primer and random primer were used to synthesize cDNA by reverse transcription in a 20-μL system containing 2 μg total RNA, 0.75 μL dNTP (10 mmol/L), 4 μL RT buffer (5×), 1.2 μL RT primers (1 μmol/L), 2 μL reverse transcriptase, and 0.5 μL RNase inhibitor. Reverse transcription was performed in 16°C for 30 min, followed by 42°C for 15 min and 85°C for 5 min. Using cDNA as the template, PCR amplification was pursued under the direction of TaqDNA polymerase. Primer sequences were: miR-106b RT: 5′-GTCGT ATCCA GTGCA GGGTC CGAGG TATTC GCACT GGATA CGACA TCTGC-3′; miR-106bPF: 5′-TAAAG TGCTG ACAGT GCAGA T-3′; miR-106bPR: 5′-GGTCT AGATG TGAAC TCTGG TGTTG GTGC-3′; U6PF: 5′-CTCGC TTCGG CAGCA CA-3′; U6PR: 5′-AACGC TTCAC GAATT TGCGT-3′; PTENPF: 5′-CTGGT CTGCC AGCTA AAGGT-3′; PTENPR: 5′-TCACC ACACA CAGGT AACGG-3′; MMP9PF: 5′-TGTAC CGCTA TGGTT ACACT CG-3′; MMP9PR: 5′-GGCAG GGACA GTTGC TTCT-3′; β-actinPF: 5′-GCACT CTTCC AGCCT TCC-3′; β-actinPR: 5′-AGAAA GGGTG TAACG CAACT AAG-3′. In a total 10 μL reaction system, there were 4.5 μL 2×SYBR Green Mixture, 0.5 μL of forward/reverse primer (5 μm/L), 1 μL cDNA, and 3.5 μL ddH2O. The reaction conditions were: 95°C denatured for 5 min, followed by 40 cycles each containing 95°C for 15 s and 60°C for 1 min on an ABI ViiA7 fluorescent quantitative PCR cycler. Data were quantitatively analyzed by Ct method (2–ΔΔCt). MicroRNA and mRNA levels were normalized using U6 and β-actin as internal reference genes, respectively. Each experiment was performed in triplicate.
Western blotting
Total proteins were extracted and quantified using bicinchoninic acid (BCA) assay. Proteins were separated in SDS-PAGE, and were transferred to polyvinylidene fluoride (PVDF) membrane, which was blocked in 5% defatted milk powder at room temperature. Primary antibody was applied at 4°C for overnight incubation, and was rinsed in phosphate-buffered saline with Tween 20 (PBST) (5 min each, 3 times). Secondary antibody was added for room temperature incubation for 60 min. After rinsing in PBST (5 min ×3 times), enhanced chemiluminescence (ECL) reagent was added for development and exposure and scanned for data analysis.
Flow cytometry for cell cycle assay
Cells were digested in trypsin, and were rinsed in phosphate-buffered saline (PBS) twice. After fixation in 4°C 75% ethanol overnight, cells were washed twice in PBS and 50 μg/mL RNase A was added for 30-min incubation at 37°C to quench endogenous RNase. Staining dye containing 0.1% Triton X-100 and 50 μg/mL PI was added for dark incubation at 4°C for 30 min. Samples were immediately loaded for flow cytometry assay.
CFDASE staining for cell proliferation
1 mL CDFASE marking solution was used to re-suspend 1~5×106 cells. Adding 1 mL CDFASE storage buffer (2×), cells were cultured at 37°C for 10 min, followed by centrifugation washing in 10 mL serum-containing culture medium. Cells were then incubated in 10 mL erum-containing culture medium at 37°C for 5 min. Culture medium was removed by centrifugation. Flow cytometry was then employed to detect at 488nm excitation wavelength.
Agarose clonal formation assay for cell malignant growth
1.2% low-melting point agarose was mixed with equal volume of 2× cell culture medium to prepare 0.6% low-melting point agarose, which was added into 6-well plate. After incubating at 4°C, the bottom layer of agarose gel was prepared. Cells at log-phase were digested in single-cell suspensions. 0.8% low-melting point agarose and equal volume of 2× cell culture medium were mixed to prepare 0.4% low-melting point agarose, which was mixed with cell suspension at 5000 cells per mL. 1 mL 0.4% low-melting agarose with cells were added into the bottom layer agarose. After 4°C settle down, the upper layer agarose was prepared, which was incubated in a 37°C chamber with 5% CO2. Fresh 0.4% low-melting point agarose was replenished every 7 days for 21 continuous days. The number of colonies containing more than 50 cells was counted. Colony formation rate=(clone number/initial inoculating cells) ×100%.
Scratch assay for cell migration
Cells at log-phase were seeded into 6-well plate at 2×105 per well. When reaching 80% confluence, 10 μL sterile pipette tip was used to draw 3 parallel starch lines with equal distance in each well. After PBS washing twice, cells were observed under an inverted microscope after 48 h incubation.
Transwell assay for cell invasion
Matrigel stock solution was diluted in 3 volumes of serum-free culture medium. A Transwell chamber was used for 100 μL Matrigel dilutions on 8 μm filter membranes. The chamber was incubated for 30 min for polymerization of gel. Serum-free culture medium was used to re-suspend cells at 1×106 per mL. We added 200 μL cell suspensions into the upper chamber, while 500 μL serum-containing conditional cell culture medium was added into the lower chamber for 24-h incubation. Liquids in the upper chamber were discarded, followed by washing twice with sterile PBS. After fixation in methanol for 30 min, cells were washed twice in PBS, stained by crystal violet, and observed under an inverted microscope. Five randomly selected fields were counted to determine the number of perforated cells.
Statistical analysis
SPSS18.0 software was used for data analysis. Measurement data are presented as mean ± standard deviation (SD), while enumeration data are presented as percentage. The comparison of enumeration data between groups was carried out by chi-square test. Measurement data were compared by one-way analysis of variance (ANOVA). A statistical significance was defined when p<0.05.
Results
Tissue expression of miR-106b and PTEN in pituitary adenoma tissues
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) results showed that, compared to normal pituitary tissues, miR-106b expression level in pituitary tumor patients was significantly elevated compared to that in normal tissues (Figure 1A), while PTEN mRNA/protein levels were remarkably depressed (Figure 1B, 1C). In pituitary adenoma patients, miR-106b levels were higher in invasive tumors compared to that in non-invasive ones, while PTEN mRNA/protein levels showed the opposite pattern. Correlation analysis revealed that miR-106b expression level was significantly negatively related with PTEN mRNA levels (r=−0.791, p=0.021), indicating the targeted regulation between miR-106b and PTEN, probably via targeted degradation of PTEN mRNA by miR-106b.
Figure 1.
MiR-106b and PTEN expression in pituitary tissues. (A) miR-10b expression in pituitary tissues; (B) PTEN mRNA expression in pituitary tissues; (C) PTEN protein expression in pituitary tissues.
Transfection of miR-10b inhibitor significantly inhibited intracellular expression of miR-106b
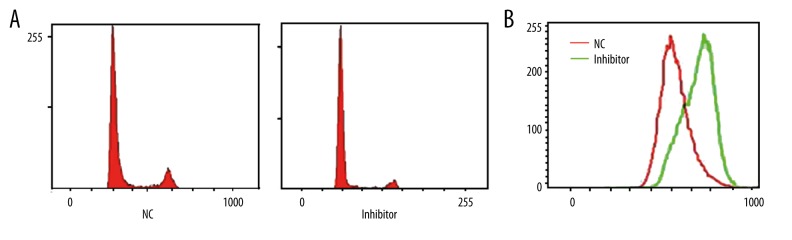
The transfection of miR-106b inhibitor significantly depressed miR-106b expression in HP75, cells by 88.6% on average (p<0.001 compared to negative control (NC) group, Figure 2), suggesting high transfection efficiency under such conditions.
Figure 2.

Transfection of miR-106b inhibitor significantly inhibited miR-106b expression.
Inhibition of miR-106b remarkably inhibited cell cycle and proliferation of HP75 cells
After transfecting anti-sense miR-106, the cycle progression of HP75 cells was significantly inhibited, as shown by decreased cell percentage at S phase and G2/M phase, accompanied with elevated G0/G1 phase (Table 1, Figure 3A). CFDASE staining results also showed weakened proliferation ability of HP75 cells (Figure 3B).
Table 1.
Cell cycle distribution (in%) of HP75 cells.
| Cell phase | NC | Inhibitor | χ2 | P |
|---|---|---|---|---|
| G0/G1 | 56.0 | 73.3 | 6.649 | 0.036 |
| S | 25.9 | 13.8 | ||
| G2/M | 18.1 | 12.9 |
Figure 3.
Flow cytometry for measuring cell cycle (A) and proliferation ability (B).
Inhibition of miR-106b suppressed malignant growth of cells
As shown by Table 2, the transfection of anti-sense miR-106b significantly depressed malignant proliferation potency of HP75 cells, as shown by decreased colony formation rate (p=0.005). These results suggested that the inhibition of miR-106b expression in pituitary adenoma cells could inhibit anchorage-independent growth of cells, thus suppressing malignant phenotype.
Table 2.
Colony formation rate (mean ±SD, in%).
| Cell phase | NC | Inhibitor | t | P |
|---|---|---|---|---|
| G0/G1 | 11.8±2.2 | 4.3±0.5 | 5.758 | 0.005 |
MiR-106b inhibition decreased cell migration and invasion potency
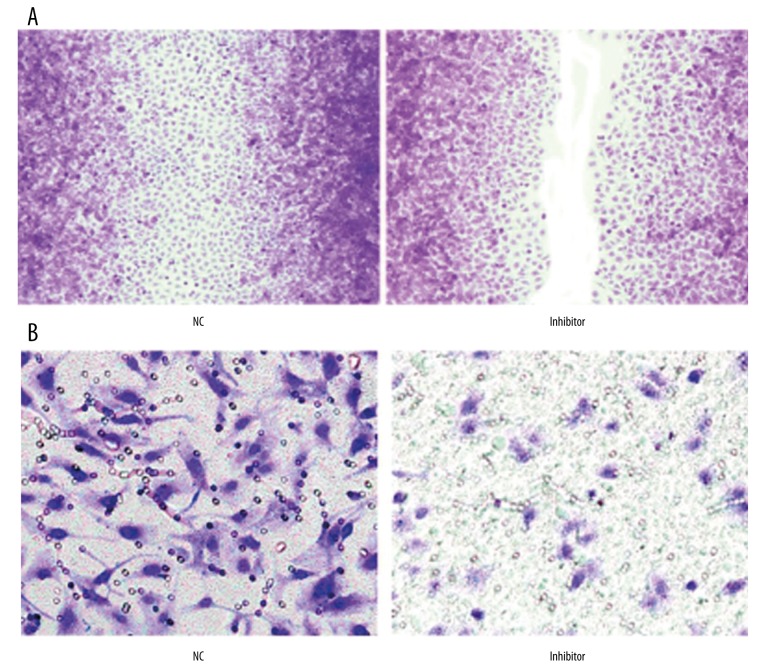
After transfecting with anti-sense miR-106b, the scratch healing of cells was significantly affected as compared to the NC group, suggesting that the inhibition of miR-106b could weaken cell migration potency (Figure 4A). Transwell assay results also revealed remarkable inhibition of cell invasion by miR-106b silencing (Figure 4B).
Figure 4.
Scratch (A) and Transwell (B) assays detecting cell migration and invasion potency.
Effect of miR-106b on PI3K/AKT signal pathway and MMP-9 expression via PTEN
The PI3K/AKT signal pathway plays a critical role in tumor migration, as it can facilitate expression of matrix mettlloproteinase-9 (MMP-9), thus enhancing tumor cell migration and invasion. As a novel tumor suppressor gene, PTEN can negatively regulate PI3K/AKT signal pathways, and decreased their regulatory roles in cell proliferation, cell cycle, migration, and invasion. Various studies have confirmed the negatively targeted regulation of PTEN expression and function by miR-106, which was a definitive target gene, and affected multiple biological functions of tumor cells. We observed significantly depressed invasion and migration anilities in those cells transfected with miR-106b inhibitor. We also investigated whether miR-106b could affect biological functions of HP75 cells via affecting PTEN-PI3K/AKT. As shown by Figure 5A, 5B, the inhibition of miR-106p increased endogenous PTEN gene expression by 265.5% and the expression of PTEN protein was also remarkably potentiated. The phosphorylation degree of PI3K and AKT proteins was, however, gradually suppressed by and accompanied with down-regulation of MMP-9 mRNA/protein.
Figure 5.
miR-106b affected PI3K/AKT signal pathway for editing PI3K/AKT signal and MMP-9 expression. (A) mRNA expression level by qRT-PCR; (B) Western blotting for protein expression.
Discussion
Pituitary adenoma is a common intracranial tumor in clinics, and accounts for about 10% of total cases [20]. Pituitary adenoma can cause a series of endocrine symptoms, including acral growth and decreased sexual function. Although most pituitary adenoma is benign without metastasis, about 40% of total cases have focal invasion and worse clinical symptoms [24]. The invasion of pituitary adenoma can destroy the structure of vascular walls, cavernous sinus, or even brain tissues around the sellar region, and may also cause CSF leakage and injury to the internal carotid artery in the sinus cavernosus segment, worsening the prognosis [25]. The further development of invasive pituitary adenoma into pituitary gland cancer creates severe consequences [26]. Therefore, the understanding of pituitary adenoma occurrence/progression and the acquirement of invasiveness is of critical importance for both diagnosis and treatment. One study showed that the occurrence and progression of pituitary adenoma might be related with the absence of tumor suppressor gene or expression of oncogenes [27]. MicroRNA is a biological marker for human tumors, and has important roles in the biological process of tumors. Its abnormal expression has tumor suppression or facilitating roles. Another study showed abnormal expression of microRNA in pituitary adenoma and pituitary gland cancer, and the correlation with tumor invasiveness and size [28]. Through literature review, some articles utilized high-throughput microarray to detect the differential expression of microRNA between invasive and non-invasive growth of pituitary adenoma [29]. In addition, a correlation was found between this differential expression and invasive growth of pituitary adenoma 24. miR-106b is a member of the miR-106b~25 gene family (including miR-106b, miR-93, and miR-25), and is an oncogenic microRNA [8]. MiR-106b has roles in regulating tumor cell proliferation, migration, and invasion, as its abnormally high expression is correlated with the occurrence of multiple tumors [9–12]. As an oncogenic microRNA molecule, the abnormally high expression of miR-106b has also been reported in pituitary adenoma tissues [20]. However, its detailed mechanism in participating in pituitary adenoma pathogenesis and its correlation with the invasiveness of tumors are clear.
This study showed abnormally elevated expression of miR-106b in pituitary adenoma tissues compared to that in normal pituitary tissues. MiR-106b expression was higher in invasive pituitary adenoma tissues compared to that in non-invasive tumors, suggesting the correlation between miR-106 high-expression and both occurrence and invasiveness of pituitary adenoma, similar to the results reported by Wei et al. [20]. Research results show that miR-106b can affect the biological function of tumor cells via affecting cell proliferation [30,31] and cycle progression [32]. The present study showed that the inhibition of endogenous miR-106 expression significantly retards the proliferation of pituitary adenoma cells, along with significant cell cycle arrest, indicating that the elevation of miR-106b expression can facilitate cell proliferation, thus playing a role in the occurrence of pituitary adenoma. Moreover, the down-regulation of miR-106b largely suppressed the anchorage-independent growth of tumor cells, suggesting that the role of miR-106b in malignant transformation of pituitary adenoma cells Ki-67 is a critical marker differentiating non-invasive and invasive pituitary adenoma mitosis, and is a critical index determining the invasiveness of pituitary adenoma [33]. Thapar et al. found a significantly higher mitotic index of invasive pituitary adenoma cells compared to that in non-invasive pituitary adenoma cells, suggesting that more active mitosis and uncontrolled tumor cell growth are important factors for the acquisition of invasiveness by pituitary adenoma [34]. Based on these findings, we speculate that high expression of miR-106b and proliferation of pituitary adenoma cells may also play certain roles in the invasiveness of pituitary adenoma, although the details of the mechanism require further study.
Some studies found that miR-106b could affect the invasion and metastasis of breast cancer [14], endometrial carcinoma [15], cervical cancer [16], hepatocellular carcinoma [17], renal carcinoma [18], and pulmonary carcinoma [19] via various mechanisms. For example, miR-106b could enhance metastasis of breast cancer via inducing TGF-β signal pathway [14]. Therefore, this study investigated the role of miR-106b in the invasiveness of pituitary adenoma. Our results found that the inhibition of intracellular miR-106b could significantly inhibit the scratch healing and invasiveness of HP75 cells, supporting the oncogenic effect of miR-106b in pituitary adenoma invasion. Phosphatase and tensin homology deleted on chromosome 10 (PTEN) locates in 10q23.3 and is a member of the protein tyrosine phosphatase family [35]. As a novel tumor suppressor gene, PTEN has drawn increasing research interest in oncology study since its discovery in 1997 [36]. As a target gene for miR-106b, the expression and function of PTEN are negatively regulated by miR-106b, thus affecting multiple biological functions of tumor cells [21,22]. This study showed significantly depressed PTEN mRNA and protein expressions in pituitary adenoma tissues compared to normal tissues, in contrast with the elevated expression of miR-106b. The most important substrate of PTEN is phosphatidylinositol-3,4,5-triphosphae (PIP3), whose contents can be decreased by dephosphorylation, and antagonizing the phosphorylation of PIP2 by phosphatidylinositol-3 kinase (PI3K), thus inhibiting the activation of AKT signal molecules and downstream pathways via inhibiting the activation by PI3K via PIP3 phosphorylation [37]. Tumor cell invasion requires 3 conditions: higher motility caused by alternation of cytoskeleton, decreased cell-to-cell adhesion and cell activation, and abundant expression of matrix metalloproteinase (MMP) to degrade extracellular matrix (ECM), thus benefiting cell migration and invasion. MMPs play a crucial role in degrading ECM and inducing tumor cell migration. MMP-9 is one of the most important members, and is expressed in most tumor cells [38]. The PI3K/AKT signal pathway plays a critical role in tumor cell invasion and migration, as it can facilitate intracellular expression of MMP-9 via various mechanisms to facilitate cell migration and invasion [39]. This study found that the transfection of miR-106b inhibitor on HP75 cells significantly enhanced the gene and protein expression of PTEN, supporting the targeted regulation between miR-106b and PTEN. Furthermore, we found that the inhibition of miR-106b significantly depressed phosphorylation of intracellular PI3K and AKT, accompanied with MMP-9 expression, suggesting that miR-106b can affect activity of the PI3K/AKT signal pathway and MMP-9 expression via targeted regulation of PTEN, thus affecting the migration and invasiveness of pituitary adenoma cells.
Conclusions
We believe that miR-106b plays a crucial role in the invasiveness of pituitary adenoma, probably via targeted regulation of PTEN to further affect the activity of the PI3K/AKT signal pathway and MMP-9 expression, thus mediating migration and invasiveness of pituitary adenoma cells. This study provides a novel strategy for explaining pathogenic mechanism of the invasiveness of pituitary adenoma, and also provides a potential strategy for the biological therapy of invasive pituitary adenoma, thus having clear value for further exploration.
Footnotes
Source of support: This work was supported by grants from the Shandong Provincial Key Research and Development Project (2015GSF118085)
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
References
- 1.Budan RM, Georgescu CE. Multiple pituitary adenomas: A systematic review. Front Endocrinol (Lausanne) 2016;7:1. doi: 10.3389/fendo.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasiljevic A, Jouanneau E, Trouillas J, Raverot G, et al. Clinicopathological prognostic and theranostic markers in pituitary tumours. Minerva Endocrinol. 2016;41(3):377–89. [PubMed] [Google Scholar]
- 3.Stache C, Hölsken A, Schlaffer SM, et al. Insights into the infiltrative behavior of adamantinomatous craniopharyngioma in a new xenotransplant mouse model. Brain Pathol. 2015;25(1):1–10. doi: 10.1111/bpa.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Zhang H, Heng X, et al. Expression of cold-inducible RNA-binding protein (CIRP) in pituitary adenoma and its relationships with tumor recurrence. Med Sci Monit. 2015;21:1256–60. doi: 10.12659/MSM.893128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaras V, Samaras E, Stergiou I, et al. Simultaneous occurrence of cerebellar medulloblastoma and pituitary adenoma: A case report. Cases J. 2008;1(1):175. doi: 10.1186/1757-1626-1-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Bai J, Hong L, et al. Low expression of secreted frizzled-related protein 2 and nuclear accumulation of beta-catenin in aggressive nonfunctioning pituitary adenoma. Oncol Lett. 2016;12(1):199–206. doi: 10.3892/ol.2016.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlikowski M, Radek M, Kunert-Radek J, et al. Overexpression of prothymosin alpha is related to pituitary adenoma recurrence but not to adenoma invasiveness and proliferation. Endokrynol Pol. 2014;65(5):382–86. doi: 10.5603/EP.2014.0053. [DOI] [PubMed] [Google Scholar]
- 8.Maimaiti A, Maimaiti A, Yang Y, Ma Y. MiR-106b exhibits an anti-angiogenic function by inhibiting STAT3 expression in endothelial cells. Lipids Health Dis. 2016;15(1):51. doi: 10.1186/s12944-016-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang GJ, Li JS, Zhou H, et al. MicroRNA-106b promotes colorectal cancer cell migration and invasion by directly targeting DLC1. J Exp Clin Cancer Res. 2015;34:73. doi: 10.1186/s13046-015-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin N, Zhou Y, Lian X, Tu Y. Expression of microRNA-106b and its clinical significance in cutaneous melanoma. Genet Mol Res. 2015;14(4):16379–85. doi: 10.4238/2015.December.9.6. [DOI] [PubMed] [Google Scholar]
- 11.Zheng R, Pan L, Gao J, et al. Prognostic value of miR-106b expression in breast cancer patients. J Surg Res. 2015;195(1):158–65. doi: 10.1016/j.jss.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Wang K, Gao W, et al. MicroRNA-106b regulates the tumor suppressor RUNX3 in laryngeal carcinoma cells. FEBS Lett. 2013;587(19):3166–74. doi: 10.1016/j.febslet.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Yu J, Han TS, et al. Functional links between clustered microRNAs: Suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37(5):1672–81. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AL, Iwanaga R, Drasin DJ, et al. The miR-106b-25 cluster targets Smad7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012;31(50):5162–71. doi: 10.1038/onc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong P, Kaneuchi M, Watari H, et al. MicroRNA-106b modulates epithelial-mesenchymal transition by targeting TWIST1 in invasive endometrial cancer cell lines. Mol Carcinog. 2014;53(5):349–59. doi: 10.1002/mc.21983. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Guo Y, Zhang Y, et al. MicroRNA-106b is involved in transforming growth factor beta1-induced cell migration by targeting disabled homolog 2 in cervical carcinoma. J Exp Clin Cancer Res. 2016;35(1):11. doi: 10.1186/s13046-016-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau WL, Lam CS, Ng L, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One. 2013;8(3):e57882. doi: 10.1371/journal.pone.0057882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slaby O, Jancovicova J, Lakomy R, et al. Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J Exp Clin Cancer Res. 2010;29:90. doi: 10.1186/1756-9966-29-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savita U, Karunagaran D. MicroRNA-106b-25 cluster targets beta-TRCP2, increases the expression of Snail and enhances cell migration and invasion in H1299 (non-small cell lung cancer) cells. Biochem Biophys Res Commun. 2013;434(4):841–47. doi: 10.1016/j.bbrc.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Wei Z, Zhou C, Liu M, et al. MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary. 2015;18(5):710–21. doi: 10.1007/s11102-015-0648-3. [DOI] [PubMed] [Google Scholar]
- 21.Fong P, Meng LR. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res. 2014;20:146–52. doi: 10.12659/MSMBR.892514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li KK, Xia T, Ma FM, et al. miR-106b is overexpressed in medulloblastomas and interacts directly with PTEN. Neuropathol Appl Neurobiol. 2015;41(2):145–64. doi: 10.1111/nan.12169. [DOI] [PubMed] [Google Scholar]
- 23.Yang TS, Yang XH, Chen X, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588(13):2162–69. doi: 10.1016/j.febslet.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Kim JH, Yang HK, Hwang JM. Preserved visual function with an orbital invasion of pituitary adenoma. Br J Neurosurg. 2016 doi: 10.3109/02688697.2016.1139051. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Bidari-Zerehpoosh F, Sharifi G, Novin K, Mortazavi N. Invasive growth hormone producing pituitary adenoma with lymphocytic infiltration: A case report and literature review. Iran J Cancer Prev. 2015;8(6):e3504. doi: 10.17795/ijcp-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong CH, Tao B, Wu Y, et al. [The Role of Cancer-associated Fibroblasts in Invasive Behavior of Pituitary Adenoma]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46(5):673–78. [in Chinese] [PubMed] [Google Scholar]
- 27.Lampichler K, Ferrer P, Vila G, et al. The role of proto-oncogene GLI1 in pituitary adenoma formation and cell survival regulation. Endocr Relat Cancer. 2015;22(5):793–803. doi: 10.1530/ERC-15-0109. [DOI] [PubMed] [Google Scholar]
- 28.Gadelha MR, Kasuki L, Dénes J, et al. MicroRNAs: Suggested role in pituitary adenoma pathogenesis. J Endocrinol Invest. 2013;36(10):889–95. doi: 10.1007/BF03346759. [DOI] [PubMed] [Google Scholar]
- 29.Sivapragasam M, Rotondo F, Lloyd RV, et al. MicroRNAs in the human pituitary. Endocr Pathol. 2011;22(3):134–43. doi: 10.1007/s12022-011-9167-6. [DOI] [PubMed] [Google Scholar]
- 30.Chen PS, Su JL, Cha ST, et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121(9):3442–55. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Han XH, Chen H, et al. Repetitive magnetic stimulation promotes neural stem cells proliferation by upregulating MiR-106b in vitro. J Huazhong Univ Sci Technolog Med Sci. 2015;35(5):766–72. doi: 10.1007/s11596-015-1505-3. [DOI] [PubMed] [Google Scholar]
- 32.Schlörmann W, Naumann S, Renner C, Glei M. Influence of miRNA-106b and miRNA-135a on butyrate-regulated expression of p21 and Cyclin D2 in human colon adenoma cells. Genes Nutr. 2015;10(6):50. doi: 10.1007/s12263-015-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, et al. Pituitary carcinoma: A clinicopathological review. Neurosurgery. 2005;56(5):1066–74. discussion 1066–74. [PubMed] [Google Scholar]
- 34.Thapar K, Kovacs K, Scheithauer BW, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery. 1996;38(1):99–106. doi: 10.1097/00006123-199601000-00024. discussion 106–7. [DOI] [PubMed] [Google Scholar]
- 35.Rizvi NA, Chan TA. Immunotherapy and oncogenic pathways: The PTEN connection. Cancer Discov. 2016;6(2):128–29. doi: 10.1158/2159-8290.CD-15-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCabe N, Kennedy RD, Prise KM. The role of PTEN as a cancer biomarker. Oncoscience. 2016;3(2):54–55. doi: 10.18632/oncoscience.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassi C, Mak TW. Regulation of the phosphatidylinositide 3-kinase pathway by the lipid phosphatase PTEN. Clin Chem. 2016;62(6):884–85. doi: 10.1373/clinchem.2015.253237. [DOI] [PubMed] [Google Scholar]
- 38.Hua J, Muschel RJ. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996;56(22):5279–84. [PubMed] [Google Scholar]
- 39.Kim D, Kim S, Koh H, et al. AKT/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15(11):1953–62. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]