Abstract
Background
Restless legs syndrome (RLS) is a common disorder in hemodialysis (HD) patients that causes sleep disturbances and diminished quality of life. Because iron deficiency has been implicated in the pathogenesis of RLS, we sought to investigate the effects of intravenous (IV) iron sucrose on symptoms of RLS in HD patients.
Material/Methods
The study was a randomized, placebo-controlled study of 1000 mg iron sucrose versus normal saline as placebo. Patients were evaluated at baseline and 2 weeks after the last injection. The severity of RLS was assessed using the International RLS Study Group rating scale (IRLS). Blood samples were taken to measure iron parameters reflecting the iron status, including serum ferritin (SF) concentration, percentage transferrin saturation (TSAT%) and hemoglobin (Hb), and other biochemical parameters as safety assessments, including creatinine (Cr), urea, intact parathyroid hormone (iPTH), and the index of urea clearance (Kt/V). Adverse events were monitored in all subjects during the period of infusion.
Results
After 2 weeks, IRLS scores decreased more in the IV-iron group (−7.38±2.03) than in the placebo group (−0.81±2.61) (P=0.000). Serum ferritin, TSAT, and hemoglobin increased more in the IV-iron group (227.63±77.64 μg/L; 26.06±7.77%; 13.98±3.62g/L, respectively) than in the placebo group (SF, p=0.000; TSAT, p=0.000; Hb, p=0.000, respectively). There were no significant differences between IV-iron and placebo groups in Cr, urea, iPTH, and Kt/V. No adverse effects were observed in the study.
Conclusions
IV iron sucrose is a safe and effective treatment for reducing RLS symptoms in HD patients over the short-term.
MeSH Keywords: Hemodialysis Units, Hospital; Iron; Restless Legs Syndrome
Background
Restless legs syndrome (RLS) is a primary disorder of the sensorimotor system characterized by considerable discomfort and irresistible desire to move the lower extremities, which leads to sleep disturbances and diminished quality of life [1,2]. The symptoms are relieved by movement, aggregated while resting, and are worse in the evening or night [3]. RLS is a common and well-recognized problem experienced by many patients with chronic kidney disease (CKD), and especially in patients with end-stage renal disease (ESRD), which affects the quality of life and survival in these patients [4,5]. The prevalence of this disorder in hemodialysis (HD) patients is 8.8–83% [6, 7] as reported in different case series, which is more than that in the general population (5–15%) [8]. The mechanisms of uremic RLS are complex and not yet fully understood. The risk factors related to the occurrence of RLS in HD patients may include anemia, iron deficiency, calcium/phosphate imbalance, inadequate dialysis, and peripheral and central nervous system abnormalities [9].
Although the pathophysiology of RLS has not been fully elucidated, decreased activity of dopamine may play a key role in RLS [10]. The relationship between iron and RLS is not completely understood but may be related to the dopamine synthesis in certain areas of the central nervous system [11,12]. Iron is a co-factor of the rate-limiting step required for conversion of tyrosine to levodopa, which is subsequently decarboxylated into dopamine [13]. Low levels of iron may induce or aggravate RLS by reducing the activity of the dopamine system [14]. Low ferritin and/or high transferrin levels in cerebrospinal fluid (CSF) may be present in RLS patients in comparison with healthy controls, even in the absence of low serum ferritin levels [15]. There is substantial evidence that iron deficiency is a contributing factor in RLS. A negative correlation exists between body iron stores, measured by serum iron and ferritin levels, and symptom severity [13,16,17].
Oral iron formulations appear to improve the symptoms of RLS, mostly in RLS patients with significant anemia or low serum ferritin [17]. However, iron absorption across the intestinal epithelium into the blood is limited, resulting in low bioavailability [18]. To circumvent this problem, it has been suggested that infusion of intravenous (IV) iron may provide significant therapeutic advantages. This contention is supported by several previous trials using IV iron [19–22]. Allen et al. conducted a multi-center, placebo-controlled preliminary clinical trial reporting that intravenous administration of ferric carboxymaltose improved significantly RLS scores and secondary outcomes including the Clinical Global Inventory of Change (CGI-1) and the self-report patient global rating of change (PGI-1) [19]. Ondo et al. found that intravenous iron dextran can produce treatment benefit for severe refractory RLS, but the results were inconsistent and not predicted by patient demographics [20]. An observational study by Lieske et al. in geriatric patients reported that intravenous iron administration was associated with a significant improvement in RLS symptoms as measured by the IRLS score after 2 weeks [21]. Tahir et al. performed a consecutive case series evaluating the effects of IV iron therapy on RLS occurring with iron deficiency anemia (IDA), suggesting that the symptoms of RLS are reduced in most cases after administration of IV iron [22].
To date, there are only 2 previous studies available that investigated the effects of iron in the treatment of RLS in HD patients [23,24]. In the first, Benz et al. reported that treatment with IV iron and erythropoietin reduced the RLS symptoms in patients on dialysis [23]. A subsequent double-blinded, placebo-controlled study by Sloand et al. was performed to investigate the effects of IV iron dextran therapy on RLS in patients with ESRD, suggesting a significant but transient reduction in RLS symptoms when using high-dose iron dextran infusion [24]. Iron sucrose showed a better safety and adverse-effects profile compared with iron dextran, and has rapidly replaced iron dextran for treatment of HD patients [25]. There have been 2 previous randomized, double-blinded, placebo-controlled trials of IV iron sucrose for RLS patients without HD [26,27]. Therefore, it is still not clear whether therapeutic effect can be obtained by IV iron sucrose in HD patients with RLS. The purpose of our study was to determine the efficacy of IV iron sucrose in this population.
Material and Methods
Patient selection
We recruited uremic patients on regular hemodialysis from the Department of Neurology at Xuanwu Hospital, Capital Medical University, from February 2014 to May 2014. Criteria for inclusion were a diagnosis of RLS, stable hemodialysis for more than 12 months, a serum ferritin (SF) concentration of <200 ng/ml, and a transferrin saturation (TSAT) of <20%. Subjects who received IV iron treatment within 6 months before entry into this study and those with a history of blood disorders, acute or chronic blood loss and infection, cancer, trauma, or immune system disorders were excluded. Participants could be excluded or withdrawn from the study at the treating physician’s discretion. The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University, and informed consent was signed by each patient.
The RLS diagnosis was made if the patient satisfied the International RLS Study Group (RLSSG) consensus criteria [28]. The severity of RLS was evaluated using the IRLSSG rating scale (IRLS), which is composed of 10 questions. Each question can be classified according to the severity as follows: none (0 points), mild (1 point), moderate (2 points), severe (3 points), and very severe (4 points). The total score can range from 0 to 40, with higher scores indicating more severe symptoms.
Treatment protocol
Eligible subjects were paired if they had similar RLS symptom severity scores [28], and were randomly assigned to an IV iron group or a placebo group. Subjects in the IV-iron group were given iron sucrose 100 mg diluted in 50 ml of normal saline 3 times per week for a total dose of 1000 mg. Placebo doses of 50-ml normal saline were similarly given 3 times per week. All patients were treated with recombinant human erythropoietin injection (CHO Cell) (rHuEpo) by IV injection after hemodialysis. The mean dose of rHuEpo was 119.44±43.21 U/Kg·W (range 30–181) in the IV-iron sucrose group and 130.31±36.92 U/Kg·W (range 67–181) in the placebo group.
During the study period, the hemodialysis prescription had to remain unchanged. Patients were dialyzed 3 times a week for 4 h using a 4008S hemodialysis machine (Fresenius) and triacetate hollow fiber dialyzer (Sureflux-130G, Nipro). Dialysis fluid contained calcium (1.25 mmol/L) and sodium (138 mmol/L). Dialysate flow rate was 500 mL/min with blood flow rate 200~250 ml/min. Heparin sodium was used as an anticoagulant.
Outcome measures
Subjects were evaluated at baseline and 2 weeks after the last injection of iron sucrose or normal saline. The IRLS score of each patient was assessed. Blood samples were taken to measure iron parameters reflecting the iron status, including serum ferritin concentration, TSAT and hemoglobin (Hb), and other biochemical parameters as safety assessments, including creatinine (Cr), urea, intact parathyroid hormone (iPTH), the index of urea clearance (Kt/V), serum calcium (SCa), and serum phosphorus (SP).
Hb, Cr, urea, serum iron, Sca, and SP were measured by an automatic biochemical analyzer. The serum ferritin was determined using a chemiluminescence microparticle immunoassay (CMIA). Total iron binding capacity (TIBC) was detected through the ferrous triazine chromogenic method. TSAT was calculated from the following formula: TSAT=(serum iron/TIBC)×100%. IPTH was measured with an electrochemiluminescent immunoassay (ECLIA). The Kt/V values were obtained by Daugirdas’s formula. Blood samples for the above parameters were taken from each patient before hemodialysis.
Adverse effects
Adverse events (e.g., allergic reaction, dizziness, low blood pressure, gastrointestinal discomfort, and fever) were monitored in all subjects during the period of infusion. If adverse events were observed, a physician/investigator was notified.
Data analyses
A two-sample independent t test was used to compare baseline characteristics and changes in IRLS scores and other outcome variables. Fisher’s exact test was used to compare the IV-iron group and control group. Results are reported as mean±standard deviation. All tests were 2-sided and P value set at <0.05 was considered statistically significant. SPSS 19.0 was used for all the analyses.
Results
Figure 1 shows the study process. Of the total of 44 patients who signed consent forms and were screened, 32 (mean age 63.90±6.47, 12 males) were fully eligible. All enrolled patients completed the study. Baseline serum ferritin was 155.94±36.29 ng/ml (range 69–198) and TSAT was 15.35±3.35% (range 7.25–19.85%). The time on HD therapy was 35.34±12.98 months (range 14–65). The mean baseline IRLS scores in these patients were 26.19±6.88 (range 14–36). The causes of ESRD were diabetic nephropathy in 12 cases, chronic glomerulonephritis in 8 cases, hypertensive renal injury in 6 cases, drug-induced renal impairment in 1 case, and unknown in 5 cases. Patient demographics are listed in Table 1.
Figure 1.
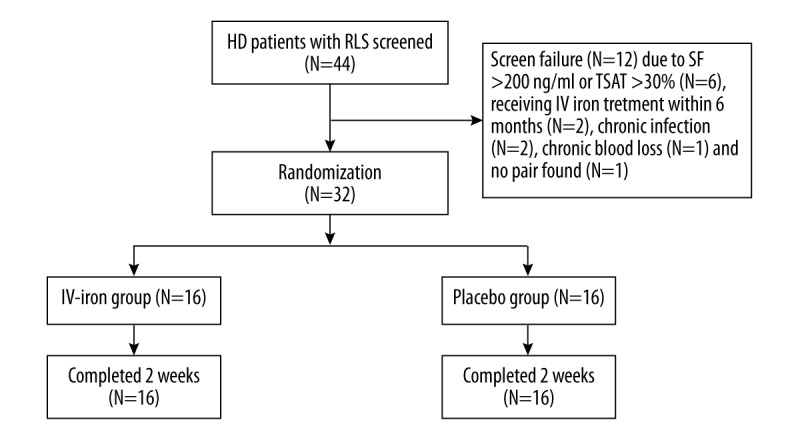
Flow chart of the present study.
Table 1.
Demographic characteristics in HD patients with RLS at baseline.
| HD patients with RLS (n=32) | |
|---|---|
| Age (year) | 63.90±6.47 |
| Gender (Female; %) | 20 (62.5%) |
| Time on hemodialysis (months) | 35.34±12.98 |
| Cause of ESRD | |
| Diabetic nephropathy | 12 |
| Chronic glomerulonephritis | 8 |
| Hypertensive renal injury | 6 |
| Drug-induced renal impairment | 1 |
| Unknown | 5 |
| IRLS score | 26.19±6.88 |
| SF (μg/L) | 155.94±36.29 |
| TSAT (%) | 15.35±3.35 |
| Hb (g/L) | 107±9.55 |
Data are present as mean ±SD. IRLS – international RLS study group rating scale for severity; SF – serum ferritin; TSAT – transferrin saturation; Hb – hemoglobin.
Sixteen patients were randomly assigned to the IV-iron sucrose group and 16 patients were assigned to the placebo group. The major baseline characteristics of both groups are listed in Table 2. As shown in Table 2, no statistically significant differences were observed between the 2 groups in terms of age, IRLS scores, iron parameters, and other biochemical parameters at baseline.
Table 2.
The major baseline characteristics between IV-iron and placebo groups.
| IV-iron group (n=16) | Control group (n=16) | P value | |
|---|---|---|---|
| Average age ±SD | 63.63±4.83 | 64.19±7.93 | 0.83 |
| IRLS score | 26.06±6.84 | 26.31±7.14 | 0.80 |
| SF (μg/L) | 154.75±38.34 | 157.13±35.33 | 0.72 |
| TSAT (%) | 16.18±4.47 | 15.76±3.58 | 0.62 |
| Hb (g/L) | 105.50±9.08 | 108.50±10.06 | 0.23 |
| Cr (μmol/L) | 927.56±156.62 | 948.44±206.03 | 0.51 |
| Urea (mmol/L) | 21.32±1.98 | 21.27±2.44 | 0.87 |
| IPTH (ng/L) | 288.06±108.26 | 292.13±107.29 | 0.76 |
| Kt/V | 1.30±0.11 | 1.32±0.12 | 0.83 |
| SCa (mmol/L) | 2.42±0.26 | 2.36±0.22 | 0.26 |
| SP (mmol/L) | 1.75±0.20 | 1.76±0.24 | 0.88 |
Data are present as mean ±SD. IRLS – international RLS study group rating scale for severity; SF – serum ferritin; TSAT – transferrin saturation; Hb – hemoglobin; Cr – creatinine; IPTH – intact parathyroid hormone; Kt/V – the index of urea clearance; SCa – serum calcium; SP – serum phosphorus.
Table 3 shows changes in IRLS score, iron status, and other laboratory parameters. The primary outcome comparison was change in IRLS score from baseline. Mean decreases in IRLS score after 2 weeks in the IV-iron group (−7.38±2.03) were significantly greater than in the placebo group (−0.81±2.61) (p=0.000). Improvement in RLS symptoms started between 1 and 2 weeks after IV iron sucrose treatment, and treatment benefits lasted from 4 weeks to 24 weeks. The mean change in serum ferritin, TSAT, and hemoglobin in the IV-iron group (227.63±77.64 μg/L; 26.06±7.77%; 13.98±3.62 g/L, respectively) was significantly greater than in the placebo group (SF, p=0.000; TSAT, p=0.000; Hb, p=0.000, respectively). When comparing other outcome variables at baseline and at week 2, no significant changes were observed in Cr, urea, iPTH, Kt/V, Sca, or and SP between the IV-iron and placebo groups. No adverse effects were observed in the study.
Table 3.
Changes in variables between baseline and week 2 within each group and between the two groups.
| Outcome measures | IV-iron group (n=16) | Control group (n=16) | P value |
|---|---|---|---|
| ΔIRLS score | −7.38±2.03 | −0.81±2.61 | 0.000 |
| ΔSF (μg/L) | 227.63±77.64 | −2.09±19.66 | 0.000 |
| ΔTSAT (%) | 26.06±7.77 | −0.58±2.47 | 0.000 |
| ΔHb (g/L) | 13.98±3.62 | 0.28±3.82 | 0.000 |
| ΔCr (μmol/L) | 11.43±62.37 | 5.63±37.6 | 0.75 |
| ΔUrea (mmol/L) | 0.50±1.02 | 0.04±0.53 | 0.13 |
| ΔIPTH (ng/L) | −0.53±54.27 | −13.01±50.89 | 0.52 |
| ΔKt/V | 0.017±0.001 | 0.017±0.001 | 0.43 |
| ΔSCa(mmol/L) | 0.03±0.30 | 0.07±0.24 | 0.50 |
| ΔSP(mmol/L) | 0.09±0.20 | −0.008±0.22 | 0.11 |
IRLS – international RLS study group rating scale for severity; SF – serum ferritin; TSAT – transferrin saturation; Hb – hemoglobin; Cr – creatinine; IPTH – intact parathyroid hormone; Kt/V – the index of urea clearance; SCa – serum calcium; SP – serum phosphorus; Δ – difference of mean score between baseline and week 2.
Discussion
Iron deficiency with abnormal dopaminergic consequences has been strongly implicated in the pathogenesis of RLS and has been shown to aggravate symptoms of RLS [11,13]. Iron deficiency is common in patients with chronic renal failure undergoing maintenance hemodialysis, mainly due to the frequent blood tests, increased blood loss from dialysis procedures, gastrointestinal blood loss, malnutrition, and increased erythropoietic demand for iron [29]. For most HD patients, the supply of oral iron is insufficient to meet the body’s demand. IV iron supplementation is a priority recommendation for HD patients. Although HD patients have a high prevalence of RLS and most of them receive iron therapy, the effect of IV iron has rarely been studied in RLS patients undergoing hemodialysis. Our results show significantly greater improvement in symptoms of RLS from baseline for IV iron sucrose than in placebo-treated subjects. The results were similar to the findings by Sloand et al., who showed reduced RLS symptoms in ESRD patients following IV iron dextran treatment [24]. Significant increases in serum ferritin, TSAT, and hemoglobin were also observed at 2 weeks following the IV iron sucrose in this study. We speculate that with the increase of body iron stores and serum ferritin levels, iron content and iron metabolism in cerebrospinal fluid (CSF) and brain tissue in HD patients would improve correspondingly. Increased hemoglobin has also been associated with improvement in RLS symptoms [30]. Iron sucrose injection was shown to be well tolerated with less adverse drug reactions, and we found no adverse reactions in our patients. HD patients require higher levels of iron stored in the body than for the general population, which provides more space for iron therapy.
In previous studies, oral or IV iron supplementation have been found to improve or resolve RLS symptoms in patients with low serum ferritin levels (≤45 ng/ml) but to be ineffective in subjects with a normal serum ferritin levels [31]. However, serum ferritin in our patients is comparable to the normal serum ferritin level, and may be higher. Our findings reported here are consistent with previous results, which showed that no significant differences were noted in the serum levels of ferritin, hemoglobin, calcium, and phosphorus in HD patients with and without RLS [32]. One would expect lower ferritin in the RLS group, but due to the acute-phase protein properties of ferritin, many HD patients with functional iron deficiency undergoing intense inflammatory states may show elevated levels of serum ferritin. This factor may obscure the association of iron stores and uremic RL; therefore, serum ferritin is not considered to be an indicator of iron sufficiency [33].
Iron status was assessed in this study using the biochemical parameters (SF, TSAT, and Hb), which are the most commonly ones in this patient population. We set 2 weeks as the evaluation period to reflect the effect of iron sucrose, mainly due to the following reasons. Firstly, several previous studies have shown that serum iron, ferritin, and CSF ferritin levels increased at 2 weeks after IV iron treatment [16,24,26]. Improvement in RLS symptoms can be observed within 1–2 weeks after IV iron, and continued for 4–22 months [19,27,30,34]. Despite the different durations of improvement reported in these studies, the effect of IV iron can be observed at 2 weeks after treatment. Moreover, HD patients need long-term treatment, and patients’ conditions change quickly. As time increases, many factors, including drug adjustment, therapeutic regimen, biochemical indicators, and physical conditions, may change, which can affect observation, analysis, and interpretation, and always increase the uncertainty of the results. Therefore, although the symptoms improvement effect lasted 4–24 weeks in these patients in our study, no further analysis was conducted due to the above factors.
Two previous trials of IV iron sucrose in adults in RLS have shown conflicting results [26,27]. A study by Earley et al. reported a small but significant increase in the cerebrospinal fluid (CSF) ferritin and a decrease in RLS severity (GRS), but other outcomes, including PLMs or brain iron index quantified by MRI imaging, showed no significant difference. The authors concluded that there were no robust changes in RLS symptoms when using high-dose IV iron sucrose [26]. By contrast, another study, by Grote et al., showed that the RLS severity scale (IRLS) score at week 11 tended to be lower in the iron sucrose group, but the difference was not statistically significant compared to placebo. However, iron sucrose was associated with a decrease in RLS severity both in the acute phase (7 weeks) and during the long-term follow-up [27]. Both used high doses (1000 mg) of iron sucrose. However, 2 different administration schedules were used in these 2 studies. A single dose of 1000 mg IV iron dextran was given into RLS patients in the first study, while repeated doses were given in the other study. Repeated small doses may be more effective than a single large dose. Moreover, the latter study limited patients to those with serum ferritin <50 mcg/l. Therefore, these 2 factors may have caused the contradictory results. Our study used low doses of iron sucrose (100 mg) repeated 3 times per week to reach a total dose of 1000 mg. In our study, we found that IV iron sucrose can provide an effective treatment for RLS in HD patients. The differences in treatment effects between this study and the earlier studies may be related to how the iron sucrose was administered.
Our study also has several limitations. First, this was not a blinded study, and participants could have been aware of the nature of the medication taken. Second, the small sample size and lack of long-term follow-up might lower the power of the present study. The patients were followed up for only 2 weeks after the last infusion of IV iron, which may be inadequate to observe the long-term changes and effects in RLS symptoms. Finally, the CSF ferritin levels reflecting the brain iron status were not measured, so the results obtained here in uremic RLS must be cautiously interpreted.
Conclusions
Our study provides some support for a reduction of RLS symptoms after IV iron sucrose administration in HD patients. The results reported in the literature and our findings both suggest that IV iron could have beneficial effects in HD patient with RLS. The effects may be more apparent for patients who fail to achieve treatment goals through the correction of anemia and replenishment of the body’s iron stores. Further studies of IV iron therapy for HD patients with RLS with larger sample sizes and longer-term follow-up are warranted to confirm and expand our findings.
Footnotes
Source of support: Departmental sources
Competing interests
The author(s) declare that they have no competing interests.
References
- 1.Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000;62:623–28. doi: 10.1002/1097-4547(20001201)62:5<623::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Merlino G, Lorenzut S, Gigli GL, et al. A case-control study on restless legs syndrome in nondialyzed patients with chronic renal failure. Mov Disord. 2010;25:1019–25. doi: 10.1002/mds.23010. [DOI] [PubMed] [Google Scholar]
- 5.Araujo SM, de Bruin VM, Nepomuceno LA, et al. Restless legs syndrome in end-stage renal disease: Clinical characteristics and associated comorbidities. Sleep Med. 2010;11:785–90. doi: 10.1016/j.sleep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Szentkiralyi A, Molnar MZ, Czira ME, et al. Association between restless legs syndrome and depression in patients with chronic kidney disease. J Psychosom Res. 2009;67:173–80. doi: 10.1016/j.jpsychores.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Tuncel D, Orhan FO, Sayarlioglu H, et al. Restless legs syndrome in hemodialysis patients: Association with depression and quality of life. Sleep Breath. 2011;15:311–15. doi: 10.1007/s11325-010-0382-z. [DOI] [PubMed] [Google Scholar]
- 8.Lipford MC, Silber MH. Long-term use of pramipexole in the management of restless legs syndrome. Sleep Med. 2012;13:1280–85. doi: 10.1016/j.sleep.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Yazdi Z, Sadeghniiat-Haghighi K, Kazemifar AM, et al. Restless leg syndrome in hemodialysis patients: A disorder that should be noticed. Saudi J Kidney Dis Transpl. 2015;26:625–50. doi: 10.4103/1319-2442.157431. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Borreguero D, Odin P, Schwarz C. Restless legs syndrome: An overview of the current understanding and management. Acta Neurol Scand. 2004;109:303–17. doi: 10.1111/j.1600-0404.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Allen R. Dopamine and iron in the pathophysiology of restless leg syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo G, Manners D, Testa C, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imagine. Mov Disord. 2013;28:1886–90. doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 13.Sun ER, Chen CA. Iron and the restless legs syndrome. Sleep. 1998;21:371–77. [PubMed] [Google Scholar]
- 14.Blake DR, Williams AC, Pall H, et al. Iron and akathisia. Br Med J. 1986;292:1393. doi: 10.1136/bmj.292.6532.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno S, Mihara T, Miyaoka T, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 16.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–35. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, O’Reilly B, Venkataraman R, et al. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–75. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach M. The role of intravenous iron for the treatment of restless legs syndrome. Am J Hematol. 2014;89:1016. doi: 10.1002/ajh.23820. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Adler CH, Du W, et al. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: A multi-centred, placebo-controlled preliminary clinical trial. Sleep Med. 2011;12:906–13. doi: 10.1016/j.sleep.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Ondo WG. Intravenous iron dextran for severe refractory restless legs syndrome. Sleep Med. 2010;11:494–96. doi: 10.1016/j.sleep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lieske B, Becker I, Schulz RJ, et al. Intravenous iron administration in restless legs syndrome: An observational study in geriatric patients. Z Gerontol Geriatr. :2015. doi: 10.1007/s00391-015-0984-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Mehmood T, Auerbach M, Earley CJ, Allen RP. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis-Ekbom disease) Sleep Med. 2014;15:1473–76. doi: 10.1016/j.sleep.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Benz RL, Pressman MR, Hovick ET, Peterson DD. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study) Am J Kidney Dis. 1999;34:1089–95. doi: 10.1016/S0272-6386(99)70015-6. [DOI] [PubMed] [Google Scholar]
- 24.Sloand JA, Shelly MA, Feigin A, et al. A double-Blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004;43:663–70. doi: 10.1053/j.ajkd.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Grim K, Lee B, Sung AY, Kotagal S. Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med. 2013;14:1100–4. doi: 10.1016/j.sleep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Earley CJ, Horská A, Mohamed MA, et al. A randomized, double-Blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10:206–11. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double-Blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24:1445–52. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- 28.Walters AS. International Restless Legs Syndrome Study Group. Toward a better definition of the restless legs syndrome. Mov Disord. 1995;10:634–42. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 29.Macdougall IC, Bircher AJ, Eckardt KU, et al. Conference Participants. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89:28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Cho YW, Allen RP, Earley CJ. Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep Med. 2013;14:274–77. doi: 10.1016/j.sleep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Lee CS, Lee SD, Kang SH, et al. Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol. 2014;21:260–66. doi: 10.1111/ene.12286. [DOI] [PubMed] [Google Scholar]
- 32.Collado-Seidel V, Kohnen R, Samtleben W, et al. Clinical and biochemical findings in uremic patients with and without restless legs syndrome. Am J Kidney Dis. 1998;31:324–28. doi: 10.1053/ajkd.1998.v31.pm9469505. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in HD patients. Kidney Int. 1999;55:648–58. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Hornyak M, Scholz H, Kiemen A, Kassubek J. Investigating the response to intravenous iron in restless legs syndrome: an observational study. Sleep Med. 2012;13:732–35. doi: 10.1016/j.sleep.2012.02.011. [DOI] [PubMed] [Google Scholar]


