Summary
Background
Stroke and hemiplegia are frequent complications of stroke. This study was performed to sonographically evaluate post-stroke hemiplegic shoulders and explore possible relationship(s) between the sonographic findings and clinical indices.
Material/Methods
Forty-five stroke patients and 45 age- and sex-matched controls were recruited. Standard sonographic examination of both shoulders was performed to assess for joint subluxation, rotator cuff tears, tendinosis, subacromial-subdeltoid bursitis or effusion and adhesive capsulitis.
Results
Hemiplegic shoulders exhibited significantly higher number of pathologies compared to the unaffected shoulders and shoulders of controls (p=0.000). One or more structural abnormalities were found in all 45 (100%) hemiplegic shoulders, 25 (55.6%) unaffected shoulders of the stroke subjects, and 39 (43.3%) control shoulders. The most frequent pathologies in the hemiplegic shoulders were the following: tendinosis of the long head of bicep tendon (48.9%), inferior shoulder subluxation (44.4%), co-existing subacromial-subdeltoid bursa/long head of bicep tendon sheath effusion (44.4%), and long head of bicep tendon sheath effusion only (40%). Tendinosis of the long head of bicep tendon was commoner in hemiplegic shoulders with poor motor status than those with good motor status.
Conclusions
Hemiplegic shoulders have significantly higher number of structural abnormalities than unaffected shoulders and the shoulders of controls. Hemiplegic stroke patients should undergo ultrasonography of the hemiplegic shoulder to define the nature and extent of soft tissue injuries prior to physical therapy.
MeSH Keywords: Hemiplegia, Shoulder Joint, Stroke, Ultrasonography
Background
Hemiplegia/hemiparesis is a common complication of stroke. It is often worsened by the presence of hemiplegic shoulder pain (HSP)/post-stroke shoulder pain (PSSP) [1]. Ultrasonography (USS) is advantageous over other imaging methods that have been used in evaluating the shoulder. Its advantages include broad availability, cost-effectiveness, real-time imaging, direct multiplanar assessment, immediate side-to-side comparison, short examination time, and lack of ionizing radiation) [2]. Furthermore, it closely rivals shoulder magnetic resonance imaging (MRI) in accurately diagnosing full thickness rotator cuff tears [3,4] and has been shown to be preferred to MRI by patients with shoulder pain [5].
This study was performed in order to sonographically describe the spectrum of structural abnormalities in post-stroke hemiplegic shoulders and to explore/analyze possible relationship(s) between ultrasound findings and a number of clinical-functional variables/parameters.
Material and Methods
This prospective, non-randomized, case-control study was conducted at the Radiology department of our institution from July 2013 to July 2014. The study was approved by the hospital Bioethical Committee and informed consent was obtained from all the participants.
The subjects were conscious, first-time stroke patients with hemiplegia recruited from the Neurology unit of the institution, who had not experienced pain in either shoulder for at least 6 months prior to developing stroke. Power of the hemiplegic shoulder was ≤4/5 as measured by the Medical Research Council’s (MRC) scale for muscle strength [6]. Subjects with previous episodes of stroke, stroke in the young (stroke in patients <40 years), patients with conditions predisposing to shoulder pathology (diabetes mellitus, cervical disk disease, and thyroid disease), previous or current history of shoulder trauma, injury, or surgery, bilateral upper limb weakness, glenohumeral osteoarthritis, inflammatory arthritis, and unconscious patients were excluded from the study.
The unaffected/contralateral/non-hemiplegic upper limbs of the stroke patients served as primary controls, while age- and sex-matched, non-hemiplegic individuals were secondary controls. The secondary controls were asymptomatic volunteers ≥40 years old, without previous injuries, surgeries or significant symptoms in the shoulder at any time. The total number of scanned shoulders was 180.
Clinical assessment
The diagnosis of stroke was based on clinical history, examination, and cross-sectional imaging (cranial CT and/or MRI). Subjects’ age, sex, hand dominance, hemiplegic side, time from onset of stroke (stroke symptom duration), and stroke type (ischemic or hemorrhagic) were recorded. Acute hemiplegics were those with stroke symptom duration <3 months, while those hemiplegics with stroke symptom duration of >3 months were classified as chronic [7–9].
HSP was diagnosed when a subject reported shoulder pain during passive range-of-motion exercise of the hemiplegic shoulder or at rest. The severity of HSP was measured using a 10-point visual assessment scale (VAS), where a score of 10 indicated the worst pain possible, and a score of 0 indicated no pain [10].
The levels of functional activity were classified as follows: Grade 0 – bedridden state; Grade 1 – has the ability to roll onto the side; Grade 2 – sits unsupported; Grade 3 – stands independently; Grade 4 – walks with assistance; Grade 5 – walks without assistance [11].
The motor status of the shoulder girdle was documented based on the MRC scale, as follows: grade 5: normal power, moves against full resistance; grade 4: reduced power, but can move against gravity and resistance; grade 3: active movement against gravity; grade 2: active movement in the absence of gravity; grade 1: flicker or trace of contraction/movement; grade 0: no contraction/movement [6]. Using the MRC scores, hemiplegic shoulders were grouped into those with poor motor status (score=0–2) and good motor status (score=3–5).
Sonographic assessment
Real-time shoulder ultrasonography was performed using linear, high-frequency (7–12 MHz) transducer of the MINDRAY® ultrasound machine: Model DC-7 (Shenzhen Mindray Bio-medical Electronics, Nanshan, Shenzhen, China). Most of the subjects underwent shoulder sonography while seated on a wheelchair. Passive movement was induced as needed with the help of an assistant. Both shoulders were examined in all subjects and controls using a scanning protocol previously described by other authors [11,12] to evaluate the long head of biceps tendon (LHBT), subscapularis (SCT) tendon, supraspinatus (SST) tendon, glenohumeral joint, infraspinatus tendon (IST), shoulder subluxation distance (measured by determining the Acromion-Greater Tuberosity Distance [AGTD]) [7,13,14], presence of adhesive capsulitis (by measuring the thickness of the coracohumeral ligament [CHL] – studies have shown that the coracohumeral ligament (CHL) is thickened in adhesive capsulitis) [15]. All examinations were performed by the first author.
Sonographic criteria
The sonographic criteria were as described by previous authors [2,16,17]:
Sonographic findings for a full-thickness rotator cuff tear included: non-visualization or absence of cuff tissue, a full-thickness hypoechoic defect (discontinuity in the normal homogeneous echogenicity of the cuff), visualization of the underlying hyaline cartilage (or naked tuberosity), heterogeneously hypoechoic cuff with bursal fluid, replacement of normal homogeneous echogenicity by a central echogenic band and herniation of the deltoid muscle or SASD bursa into the cuff.
The signs suggestive of a partial-thickness cuff tear were the following: heterogeneous tendon with hypoechoic areas (>3 mm) that did not reach both sides of the tendon or a large, linear hypoechoic/anechoic focus within the cuff substance (i.e., a purely intrasubstance tear) and a hypoechoic defect that involves the articular or bursal surface, i.e. either a hypoechoic or an anechoic cleft within the tendon with bursa or articular extension in both longitudinal and transverse planes.
Biceps tendinosis was identified by decreased echogenicity and enlargement of the biceps tendon by more than 8mm. Rotator cuff tendinosis was defined as hypoechoic and edematous changes, with a difference in tendon thickness of > 2 mm in comparison to the healthy side.
An anechoic area (>2 mm) surrounding the long head of the biceps tendon (LHBT) in transverse view or an anechoic/hypoechoic crescent deep into the LHBT in longitudinal views was interpreted as LHBT tendon sheath effusion.
Fluid in the SASD bursa with thickness of >2 mm indicated bursitis.
AGTD for shoulder subluxation was compared to the contralateral shoulder. Normal AGTD is 1.91–2.84 cm with average difference between shoulders amounting to 0.21 cm (range=0–0.36 cm) [18]. AGTD difference >0.4 cm indicated presence of subluxation.
CHL thickness >3 mm indicated presence of adhesive capsulitis. Literature reports normal thickness as 1–3 mm [15,19].
Each abnormal ultrasound (USS) finding was assigned a score of one (1) if present or zero (0) if absent. Thus, LHBT effusion, SASD effusion, subluxation, and adhesive capsulitis scored one each when present. Tendon tear, tendinosis, and tendon degeneration were similarly scored for each of the four examined tendons per shoulder. The sum of these scores yielded a raw ultrasound (USS) score, such that the minimum score was zero (normal examination) while the maximum score amounted to 16. The raw USS scores were further grouped into graded USS scores, such that scores of 0, 1–2, 3–4, 5–6, and more than 6 abnormal sonographic findings represented normal shoulder, mild damage, moderate damage, severe damage, and intense damage, respectively.
Chi square analysis was performed to detect the differences in prevalence of abnormal sonographic findings between hemiplegics and controls. One-way analysis of variance (ANOVA) was used to compare means of variables across age groups. McNemar’s Chi square test was applied to compare the prevalence of abnormal findings in the hemiplegic and unaffected shoulders of hemiplegics. Statistical significance was defined as P<0.05. Analysis was performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) software, version 20.0 for Windows.
Results
The study population comprised 45 hemiplegic/hemiparetic stroke patients and 45 age- and sex-matched controls. There was no statistically significant difference in the mean age of hemiplegics (62.0±11.3 years) and controls (65.8±11.3 years), P=0.115. Both the hemiplegic and control groups comprised 24 males and 21 females.
Stroke symptom duration was <3 months (acute stage) in 28 (62.2%) hemiplegics and >3 months (chronic stage) in 17 (37.8%) hemiplegics. Thirty-nine (86.7%) hemiplegics suffered from ischemic stroke while 6 (13.3%) had hemorrhagic stroke. Twenty-six (57.8%) hemiplegics had right shoulder hemiplegia while 19 (42.2%) suffered left shoulder hemiplegia. Hemiplegia affected the dominant and non-dominant hands in 27 (60%) and 18 (40%) hemiplegics, respectively. Sixteen (35.6%) hemiplegics had hemiplegic shoulder pain (HSP), while 29 (64.4%) did not complain of any pain (HNSP). Other relevant clinical features of the hemiplegics are summarized in Table 1.
Table 1.
Other clinical features of the hemiplegics.
| Variables | Total=45 | χ2 | df | P value |
|---|---|---|---|---|
| n (%) | ||||
| Level of functional activity | ||||
| Able to roll to one side | 2 (4.4) | 1.326 | 4 | 0.857 |
| Sits unsupported | 19 (42.2) | |||
| Stands independently | 1 (2.2) | |||
| Walks with assistance | 8 (17.8) | |||
| Walks without assistance | 15 (33.3) | |||
| Power grade (MRC) | ||||
| Grade 0 | 6 (13.3) | 0.486 | 4 | 0.975 |
| Grade 1 | 6 (13.3) | |||
| Grade 2 | 3 (6.7) | |||
| Grade 3 | 7 (15.6) | |||
| Grade 4 | 23 (51.1) |
χ2=Chi-square analysis applied.
None of the hemiplegic shoulders was sonographically normal, while 20 (44.4%), and 51 (56.7%) unaffected/contralateral shoulders and control shoulders had normal sonographic image, respectively (Table 2). Hemiplegic shoulders had significantly higher mean USS scores than controls, with approximately three abnormalities per hemiplegic shoulder compared to approximately one abnormality per control shoulder. Severe shoulder damage (5–6 sonographic abnormalities) was seen in hemiplegic shoulders only. The hemiplegic shoulders also had significantly higher USS scores than the unaffected shoulders. Twenty-nine (64.4%) hemiplegic shoulders had moderate to severe damage compared to 1 (2.2%) unaffected shoulder, p=0.000. There were no statistically significant differences between the USS scores of the unaffected/contralateral shoulders of the hemiplegics and the control shoulders (Table 3).
Table 2.
Differences in the USS Scores of the hemiplegic shoulders, unaffected shoulders, and shoulders of controls.
| N | Mean Rrw USS score | SD | P value* | |
|---|---|---|---|---|
| Hemiplegic side | 45 | 2.98 | 1.29 | 0.000 |
| Unaffected side | 45 | 0.84 | 0.88 | |
| 90 control shoulders | 90 | 0.62 | 0.92 | |
| Graded Score | Hemiplegic side | Unaffected side | 90 control shoulders | Total |
| n (%) | n (%) | n (%) | n (%) | |
| Normal shoulder | 0 (0.0) | 20 (44.4) | 51 (56.7) | 71 (39.4) |
| Mild damage | 16 (35.6) | 24 (53.3) | 35 (38.9) | 75 (41.7) |
| Moderate damage | 24 (53.3) | 1 (2.2) | 4 (4.4) | 29 (16.1) |
| Severe damage | 5 (11.1) | 0 (0.0) | 0 (0.0) | 5 (2.8) |
| χ2=93.532, df=6; P=0.000 | ||||
Kruskal-Wallis test applied; χ2=Chi-square analysis applied.
Table 3.
Differences in the USS Scores of the unaffected shoulders of the hemiplegics and both shoulders of controls.
| Category | Unaffected side of hemiplegics | Right control shoulder | Left control shoulder | Statistic |
|---|---|---|---|---|
| Mean ±SD | Mean ±SD | Mean ±SD | ||
| Raw Score | 0.84±0.88 | 0.69±1.06 | 0.56±0.76 | P=0.217* |
| n (%) | n (%) | n (%) | ||
| Normal | 20 (44.4) | 25 (55.6) | 26 (57.8) | χ2=3.931, df= 4, p=0.415 |
| Mild | 24 (53.3) | 17 (37.8) | 18 (40.0) | |
| Moderate | 1 (2.2) | 3 (6.7) | 1 (2.2) |
Kruskal-Wallis test applied; χ2=Chi-square analysis applied.
LHBT sheath effusion only and LHBT sheath effusion coexisting with SASD bursa effusion were significantly more common in the hemiplegic shoulders than control shoulders. LHBT sheath effusion only was observed in 18 (40.0%) hemiplegic shoulders and 11 (12.2%) controls (p=0.000). LHBT sheath effusion coexisting with SASD bursa effusion was seen in 20 (44.4%) hemiplegic shoulders and 5 (5.6%) controls (p=0.000). However, occurrence of SASD bursa effusion only did not differ significantly between the hemiplegics and controls.
Co-existing LHBT and SASD effusion was seen in 20 (44.4%) hemiplegic shoulders compared to 2 (4.4%) unaffected/non-hemiplegic/contralateral shoulders (p=0.000). There were no significant differences in the occurrence of LHBT effusion only and SASD effusion only between the hemiplegic and the unaffected shoulders of hemiplegics (Table 4).
Table 4.
Differences in sonographic findings between both shoulders in the hemiplegics.
| Sonographic Findings | Hemiplegic side | Unaffected side | P value* |
|---|---|---|---|
| n (%) | n (%) | ||
| SASD effusion only | 5 (11.1) | 6 (13.3) | 1.000 |
| LHBT effusion only | 18 (40.0) | 11 (24.4) | 0.167 |
| SASD & LHBTeffusion | 20 (44.4) | 2 (4.4) | 0.000 |
| Supraspinatus tendon tear | 8 (17.8) | 8 (17.8) | 1.000 |
| LHBT tendinosis | 22 (48.9) | 0 (0.00) | NA |
| Subscapularis tendinosis | 6 (13.3) | 0 (0.00) | NA |
| Supraspinatus tendinosis | 8 (7.8) | 1 (2.2) | 0.039 |
| Infraspinatus tendinosis | 0 (0.0) | 1 (2.2) | NA |
| LHBT degeneration | 1 (2.2) | 0 (0.0) | NA |
| Subscapularis tendon degeneration | 1 (2.2) | 2 (4.4) | 1.000 |
| Supraspinatus tendon degeneration | 0 (0.0) | 1 (2.2) | NA |
McNemar’s chi-square test applied; NA – not applicable.
LHBT tendinosis, SCT tendinosis, and SST tendinosis were seen in 22 (48.9%), 6 (13.3%) and 8 (17.8%) hemiplegic shoulders respectively, compared to nil, nil, and one (1.1%), respectively, in control shoulders, p=0.000. There were no significant differences with regard to the occurrence of tendon tears and tendon degeneration between hemiplegic shoulders and control shoulders. Neither the hemiplegics nor controls presented with LHBT or IST tendon tear.
Supraspinatus tendinosis was present in 8 (7.8%) hemiplegic shoulders and 1 (2.2%) unaffected shoulder (p=0.039). LHBT tendinosis was present in twenty-two (48.9%) hemiplegic shoulders but was not seen in any of the contralateral shoulders (Table 4).
Stroke patients with hemiplegia affecting the non-dominant upper limb demonstrated significantly thicker LHBT and significantly higher prevalence of LHBT and SCT tendinosis than those with dominant hand hemiplegia.
Poor motor status was observed in 13 (46.4%) acute hemiplegics and 2 (11.7%) chronic hemiplegics, p=0.017. LHBT tendinosis was significantly more prevalent in hemiplegic shoulders with poor motor status than those with good motor status (p=0.020).
Hemiplegic shoulder subluxation was present in 20 (44.4%) hemiplegics. In fifteen (75%) of those patients with persistence of stroke symptoms of <3 months compared to 5 (25%) patients with stroke symptoms lasting >3 months; p=0.025. The subluxed hemiplegic shoulders had significantly higher raw USS scores (3.55±1.32) than non-subluxed, hemiplegic shoulders (2.52±1.09), p=0.006.
There were no significant sonographic differences between hemiplegics with shoulder pain (HSP) and hemiplegics without shoulder pain (HNSP). Similarly, there were no statistically significant gender differences in the sonographic features of the hemiplegics.
Adhesive capsulitis was not detected in any of the hemiplegic shoulders.
Figures 1–4 depict some of the structural abnormalities seen in the hemiplegic shoulders.
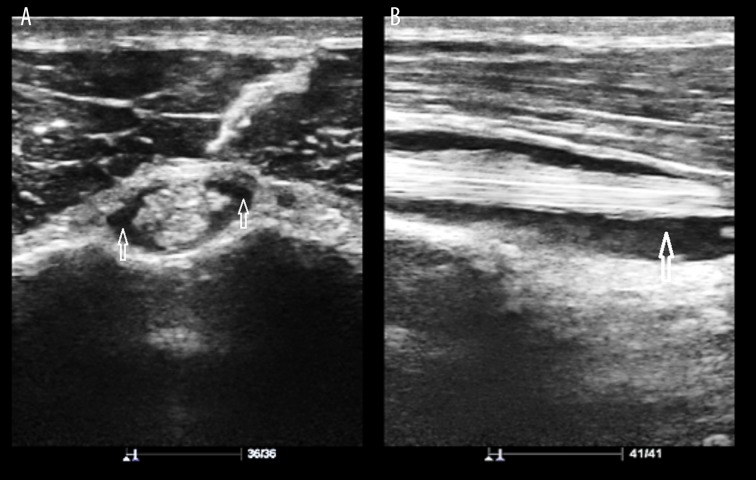
Figure 1.
(A) LHBT sheath effusion in a 60-year-old man. Sonogram (Short axis view) shows hypoechoic collection surrounding the LHBT (arrows). (B) LHBT sheath effusion in a 60-year-old man. Sonogram (Long axis view) shows hypoechoic crescentic collection deep in the LHBT (arrow).
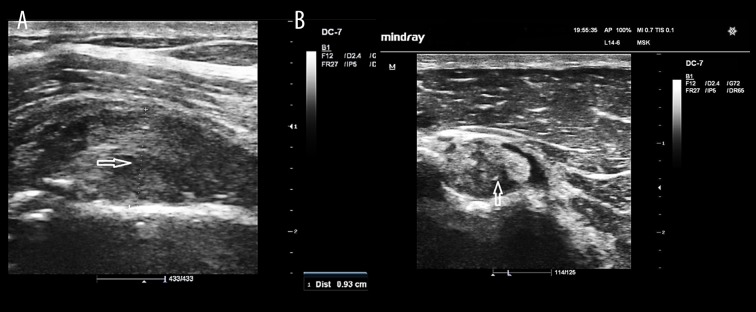
Figure 2.
SASD bursa effusion in a hemiplegic 62-year-old woman. Sonogram shows fluid collection in the SASD bursa (arrow).
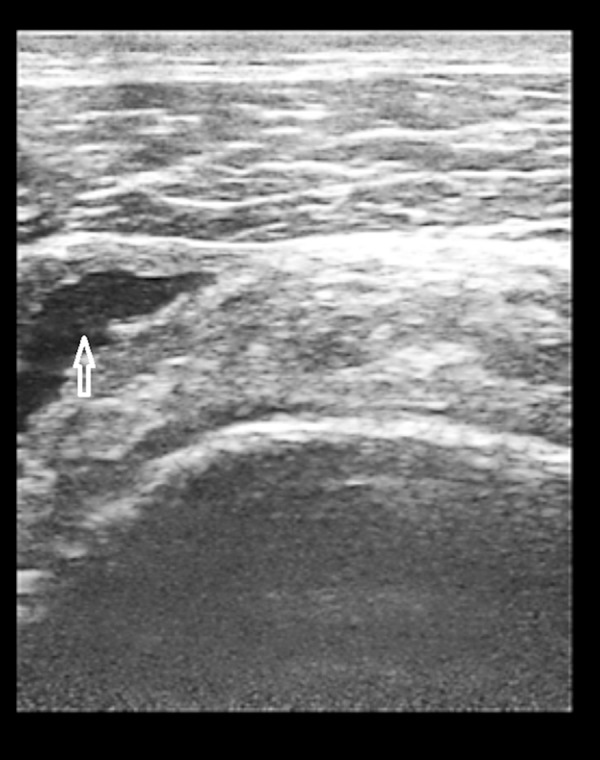
Figure 3.
(A) LHBT tendinosis in a 75-year-old man. Sonographic image (Long axis view) shows swollen, heterogeneous tendon (arrows) 9.3 mm in thickness. (B) LHBT tendinosis in a 75-year-old man. Sonographic image (Short axis view) shows swollen, heterogeneous tendon (arrow) with moderate hypoechoic fluid (effusion) around it.
Figure 4.
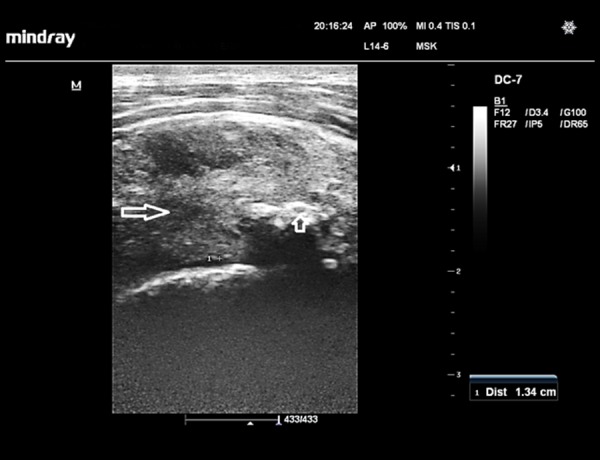
Supraspinatus tendinosis in a hemiplegic 58-year-old woman. Sonographic image of the supraspinatus tendon (short axis view) shows a swollen, hypoechoic tendon (long arrow),13.4-mm thick, which also contains foci of calcifications (short arrow)
Discussion
Hemiplegia is a common residual deficit in stroke survivors. The hemiplegic shoulders exhibited significantly more sonographic abnormalities than the unaffected shoulders and the control shoulders, which is in agreement with the findings of previous studies [10,17,20,21]. These results show that hemiplegia caused significant shoulder soft tissue damage in the recruited stroke survivors, with a mean of approximately three sonographic abnormalities per hemiplegic shoulder, which was not observed in the age- and sex-matched controls.
All of the examined hemiplegic shoulders (100%) demonstrated at least one structural abnormality. This rate (100%) is much higher than the 60.5–85% reported by other studies [9,17,20,22], and may be due to the fact that some of these studies recruited acute hemiplegics only [9]. Moreover, subluxation was not included among their sonographic variables, which was seen in 44% of hemiplegic shoulders in the present study. Furthermore, this may point to possible racial difference(s) in the severity of soft tissue injuries associated with post-stroke hemiplegia.
The commonest sonographic abnormalities in the hemiplegic limbs were LHBT tendinosis (49%), coexisting LHBT/SASD effusion (44%), inferior shoulder subluxation (44%), and isolated LHBT effusion (40%). These findings are generally in agreement with the works of previous researchers [16,17,20–24] but differ from others who reported SASD effusion [2,9,13] and adhesive capsulitis [25–27] as their main findings. The differences could be due partly to the fact that those with coexisting LHBT/SASD effusions were not separated from those with isolated SASD effusion and isolated LHBT effusion during the data analysis, possibly increasing the frequency of both findings. The authors who reported adhesive capsulitis as their dominant finding carried out MRI or arthrographic studies. Lee et al. [16] reported that LHBT sheath effusion was mostly located in the distal part of the tendon rather than its intra-articular part. This pattern was also observed in this study, and is probably due to the fact that the distal part is the dependent portion.
HSP was present in 36% of patients in this study, which agrees with published values of 17–70% [1,23,28]. However, there were no statistically significant sonographic differences between HSP and HNSP, though coexisting LHBT/SASD effusion was more frequent/prevalent in HSP (56%) than HNSP (38%), but this did not reach statistical significance (p=0.236). Moreover, HSP did not correlate significantly with any sonographic variables. These observations are in line with the findings of other workers [16,21,23,26] but differ from Pong et al., [17] who found that HSP was associated with LHBT effusion and tendinosis as well as SST tendinosis in chronic hemiplegics. However, their research was a longitudinal study, in which the same set of hemiplegics were scanned on admission (acute stage) and at 6 months after discharge (chronic stage) rather than including a random selection of different stroke patients with different duration of hemiplegia. Moreover, they used a qualitative criterion only for the diagnosis of LHBT tendinosis (which could be subjective) rather than both quantitative (thickness >8 mm) and qualitative criteria (diffuse tendon swelling, decreased echogenicity, and increased interfibrillar distance) as was performed in this study.
Shoulder subluxation was demonstrated sonographically in 44% of hemiplegic shoulders in this study. This falls within the reported incidence of 17–84% [7,13,14]. Acute hemiplegics with subluxation (75%) were significantly more frequent than chronic hemiplegics with subluxation (15%). This agrees with previous reports that shoulder subluxation in hemiplegia is predominantly seen in the acute phase [23,26,27], which has been attributed to its self-limiting nature [27]. The subluxed hemiplegic shoulders were characterized by significantly higher raw USS score (approximately 4 abnormalities per subluxed shoulder) than the non – subluxed hemiplegic shoulders (about 2 abnormalities per non-subluxed shoulder), suggesting that presence of subluxation is associated with more soft tissue injuries. This is in agreement with the findings of Ko et al. [22] who reported that hemiplegic shoulders with abnormal sonographic findings suffered more severe subluxation. Other investigators have also identified subluxation as an important finding in hemiplegic shoulders [23,27].
Adhesive capsulitis was not seen in any of the hemiplegic shoulders in this study. This is in agreement with previous sonographic studies [2,9]. However, adhesive capsulitis was the dominant finding in some studies that examined hemiplegic shoulders with conventional arthrography [28], contrast enhanced MRI [26,27], and magnetic resonance arthrography [29]. This discrepancy between USS and other imaging techniques could potentially be addressed in future studies by combining all of the various sonographic methods of detecting adhesive capsulitis reported in English literature thus far [15,30–34] in order to improve detection rate. Only CHL thickness was evaluated in this study.
Rotator cuff tears (RCTs) were found in 39.8% of the hemiplegic shoulders in this study, affecting the subscapularis and supraspinatus tendons only. This is close to the 34–38.5% reported by some studies [7,8], lower than 59% reported by Yi et al. [35], and higher than the 0–30% reported by other researchers [2,9,3,16,17,23,25]. The different rates of rotator cuff tears could possibly be due to differences in the degree of motor weakness among different study populations. Yi et al. [35] demonstrated that the occurrence of RCT in hemiplegia depended on the degree of motor weakness. Therefore, it is likely that studies whose participants had more severe paresis had higher prevalence of RCT and vice versa. On the other hand, the higher prevalence of RCTs (59%) in the study by Yi et al. [35] could be due partly to the fact that they did not exclude diabetic stroke patients who constituted 36% of their sample size. Rotator cuff tears are more prevalent in diabetics than in the general population [26]. Hence, different inclusion/exclusion criteria may also partly account for the disparity in results.
There were no statistically significant sonographic differences between shoulders of subjects with hemiplegia of less than 3 months duration and those with hemiplegia lasting more than 3 months. Similar findings were reported by Lee et al. [16].
No statistically significant differences were noted in the sonographic abnormalities of hemiplegic shoulders of male and female subjects. This agrees with the results of previous studies [10,13,21] and is probably due to the fact that shoulder anatomy in men and women is very similar. Therefore, changes due to aging or secondary to lesions such as stroke often present the same basic clinical or imaging pattern(s) in both sexes.
Hemiplegics with paralysis of the non-dominant upper limb had significantly thicker LHBT and significantly higher prevalence of LHBT tendinosis and SCT tendinosis than those with dominant upper limb paralysis. This is in agreement with the findings of Adunsky et al. [21] but differs from that of Cho et al. [10] who reported no significant differences between the two groups. These conflicting results may be due to the fact that, almost invariably, the different study populations were at different stages of anatomical recovery, functional recovery, and rehabilitation.
Poor motor status (MRC power score=0–2) was found in a significantly greater number of acute hemiplegics than chronic hemiplegics. This is expected in view of the fact that gradual recovery of motor function (of varying degrees) often occurs with time in hemiplegic limbs. LHBT tendinosis was significantly more prevalent in hemiplegics with poor motor status than those with good motor status (MRC power score=3–5). This finding is in consonance with the results of researchers elsewhere [17,20,24] who reported that acute stroke patients with poor upper limb motor function were more vulnerable to soft tissue injuries during rehabilitation. These findings are, however, at variance with the results of other studies [2,21] that found no significant relationship between motor status and USS findings. This disparity may be due to differences in use, administration, and interpretation of different clinical scales for the assessment of motor function.
A unique feature of this study is the enrolment of age- and sex-matched (secondary) controls in addition to the primary controls (contralateral, non-hemiplegic shoulders of subjects). Virtually all the previous studies used only the contralateral, non-hemiplegic shoulders as controls.
Limitations of this study include not evaluating for the presence of impingement, muscle atrophy, and capsular hypertrophy in the subjects. The ultrasound operator was also not completely blinded to all of the subjects’ clinical details.
Conclusions
In conclusion, post-stroke hemiplegic shoulders had significantly more structural abnormalities than the unaffected shoulders and the shoulders of age – and sex – matched controls. Sonography of post-stroke hemiplegic shoulders is recommended to assess for the soft tissue injuries with a perspective for implementation in individualized rehabilitation.
References
- 1.Lindgren I, Jönsson AC, Norrving B, Lindgren A. Shoulder pain after stroke: A prospective population-based study. Stroke. 2007;38:343–48. doi: 10.1161/01.STR.0000254598.16739.4e. [DOI] [PubMed] [Google Scholar]
- 2.Lee IS, Shin YB, Moon T, et al. Sonography of patients with hemiplegic shoulder pain after stroke: correlation with motor recovery stage. Am J Roentgenol. 2009;192:W40–44. doi: 10.2214/AJR.07.3978. [DOI] [PubMed] [Google Scholar]
- 3.De Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: A meta-analysis. Am J Roentgenol. 2009;192:1701–7. doi: 10.2214/AJR.08.1241. [DOI] [PubMed] [Google Scholar]
- 4.Lazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. Am J Roentgenol. 2008;190:1621–26. doi: 10.2214/AJR.07.3385. [DOI] [PubMed] [Google Scholar]
- 5.Middleton WD, Payne WT, Teefey SA, et al. Sonography and MRI of the shoulder: Comparison of patient satisfaction. Am J Roentgenol. 2004;183:1449–52. doi: 10.2214/ajr.183.5.1831449. [DOI] [PubMed] [Google Scholar]
- 6.Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40:665–71. doi: 10.2340/16501977-0235. [DOI] [PubMed] [Google Scholar]
- 7.Huang SW, Liu SY, Tang HW, et al. Relationship between severity of shoulder subluxation and soft-tissue injury in hemiplegic patients. J Rehabil Med. 2012;44:733–39. doi: 10.2340/16501977-1026. [DOI] [PubMed] [Google Scholar]
- 8.Shah RR, Haghpanah S, Elovic EP, et al. MRI findings in the painful post-stoke shoulder. Stroke. 2008;39:1808–13. doi: 10.1161/STROKEAHA.107.502187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaiton F, Hazim IT, Khaled AME, Ghada S. Painful post-stroke shoulder: Comparison of magnetic resonance imaging and high frequency ultrasonography. Egypt J Radiol Nucl Med. 2011;42:47–55. [Google Scholar]
- 10.Cho HK, Kim HS, Joo SH. Sonography of affected and unaffected shoulders in hemiplegic patients: Analysis of the relationship between sonographic imaging data and clinical variables. Ann Rehabil Med. 2012;36:828–35. doi: 10.5535/arm.2012.36.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teefey SA. Shoulder sonography. Why we do it. J Ultrasound Med. 2012;31:1325–31. doi: 10.7863/jum.2012.31.9.1325. [DOI] [PubMed] [Google Scholar]
- 12.Gaitini D. Shoulder ultrasonography: Performance and common findings. J Clin Imaging Sci. 2012;2:1–8. doi: 10.4103/2156-7514.99146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park GY, Kim JM, Sohn S, Shin IH, Lee MY. Ultrasonographic measurement of shoulder subluxation in patients with post-stroke hemiplegia. J Rehabil Med. 2007;39:526–30. doi: 10.2340/16501977-0099. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Bradley M, Gray S, Swinkels A. Reliability and validity of ultrasonographic measurements of acromion-greater tuberosity distance in post-stroke hemiplegia. Arch Phys Med Rehabil. 2011;92:731–36. doi: 10.1016/j.apmr.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Homsi C, Bordalo-Rodrigues M, da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: Is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006;35:673–78. doi: 10.1007/s00256-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 16.Lee CL, Chen TW, Weng MC, et al. Ultrasonographic findings in hemiplegic shoulders of stroke patients. Kaohsiung J Med Sci. 2002;18:70–76. [PubMed] [Google Scholar]
- 17.Pong YP, Wang LY, Huang YC, et al. Sonography and physical findings in stroke patients with hemiplegic shoulders: A longitudinal study. J Rehabil Med. 2012;44:553–57. doi: 10.2340/16501977-0987. [DOI] [PubMed] [Google Scholar]
- 18.Cholewinski JJ, Kusz DJ, Wojciechowski P, et al. Ultrasound measurement of rotator cuff thickness and acromio-humeral distance in the diagnosis of subacromial impingement of the shoulder. Knee Surg Sports Traumatol Arthrosc. 2008;16:408–14. doi: 10.1007/s00167-007-0443-4. [DOI] [PubMed] [Google Scholar]
- 19.Martinoli C, Bianchi S, Prato N, et al. US of the shoulder: Non-rotator cuff disorders. Radiographics. 2003;23:381–401. doi: 10.1148/rg.232025100. [DOI] [PubMed] [Google Scholar]
- 20.Huang YC, Liang PJ, Pong YP, et al. Physical findings and sonography of hemiplegic shoulder in patients after acute stroke during rehabilitation. J Rehabil Med. 2010;42:21–26. doi: 10.2340/16501977-0488. [DOI] [PubMed] [Google Scholar]
- 21.Adunsky A, Mizrahi E, Arad M, et al. Ultrasonography and clinico-functional parameters of hemiplegic upper extremity in a rehabilitation setting. J Musculoskelet Res. 2009;12:53–58. [Google Scholar]
- 22.Ko MH, Kim JY, Park SH, et al. Comparison of ultrasonographic findings with clinical findings in hemiplegic shoulder. J Korean Acad Rehabil Med. 2006;30:213–18. [Google Scholar]
- 23.Aras MD, Gokkaya NK, Comert D, et al. Shoulder pain in hemiplegia: Results from a national rehabilitation hospital in Turkey. Am J Phys Med Rehabil. 2004;83:713–19. doi: 10.1097/01.phm.0000138739.18844.88. [DOI] [PubMed] [Google Scholar]
- 24.Pong YP, Lin-Yi Wang, Wang L, et al. Sonography of the shoulder in hemiplegic patients undergoing rehabilitation after a recent stroke. J Clin Ultrasound. 2009;37:199–205. doi: 10.1002/jcu.20573. [DOI] [PubMed] [Google Scholar]
- 25.Lo SF, Chen SY, Lin HC, et al. Arthrographic and clinical findings in patients with hemiplegic shoulder pain. Arch Phys Med Rehabil. 2003;84:1786–91. doi: 10.1016/s0003-9993(03)00408-8. [DOI] [PubMed] [Google Scholar]
- 26.Pompa A, Clemenzi A, Troisi E, et al. Enhanced-MRI and ultrasound evaluation of painful shoulder in patients after stroke: A pilot study. Eur Neurol. 2011;66:175–81. doi: 10.1159/000330657. [DOI] [PubMed] [Google Scholar]
- 27.Tavora DGF, Gama RL, Bomfim RC, et al. MRI findings in the painful hemiplegic shoulder. Clin Radiol. 2010;65:789–94. doi: 10.1016/j.crad.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Sackley C, Brittle N, Patel S, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke. 2008;39:3329–34. doi: 10.1161/STROKEAHA.108.518563. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Park J, Song SW. Correlation of MR arthrographic findings and range of shoulder motions in Patients with frozen shoulder. Am J Roentgenol. 2012;198:173–79. doi: 10.2214/AJR.10.6173. [DOI] [PubMed] [Google Scholar]
- 30.Lee JC, Sykes C, Saiffudin A, Connell D. Adhesive capsulitis: Sonographic changes in the rotator cuff interval with arthroscopic correlation. Skeletal Radiol. 2005;34:522–27. doi: 10.1007/s00256-005-0957-0. [DOI] [PubMed] [Google Scholar]
- 31.Lan HHC, Lee S, Keng CY, et al. Adhesive capsulitis of the shoulder: Ultrasonography and elastography evaluation of the coracohumeral ligament. Ultrasound Med Biol. 2011;37:140. [Google Scholar]
- 32.Ryu KN, Lee SW, Rhee YG, Lim JH. Adhesive capsulitis of the shoulder joint: Usefulness of dynamic sonography. J Ultrasound Med. 1993;12:445–49. doi: 10.7863/jum.1993.12.8.445. [DOI] [PubMed] [Google Scholar]
- 33.Lee G, Briggs L, Murrell G. Ultrasound measurement of shoulder capsule thickness for diagnosing frozen shoulder. J Sci Med Sport. 2010;13(Suppl 1):e75. [Google Scholar]
- 34.Smith MB, Stitik TP, Clark TB. Is this shoulder really frozen? Sonographic measurement of glenohumeral joint capsule thickness may aid in the diagnosis of adhesive capsulitis: A case series. Phys Med Rehabil. 2013;5(Suppl):214. [Google Scholar]
- 35.Yi Y, Shim JS, Kim K, et al. Prevalence of the rotator cuff tear increases with weakness in hemiplegic shoulder. Ann Rehabil Med. 2013;37:471–78. doi: 10.5535/arm.2013.37.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]