Abstract
Several studies have demonstrated that increased apoptosis plays an essential role in neurodegenerative disorders. It has been demonstrated that lipopolysaccharide (LPS) induces apoptosis largely through the production of intracellular reactive oxygen species (ROS) and inflammatory mediators. In this study, we investigated the potential protective mechanisms of naringin (Nar), a pummelo peel extract, on LPS-induced PC12 cell apoptosis. Nar pre-conditioning prior to stimulation with LPS for 18 h was a prerequisite for evaluating PC12 cell viability and the protective mechanisms of Nar. Nar significantly improved cell survival in a time- and concentration-dependent manner. On the one hand, Nar downregulated cytochrome P450 2E1 (CYP2E1), inhibited the release of ROS, mitigated the stimulation of oxidative stress, and rectified the antioxidant protein contents of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), superoxide dismutase (SOD)2 and glutathione synthetase (GSS). On the other hand, Nar down-regulated inflammatory gene and protein expression, including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, HMGB1, high mobility group box 1 protein (HMGB1), cyclo-oxygenase-2 (COX-2), the Toll-like receptor 4 (TLR4)-myeloid differentiation factor 88 (MyD88)-TNF receptor-associated factor 6 (TRAF6) path way and downstream mitogen activated protein kinase (MAPK) phosphorylation, activator protein transcription factor-1 (AP-1) and nuclear factor (NF)-κB. Moroever, Nar markedly attenuated the cytochrome c shift from the mitochondria to the cytosol and regulated caspase-3-related protein expression. To the best of our knowledge, this is the first study to report the antioxidant, anti-inflammatory and anti-apoptotic effects of Nar in neuronal-like PC12 cells. These results suggest that Nar can be utilized as a potential drug for the treatment of neurodegenerative disorders.
Keywords: naringin, neuroprotection, oxidative stress, inflammation, apoptosis
Introduction
With the progress made in the study of nervous system diseases, considerable evidence has been provided to support the hypothesis that oxidative stress, inflammation and apoptosis are closely associated with the development of neurodegenerative diseases (1–3). The use of the rat pheochromocytoma cell line, PC12, as an in vitro model system is quite acceptable for neurological and neurochemical studies (4,5). Moreover, apoptosis can be induced by a variety of methods, including the use of lipopolysaccharide (LPS), a significant component of the Gram-negative bacteria cell wall, and it has been widely used in the study of neuronal apoptosis (6). The Toll-like receptor (TLR)4 can specifically bind with LPS and can thus trigger the release of inflammatory factors, free radicals and cysteinyl aspartate specific proteinases (known as caspases) that subsequently cause apoptosis (7–9). Hence, the development of a novel drug to reverse neurodegeneration through the inhibition of apoptosis is feasible. The exploration of new chemicals with high efficiency and low toxicity for the treatment of neurodegenerative diseases associated with oxidative stress, inflammation and apoptosis is of utmost importance.
Bioflavonoids, a group of polyphenolic substances, are found in most plants and are a sustainable supplement for human consumption (10). Due to their widespread availability, coupled with their low toxicity, they can be developed for use as therapeutic materials (11–14). Naringin (Nar; 4′, 5,7-trihydroxyflavanone-7-rhamnoglucoside) is a proverbial flavanone glycoside, which is found in abundance in citrus fruit, grapefruit and juices (15). Nar has been shown to have multiple biological and pharmacological properties, including anti-inflammatory, anti-carcinogenic, lipid-lowering and antioxidant activities (16–18).
In the study of pharmaceuticals for the treatment of central nervous system diseases, the critical threshold depends on whether or not these agents can cross the blood-brain barrier (19). Naringenin (4′,5,7-trihydroxyflavanone), a metabolic product of Nar, can easily cross the blood brain barrier (20), and due to this fact, the study of Nar instantly acquires more importance.
All in all, the mechanisms responsible for the protective effects of Nar against LPS-stimulated PC12 cell damage are not well understood. In the present study, we demonstrate that Nar protects PC12 cells from LPS-induced apoptosis by exerting antioxidant, anti-inflammatory and anti-apoptotic effects. Firstly, Nar reduces the level of intracellular reactive oxygen species (ROS) through the downregulation of cytochrome P450 2E1 (CYP2E1) expression directly, rather than through the upregulation of antioxidant-related protein expression, progressively maintaining the balance of the pro-oxidant and antioxidant enzyme system. Nar also attenuates the inflammatory response through the downregulation of the TLR4 pathway. Finally, we also explore the underlying anti-apoptotic mechanisms in PC12 cells.
Materials and methods
Materials
PC12 rat pheochromocytoma cells were obtained from Shanghai Biochemistry Co., Ltd (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), RPMI-1640 medium, fetal bovine serum (FBS), penicillin and streptomycin, were obtained from Sigma Chemical Co. (St. Louis, MO, USA). 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), acridine orange (AO) and ethidium bromide (EB) fluorescent dyes, 4′,6-damidino-2-phenylindole (DAPI) and TRIzol reagent were from Nanjing KeyGen Biotech Co. Ltd. (Nanjing, China). The reactive oxygen assay kit and DCFH-DA were provided by Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). The Annexin V/propidium iodide (PI) apoptosis detection kit was obtained from Invitrogen Life Technologies, Inc. (Carlsbad, CA, USA).
Cell culture and treatment
The PC12 cells have been diffusely used as an analog neuron model in research (21). In this study, the cell culture medium contained RPMI-1640 with 5% FBS, and appropriate penicillin and streptomycin. In the process of cell culture, the culture medium was changed 3 times a week.
MTT assay and cell viability
In order to determine the efficacy and dose, as well as optimal treaqtment time, the PC12 cells were treated with various concentrations (0–2,000 ng/ml) of Nar for 0.5, 1 and 2 h before being exposed to 400 µg/ml LPS for 18 h at 37°C with 5% CO2. MTT solution was added to terminate the drug reaction process, and DMSO was then utilized to dissolve the crystals. The results were gauged by a POLARstar OPTIMA multi-detection microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm.
Morphological inspection
AO can penetrate viable cells, and is embedded in nuclear DNA to emit a bright green fluorescence. EB can only penetrate damaged cells, and is embedded in nuclear DNA to emit orange fluorescence. DAPI, as a fluorescent dye, can penetrate the cell membrane and nucleus of the double-stranded DNA. Thus, we used AO, EB, DAPI as dyes. The half lethal concentrations of LPS and the optimal protective concentrations of Nar were determined by MTT assays, and these were then used in subsequent experiments. The cells were treated with Nar (200, 600 and 1,000 ng/ml) for 1 h before being exposed to 400 µg/ml LPS for 18 h at 37°C with 5% CO2. The treated PC12 cells were inoculated into 6-well plates and stained with AO, EB and DAPI (the final concentration of AO and EB was 100 mg/ml, and that of DAPI was 100 ng/ml; these were dissolved in PBS). Staining was carried out at room temperature for 2–5 min, and the cells were then rinsed well with PBS. Finally, images of the cells were acquired utilizing an inverted fluorescence microscope (Olympus BX63; Olympus, Tokyo, Japan).
Transmission electron microscopy (TEM)
The PC12 cells were subjected to various treatments as described above. The cells were collected in 1.5 ml Eppendorf tubes, and 4% glutaraldehyde was then slowly added along the tube wall. The tubes were then placed in a refrigerator at 4°C for preservation. The the process of dehydration, infiltration, embedding, slicing and heavy metal staining was carried out in accordance with conventional TEM techniques. A transmission electron microscope was used to obtain microscopic images.
Determination of apoptosis
The PC12 cells were treated as described above. In this assay, we used the Annexin V/PI apoptosis detection kit (Invitrogen Life Technologies, Inc.) and a flow cytometer (FACSCalibur, Becton-Dickson, San Diego CA, USA). The cells (1×105/sample) were washed twice with PBS, followed by centrifugation at 300 × g at 4°C for 5 min. The cells were then resuspended in the 1X binding buffer (100 µl), followed by the addition of Annexin V (5 µl) and PI staining solution (5 µl). Finally, the cells were exposed to room temperature for 10 min, avoiding cell-cell contact, and 400 µl 1X binding buffer was then added. Apoptosis was detected by flow cytometry. The third quadrant in the flow cytometric plots rerepsents the distribution of viable cells. The second and fourth quadrant are considered to indicate the distribution of apoptotic cells.
Intracellular ROS assay
The PC12 cells were treated as described above. The cells (1×105/sample) were washed twice with PBS, followed by centrifugation at 300 × g at 4°C for 5 min. DCFH-DA solution was then added followed by incubation for 30 min in room temperature. The density distribution of ROS was analyzed by flow cytometry.
Detection of cytochrome c (Cyto c) release
The PC12 cells were subjected to various treatments as described above. The cells were washed with PBS, fixed with 4% paraformaldehyde for 15 min at 4°C, washed with PBS again, and then permeabi-lized with 0.2% Trixon-100 for 8 min. Non-specific binding was blocked by incubating the cells in 3% BSA for 45 min at 37°C, and the cells were then incubated with the primary antibody overnight at 4°C. The cells were then washed twice with PBS. The cells were incubated with the secondary antibody for 1 h at 37°C, washed with PBS, and then stained with DAPI (1 µg/ml) for 5 min. A laser scanning confocal microscope (TCS SP5, Leica Microsystems CMS GmbH., Mannheim, Germany) was used to acquire images. Cyto c was combined with the antibody to emit green light, and DAPI was used to stain the nuclei blue. In addition, we respectively extracted the cytoplasmic and mitochondrial proteins for use in western blot analysis.
Reverse transcrtiption-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using TRIzol reagent. One microgram total RNA from each sample was then converted into complementary DNA (cDNA) by reverse transcription [using the PrimeScript™ RT reagent kit (Takara, Dalian, China)]. Quantitative PCR (qPCR) was performed to gauge the mRNA levels using the 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The PCR cycling conditions were as follows: 94°C pre-degeneration for 4 min, 94°C degeneration for 30 sec, 60°C annealing for 30 sec, 72°C extension for 2 min, for 35 cycles. The data were analyzed using System SDS software (Applied Biosystems). The expression level of the GAPDH gene was considered as a standard. The relative gene expression levels of interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), fatty acid synthase (Fas), Fas ligand (FasL) and Bcl-2-associated X protein (Bax) were expressed as a percentage of the expression of GAPDH. Respective primers (Table I) were applied by calculating target gene expression.
Table I.
The primer sequences used for RT-qPCR.
| Genes | GenBank accession no. |
Forward primers (5′→3′) | Reverse primers (5′→3′) |
|---|---|---|---|
| GAPDH | NM_017008.3 | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| IL-1β | NM_031512.2 | CCCTGAACTCAACTGTGAAATAGCA | CCCAAGTCAAGGGCTTGGAA |
| IL-6 | NM_012589.1 | ATTGTATGAACAGCGATGATGCAC | CCAGGTAGAAACGGAACTCCAGA |
| TNF-α | NM_012675.3 | TCAGTTCCATGGCCCAGAC | GTTGTCTTTGAGATCCATGCCATT |
| Fas | NM_139194.2 | CACAGCATTCAGTCCTATCCACAGA | CACAGCCAACCAGATGCTTCA |
| FasL | NM_012908.1 | CACCAACCACAGCCTTAGAGTATCA | CACTCCAGAGATCAAAGCAGTTCC |
| Bax | NM_017059.2 | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC |
IL, interleukin; TNF, tumor necrosis factor; Fas, fatty acid synthase; FasL, Fas ligand; Bax, Bcl-2-associated X protein.
Western blot analysis
We used lysis buffer to extract the total proteins, and the extracted proteins were then quantified using the Bradford assay kit (Blossom Bio Inc., Hangzhou, China). The cells were lysed using lysis buffer containing phenylmethanesulfonyl fluoride (PMSF) on ice for 30 min, The lysates were then centrifuged at 20,000 × g at 4°C for 5 min. The supernatant was placed in −20°C. A total of 50 µg protein was placed on a 12% SDS-PAGE gel and separated electrophoretically. The target proteins were then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking the PVDF membranes in 5% dried skim milk (Boster Biological Technology, Wuhan, China) for 3 h at room temperature, the membranes were incubated overnight at 4°C with primary antibodies (Table II). Subsequently, the membranes were incubated at room temperature for 2 h with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibodies at a 1: 2,000 dilution (Beyotime Institute of Biotechnology, Beijing, China). Protein detection was performed by enhanced chemiluminescence (ECL) and imaging was carried out using a BioSpectrum Gel Imaging System (HR410, UVP, USA). In order to eliminate the variations, the expression level of the GAPDH gene was considered as a standard. It should be noted that all protein extractions were carried out once, and thus the internal reference was same in this experiment. Moreover, in order to detect the intra- and extranuclear content of Cyto c, we used the Nuclear and Cytoplasmic Protein Extraction kit (Blossom Bio Inc.) to extract the protein, and the remaining process was carried out as described above..
Table II.
Information for the antibodies used in the present study.
| Antibodies | Sources | Dilutions | Companies |
|---|---|---|---|
| GAPDH | Rat | 1:5,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Nrf2 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| HO-1 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| SOD2 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| GSS | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| CYP2E1 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| HMGB1 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| COX-2 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| TLR4 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| MyD88 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| TRAF6 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| NF-κB | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| AP-1 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Bak | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Bcl-2 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Bcl-xL | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Caspase-9 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Cyto c | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Caspase-8 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| Caspase-3 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| p53 | Rabbit | 1:1,000 | Proteintech Group, Inc., Chicago, IL, USA |
| p-p38 | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
| p-38 | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
| p-ERK | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
| ERK | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
| p-JNK | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
| JNK | Rabbit | 1:1,000 | Bioworld Technology, Inc., St. Louis Park, MN, USA |
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; SOD2, superoxide dismutase 2; GSS, glutathione synthetase; CYP2E1, cytochrome P450 2E1; HMGB1, high mobility group box 1 protein; COX-2, cyclooxygenase-2; TLR4, toll-like receptor 4; My88, myeloid differentiation factor 88; TRAF6, TNF receptor-associated factor 6; NF-κB, nuclear factor-κ-light-chain-enhancer of activated B cells; AP-1, activator protein transcription factor-1; Bcl-2, B-cell lymphoma 2; Bcl-xL, anti-apoptotic Bcl-2 family protein; caspase, cysteinyl aspartate specific proteinase; cytochrome c, Cyto c; ERK, extracellular signal-related protein kinase; JNK, c-Jun N-terminal kinase.
Statistical analysis
All data are presented as the means ± standard deviation (SD). The data between groups were compared by one-way analysis of variance (ANOVA) and inter-group differences were compared using a t-test. A value of p<0.05 was considered to indicate a statistically significant difference.
Results
Effects of Nar on LPS-induced cell death
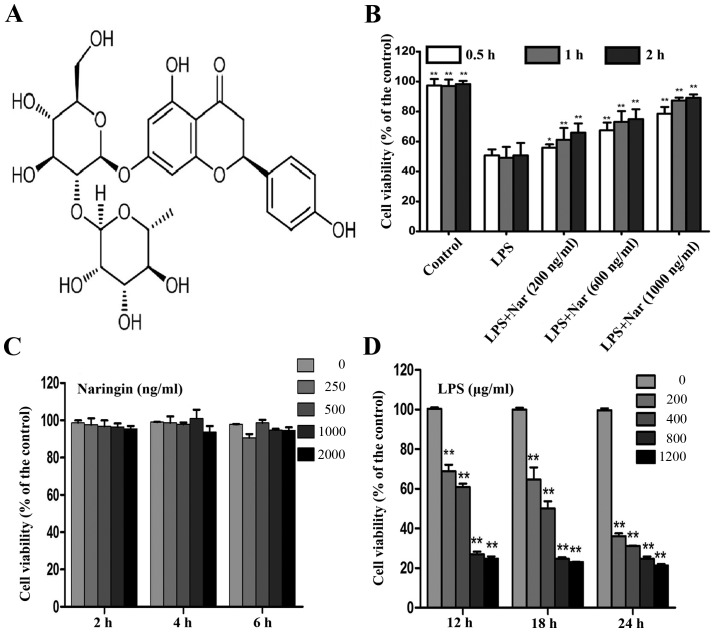
The chemical structures of Nar is shown in Fig. 1A. MTT assay is widely used in pharmacodynamics experiments (22,23). Treatment with various concentrations (200, 600 and 1,000 ng/ml) of Nar for 1 h prior to treatment with LPS (400 µg/ml, 18 h) induced a time and dose-dependent increase in the cell survival rate (cell viability increased to 61.2±1.3, 73.1±1.4 and 87.36±1.8%, respectively) (Fig. 1B).
Figure 1.
(A) Chemical structure of naringin (Nar). (B) Pre-treatment with Nar at various concentrations (200, 600 and 1,000 ng/ml) and for different periods of time (0.5, 1 and 2 h) mitigated lipopolysaccharide (LPS)-induced cell death. (C) Safe dose of Nar to maintain cell viability. (D) Dose- and time-dependent effects of LPS on cell viability. Data are presented as the means ± SD (n=5 treatment groups). *p<0.05 and **p<0.01 compared with the LPS group.
Treatment with Nar at the range of 0–2,000 ng/ml had no obvious effect on the survival rate of the PC12 cells (Fig. 1C). Therefore, the Nar concentrations of 200, 600 and 1,000 ng/ml used in the subsequent experiments were not considered to be toxic to the cells. In addition, the viability of the PC12 cells exposed to LPS (400 µg/ml, 18 h) was decreased to 50.15±1.1% (Fig. 1D); thus, LPS at the concentration of 400 µg/ml was selected for use in the subsequent experiments to induce cell damage. Our results demonstrated the potential protective effects of Nar on LPS-induced PC12 cell viabiltiy in a time- and dose-dependent manner.
Cell morphology
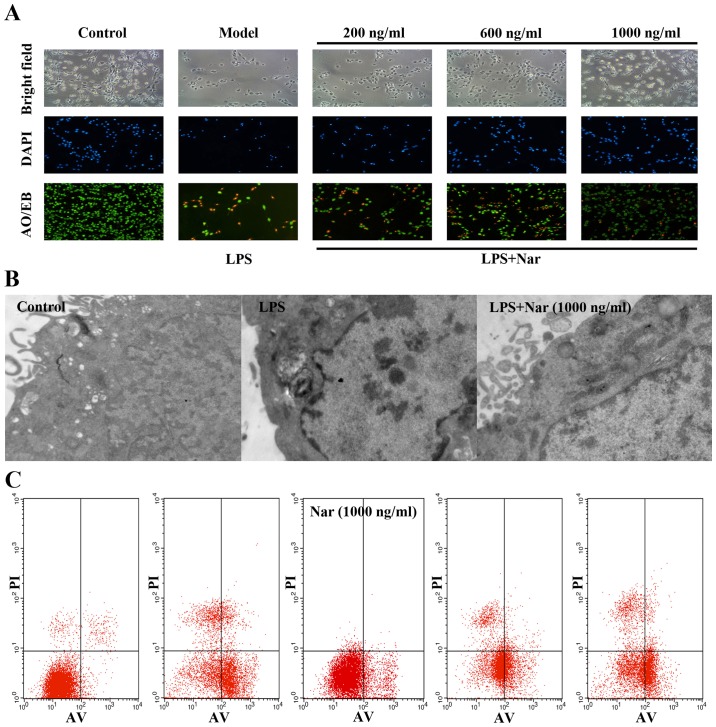
Black and white microscopic images and images of AO/EB and DAPI staining provided morphological evidence to confirm our results. As shown in Fig. 2A, the control PC12 cells exhibited green fluorescence, whereas the model group cells exposed to LPS exhibited yellow-stained cells, indicating apoptosis. The apoptotic cells were significantly decreased by pre-treatment with Nar (1,000 ng/ml, 1 h), as evidenced by the decreased number of yellow-stained cells.
Figure 2.
(A) Morphological and fluorescence images of PC12 cells stained with acridine orange and ethidium bromide AO/EB and DAPI (×100 magnification). (B) Protective effect of the naringin (Nar) on the ultra-structure of PC12 cells (×40,000 magnification). (C) Detection of apoptosis by flow cytometry. Data are presented as the means ± SD (n=5 treatment groups). *p<0.05 and **p<0.01 compared with model group.
In order to further confirm our results, the changes in the ultrastructure of the PC12 cells were observed under a transmission electron microscope. As shown in Fig. 2B, the control group cells exhibited intact cell membranes, intact nuclei, and a large number of villi structures; however, the cells exposed to LPS exhibited distinct changes, including cytoplasmic vacuoles, as well as the disappearance of microvilli structures. Compared with the model group exposed to LPS, pre-treatment with Nar markedly reversed the above-mentioned changes.
Finally, flow cytometry was used to measure the amount of apoptotic cells (Fig. 2C). In relation to the condition, LPS provoked a 14-fold increase in the number of apoptotic cells (from 3.9% of cells in the control condition to 55% of cells following exposure to LPS). However, pre-treatment with Nar decreased the apoptotic rate significantly (Fig. 2C).
Effects of Nar on intracellular ROS levels
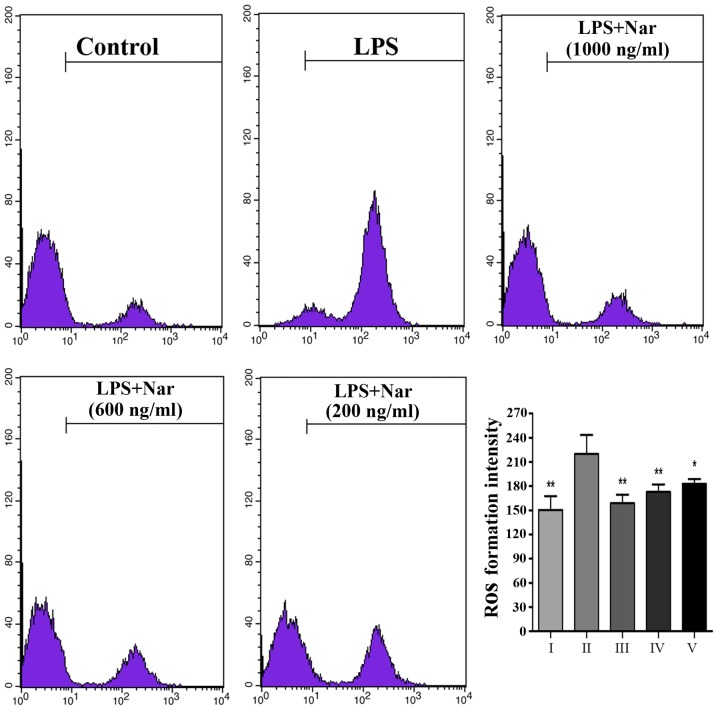
Exposure to LPS prompted the release of a large number of ROS from the cells (Fig. 3); however, ROS production was markedly inhibited by pre-treatment with Nar, particularly pre-treatment Nar at 1,000 ng/ml. Our findings demonstrated that the LPS-induced PC12 cell apoptosis may be associated with oxidative stress, as also previously demonstrated (4,24). However, Nar protected the PC12 cells from apoptosis and attenuated the production of intracellular ROS.
Figure 3.
Level of intracellular reactive oxygen species (ROS) detected by flow cytometry and the intracellular ROS formation intensity. Data are presented as the means ± SD (n=5 treatment groups). *p<0.05 and **p<0.01 compared with the LPS group. For the bar chart: I, control; II, LPS only; III, Nar at 1,000 ng/ml; IV, Nar at 600 ng/ml; and V, Nar at 200 ng/ml.
Suppression of Cyto c release
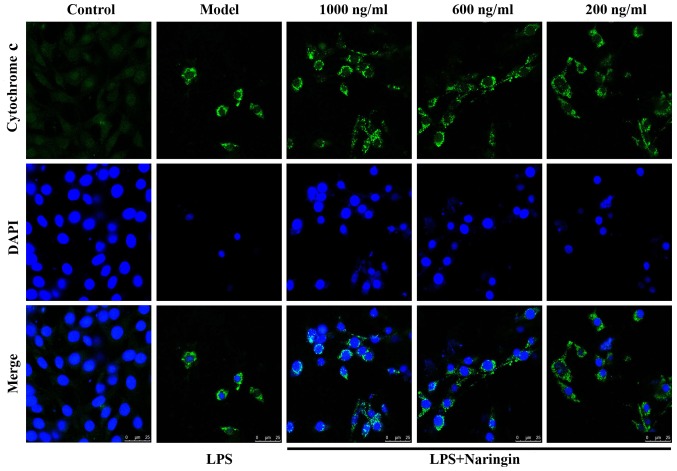
As is known, Cyto c plays a vital role in the process of apoptosis (25,26). In Fig. 4, green fluorescence reflects staining with Cyto c fluorescent antibody, and blue fluorescence reflects staining with DAPI, which directly reflects nuclear staining. As shown in the merge image of Fig. 4, little fluorescence was observed in the control group, whereas in the LPS group, even though the number of viable cells was significantly decreased, the cell size was reduced and the nuclear staining also decreased, the content of extranuclear Cyto c was still evident. Thus, there was a mass of diffuse cytoplasmic fluorescence. As also shown in Fig. 8G and H, Nar effectively abated the transfer of Cyto c from the mitochondria to the cytoplasm. Therefore, it exerted an anti-apoptotic effect.
Figure 4.
Immunofluorescence of cytochrome c (Cyto c) (×800 magnification). Green fluorescence reflects staining with cytochrome c fluorescent antibody, and blue fluorescence reflects staining with DAPI, which directly reflects nuclear staining. Compared with the LPS group, with the increasing concentration of naringin, the cell nuclei returned to their normal form, the number of viable cells increased, and staining for extracellular cytochrome c decreased.
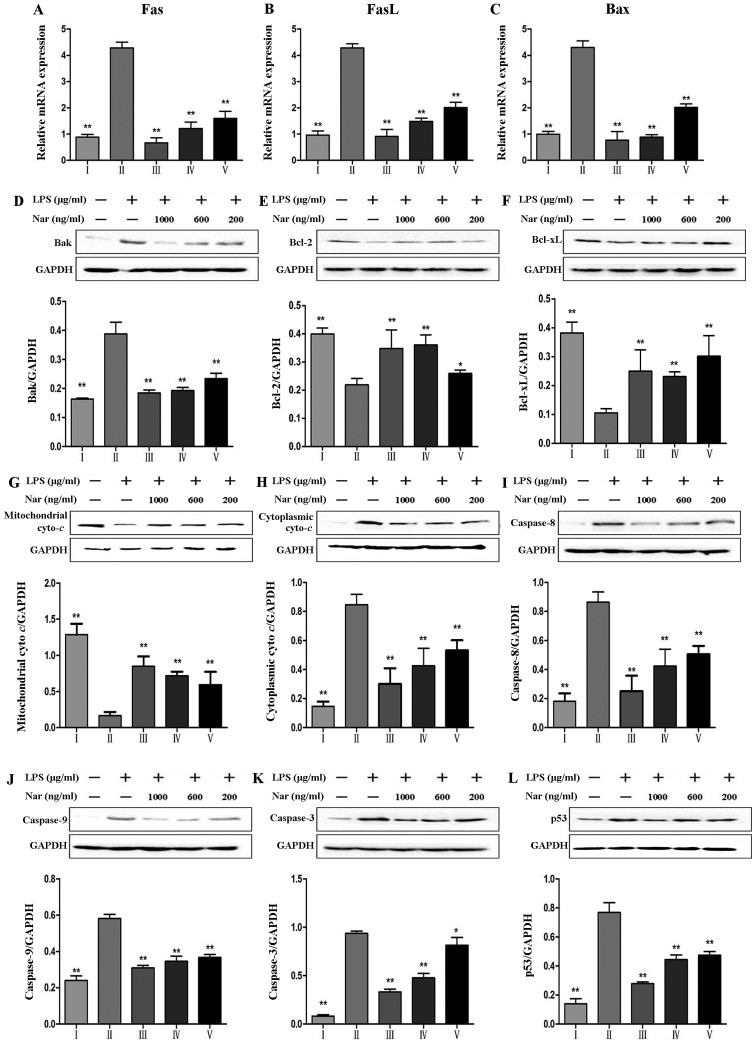
Figure 8.
Effects of naringin (Nar) on the expression of apoptosis-related genes and proteins. mRNA expression of (A) fatty acid synthase (Fas), (B) Fas ligand (FasL), (C) Bcl-2-associated X protein (Bax), and (D) protein expression of Bak, (E) Bcl-2, B-cell lymphoma 2 (Bcl-2), (F) Bcl-xL, (G) mitochondrial cytochrome c (Mito Cyto c) (H) cytoplasmic cytochrome c (Cyto Cyto c), (I) caspase-8, (J) capase-9, (K) caspase-3 and (L) p53. *p<0.05 and **p<0.05 compared with the LPS group. For the bar charts: I, control; II, LPS only; III, Nar at 1,000 ng/ml; IV, Nar at 600 ng/ml; and V, Nar at 200 ng/ml.
Effects of the Nar on the expression levels of oxidative stress-related markers
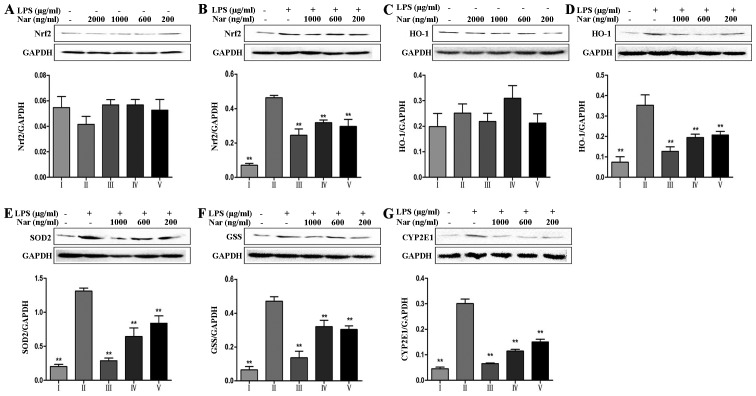
It is worth noting that nuclear factor E2-related factor 2 (Nrf2) can mediate antioxidant gene expression (27). As shown in Fig. 5A and B, in the absence of LPS, treatment of the PC12 cells with Nar at any concentration did not alter the total Nrf2 protein level compared with the untreated control group. However, in the cells exposed to LPS, the total Nrf2 protein level was significantly increased, indicating the resistance to the oxidative stress reaction through compensatory mechanisms. Pre-treatment of the cells with Nar improved the cell survival rate and gradually corrected the balance of the intracellular total Nrf2 levels. In addition, other oxidative-related proteins, including heme oxygenase-1 (HO-1), superoxide dismutase 2 (SOD2) and glutathione synthetase (GSS) also exhibited the same trend in expression (Fig. 5C–F).
Figure 5.
Effects of naringin (Nar) on the levels of oxidative stress-related proteins. (A and B) Nuclear factor E2-related factor 2 (Nrf2), (C and D) heme oxygenase-1 (HO-1), (E) superoxide dismutase 2 (SOD2), (F) glutathione synthetase (GSS) and (G) cytochrome P450 2E1 (CYP2E1). Values are expressed as the means ± SD (n=5 treatment groups). *p<0.05 and **p<0.01 compared with the LPS group. For the bar charts: I, control; II, LPS only; III, Nar at 1,000 ng/ml; IV, Nar at 600 ng/ml; and V, Nar at 200 ng/ml.
It is worth emphasizing that CYP2E1 plays an essential role in the production of ROS (28). As shown in Fig. 5G, relative to the specific condition, LPS provoked a 6.7-fold increase in the CYP2E1 levels (from 4.5% of cells in the control condition to 30.1% of cells exposed to LPS). However, pre-treatment with Nar significantly decreased the levels of CYP2E1.
Effects of the Nar on the expression levels of inflammatory cytokines and TLR4-related proteins
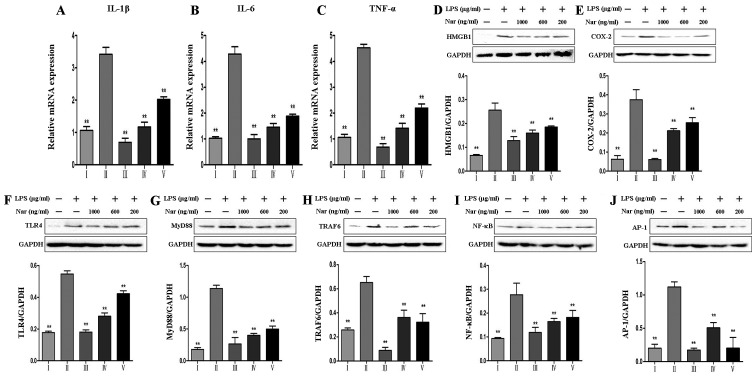
As shown in Fig. 6, we detected the expression levels of inflammation-related genes and proteins, including IL-1β, IL-6, TNF-α, high mobility group box 1 protein (HMGB1) and cyclooxygenase-2 (COX-2), as well as TLR4-related proteins, including TLR4, myeloid differentiation factor 88 (MyD88), TNF receptor-associated factor 6 (TRAF6), nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and activator protein transcription factor-1 (AP-1), and the results revealed that LPS promoted the overexpression of inflammation-related proteins, whereas pre-treatment with Nar decreased the levels of inflammation-related proteins in a dose-dependent manner.
Figure 6.
Effects of naringin (Nar) on the expression of inflammation-related markers. mRNA levels of (A) interleukin (IL)-1β, (B) IL-6, (C) tumor necrosis factor-α (TNF-α), and protein expressions of (D) high mobility group box 1 protein (HMGB1), (E) cyclooxygenase-2 (COX-2), (F) Toll-like receptor 4 (TLR4), (G) myeloid differentiation factor 88 (MyD88), (H) TNF receptor-associated factor 6 (TRAF6), (I) nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and (J) activator protein transcription factor-1 (AP-1). Values are expressed as the means ± SD (n=5 treatment groups). **p<0.01 compared with the LPS group. For the bar charts: I, control; II, LPS only; III, Nar at 1,000 ng/ml; IV, Nar at 600 ng/ml; and V, Nar at 200 ng/ml.
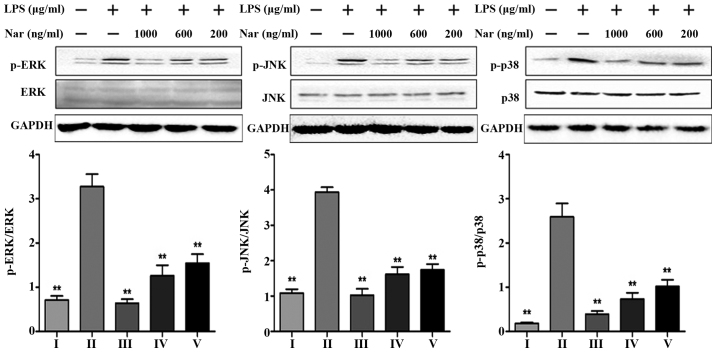
Effects of Nar on the expression levels of mitogen-activated protein kinase (MAPK) phosphorylation
Consistent with the in vitro experimental results of the TLR4 pathway (Fig. 6F–J), we detected the levels of MAPK phosphorylation at the same time. LPS markedly increased the protein expression levels (p-ERK, p-JNK, p-p38) by 4.6-, 3.6- and 14.3-fold. However, Nar markedly decreased MAPK phosphorylation (Fig. 7). On the whole, pre-treatment with Nar significantly inhibited the LPS-induced activation of extracellular signal-related protein kinase (ERK), c-Jun N-terminal kinase (JNK) and the p38 signaling pathways. Furthermore, our results demonstrated that the anti-inflammatory effects of Nar are associated with the inhibition of the TLR4 pathway.
Figure 7.
Effects of naringin (Nar) on the phosphorylation levels of mitogen activated protein kinases (MAPKs). Values are expressed as the means ± SD (n=5 treatment groups). **p<0.01 compared with the LPS group. For the bar charts: I, control; II, LPS only; III, Nar at 1,000 ng/ml; IV, Nar at 600 ng/ml; and V, Nar at 200 ng/ml.
Effects of Nar on the expression levels of apoptosis-related genes and proteins
As shown in Fig. 8, LPS increased the expression levels of Fas, FasL and Bax (Fig. 8A–C). Furthermore, LPS increased the protein levels of Bak, caspase-9, caspase-8, caspase-3 and p53, and decreased the levels of the anti-apoptotic proteins, Bcl-xL, B-cell lymphoma 2 (Bcl-2). However, pre-treatment with Nar markedly decreased the levels of Fas, FasL, Bax, Bak, caspase-9, caspase-3 and p53. The levels of Bcl-2 and Bcl-xL were evidently upregulated by 1.9-fold (from 21.9 to 42.5%) and 3.0-fold (from 10.5 to 31.7%) (p<0.01) by pre-treatment with Nar (1,000 ng/ml).
Discussion
Nar, a flavanone glycoside of plants and fruits, has been shown to possess potent antioxidant (29), anti-inflammatory proper ties (30–32). As it has been previously reported, the long-term consumption of Nar improves memory in animal models of neurodegenerative diseases (33–35). To date, to the best of our knowedge, in vitro research on the effects of Nar on neurons is limited. Thus, in this study, in order to explore and confirm the potential neuroprotective effects of Nar, we examined the effects of Nar on the LPS-induced apoptosis of PC12 cells in vitro. In addition, we investigated the potential underlying mechanisms.
On the one hand, chronic toxicological assessment analysis of Nar has indicated that Nar is a substance with low toxicity (36), and our results also demonstrated this fact. On the other hand, naringenin, a metabolic product of Nar, can easily cross the blood brain barrier to protect brain cells (20). All these data make the research of Nar feasible.
Oxidative stress is closely associated with neurodegenerative diseases (37). ROS play an essential role in oxidative stress. In the present study, LPS markedly increased the release of intracellular ROS from PC12 cells; however, pre-treatment with various concentrations of Nar decreased the intracellular ROS levels. It should be noted that CYP2E1 is also associated with LPS-induced oxidative stress and ROS production (38). Our results from western blot analysis demonstrated this view, which also revealed that the protective effects of Nar against LPS-induced PC12 cell apoptosis may be associated with its specific inhibition of CYP2E1.
In addition, the enhanced tolerance capacity against oxidetive stress gained our attention (Fig. 5). The protein levels of Nrf2, HO-1, SOD2 and GSS increased following exposure to LPS. Of note, the levels of these proteins were not altered when Nar treatment was used alone. Therefore, we speculate that Nar can not only enhance the antioxidant capacity through the downregulation of CYP2E1 protein expression, but can also maintain the balance of intracellular antioxidant protein expression.
LPS is a major activator of inflammation and a ligand for TLR4 (39). Upon LPS stimulation, inflammation is generated by TLR4 through the activation of downstream proteins, such as MyD88, TRAF6, NF-κB, MAPKs and AP-1, resulting in the production of a wide range of pro-inflammatory cytokines and chemokines (40,41). Moreover, NF-κB is an inducible transcription factor, which can be sensitized by TNF-α, IL-1β, LPS and ROS (42–44). The present study demonstrated that LPS has the ability to cause the robust transcriptional activity of NF-κB and to markedly increase the expression of pro-inflammatory cytokines, leading to PC12 cell apoptosis. However, all of changes were reversed by pre-treatment with Nar. In addition, our results of western blot analysis demonstrated that the phosphorylation of ERK, p38, JNK was inhibited in the presence of Nar. Thus, it is possible that Nar mediates the TLR4-related inflammatory pathway, and thereby alleviate inflammation in PC12 cells.
Apoptosis is accompanied by increased mRNA levels of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α (45). COX-2 is one of inflammatory early-response proteins (46), and its overexpression is associated with the stimulation of HMGB1. HMGB1 is a member of the damage-associated molecular pattern (DAMP) family of proteins that is secreted by necrotic brain cells (47). HMGB1 can bind to its receptor (TLR4) for mediating the inflammatory reaction (48); however, the potential mechanisms of action of the HMGB1-TLR4 pathway remain unclear. Nevertheless, in this study, Nar downregulated the levels of HMGB1 and TLR4-related proteins, and these results indicated that Nar effectively mitigated the secretion of inflammatory cytokines.
Finally, the death of PC12 cells induced by LPS is considered the common effect of oxidative stress, and inflammatory and apoptosis reactions. Bcl-2 is a critical unit for pro-survival function by maintaining the mitochondrial membrane. p53 is a critical suppressor molecule for Bcl-2. Upon oxidative or inflammatory stimulation, p53 is activated and associates with Bcl-2 by direct binding. The association of p53 with Bcl-2 can promote Bcl-2 dissociation from the mitochondria and accelerate the permeabilization of the mitochondrial membrane. This causes Cyto c release from the mitochondria to the cytoplasm and activates downstream of caspase apoptotic cascades (49,50). Caspase-3 is considered to be a unique factor of apoptosis in this cascade reaction. On the one hand, Fas/FasL and caspase-8 are the upstream regulators of caspase-3 (51), and LPS can upregulate caspase-3 through Fas/FasL-caspase-3 pathway; on the other hand, upon LPS stimulation, LPS can downregulate Bcl-2, Bcl-xL, and upregulate Bax, Bak, cytoplasmic Cyto c and capase-9 (52). Finally, the caspase-3-related pathway is activated which eventually leads to apoptosis. The present study demonstrated that Nar markedly downregulate the protein expression levels of p53, Bak, cytoplasmic Cyto c, caspase-9, caspase-8 and caspase-3, and the mRNA expression levels of Fas, FasL and Bax; Nar increased the expression of Bcl-2 and Bcl-xL. Our results demonstrated that the Nar suppressed LPS-induced PC12 cell apoptosis by mediating the expression of a series of caspase-3-related proteins.
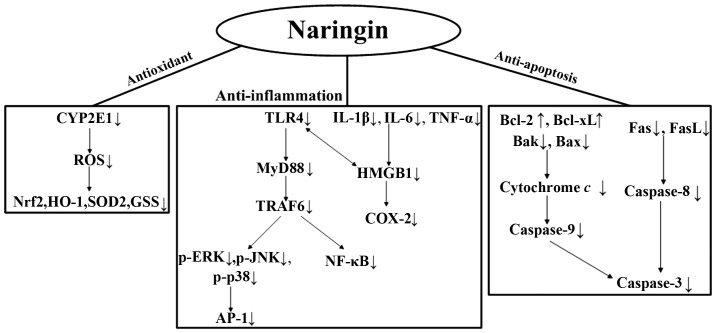
In conclusion, our results demonstrate for the first time, at least to the best of our knowledge, that Nar reverses the oxidative stress, inflammation and the apoptosis of PC12 cells resulting from exposure to LPS. In Fig. 9, we summarize the underlying mechanisms responsible for the protective effects of Nar. As neuroinflammation is involved in the development of neurodegenerative diseases, Nar seems to act as a possible neuroprotective substance. Further studies are warranted to fully determine the protective effects of Nar and to elucidate all the underlying molecular mechanisms.
Figure 9.
The conceivable mechanisms responsible for the protective effects of naringin (Nar) against lipopolysaccharide (LPS)-induced PC12 cell apoptosis.
Acknowledgments
We would like to thank all colleagues within the Department of Pharmaceutical Analysis and correlative laboratory technicians from the Science Research Center of Dalian Medical University for their kind help and support.
Abbreviations
- LPS
lipopolysaccharide
- CYP
cytochrome P450 superfamily
- CYP2E1
cytochrome P450 2E1
- ROS
reactive oxygen species
- Nrf2
nuclear factor erythroid 2-related factor 2
- HO-1
heme oxygenase-1
- SOD
superoxide dismutase
- GSS
glutathione synthetase
- IL-1
interleukin-1
- TNF-α
tumor necrosis factor-α
- HMGB1
high mobility group box 1 protein
- COX-2
cyclooxygenase-2
- TLR4
Toll-like receptor 4
- MyD88
myeloid differentiation factor 88
- TRAF6
TNF receptor-associated factor 6
- NF-κB
nuclear factor κ- light-chain-enhancer of activated B cells
- ERK
extracellular signal-related protein kinase
- JNK
c-Jun N-terminal kinase
- MAPKs
mitogen activated protein kinases
- AP-1
activator protein transcription factor-1
- Fas
fatty acid synthase
- FasL
Fas ligand
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- Bax
Bcl-2- associated X protein
- Mito Cyto c
mitochondrial cytochrome c
- Cyto Cyto c
cytoplasmic cytochrome c
- LD 50
median lethal dose
References
- 1.Zhou WW, Lu S, Su YJ, Xue D, Yu XL, Wang SW, Zhang H, Xu PX, Xie XX, Liu RT. Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radic Biol Med. 2014;74:50–63. doi: 10.1016/j.freeradbiomed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil WK, Assaf N, ElShebiney SA, Salem NA. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem Int. 2015;80:79–86. doi: 10.1016/j.neuint.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Ansari N, Khodagholi F, Amini M, Shaerzadeh F. Attenuation of LPS-induced apoptosis in NGF-differentiated PC12 cells via NF-κB pathway and regulation of cellular redox status by an oxazine derivative. Biochimie. 2011;93:899–908. doi: 10.1016/j.biochi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 5.He AY, Qiu LJ, Gao Y, Zhu Y, Xu ZW, Xu JM, Zhang ZH. The role of oxidative stress in neuromelanin synthesis in PC12 cells. Neuroscience. 2011;189:43–50. doi: 10.1016/j.neuroscience.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Shaerzadeh F, Ansari N, Amini M, Khodagholi F. Neuroprotective effects of oxazine derivative against LPS-induced oxidative stress via attenuation of NF-κB pathway and regulation of cellular redox status in PC12 cells. Alzheimers Dement. 2011;7:S616–S617. doi: 10.1016/j.jalz.2011.05.1757. [DOI] [PubMed] [Google Scholar]
- 7.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song X, Guo M, Wang T, Wang W, Cao Y, Zhang N. Geniposide inhibited lipopolysaccharide-induced apoptosis by modulating TLR4 and apoptosis-related factors in mouse mammary glands. Life Sci. 2014;119:9–17. doi: 10.1016/j.lfs.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Gadad BS, Britton GB, Rao KS. Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide. J Alzheimers Dis. 2011;24(Suppl 2):223–232. doi: 10.3233/JAD-2011-110182. [DOI] [PubMed] [Google Scholar]
- 10.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 11.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y, Qi Y, Han X, Peng J. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food Chem Toxicol. 2013;59:657–669. doi: 10.1016/j.fct.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Peng J. Response to: 'hormetic effect of Rosa laevigata Michx in CCl4-induced hepatotoxicity and the presumptive role of PPARs'. Food Chem Toxicol. 2013;57:389. doi: 10.1016/j.fct.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, Yin L, Sun H, Liu K, Peng J. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol. 2013;59:118–128. doi: 10.1016/j.fct.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Dandekar DV, Toledo RT, Singh RK, Patil BS. Supercritical fluid extraction of limonoids and naringin from grapefruit (Citrus paradisi Macf.) seeds. Food Chem. 2007;105:1026–1031. doi: 10.1016/j.foodchem.2007.04.062. [DOI] [Google Scholar]
- 16.Choi MS, Do KM, Park YS, Jeon SM, Jeong TS, Lee YK, Lee MK, Bok SH. Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin E. Ann Nutr Metab. 2001;45:193–201. doi: 10.1159/000046729. [DOI] [PubMed] [Google Scholar]
- 17.Raza SS, Khan MM, Ahmad A, Ashafaq M, Islam F, Wagner AP, Safhi MM, Islam F. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Sachdeva AK, Kuhad A, Chopra K. Naringin ameliorates memory deficits in experimental paradigm of Alzheimer's disease by attenuating mitochondrial dysfunction. Pharmacol Biochem Behav. 2014;127:101–110. doi: 10.1016/j.pbb.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Ding G, Zhang Z, Chopp M, Li L, Zhang L, Li Q, Wei M, Jiang Q. MRI evaluation of BBB disruption after adjuvant AcSDKP treatment of stroke with tPA in rat. Neuroscience. 2014;271:1–8. doi: 10.1016/j.neuroscience.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 21.Rogers SW, Mandelzys A, Deneris ES, Cooper E, Heinemann S. The expression of nicotinic acetylcholine receptors by PC12 cells treated with NGF. J Neurosci. 1992;12:4611–4623. doi: 10.1523/JNEUROSCI.12-12-04611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Cong X, Zheng L, Xu L, Yin L, Peng J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol Lett. 2012;214:69–80. doi: 10.1016/j.toxlet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y, Ji L, Zhang S, Xu L, Yin L, Li L, Zhao Y, Peng J. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem Toxicol. 2012;50:3133–3141. doi: 10.1016/j.fct.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 24.Omata Y, Saito Y, Fujita K, Ogawa Y, Nishio K, Yoshida Y, Niki E. Induction of adaptive response and enhancement of PC12 cell tolerance by lipopolysaccharide primarily through the upregulation of glutathione S-transferase A3 via Nrf2 activation. Free Radic Biol Med. 2008;45:1437–1445. doi: 10.1016/j.freeradbiomed.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Balachandran C, Sangeetha B, Duraipandiyan V, Raj MK, Ignacimuthu S, Al-Dhabi NA, Balakrishna K, Parthasarathy K, Arulmozhi NM, Arasu MV. A flavonoid isolated from Streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer A549 cells through P53 and cytochrome c release caspase dependant pathway. Chem Biol Interact. 2014;224:24–35. doi: 10.1016/j.cbi.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Blatt NB, Boitano AE, Lyssiotis CA, Opipari AW, Jr, Glick GD. Bz-423 superoxide signals apoptosis via selective activation of JNK, Bak, and Bax. Free Radic Biol Med. 2008;45:1232–1242. doi: 10.1016/j.freeradbiomed.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YT, Shi D, Yang D, Yan B. Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase-1 through a novel element transactivated by nuclear factor-E2 related factor-2. Biochem Pharmacol. 2012;84:864–871. doi: 10.1016/j.bcp.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valencia-Olvera AC, Morán J, Camacho-Carranza R, Prospéro-García O, Espinosa-Aguirre JJ. CYP2E1 induction leads to oxidative stress and cytotoxicity in glutathione-depleted cerebellar granule neurons. Toxicol In Vitro. 2014;28:1206–1214. doi: 10.1016/j.tiv.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol. 2010;48:626–632. doi: 10.1016/j.fct.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Golechha M, Chaudhry U, Bhatia J, Saluja D, Arya DS. Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol Pharm Bull. 2011;34:360–365. doi: 10.1248/bpb.34.360. [DOI] [PubMed] [Google Scholar]
- 31.Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219:101–112. doi: 10.1016/j.cbi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Dong D, Xu L, Yin L, Qi Y, Peng J. Naringin prevents carbon tetrachloride-induced acute liver injury in mice. J Funct Foods. 2015;12:179–191. doi: 10.1016/j.jff.2014.11.020. [DOI] [Google Scholar]
- 33.Wang D, Gao K, Li X, Shen X, Zhang X, Ma C, Qin C, Zhang L. Long-term naringin consumption reverses a glucose uptake defect and improves cognitive deficits in a mouse model of Alzheimer's disease. Pharmacol Biochem Behav. 2012;102:13–20. doi: 10.1016/j.pbb.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, Arya DS. Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: possible mechanisms of neuroprotection. Epilepsy Behav. 2014;41:98–102. doi: 10.1016/j.yebeh.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 35.Mani VM, Sadiq AMM. Naringin modulates the impairment of memory, anxiety, locomotor, and emotionality behaviors in rats exposed to deltamethrin; a possible mechanism association with oxidative stress, acetylcholinesterase and ATPase. Biomed Prev Nutr. 2014;4:527–533. doi: 10.1016/j.bionut.2014.08.006. [DOI] [Google Scholar]
- 36.Li P, Wang S, Guan X, Cen X, Hu C, Peng W, Wang Y, Su W. Six months chronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem Toxicol. 2014;66:65–75. doi: 10.1016/j.fct.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Han Y, Zhang T, Yang Z. Protective effect of trifluoperazine on hydrogen peroxide-induced apoptosis in PC12 cells. Brain Res Bull. 2011;84:183–188. doi: 10.1016/j.brainresbull.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Pérez MJ, Cederbaum AI. Antioxidant and pro-oxidant effects of a manganese porphyrin complex against CYP2E1-dependent toxicity. Free Radic Biol Med. 2002;33:111–127. doi: 10.1016/S0891-5849(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Lian LH, Bai T, Wu YL, Wan Y, Xie WX, Jin X, Nan JX. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int Immunopharmacol. 2011;11:1871–1876. doi: 10.1016/j.intimp.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Lu SM, Yu CJ, Liu YH, Dong HQ, Zhang X, Zhang SS, Hu LQ, Zhang F, Qian YN, Gui B. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD 88 pathway. Brain Behav Immun. 2015;44:221–234. doi: 10.1016/j.bbi.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-κB activation. Free Radic Biol Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Guo Z, He W, Yang Y, Li Y, Zheng A, Li P, Zhang Y, Ma J, Wen M, et al. Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines. Int Immunopharmacol. 2012;13:46–53. doi: 10.1016/j.intimp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–1381. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelazeem AH, Abdelatef SA, El-Saadi MT, Omar HA, Khan SI, McCurdy CR, El-Moghazy SM. Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. Eur J Pharm Sci. 2014;62:197–211. doi: 10.1016/j.ejps.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 48.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 49.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng KW, Liao LX, Zhao MB, Song FJ, Yu Q, Jiang Y, Tu PF. Protosappanin B protects PC12 cells against oxygen-glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent P53 protein degradation. Eur J Pharmacol. 2015;751:13–23. doi: 10.1016/j.ejphar.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 51.Miller DK. The role of the Caspase family of cysteine proteases in apoptosis. Semin Immunol. 1997;9:35–49. doi: 10.1006/smim.1996.0058. [DOI] [PubMed] [Google Scholar]
- 52.Islam Z, Amuzie CJ, Harkema JR, Pestka JJ. Neurotoxicity and inflammation in the nasal airways of mice exposed to the macrocyclic trichothecene mycotoxin roridin a: kinetics and potentiation by bacterial lipopolysaccharide coexposure. Toxicol Sci. 2007;98:526–541. doi: 10.1093/toxsci/kfm102. [DOI] [PubMed] [Google Scholar]