Abstract
Fisetin (3,3′,4′,7-tetrahydroxyflavone) is a plant flavonol found in fruits and vegetables that has been reported to inhibit migration and proliferation in several types of cancer. Reactive astrogliosis involves astrocyte migration and proliferation, and contributes to the formation of glial scars in central nervous system (CNS) disorders. However, the effect of fisetin on the migration and proliferation of astrocytes remains unclear. In this study, we found that fisetin inhibited astrocyte migration in a scratch-wound assay and diminished the phosphorylation of focal adhesion kinase (FAK; Tyr576/577 and paxillin (Tyr118). It also suppressed cell proliferation, as indicated by the decreased number of 5-ethynyl-2′-deoxyuridine (EdU)-positive cells, induced cell cycle arrest in the G1 phase, reduced the percentage of cells in the G2 and S phase (as measured by flow cytometry), and decreased cyclin D1 expression, but had no effect on apoptosis. Fisetin also decreased the phosphorylation levels of Akt and extracellular signal-regulated kinase (Erk)1/2, but had no effect on the phosphorylation of p38 mitogen-activated protein kinase (MAPK). These results indicate that fisetin inhibits aggressive cell phenotypes by suppressing cell migration and proliferation via the Akt/Erk signaling pathway. Fisetin may thus have potential for use as a therapeutic strategy targeting reactive astrocytes, which may lead to the inhibition of glial scar formation in vitro.
Keywords: fisetin, astrocytes, migration, proliferation
Introduction
Astrocytes are the most abundant glial subtype in the central nervous system (CNS) and play important roles in interacting with neurons and communicating with other neural cell types (1–4). In spinal cord injury (SCI), astrocytes proximal to lesion zones become reactive. The process of reactive astrogliosis involves the migration and proliferation of astrocytes, contributing to glial scar formation, which is a major impediment to axonal regeneration. Overcoming this physical and biochemical barrier may be crucial for axonal regeneration and functional compensation during the progression of SCI (5,6). Although extensive investigations have been conducted on SCI (7), no effective therapy has yet been established. The lack of effective interventions is at least partially attributed to the complex process and the failure of axonal regeneration due to glial scaring (7). There is therefore a need to develop therapeutic strategies with which to prevent reactive astrogliosis.
Fisetin (3,3′,4′,7-tetrahydroxyflavone) is a dietary flavonoid found in fruits and vegetables, including strawberries, persimmons, grapes, cucumbers and onions (8). It has extensive physiological and pharmacological activities, including antioxidant, anti-inflammatory, anti-neoplastic and neuroprotective effects (8–10). It has also been shown that fisetin enhances behavioral performance and attenuates reactive gliosis and inflammation during aluminum chloride–induced neurotoxicity (9). Furthermore, fisetin suppresses oxidative and neuroinflammatory responses in microglial cells (10). However, the effects of fisetin on reactive astrocytes remain unclear. In the present study, we examined whether fisetin inhibits reactive astrogliosis in an in vitro scratch-wound model, and then characterized its underlying mechanisms of action.
Materials and methods
Materials and reagents
Fisetin (Fig. 1A) (molecular weight, 286.24; Sigma-Aldrich, St. Louis, MO, USA) was used at a purity of >98%. The molecular structure has been deposited in a publicly accessible database in PubChem with research number: CID 5281614. Stock solutions of fisetin were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored at −20°C.
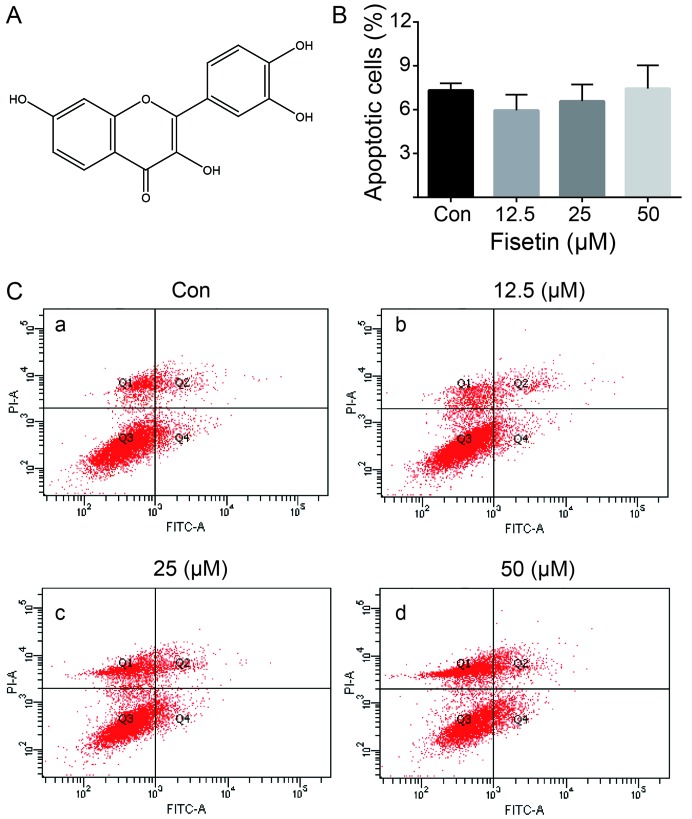
Figure 1.
Structure of fisetin and effects on cell migration and apoptosis in primary cultured astrocytes. (A) Chemical structure of fisetin. (B) Quantification of the astrocyte apoptotic rate following treatment with various concentrations of fisetin. (C) Apoptosis of astrocytes treated with or without fisetin for 24 h as detected by flow cytometry. Fisetin at concentrations ranging from 12.5–50 µM (panels b–d) did not significantly affect cell apoptosis following treatment for 24 h compared to the control group (panel a).
Cell culture
Primary astrocyte cultures were prepared from 1-day-old Sprague-Dawley rat brains (Animal Experiment Center, Southern Medical University, Guangzhou, China), as previously described (11,12). Ethics approval was granted by the Medical Ethics Committee of Southern Medical University, Guangzhou, China. Briefly, a total of 12 rats (n=3/group) were deeply anesthetized with a mixture of ketamine and xylazine (70:6 mg/kg, intramuscular injection) and then decapitated, the striatums were dissected, and the meninges were removed. The cerebral hemisphere was freed of the meninges and cut into 1 mm3 cubes in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies, Fresno, CA, USA). The tissue was dissociated by vortex mixing for 60 sec, and the cell suspension was passed through 70 and 20 µm sterile mesh nylon filters. A volume of cell suspension containing approximately 4.5×105 cells was seeded in a 35 mm2 Falcon tissue culture dish (Corning, Albany, NY, USA). Primary astrocytes were cultured in DMEM containing 10% fetal bovine serum (FBS) and 0.15% penicillin-streptomycin reagent (both from Gibco Life Technologies) at 37°C with 5% CO2. The medium was changed twice a week. Cultures of at least 4 weeks were used in the experiments.
Scratch-wound assay
The capacity of the astrocytes to migrate was examined by scratching confluent astrocyte monolayers to induce natural cell migration, as previously reported (12,13). Astrocyte monolayers were wounded by dragging a sterile 200-µl pipette tip across the surface. The detached cells and debris were removed immediately with 3 washes of phosphate-buffered saline (PBS). The cells were then maintained for an additional 24 h in culture medium with 1% FBS and various concentrations of fisetin (25 and 50 µM) or 0.1% DMSO as the control group. Images of the closing wound were acquired under an inverted microscope (Olympus TH4-200; Olympus, Tokyo, Japan) and analyzed using NIS Elements D3.2 (Nikon NT-88-V3; Nikon, Tokyo, Japan).
Cell apoptosis assay
The astrocytes were pre-treated with fisetin (12.5, 25 and 50 µM) or 0.1% DMSO as the control group for 24 h. The cells were then digested with 0.125% EDTA-free trypsin and collected by centrifugation at 2,000 rpm for 5 min. The cells were washed twice with PBS, resuspended in 500 µl binding buffer, and incubated in the dark for 10 min at room temperature with 5 µl of Annexin V-Fluor 488 and 5 µl of propidium iodide (PI) (product code: KGAV103; KeyGen, Nanjing, China). The samples were assessed by flow cytometry (BD Biosciences, Mountain View, CA, USA).
Click-iT 5-ethynyl-2′-deoxyuridine (EdU) test
The astrocytes were treated simultaneously with various concentrations of fisetin (12.5–50 µM) or 0.1% DMSO as the control group and EdU (10 µM), using the Cell-Light™ EdU Apollo 567 in vitro flow cytometry kit (product code: C10338-3; Guangzhou RiboBio Co., Ltd., Guangzhou, China) for 24 h. The cells were then stained according to the following protocol: the cells were collected by centrifugation at 1,500 rpm for 5 min, 4% paraformaldehyde was added at room temperature for 30 min to fix the cells, followed by washing with PBS, then the addition of 0.5% Triton X-100 for 15 min, 2 washes with PBS, and incubation with click reaction buffer (100 mM Tris-HCl, pH 8.5; 1 mM CuSO4; 100 µM Apollo 550 fluorescent azide; and 100 mM ascorbic acid) for 30 min in the dark, washing with 0.5% Triton X-100, and a final addition of 500 µl PBS for the flow cytometry analysis, within 1 h.
Cell cycle assay
The astrocytes were synchronized by starved for 12 h in serum-free medium and treated subsequently with different concentrations of fisetin (12.5–50 µM) or 0.1% DMSO as the control group for 24 h prior to analysis. Cell cycle analysis was performed by PI staining. The astrocytes were trypsinized and washed with ice-cold PBS (pH 7.4) and fixed in ice-cold 75% ethanol for >2 h. The cells were then washed with PBS, treated with 100 µl RNase A at 37°C for 30 min, and finally stained with 400 µl PI in the dark at 4°C for 30 min. Flow cytometric analysis was then carried out within 1 h. The analysis was performed using a Cell Cycle Detection Test kit (product code: KGA512; KeyGen).
Western blot analysis
Western blot analysis was conducted as previously described (6). The astrocytes were treated simultaneously with various concentrations of fisetin (12.5–50 µM) or 0.1% DMSO as the control group for 24 h prior to analysis. The cells were then washed with ice-cold PBS, and total proteins were extracted using the radioimmunoprecipitation assay (RIPA; Sigma-Aldrich) buffer supplemented with phosphatase inhibitors. The concentration of proteins was determined by the bicinchoninic acid (BCA) method with bovine serum albumin (BSA) as a standard using the protein quantification kit (product code, 51254; Sigma-Aldrich). A total of 30 µg of protein from each sample was analyzed by SDS/PAGE and transferred by electrophoresis onto polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Billerica, MA, USA). The membranes were blocked for 1 h at room temperature by using 5% (w/v) BSA Tris-buffered saline with Tween-20 (TBST) buffer (20 mM Tris pH 7.5 and 150 mM NaCl). The membranes were then incubated with primary antibodies to the following: phosphorylated (p-)focal adhesion kinase (FAK; Tyr 576/577; product code: 3281; diluted 1:1,000), FAK (D2R2E; product code: 13009; diluted 1:1,000), p-paxillin (Tyr118; product code: 2541; diluted 1:1,000), paxillin (product code: 12065; diluted 1:1000), cyclin D1 (product code: 2922; diluted 1:1,000), p-Akt (s473; product code: 4060; diluted 1:2,000), Akt (pan; product code: 4691; diluted 1:1,000), p-p44/42 mitogen-activated protein kinase [MAPK; extracellular signal-regulated kinase (Erk1/2); Thr202/Tyr204; product code: 4370, diluted 1:2,000), p44/42 MAPK (Erk1/2; 137F5; product code: 4695, diluted 1:1,000), p-p38 MAPK (Thr180/Tyr182; D3F9; product code: 4511, diluted 1:1,000), p38 MAPK (D13E1; product code: 8690, diluted 1:1,000) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; product code: 5174, diluted 1:1,000. All antibodies were from Cell Signaling Technology, Inc., (Beverly, MA, USA), and were used at 4°C overnight. The blots were then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (product code: 7071; all diluted 1:4,500; Cell Signaling Technology, Inc.) for 1 h at room temperature. Finally, the signals were developed using the enhanced chemiluminescence (ECL) kit (Millipore Corp.), and analyzed using Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein levels were expressed as gray values (GVs) relative to the background controls.
Quantitative analysis
The evaluation of the western blot analysis data was carried out using the following steps: i) The GV of the western blot bandings was examined using Image-Pro Plus (IPP) software in the control and experimental group; ii) in each group, the GV of the protein of interest was divided by the GV of the internal reference; iii) the results of the experimental group from 'step ii' were divided by the results of the control group from 'step ii'; and iv) the final results were presented as the 'fold change'.
Statistical analysis
Data are representative of at least 3 independent experiments. Each experiment was carried out in triplicate. Data were plotted using GraphPad 'PRISM' soft ware (version 3.0; GraphPad Software, Inc., La Jolla, CA, USA). The significance of the differences was evaluated by one-way ANOVA followed by the Student-Newman-Keuls post hoc test. Error bars represent the standard error of the mean (SEM) and values of P<0.05 and P<0.01 were considered to indicate statistically significant differences.
Results
Effect of fisetin on astrocyte apoptosis
As shown in Fig. 1B and C, we examined the effect of fisetin on astrocyte cell apoptosis using flow cytometry analysis. Fisetin, at concentrations ranging from 12.5–50 µM, did not significantly affect cell apoptosis following treatment for 24 h.
Fisetin suppresses astrocyte migration
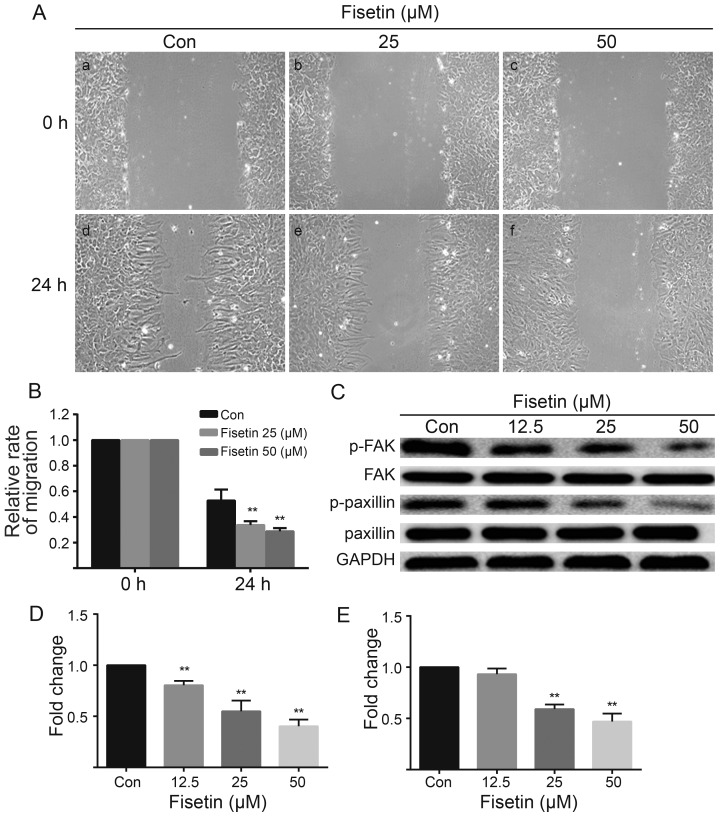
The effect of fisetin on astrocyte migration was determined using an in vitro scratch-wound assay, in which the loss of cell contact induces local chemokine release, which in turn stimulates the cells to close the scratch (12,13). Our results revealed that fisetin significantly decreased the ability of astrocytes to migrate into the empty space (Fig. 2A and B). In the control astrocytes, the wound gap was reduced at 24 h (Fig. 2A, panel d), while the gap was larger at 24 h in the fisetin-treated astrocytes (Fig. 2A, panels e and f). Examination of the leading edge of the migrating cells revealed multiple protrusions and extensions in the control astrocytes, but a relatively uniform flat edge in the astrocytes in the fisetin group. To gain further insight into the effects of fisetin on the migration of primary cultured astrocytes, we examined the expression of p-FAK and p-paxillin in vitro. The results of western blot analysis revealed that the expression levels of both p-FAK (Fig. 2C and D) and p-paxillin (Fig. 2C and E) were decreased in the fisetin-treated astrocytes. Taken together, these results indicate that fisetin suppresses astrocyte migration.
Figure 2.
Fisetin suppresses astrocyte migration. (A) Migration profiles showing the inhibition of scratch wound closure by fisetin. (A and B) Fisetin significantly reduced the ability of astrocytes to migrate into the empty space. Control astrocytes reduced the gap at 24 h (panel d), while the gap was larger at 24 h in fisetin-treated astrocytes (panels e and f). (B) Quantification of migration rates as a percentage of cell free width. (C) Western blot analysis results showing that both (C and D) p-FAK and (C and E) p-paxillin expression decreased. (B,D and E) The values are shown as the means ± SEM of data from independent experiments. **P<0.01 vs. control.
Fisetin inhibits astrocyte proliferation and cell cycle progression
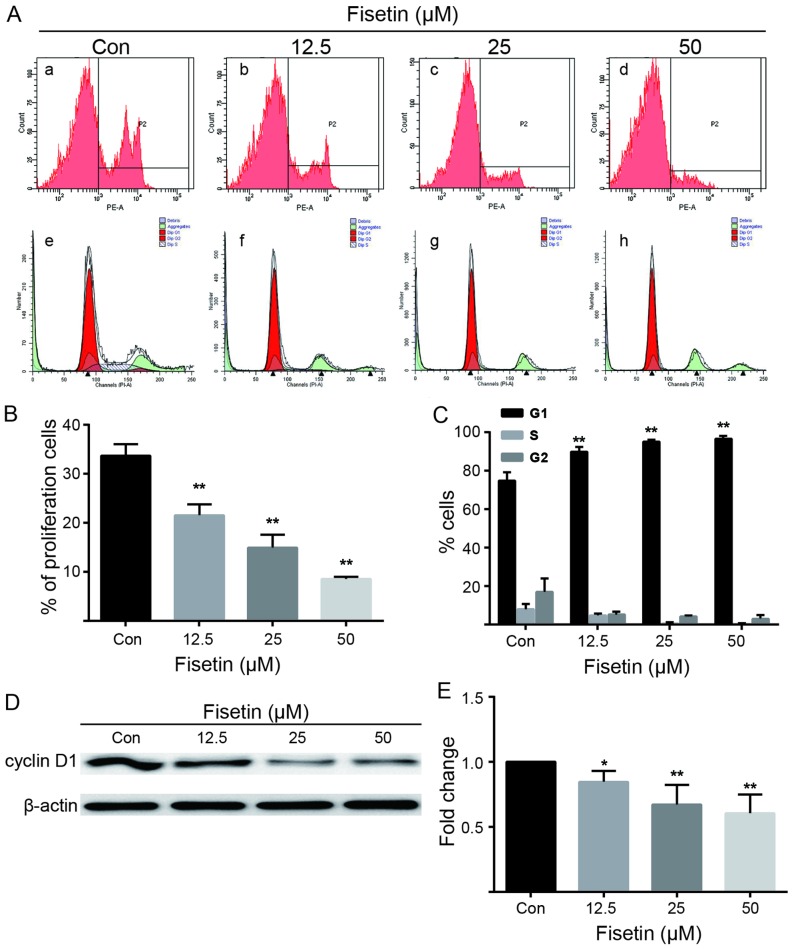
Astrocyte proliferation was detected using EdU as a marker of dividing cells (Fig. 3A). The proportion of EdU(+) cells was markedly reduced in the fisetin-treated group (Fig. 3A, panels b–d) compared with the control group (Fig. 3A, panel a), in a dose-dependent manner (Fig. 3A and B). Similarly, fisetin increased the percentage of cells in the G1/G0 phase and decreased the percentage of cells in the G2 and S phase (Fig. 3A, panels f–h) compared with the control group (Fig. 3A, panel e), as demonstrated by flow cytometry, and also in a dose-dependent manner (Fig. 3A and C). Accordingly, the results of western blot analysis revealed that fisetin downregulated cyclin D1 protein expression (Fig. 3D and E). Taken together, these data indicated that treatment with fisetin inhibited astrocyte proliferation and arrested the cell cycle at the G1 phase.
Figure 3.
Fisetin inhibits astrocyte proliferation and arrests the cell cycle at the G1 phase in a dose-dependent manner. (A) Astrocyte proliferation was measured using the Click-iT EdU test by flow cytometry. (A and B) The proportion of EdU(+) cells was markedly reduced in the fisetin-treated group (panels b–d) compared with the control group (panel a), in a dose-dependent manner. P2 is the percentage of EdU(+) cells. Fisetin increased the ratio cells in the G1 phase and reduced the ratio of cells in the G2 and S phase (panels e–h). (B) Quantification of astrocyte proliferation ratio with various concentrations of fisetin. (C) Quantification of cell cycle phases in astrocytes treated with various concentrations of fisetin. (D and E) Cyclin D1 expression was downregulated by fisetin in a dose-dependent manner. (B,C and E) The values are shown as the means ± SEM of data from independent experiments. *P<0.05, **P<0.01 vs. control.
Fisetin suppresses the Akt/Erk signaling pathway
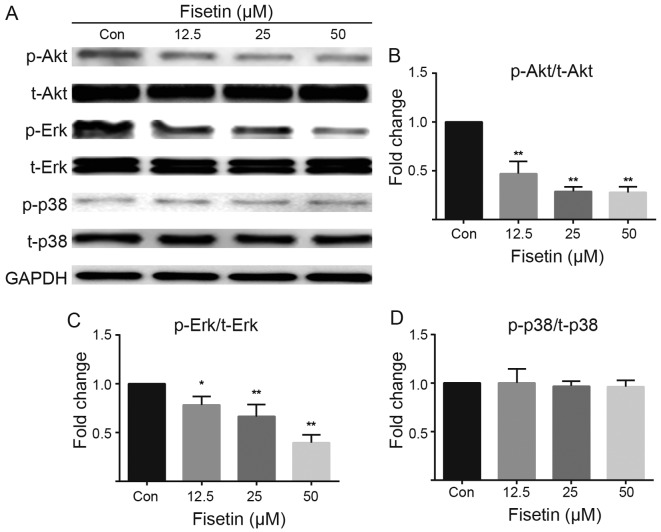
Previous studies have demonstrated that fisetin regulates the phosphoinositide 3-kinase (PI3K)/Akt and Erk signaling pathways (14–16). It has also been demonstrated that astrocyte migration is modulated by Rho GTPase/p21-activated kinase (PAK)/Erk-dependent signaling (17). In the present study, the results of western blot analysis revealed that fisetin downregulated the phosphorylation of Akt (Fig. 4A and B) and Erk (Fig. 4A and C) in a dose-dependent manner, but had no effect on total levels of Akt and Erk. Furthermore, fisetin had no effect on the expression of p38 MAPK or p38 phosphorylation levels compared with the control group (Fig. 4A and D).
Figure 4.
Effect of fisetin on phosphorylated (p-)Akt and total Akt, p-Erk1/2 and total Erk1/2, and p-p38 and total p38 assessed by western blot analysis. (A) Fisetin reduced Akt and Erk1/2 phosphorylation in a dose-dependent manner post-fisetin treatment, but had no effect on p38 phosphorylation. (B,C and D) The values of the quantification of Akt, Erk1/2, and p38 phosphorylation levels are shown as the means ± SEM of data from independent experiments. *P<0.05, **P<0.01 by one-way ANOVA followed by the Student-Newman-Keuls post hoc test.
Discussion
Astrocytes are ubiquitous glia with finely branching processes that envelope all the cellular components throughout the CNS (5). Under normal physiological conditions, astrocytes provide essential support for neuronal activities, such as maintaining the homeostasis of the extracellular ionic environment and pH, the clearance and release of extracellular glutamate (18), and provisions for metabolic substrates for neurons (19). SCI is a common and devastating CNS insult that leads to the destruction of the cord microenvironment, followed by limited neuronal regeneration and poor functional recovery in adult mammals. Following severe SCI, and in response to changes in the local microenvironment, astrocytes, the most abundant glial cells in the CNS, transform into reactive astrocytes (20). Reactive astrogliosis involves cell division, migration, hypertrophy, proliferation and changes in gene expression that contribute to glial scar formation. Compelling evidence suggests that reactive astrogliosis has beneficial effects, including restricting the spread of inflammation, protecting nerves from secondary injuries, and restoring tissue integrity in the acute phase of CNS insults (21,22). However, studies have also demonstrated the detrimental effects of reactive astrogliosis in terms of interfering with the function of residual neuronal circuits by preventing axonal remyelination and inhibiting axonal regeneration (23,24), thus blocking functional recovery.
Fisetin is a flavonoid and an important natural product derived from many fruits and herbal sources. It has low cytotoxicity and has antitumor effects in various cancer cells, in addition to inducing cell cycle arrest (25). Although previous studies have revealed that fisetin exerts both antioxidant and anti-inflammatory effects in astrocytes (9,10,26), its other activities in astrocytes are unclear.
Astrocyte migration and proliferation in SCI is a fundamental component of glial scarring. In the present study, we demonstrated that fisetin inhibited astrocyte migration in an in vitro scratch-wound model and decreased FAK and paxillin phosphorylation levels in a dose-dependent manner. FAK is a widely expressed cytoplasmic protein tyrosine kinase involved in integrin-mediated signal transduction. It plays an important role in the control of several biological processes, including cell spreading, migration and survival (27). FAK serves as a unique regulator of focal adhesion (FA) assembly and disassembly, processes that are fundamental for efficient directional cell movement (28,29). FAK is a leading edge organizer. Nascent FAs are formed at the cell periphery by integrin and extracellular matrix (ECM) interactions. Paxillin is another important cytoskeletal and scaffolding protein recruited early to nascent FAs at cell fronts, and is necessary for FA turnover (e.g., adhesion disassembly at cell fronts) during cell migration (29,30). Our results indicated that fisetin inhibited healing in the scratch wound model of primary astrocytes and decreased the phosphorylation levels of FAK and paxillin, thus indicating that is plays an essential role in the migratory ability of astrocytes. Furthermore, emerging data indicate that fisetin possesses potent anti-proliferative activity against various cancer cells (25,31,32). Another study reported that fisetin perturbed spindle checkpoint signaling, which may contribute to the anti-proliferative effects of the compound (33). In a previous study, using a cell-based high throughput screen for small molecules that can override chemically-induced mitotic arrest, fisetin was identified as an anti-mitotic compound. It was demonstrated that fisetin rapidly compromised the microtubule drug-induced mitotic block in a proteasome-dependent manner in several human cell lines (33). In this study, we found that fisetin suppressed astrocyte proliferation by inhibiting DNA synthesis, which was shown by EdU, and induced cell cycle arrest in the G1 phase. We also examined the cell cycle-associated protein cyclin D1 by western blot analysis. Cyclin D1 is expressed in the G1 phase and binds to and activates cyclin-dependent kinase (CDK)4 and 6, followed by the activation of the cyclin E-dependent kinase, CDK2.
The MAPK and Akt signaling pathways are crucial for cellular responses including proliferation, differentiation, survival and apoptosis. Accumulating evidence suggests an important role for the MAPK and PI3K/Akt pathways in promoting these biological functions; however, the findings are complex and controversial, and depend on the specific characteristics of the cell type (34,35). A recent study found that the Erk1/2 signaling pathway modulated the cytoskeleton dynamics of astrocytes in a scratch-wound model (17). Fisetin has been shown to play an important role in regulating the PI3K/Akt and Erk pathways (14,16,36). In the present study, fisetin downregulated the Akt and Erk phosphorylation levels in astrocytes in a dose-dependent manner, but had no effect on p38 expression and phosphorylation. The p38 protein has been implicated as a key regulator of apoptosis (37), and these results were consistent with the apparent lack of effect of fisetin on apoptosis.
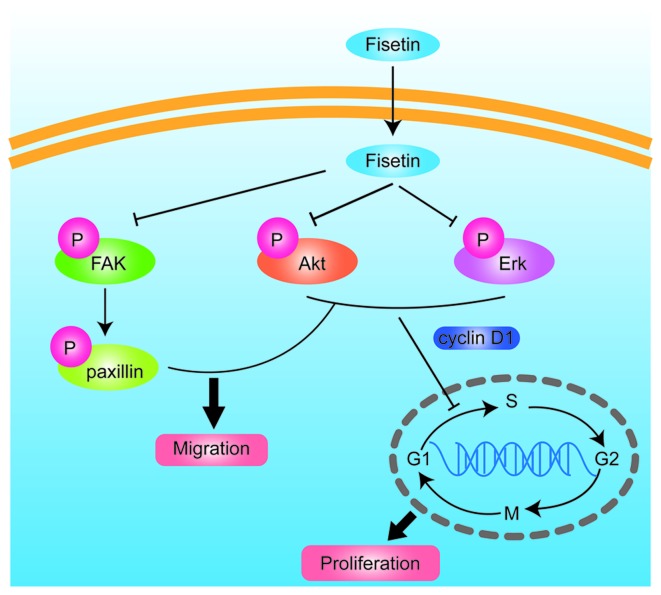
In conclusion, the findings of the present study indicate that fisetin inhibits astrocyte migration, as shown by a scratch-wound model and induced cell cycle arrest in the G1 phase. Fisetin inhibited astrocyte proliferation via the PI3K/Akt- and Erk-dependent signaling pathways, and further decreased glial scar formation. The mechanisms involved ares shown in Fig. 5. Fisetin may therefore have potential for use in the treatment of glial scar formation in SCI.
Figure 5.
Schematic representation of the possible cellular targets of fisetin action in astrocytes after the scratch wound injury.
Acknowledgments
The present study was supported by the Presidential Foundation of Nan Fang Hospital, Southern Medical University, China (grant no. 2013C003)
References
- 1.Yang Q, Wang EY, Huang XJ, Qu WS, Zhang L, Xu JZ, Wang W, Tian DS. Blocking epidermal growth factor receptor attenuates reactive astrogliosis through inhibiting cell cycle progression and protects against ischemic brain injury in rats. J Neurochem. 2011;119:644–653. doi: 10.1111/j.1471-4159.2011.07446.x. [DOI] [PubMed] [Google Scholar]
- 2.Binder DK, Steinhäuser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 3.Fuller S, Münch G, Steele M. Activated astrocytes: a therapeutic target in Alzheimer's disease? Expert Rev Neurother. 2009;9:1585–1594. doi: 10.1586/ern.09.111. [DOI] [PubMed] [Google Scholar]
- 4.Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 6.Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem Int. 2015;83–84:9–18. doi: 10.1016/j.neuint.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wang YF, Zu JN, Li J, Chen C, Xi CY, Yan JL. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett. 2014;560:51–56. doi: 10.1016/j.neulet.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Adhami VM, Syed DN, Khan N, Mukhtar H. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol. 2012;84:1277–1281. doi: 10.1016/j.bcp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash D, Gopinath K, Sudhandiran G. Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. Neuromolecular Med. 2013;15:192–208. doi: 10.1007/s12017-012-8210-1. [DOI] [PubMed] [Google Scholar]
- 10.Chuang JY, Chang PC, Shen YC, Lin C, Tsai CF, Chen JH, Yeh WL, Wu LH, Lin HY, Liu YS, Lu DY. Regulatory effects of fisetin on microglial activation. Molecules. 2014;19:8820–8839. doi: 10.3390/molecules19078820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu BY, Yu AC. Quercetin inhibits c-fos, heat shock protein, and glial fibrillary acidic protein expression in injured astrocytes. J Neurosci Res. 2000;62:730–736. doi: 10.1002/1097-4547(20001201)62:5<730::AID-JNR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Z, Yao F, Hu Z, Sun S, Wu B. Quercetin inhibits the migration and proliferation of astrocytes in wound healing. Neuroreport. 2015;26:387–393. doi: 10.1097/WNR.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 13.Cory G. Scratch-wound assay. Methods Mol Biol. 2011;769:25–30. doi: 10.1007/978-1-61779-207-6_2. [DOI] [PubMed] [Google Scholar]
- 14.Chen PY, Ho YR, Wu MJ, Huang SP, Chen PK, Tai MH, Ho CT, Yen JH. Cytoprotective effects of fisetin against hypoxia-induced cell death in PC12 cells. Food Funct. 2015;6:287–296. doi: 10.1039/C4FO00948G. [DOI] [PubMed] [Google Scholar]
- 15.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang HM, Chan SY, Chu Y, Wen KC. Fisetin ameliorated photodamage by suppressing the mitogen-activated protein kinase/matrix metalloproteinase pathway and nuclear factor-κB pathways. J Agric Food Chem. 2015;63:4551–4560. doi: 10.1021/jf502500t. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein MP, Carriba P, Baltrons MA, Wojciak-Stothard B, Peterson JR, García A, Galea E. Secretase-independent and RhoGTPase/PAK/ERK-dependent regulation of cytoskeleton dynamics in astrocytes by NSAIDs and derivatives. J Alzheimers Dis. 2010;22:1135–1155. doi: 10.3233/JAD-2010-101332. [DOI] [PubMed] [Google Scholar]
- 18.Mazzanti M, Sul JY, Haydon PG. Glutamate on demand: astrocytes as a ready source. Neuroscientist. 2001;7:396–405. doi: 10.1177/107385840100700509. [DOI] [PubMed] [Google Scholar]
- 19.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- 20.Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–435. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 22.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 23.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/S0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen CM, Hsieh YH, Hwang JM, Jan HJ, Hsieh SC, Lin SH, Lai CY. Fisetin suppresses ADAM9 expression and inhibits invasion of glioma cancer cells through increased phosphorylation of ERK1/2. Tumour Biol. 2014;36:3407–3415. doi: 10.1007/s13277-014-2975-9. [DOI] [PubMed] [Google Scholar]
- 26.Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, Maher P. Modulation of P25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer's disease transgenic mice. Aging Cell. 2014;13:379–390. doi: 10.1111/acel.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of P60s rc. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/MCB.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep. 2014;4:6024. doi: 10.1038/srep06024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed DN, Suh Y, Afaq F, Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19:151–162. doi: 10.1089/ars.2012.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmela AL, Pouwels J, Varis A, Kukkonen AM, Toivonen P, Halonen PK, Perälä M, Kallioniemi O, Gorbsky GJ, Kallio MJ. Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint. Carcinogenesis. 2009;30:1032–1040. doi: 10.1093/carcin/bgp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 36.Barbakadze T, Natsvlishvili N, Mikeladze D. Thyroid hormones differentially regulate phosphorylation of ERK and Akt via integrin αvβ3 receptor in undifferentiated and differentiated PC-12 cells. Cell Biochem Funct. 2014;32:282–286. doi: 10.1002/cbf.3013. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zhang S, Su D, Liu J, Cheng Y, Zou L, Li W, Jiang Y. Inhibiting (pro)renin receptor-mediated P38 MAPK signaling decreases hypoxia/reoxygenation-induced apoptosis in H9c2 cells. Mol Cell Biochem. 2015;403:267–276. doi: 10.1007/s11010-015-2356-8. [DOI] [PubMed] [Google Scholar]