Abstract
WNK1 belongs to a unique family of Ser/Thr kinases that have been implicated in the control of blood pressure. Intronic deletions in the WNK1 gene result in its overexpression and lead to pseudohypoaldosteronism type II, a disease with salt-sensitive hypertension and hyperkalemia. How overexpression of WNK1 leads to Na+ retention and hypertension is not entirely clear. Similarly, there is no information on the hormonal regulation of expression of WNK kinases. There are two main WNK1 transcripts expressed in the kidney: the originally described “long” WNK1 and a shorter transcript that is specifically expressed in the kidney (KS-WNK1). The goal of this study was to determine the effect of aldosterone, the main hormonal regulator of Na+ homeostasis, on the transcription of WNK1 isoforms in renal target cells, by using an unique mouse cortical collecting duct cell line that stably expresses functional mineralocorticoid receptors. Our results demonstrate that aldosterone, at physiological concentrations, rapidly induces the expression of the KS-WNK1 but not that of the long-WNK1 in these cells. Importantly, stable overexpression of KS-WNK1 significantly increases transepithelial Na+ transport in cortical collecting duct cells. Similarly, coexpression of KS-WNK1 and the epithelial Na+ channel in Fischer rat thyroid epithelial cells also stimulates Na+ current, suggesting that KS-WNK1 affects the subcellular location or activity but not the expression of epithelial Na+ channel. These observations suggest that stimulation of KS-WNK1 expression might be an important element of aldosterone-induced Na+ retention and hypertension.
Keywords: hypertension, collecting duct
High blood pressure affects 20–25% of the adult population in industrialized countries; yet the etiology of the majority of cases is poorly understood. In recent years, however, the molecular mechanism of several monogenic forms of hypertension showing Mendelian inheritance has been identified. Interestingly, the mutations in these forms of hypertension converge on a common pathway, i.e., the aldosterone-regulated renal sodium reabsorption. For instance, Liddle's syndrome, a disease mimicking hyperaldosteronism, is caused by gain-of-function mutations in the epithelial sodium channel (ENaC), which leads to abnormally high renal Na+ reabsorption, low-renin hypertension, and hypokalemic alkalosis (1). In contrast, the features of another autosomal dominant form of familial hypertension, pseudohypoaldosteronism type II (PHAII) or Gordon's syndrome are more complex than Liddle's syndrome. In PHAII, the hypertension is associated with chloride-dependent sodium retention, hyperkalemia, and metabolic acidosis despite normal glomerular filtration rate (2). PHAII patients are markedly sensitive to thiazide diuretics (3). Despite the fact that these features indicate an overactive thiazide-sensitive Na-Cl cotransporter (NCC), no mutations in this transporter have been found in affected patients.
WNKs (with no lysine [K]) are Ser/Thr kinases that lack a key lysine in the catalytic domain that is well conserved in other protein kinases (4). Recently, mutations in WNK1 and WNK4 genes were identified as the cause of PHAII. Both WNK1 and WNK4 are highly expressed in the renal distal convoluted tubule, connecting tubule, and cortical collecting duct (CCD) (5). Oocyte expression studies demonstrated that WT WNK4 inhibits the surface expression and thereby the activity of the NCC, and one of the four missense mutations in WNK4 that cause human hypertension attenuates this inhibitory effect (6, 7). This action could explain, at least in part, the increased salt retention and resulting hypertension in PHAII (3). The observations that WNK4 is strongly expressed in nephron segments other than the distal convoluted tubule (the exclusive site of NCC expression) and in extrarenal tissues (5, 8), suggest that the NCC is not the sole target of WNK4 action. Indeed, recent studies by Lifton and coworkers (9) demonstrated that when coexpressed in Xenopus oocytes, WNK4 inhibits the surface expression of several renal transporters including the secretory K+ channel, ROMK, and the Cl/base exchanger SLC26A6 (8). These effects might contribute to the hyperkalemia and acidosis in PHAII. In addition, Madin–Darby canine kidney (MDCK) cells overexpressing a disease-causing mutant WNK4 have increased paracellular Cl permeability (10). These studies strongly implicate WNK4 as a multifunctional regulator of Na, Cl, and K transport.
Much less is known about the function and regulation of WNK1. Unlike the missense mutations in WNK4 that affect the coding region, hypertension-causing mutations in WNK1 are large intronic deletions that result in increased WNK1 expression, suggesting that in contrast to WNK4, the WT WNK1 stimulates Na+ retention. Consistent with an important role in regulating blood pressure, recently it was shown that WNK1-deficient mice display a significant decrease in blood pressure (11).
The mechanism by which increased WNK1 expression leads to hypertension is unknown. In oocytes, WNK1 has no direct effect on NCC activity, but it attenuates the inhibitory effect of WNK4 (7), suggesting that gain-of-function mutations in WNK1, by opposing the inhibitory effect of WNK4 on NCC, could contribute to Na+ retention. However, because WNK1 is widely expressed (5, 12), it is quite possible that it has additional effects on Na+ and Cl transport besides affecting NCC. For example, its expression in the collecting duct raises the possibility that it could regulate ENaC. Whether WNK1 also opposes the inhibitory action of WNK4 on other transporters such as ROMK and SLC26A6 is unknown.
Both in human and in mouse, multiple WNK1 mRNA species are expressed that arise by alternative promoter usage and splicing (13, 14). Most intriguing is the observation that the expression pattern of the different transcripts is tissue- and developmental stage-specific. In the kidney, in addition to the originally described “long” WNK1 (L-WNK1) (4), the most prominently expressed WNK1 is a shorter transcript (13, 14). Surprisingly, this kidney-specific WNK1 (KS-WNK1) is initiated at an alternative promoter and lacks the first four exons that include the kinase domain of WNK1 (13).
Although the central role of aldosterone in the control of renal Na+ transport has been known for decades, the exact molecular steps of this regulation are just beginning to be uncovered. Because both gain-of-function mutations in the aldosterone pathway as well as increased WNK1 expression (in PHAII) lead to hypertension, in this study we tested the effect of aldosterone on the expression of the two WNK1 isoforms in the main mineralocorticoid target cells, i.e., renal collecting duct cells. We report here that aldosterone rapidly induces the expression of the KS-WNK1 in an unique mouse CCD cell line that expresses functional mineralocorticoid receptors (MRs). Furthermore, we show that ectopic expression of KS-WNK1 in mammalian epithelial cells results in a significant increase of ENaC-associated Na+ current.
Methods
Generation of M1 Cell Lines Stably Expressing MRs or the KS-WNK1. The rat MR was subcloned into the retroviral vector pLNCX, and retroviruses were generated by transient transfection into amphotropic Phoenix cells by using Lipofectamin PLUS reagent (Invitrogen). M1 cells were cultured and infected with retroviruses as described (15), and clones stably expressing MR were selected with G418 (500 μg/ml). These cell lines will be referred to as M1-MR+ cells. Individual clones were expanded and tested for 3H-aldosterone binding. To determine a number of MR/cell, parent M1 cells, and M1-MR+ clones were incubated at a saturating concentration (4 nM; determined in pilot experiments) of [1,2-3H]aldosterone [Amersham Pharmacia; specific activity 36 Ci/mmol in the presence (nonspecific binding) or absence (total binding) of a 1,000-fold excess of unlabeled aldosterone].
Because a full-length KS-WNK1 clone is not available, to generate cell lines stably expressing the KS-WNK1, first we made a chimeric construct encoding exon 4A of the human KS-WNK1 (13) fused to the rat WNK1 (4) at the PacI site in exon 5 (distal from exon 4, the sequences of the KS-WNK1 and L-WNK1 are identical). We used an EST clone that contains the full exon 4A of human KS-WNK1 (GenBank accession no. AI768512) and PCR-amplified a 650-nt fragment by using KS-WNK1-specific primer located in the 5′-UTR region (5′-CTG TTT AAG CTT CCA CCA TGG ATA TTA AAA AGA AAG ATT TTT GC-3′), which generates a HindIII site and a vector primer (5′-GGG TTT TCC CAG TCA CGA CG-3′). The 650-bp product was digested with PacI and HindIII and ligated into rat WNK1 (a gift of Melanie Cobb, University of Texas Southwestern Medical Center, Dallas), which was previously subcloned into a modified retroviral vector. M1 cells were infected with retroviruses and clones stably expressing KS-WNK1 selected based on G418 resistance. In addition to individual clones, we generated three lines of pooled clones, each one containing >500 individual clones. The expression of the KS-WNK1 was verified by RT-PCR by using primers specific for the 3′ end.
M1-MR+ Cell Culture and Electrophysiology. Parent M1 cells and M1-MR+ clones were seeded onto Millicell permeable membranes (Millipore) at a density of 4 × 105 cells per filter. After seeding, cells were bathed in complete PC1 medium (BioWhit-taker, Walkersville, MD) with 5% FBS until they formed confluent monolayers (≈3–4 days). Transepithelial voltage (VTE) and resistance (RTE) values were determined with an epithelial voltohmmeter (World Precision Instruments, Sara-sota, FL), and equivalent short-circuit current was (Isc) calculated. We previously demonstrated that, in M1 cells, Isc mainly represents transepithelial sodium current (15). This was verified by treating cells with 1 μM amiloride from the apical side, which resulted in an almost total elimination of the lumen negative VTE and associated Isc.
To determine the effects of aldosterone, after reaching confluence, cells were maintained in steroid-free medium (DMEM/F12/5% FBS stripped twice with charcoal/15 mM Hepes/2mM glutamine/antibiotics) for 2 days, then treated with 0.1–10 nM aldosterone for different periods. The receptor specificity of aldosterone was determined by testing its effects in the presence of 1 μM ZK91587, a specific MR antagonist or 1 μM 486, a glucocorticoid receptor (GR)-antagonist.
The effect of protein synthesis inhibitors was tested by pre-incubating M1-MR+ cells for 30 min with vehicle, 5 μg/ml cycloheximide (CHX), or 10 μM anisomycin. The phosphatidylinositol 3-kinase (PI3-kinase) inhibitor LY294002 was added at 50 μM with or without 1 nM aldosterone, and cells were incubated for an additional 4 h before RNA was prepared.
Semiquantitative RT-PCR. To determine the relative abundance of the KS-WNK1 and the L-WNK1 mRNAs in control and corticosteroid-treated M1 and M1-MR+ cells, we used semiquantitative RT-PCR as described earlier (16–18). Cells were maintained in steroid-free medium for 48 h, treated with 1 nM aldosterone or 5 nM dexamethasone for 30 min to 24 h, and then lysed in TRI-reagent. Total RNA was prepared, and cDNA was synthesized as described (16–18). Sense (5′-CCG AAA CCA CTG TGG AAG TCG C-3′) and antisense (5′-ATC TAC CGT CGA GTG ACC AGT G-3′) PCR primers for the mouse L-WNK1 and sense (5′-TTG CCT TTT CTG ATG GAT T-3′) and antisense (5′-TCC TTT TGA GAA CAG CAG C-3′) primers for the mouse KS-WNK1 were selected based on the published sequences (14). PCR reactions were performed with four different amounts (20, 5, 1.25, and 0.3125 ng) of cDNA originating from control or steroid-treated cells. After a 2-min denaturation at 96°C, PCR for the L-WNK1 was carried out for 27 cycles (96°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min). For the KS-WNK1 PCR, we used touchdown conditions: five cycles each at annealing temperature of 59°C, 55°C, and 52°C, and then 15 cycles at annealing temperature of 49°C. The identity of the PCR products was verified by sequencing. The relative abundance of β-actin mRNA in each sample was determined by using primers and conditions as described (16–18). cDNA samples derived from control and steroid-treated cells were always amplified simultaneously. The PCR products were separated on a 5% polyacrylamide gel, stained in ethidium bromide, and quantitated with a FluorImager 575 (Molecular Dynamics). The slope of the amount of PCR products vs. amount of template cDNA was derived by linear regression. These values were normalized for the amount of β-actin mRNA. SGK1 mRNA levels were determined by RT-PCR as in ref. 18.
Expression of ENaC and KS-WNK in Fischer Rat Thyroid (FRT) Cells. FRT epithelia were cultured on permeable filter supports (Millicell PCF, 0.4-μm pore size, 12-mm diameter) in F-12 Coon's medium (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin, as described (19). One day after seeding, the cells were transfected with human α, β, and γ ENaC (WT or αY644A, βY620A, γY627A) (in pMT3, 0.07 μg of each) along with KS-WNK1 (0–0.8 μg). Total cDNA was held constant by using GFP cDNA. Amiloride-sensitive short-circuit current was measured 2 days after transfection in modified Ussing chambers (Warner Instruments, Hamden, CT). The apical and basolateral membranes were bathed in 135 mM NaCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 2.4 mM K2HPO4, 0.6 mM KH2PO4, and 10 mM Hepes (pH 7.4) at 37°C. Amiloride-sensitive current was determined by the addition of 100 μM amiloride to the apical bathing solution.
Results
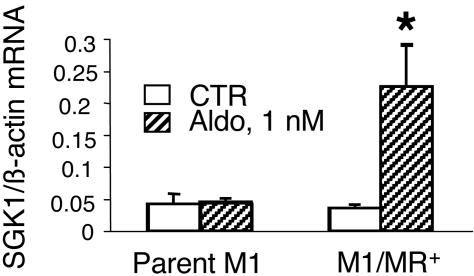
Biochemical and Functional Characterization of the M1-MR+ Cell Lines. M1 cells that originate from mouse CCD (20), like most other cultured CCD cell lines (21–26), do not express functional MRs. Therefore, to study the physiological effects of aldosterone mediated through MRs and not the ubiquitously expressed GR, we first generated M1 cells stably expressing MRs. We isolated several clonal M1 cell lines expressing 16,000–70,000 high-affinity (Kd in the nanomolar range) MRs/cell (the parent M1 cells did not have measurable MR binding). It should be noted that the reported number of MRs/cell in vivo is ≈10,000 (27, 28). Thus, M1/MR+ cells are moderately overexpressing MR, compared with physiological aldosterone target cells. For further studies, we selected four clonal cell lines with different MR numbers. All four M1-MR+ cell lines, but not the parent M1 cells, responded to physiological concentrations of aldosterone (1 nM) with a 2- to 4-fold increase in the expression of SGK1, an early aldosterone-induced gene in the CCD (18, 29). Data for parent M1 cells and M1-KS-WNK1 clonal line 2 are shown in Fig. 1. These data also indicate that the effect is not mediated through GRs that are expressed in parent M1 cells. The effect of aldosterone on SGK1 gene expression was very similar in each cell line tested, and there was no obvious correlation between the magnitude of the effect and the number of MRs per cell (data not shown).
Fig. 1.
Effect of aldosterone on SGK1 mRNA expression in parent M1 cells and in cell lines stably expressing MR. Aldosterone (1 nM) significantly induces SGK1 expression in M1-MR+ clone 2 (P < 0.05 vs. control, one-tailed unpaired t test) but not in parent M1 cells (n = 3). Cells were grown for 48 h in steroid-free medium, and then medium was changed to the same (control) or supplemented with 1 nM aldosterone, for 4 h.
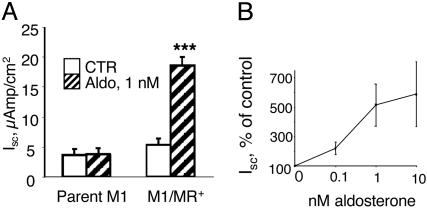
To verify that the stably expressed MRs are fully functional, we tested the effect of aldosterone on transepithelial Na+ transport (Isc). Aldosterone, at physiological concentrations, significantly increased Isc in a time-dependent fashion in all cell lines. Data for clonal cell line 1 are shown in Fig. 2. The increase in Isc was completely blocked by the apical addition of 1 μM amiloride (data not shown), indicating that it was due to Na+ currents through ENaC. Aldosterone stimulation of Isc was observed after 4 h, and this effect was maintained for the duration of the experiment (48 h). The effect was near maximal at 1 nM of aldosterone (Fig. 2B) and was completely prevented by the MR-antagonist ZK 91857 (100 nM) but not by the GR-antagonist, RU 486 (data not shown), indicating that this effect is specifically mediated via the MR.
Fig. 2.
Aldosterone increases transepithelial Na current (Isc) in M1-MR+ cells. (A) M1-MR+ clone 1 cells (n = 11) and parent M1 cells (n = 4) were grown on Millicell filters, and Isc values were determined after 24-h incubation without (CTR, empty bars) or with 1 nM aldosterone (striped bars). ***, P < 0.001 vs. control cells (one-tailed t test). Values are means ± SE. (B) Concentration dependence of the effect of aldosterone. Values were obtained after 24 h. P < 0.05 for each concentration vs. control (no aldosterone) by using one-tailed unpaired t test.
Aldosterone Increases the Expression of the KS-WNK1 Isoform. There are two main WNK1 transcripts expressed in both the human and mouse kidney. The larger (≈10-kb) transcript has a wider distribution and is expressed at lower levels (13, 14). The shorter and more abundant (≈8.5-kb) transcript KS-WNK1 is specifically expressed in the distal portion of the nephron (14).
Remarkably, KS-WNK1 lacks the N-terminal region of WNK1 that includes the kinase domain. First, we examined the expression of these two WNK1 transcripts in M1-MR+ cells by using primer pairs specific for the two transcripts. These pilot studies revealed that both WNK1 transcripts are expressed in M1-MR+ cells.
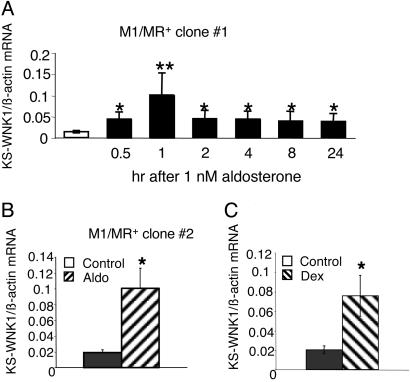
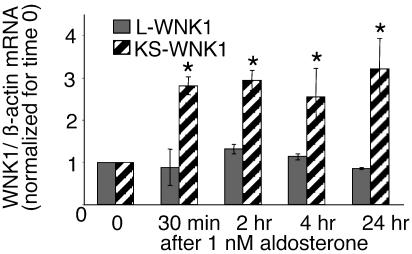
We next examined the effect of 1 nM aldosterone on the expression of the WNK1 transcripts. This low concentration of aldosterone occupies only the MR but not the GR. As shown in Fig. 3A, aldosterone rapidly increased the expression of the KS-WNK1 in clonal cell line 1. The increase was observed as early as 30 min after hormone treatment, and mRNA levels remained elevated for the duration of the experiment. This effect could be observed in all M1-MR+ cell lines tested; data for clonal line 2 are shown in Fig. 3B.
Fig. 3.
Aldosterone increases the expression of the KS-WNK1 transcript in M1-MR+ cells. (A) Time course of aldosterone effect in M1-MR+ clone 1. (B) The effect of a 4-h incubation in clone 2. KS-WNK1 mRNA levels were determined by using semiquantitative RT-PCR in cells at different times after treatment with 1 nM aldosterone or vehicle. mRNA levels were normalized for the levels of β-actin mRNA, determined from the same samples. n = 3 for each time point for clone 1, n = 2 for clone 2. *, P < 0.05; **, P < 0.01, one-tailed t test, vs. cells not treated with aldosterone. (C) Dexamethasone (5 nM) increases the expression of KS-WNK1 isoform in M1-MR+ clone 1 (4-h incubation). n = 4, *, P < 0.05, one-tailed t test.
Because in the CCD most genes that are induced by the MR can also be up-regulated by the GR (18, 21, 25, 29, 30), we next tested the effects of dexamethasone (5 nM), a synthetic glucocorticoid that saturates the GR but does not bind to the MR at such low concentration. As expected, dexamethasone also induced the expression of the KS-WNK1; the effect of a 4-h treatment is shown in Fig. 3C.
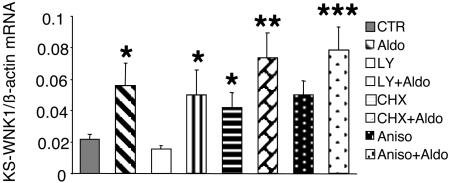
The rapid time course of aldosterone induction of KS-WNK1 suggested that it is probably due to direct transcriptional regulation through binding of MRs to a glucocorticoid response element (GRE) in the promoter of the KS-WNK1. Nevertheless, we considered the possibility that SGK1, which is induced by aldosterone as early as 15–30 min (18, 29) and is known to stimulate ENaC-mediated Na+ transport, could indirectly mediate KS-WNK1 induction by aldosterone. Because activity of the PI3-kinase pathway is necessary for SGK function, we tested the effect of aldosterone in the presence of the specific PI3-kinase inhibitor LY294002. This inhibitor, however, did not prevent the induction of KS-WNK1 (Fig. 4). To examine whether de novo synthesis of other aldosterone-induced proteins is necessary for KS-WNK1 induction, we determined the effects of aldosterone in the presence of two structurally different inhibitors of protein synthesis (CHX and anisomycin). Data in Fig. 4 indicate that when cells were treated with aldosterone plus these protein synthesis inhibitors, the levels of KS-WNK were still significantly higher than those in untreated control cells and were not significantly different from levels obtained with aldosterone alone. It has to be noted, however, that one of the inhibitors, CHX, in itself increased KS-WNK1 mRNA levels (P < 0.05 vs. control samples, and KS-WNK1 mRNA levels were not significantly induced by aldosterone when CHX was present (P = 0.07 as compared to CHX alone). This apparent induction of KS-WNK1 by CHX is probably due to the well known “superinduction” phenomenon. Therefore, although the rapid time course, the presence of a GRE in the KS-WNK1 promoter and data obtained with anisomycin suggest that the effect of aldosterone is direct, based on the results obtained with CHX, a partial indirect effect cannot be excluded.
Fig. 4.
The effect of PI3-kinase and protein synthesis inhibition on the induction of KS-WNK1 by aldosterone. M1-MR+ clone 1 cells were grown in steroid-free medium for 2 days and then preincubated for 30 min with vehicle CHX (5 μg/ml) or anisomycin (aniso; 10 μM). Aldosterone (1 nM) was given (or not) at 30 min, and cells were incubated for an additional 4 h before RNA was prepared. KS-WNK1 and β-actin mRNA levels were determined by RT-PCR. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. control, two-tailed unpaired t test. Comparisons were also made between values for each treatment obtained with and without aldosterone; P < 0.05 for vehicle vs. aldosterone; P < 0.05 for LY vs. LY + aldosterone; P = 0.07 for CHX vs. CHX + aldosterone; P < 0.05 for anisomycin vs. anisomycin + aldosterone (using one-tailed unpaired t tests). The values of KS-WNK1 mRNA levels in the presence aldosterone alone were not significantly different from those in cells incubated with aldosterone and LY or CHX or anisomycin (using two-tailed unpaired t tests). LY, LY294002.
Aldosterone and Dexamethasone Do Not Induce the Expression of the L-WNK1. Next, we asked the question whether the aldosterone induction is specific for the KS-WNK1 or is the L-WNK1 also up-regulated. By using primers specific for the third exon in the mouse L-WNK1 (which is absent in the KS-WNK1), we determined expression levels of the L-WNK1 isoform from the same samples used for measuring the KS-WNK1 transcript levels. Surprisingly, neither aldosterone (Fig. 5) nor dexamethasone (data not shown) changed the expression of the L-WNK1 isoform significantly at either early or late time points. Thus, the steroid regulation seems to be specific for the KS-WNK1 isoform.
Fig. 5.
Aldosterone does not induce the expression of the L-WNK1 transcript in. M1-MR+ clone 1 cells were incubated with vehicle or 1 nM aldosterone for the indicated times. Values shown are normalized for control cultures. n = 3–5. As a comparison, the levels of KS-WNK1 mRNA, determined from the same samples, are also shown. *, P < 0.05 vs. control by using one-tailed unpaired t test.
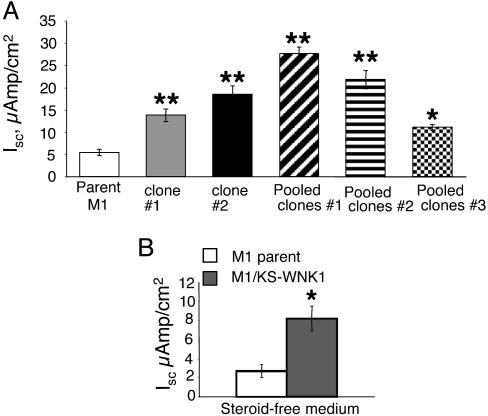
Expression of the KS-WNK1 Isoform Increases Transepithelial Na+ Current in M1 Cells. To determine the functional consequences of up-regulating the levels of KS-WNK1, we have generated several M1 lines overexpressing KS-WNK1. The expression of the KS-WNK1 construct was verified by RT-PCR by using sequence-specific primers present only in the construct but not in the endogenous mouse KS-WNK1 (data not shown). We selected three lines consisting of pooled clones (each containing >500 clones) as well as two individual clonal cell lines for further studies. To compare the effects of KS-WNK1 on electrophysiological parameters, parent M1 cells and cell lines stably expressing KS-WNK1 were seeded at the same cell densities on Millicell permeable membranes and maintained in complete growth medium. After reaching confluence, RTE and VTE were determined and Isc values were calculated. As shown in Fig. 6A, all cell lines expressing the KS-WNK1 had significantly higher Isc values than the parent M1 cells when cells were grown in a complete growth medium containing growth factors and steroids. Fig. 6B illustrates Na+ current values of a combined pool of all cell lines vs. parent M1 cells when grown in steroid-free medium. Under these conditions, Na+ current also was significantly higher in cells expressing the KS-WNK1 than in parent M1 cells. In a few cultures, we also determined the effect of activation of endogenous GRs on Isc. As expected, dexamethasone (100 nM) increased Isc in parent M1 cells (from 2.93 ± 1.69 to 6.01 ± 2.7 μAmp/cm2 in steroid-free medium vs. DEX; n = 3, P < 0.05) as well as in KS-WNK1-expressing M1 cells (from 7.82 ± 2.2 to 10.6 ± 5 μAmp/cm2; n = 6; P < 0.05). This response to DEX is not due a further elevation of the ectopically expressed WNK1 (which is not driven by a steroid-responsive promoter) but is probably mediated through other steroid-regulated pathways.
Fig. 6.
Stable expression of the KS-WNK1 increases transepithelial Na+ current in M1 cells. (A) Individual clonal lines (clones 1 and 2) as well as cell lines containing pooled clones (1, 2, and 3) were grown in complete growth medium (PC1) to confluence on Millicell membranes. *, P < 0.05, **, P < 0.01 vs. parent M1 cells, two-tailed unpaired t test. (B) Values for KS-WNK1-expressing cells shown were pooled from the two clonal cell lines and mixed clonal lines 1–3. Cells were grown in complete medium for 3 days after reaching confluence, and then medium was changed to DMEM/F12 containing 5% 2x-stripped FBS (steroid-free, SF) and electrical measurements taken 24 and 48 h later, n = 12 for parent M1; n = 23 for KS-WNK1 cells, *, P < 0.001, vs. parent M1 cells, two-tailed unpaired t test.
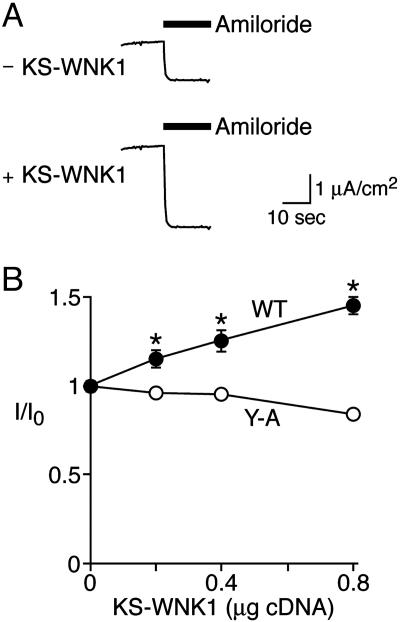
KS-WNK1 Stimulates ENaC in FRT Epithelia. To further test the hypothesis that KS-WNK1 regulates ENaC, we coexpressed them in FRT epithelia, which lack endogenous Na+ currents (19). Transfection of FRT cells with α, β, and γ ENaC generated amiloride-sensitive transepithelial Na+ current (Fig. 7A). Co-transfection with KS-WNK1 increased ENaC current in a dose-dependent manner (Fig. 7), indicating that KS-WNK1 stimulates ENaC.
Fig. 7.
Transepithelial Na current in FRT epithelia coexpressing ENaC and KS-WNK1. FRT epithelia were transfected with ENaC (WT or αY644A, βY620A, γY627A, 0.07 μg of each subunit) and KS-WNK1 (0–0.8 μg, total cDNA held constant with GFP cDNA). (A) Representative short-circuit current tracings for ENaC coexpressed with GFP (“–KS-WNK1”) or KS-WNK1 (0.8 μg). Amiloride (100 μM) was added to apical bathing solution as indicated by bar. (B) Plots of amiloride-sensitive short-circuit current (relative to 0 μg KS-WNK1 for each group) vs. quantity of KS-WNK1 cDNA for WT ENaC and αY644A, βY620A, γY627A (“Y-A”) (mean ± SEM, n = 11–19; asterisks indicate P < 0.02 vs. 0 μg of KS-WNK1).
ENaC surface expression is controlled in part by proline tyrosine (PY) motifs located in the C terminus of each ENaC subunit (reviewed in refs. 31–33). These motifs are binding sites for Nedd4-2, an E3 ubiquitin-protein ligase that targets ENaC for degradation. We asked whether the PY motifs contribute to ENaC regulation by KS-WNK1. When we mutated the PY motifs to Ala in all three subunits (αY644A, βY620A, and γY627A), KS-WNK1 did not increase ENaC current (Fig. 7B). Thus, ENaC regulation by KS-WNK1 depends on the ENaC PY motifs.
Discussion
The main findings of this study are that (i) aldosterone, at physiological concentration, rapidly and specifically induces the expression of the KS-WNK1 isoform in renal CCD cells; and (ii) overexpression of the KS-WNK1 in mammalian epithelial cells results in a significant increase in transepithelial Na+ transport by stimulating ENaC activity. These data suggest that the induction of KS-WNK1 transcription might be part of the mechanism by which aldosterone increases Na+ reabsorption in the collecting duct.
WNK kinases have been recently implicated in the pathogenesis of hypertensive hyperkalemia associated with PHAII (Gordon's syndrome; ref. 5). WNKs are highly expressed in the distal convoluted and connecting tubules as well as the collecting duct system of the kidney (5) where the final regulation of salt balance occurs. However, the mechanism of action of WNKs remains largely unknown. Because the same phenotype results from gain-of-function mutations in WNK1 and loss-of-function mutations in WNK4 (5), it is likely that both genes act in the same pathway but have opposing actions.
Because one of the hallmarks of PHAII is marked thiazide sensitivity, one likely mechanism that could lead to increased distal Na+ reabsorption in this disease is overactivity of NCC. Indeed, recent studies showed that in Xenopus oocytes WNK4 inhibits NCC activity whereas WNK1 relieves this inhibitory effect resulting in higher thiazide-sensitive Na transport (6, 7). Deletions in the first intron in the WNK1 gene in affected patients lead to elevated expression of WNK1 (5). The excess WNK1 could neutralize the inhibitory effect of WNK4 on NCC, which in turn leads to increased Na+ reabsorption in the distal nephron.
The role of the KS-WNK1 in the regulation of renal ion transport has not been previously investigated. Because hyperaldosteronism results in Na+ retention and hypertension, we hypothesized that at least part of the effect of aldosterone could be mediated by increasing the transcription of KS-WNK1. The striking differences in tissue- and cell-specific expression of the different WNK1 transcripts raise the possibility that these isoforms have different actions and are differentially regulated. Indeed, our results indicate that the KS-WNK1 and the more broadly expressed L-WNK1 isoforms are differentially regulated by corticosteroids in renal CCD cells. These two WNK1 isoforms are generated via alternative promoter usage (13), and the promoter of the KS-WNK1 transcript contains a putative GRE (identified by the tess transcription factor binding site search program) immediately 5′ from the first ATG (13). On the other hand, transcription factor binding site computer programs failed to identify classical GREs in the L-WNK1 promoters (13). Thus, the rapid induction of KS-WNK1 by aldosterone is probably a direct effect through binding of MRs to the GRE (13).
What is the biological function of the KS-WNK1 isoform? Our findings that it is induced by physiological concentrations of aldosterone and increases Na+ transport in CCD cells indicate that it might be an important regulator of ENaC function. This isoform seems to be physiologically more relevant for Na+ transport than the L-WNK1, which is expressed in many other tissues that do not participate in the regulation of Na+ balance. At this point, we can only speculate on the possible mechanisms of its action. Because the KS-WNK1 lacks the kinase domain, its mechanism of action probably involves protein–protein interactions rather than Ser/Thr phosphorylation. The unique sequence in KS-WNK1 (exon 4A) contains a Cys-rich region. Perhaps this region alters the function of a protein involved in Na+ transport by forming intermolecular disulfide bonds. That kinase activity of WNKs is not always required for biological action is supported by the recent observation that the inhibitory action of WNK4 on ROMK is independent of its kinase activity (9).
It is interesting to note that recently WNK1 was shown to modulate the activity of synaptotagmins that are important regulators of membrane trafficking and vesicle fusion, suggesting that WNK1 might regulate ion transport by increasing the number of transporters in the plasma membrane (34). We speculate that the KS-WNK1 might have similar effects. Another potential target for KS-WNK1 is WNK4, as predicted from previous work (7). Because M1 cells express significant levels of WNK4 (A.N.-F.-T., unpublished data), in theory, KS-WNK1 could counteract the effects of WNK4 thereby stimulating Na+ reabsorption in the distal nephron. Whether there is a direct interaction between KS-WNK1 and WNK4 has not yet been studied.
Because Nedd4-2 is a key regulator of ENaC (35–37), another possible target for KS-WNK1 might be Nedd4-2. Consistent with this possibility is our finding that the ENaC PY motifs are required for KS-WNK1 to stimulate ENaC in FRT epithelia. Importantly, mutations in the ENaC PY motifs also cause hypertension (Liddle's syndrome). In theory, KS-WNK1 could also increase Na+ transport by increasing the transcription or synthesis of ENaC. The findings, however, that KS-WNK1 enhances ENaC currents when ENaC is ectopically expressed in FRT cells and that ENaC mutations abolish this effect argue against this mechanism. We hypothesize that WNK1 mutations increase renal Na+ reabsorption and cause hypertension through two mechanisms: (i) increased activity of the thiazide-sensitive NCC in the distal convoluted tubule, which is suggested by oocyte expression experiments (7); and (ii) increased Na reabsorption in the CCD via ENaC (data not shown).
In summary, our results show that aldosterone rapidly induces the expression of the KS-WNK1 transcript. Even more importantly, overexpression of KS-WNK1 in two types of mammalian epithelia results in a significant elevation of transepithelial Na+ transport. Thus, KS-WNK1 might be an important element of aldosterone-induced Na+ retention and hypertension.
Acknowledgments
We thank Jessica Armstrong and Jennifer C. Steines for excellent technical assistance. This study was supported by National Institutes of Health Grants DK41841 (to A.N.-F.-T.), DK58898 (to G.F.-T.), and HL58812 (to P.M.S.).
Author contributions: A.N.-F.-T., P.M.S., and G.F.-T. designed research, performed research, analyzed data, and wrote the paper; and A.N.-F.-T. and G.F.-T. contributed new reagents/analytic tools.
Abbreviations: ENaC, epithelial sodium channel; MR, mineralocorticoid receptor; PI3-kinase, phosphatidylinositol 3-kinase; PHAII, pseudohypoaldosteronism type II; NCC, Na-Cl cotransporter; CCD, cortical collecting duct; FRT, Fischer rat thyroid; GR, glucocorticoid receptor; GRE, glucocorticoid response element; CHX, cycloheximide; KS-WNK1, kidney-specific WNK1; L-WNK, “long” WNK1; PY, proline tyrosine.
References
- 1.Shimkets, R. A., Warnock, D. G., Bositis, C. M., Nelson-Williams, C., Hansson, J. H., Schambelan, M., Gill, J. R., Jr., Ulick, S., Milora, R. V., Findling, J. W., et al. (1994) Cell 79, 407–414. [DOI] [PubMed] [Google Scholar]
- 2.Gordon, R. D., Geddes, R. A., Pawsey, C. G. & O'Halloran, M. W. (1970) Australas. Ann. Med. 19, 287–294. [DOI] [PubMed] [Google Scholar]
- 3.Mayan, H., Vered, I., Mouallem, M., Tzadok-Witkon, M., Pauzner, R. & Farfel, Z. (2002) J. Clin. Endocrinol. Metab. 87, 3248–3254. [DOI] [PubMed] [Google Scholar]
- 4.Xu, B., English, J. M., Wilsbacher, J. L., Stippec, S., Goldsmith, E. J. & Cobb, M. H. (2000) J. Biol. Chem. 275, 16795–16801. [DOI] [PubMed] [Google Scholar]
- 5.Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Williams, C., Desitter, I., Gunel, M., Milford, D. V., Lipkin, G. W., Achard, J. M., et al. (2001) Science 293, 1107–1112. [DOI] [PubMed] [Google Scholar]
- 6.Wilson, F. H., Kahle, K. T., Sabath, E., Lalioti, M. D., Rapson, A. K., Hoover, R. S., Hebert, S. C., Gamba, G. & Lifton, R. P. (2003) Proc. Natl. Acad. Sci. USA 100, 680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, C. L., Angell, J., Mitchell, R. & Ellison, D. H. (2003) J. Clin. Invest. 111, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahle, K. T., Gimenez, I., Hassan, H., Wilson, F. H., Wong, R. D., Forbush, B., Aronson, P. S. & Lifton, R. P. (2004) Proc. Natl. Acad. Sci. USA 101, 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahle, K. T., Wilson, F. H., Leng, Q., Lalioti, M. D., O'Connell, A. D., Dong, K., Rapson, A. K., MacGregor, G. G., Giebisch, G., Hebert, S. C., et al. (2003) Nat. Genet. 35, 372–376. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi, K., Rai, T., Kobayashi, K., Sohara, E., Suzuki, T., Itoh, T., Suda, S., Hayama, A., Sasaki, S. & Uchida, S. (2004) Proc. Natl. Acad. Sci. USA 101, 4690–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambrowicz, B. P., Abuin, A., Ramirez-Solis, R., Richter, L. J., Piggott, J., BeltrandelRio, H., Buxton, E. C., Edwards, J., Finch, R. A., Friddle, C. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choate, K. A., Kahle, K. T., Wilson, F. H., Nelson-Williams, C. & Lifton, R. P. (2003) Proc. Natl. Acad. Sci. USA 100, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaloy, C., Lu, J., Houot, A. M., Disse-Nicodeme, S., Gasc, J. M., Corvol, P. & Jeunemaitre, X. (2003) Mol. Cell. Biol. 23, 9208–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly, M., Marshall, E., Speirs, H. J. & Brown, R. W. (2003) J. Am. Soc. Nephrol. 14, 2447–2456. [DOI] [PubMed] [Google Scholar]
- 15.Helms, M. N., Fejes-Tóth, G. & Náray-Fejes-Tóth, A. (2003) Am. J. Physiol. 284, F480–F487. [DOI] [PubMed] [Google Scholar]
- 16.Fejes-Tóth, G., Chen, W. R., Rusvai, E., Moser, T. & Náray-Fejes-Tóth, A. (1994) J. Biol. Chem. 269, 26717–26721. [PubMed] [Google Scholar]
- 17.Fejes-Tóth, G. & Náray-Fejes-Tóth, A. (1995) Kidney Int. 48, 1420–1426. [DOI] [PubMed] [Google Scholar]
- 18.Náray-Fejes-Tóth, A., Canessa, C., Cleaveland, E. S., Aldrich, G. & Fejes-Tóth, G. (1999) J. Biol. Chem. 274, 16973–16978. [DOI] [PubMed] [Google Scholar]
- 19.Snyder, P. M. (2000) J. Clin. Invest. 105, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoos, B. A., Náray-Fejes-Tóth, A., Carretero, O. A., Ito, S. & Fejes-Tóth, G. (1991) Kidney Int. 39, 1168–1175. [DOI] [PubMed] [Google Scholar]
- 21.Robert-Nicoud, M., Flahaut, M., Elalouf, J. M., Nicod, M., Salinas, M., Bens, M., Doucet, A., Wincker, P., Artiguenave, F., Horisberger, J. D., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 2712–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summa, V., Mordasini, D., Roger, F., Bens, M., Martin, P. Y., Vandewalle, A., Verrey, F. & Feraille, E. (2001) J. Biol. Chem. 276, 47087–47093. [DOI] [PubMed] [Google Scholar]
- 23.Rafestin-Oblin, M. E., Fagart, J., Souque, A., Seguin, C., Bens, M. & Vandewalle, A. (2002) Mol. Pharmacol. 62, 1306–1313. [DOI] [PubMed] [Google Scholar]
- 24.Auberson, M., Hoffmann-Pochon, N., Vandewalle, A., Kellenberger, S. & Schild, L. (2003) Am. J. Physiol. 285, F459–F471. [DOI] [PubMed] [Google Scholar]
- 25.Verrey, F., Summa, V., Heitzmann, D., Mordasini, D., Vandewalle, A., Feraille, E. & Zecevic, M. (2003) Ann. N.Y. Acad. Sci. 986, 554–561. [DOI] [PubMed] [Google Scholar]
- 26.Summa, V., Camargo, S. M., Bauch, C., Zecevic, M. & Verrey, F. (2004) J. Physiol. (London) 555, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Náray-Fejes-Tóth, A., Rusvai, E. & Fejes-Tóth, G. (1994) Am. J. Physiol. 266, F76–F80. [DOI] [PubMed] [Google Scholar]
- 28.Schulman, G., Robertson, N. M., Elfenbein, I. B., Eneanya, D., Litwack, G. & Bastl, C. P. (1994) Am. J. Physiol. 266, C729–C740. [DOI] [PubMed] [Google Scholar]
- 29.Chen, S., Bhargava, A., Mastroberardino, L., Meijer, O. C., Wang, J., Buse, P., Firestone, G. L., Verrey, F. & Pearce, D. (1999) Proc. Natl. Acad. Sci. USA 96, 2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Náray-Fejes-Tóth, A., Fejes-Tóth, G., Volk, K. A. & Stokes, J. B. (2000) J. Steroid Biochem. Mol. Biol. 75, 51–56. [DOI] [PubMed] [Google Scholar]
- 31.Rotin, D., Kanelis, V. & Schild, L. (2001) Am. J. Physiol. 281, F391–F399. [DOI] [PubMed] [Google Scholar]
- 32.Snyder, P. M. (2002) Endocr. Rev. 23, 258–275. [DOI] [PubMed] [Google Scholar]
- 33.Kamynina, E. & Staub, O. (2002) Am. J. Physiol. 283, F377–F387. [DOI] [PubMed] [Google Scholar]
- 34.Lee, B. H., Min, X., Heise, C. J., Xu, B. E., Chen, S., Shu, H., Luby-Phelps, K., Goldsmith, E. J. & Cobb, M. H. (2004) Mol. Cell 15, 741–751. [DOI] [PubMed] [Google Scholar]
- 35.Kamynina, E., Debonneville, C., Bens, M., Vandewalle, A. & Staub, O. (2001) FASEB J. 15, 204–214. [DOI] [PubMed] [Google Scholar]
- 36.Debonneville, C., Flores, S. Y., Kamynina, E., Plant, P. J., Tauxe, C., Thomas, M. A., Munster, C., Chraibi, A., Pratt, J. H., Horisberger, J. D., et al. (2001) EMBO J. 20, 7052–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder, P. M., Olson, D. R. & Thomas, B. C. (2002) J. Biol. Chem. 277, 5–8. [DOI] [PubMed] [Google Scholar]