Abstract
Objectives
Adaptive interaction with the environment requires the ability to predict both human and non-biological motion trajectories. Prior accounts of the neurocognitive basis for prediction of these two motion classes may generally be divided into those that posit that non-biological motion trajectories are predicted using the same motor planning and/or simulation mechanisms used for human actions, and those that posit distinct mechanisms for each. Using brain lesion patients and healthy controls, this study examined critical neural substrates and behavioral correlates of human and non-biological motion prediction.
Methods
Twenty-seven left hemisphere stroke patients and 13 neurologically intact controls performed a visual occlusion task requiring prediction of pantomimed tool use, real tool use, and non-biological motion videos. Patients were also assessed with measures of motor strength and speed, praxis, and action recognition.
Results
Prediction impairment for both human and non-biological motion was associated with limb apraxia and, weakly, with the severity of motor production deficits, but not with action recognition ability. Furthermore, impairment for human and non-biological motion prediction was equivalently associated with lesions in the left inferior parietal cortex, left dorsal frontal cortex, and the left insula.
Conclusions
These data suggest that motor planning mechanisms associated with specific loci in the sensorimotor network are critical for prediction of spatiotemporal trajectory information characteristic of both human and non-biological motions.
Keywords: Simulation, Motor planning, Limb apraxia, Hemiparesis, Forward model, Premotor, Parietal, Insula, Domain-specific, Domain-general, Biological motion
INTRODUCTION
Imagine going for a walk in the park on a sunny Saturday morning. As you stroll along, you notice many other people who are likewise strolling about. However, you are not just passively observing them; instead, you are continuously trying to anticipate or predict what they are about to do, so as to avoid any awkward collisions. As you exit the park and cross the street busy with cars, you are again engaged in prediction, this time to avoid more serious collision. As this scenario makes clear, adaptive interaction with the environment requires prediction of both human actions and non-biological motion, and implementing these tasks is, therefore, a central function of the human brain (Bubic, von Cramon, & Schubotz, 2010; Clark, 2015; see also Fuster & Bressler, 2015).
According to one influential account, action prediction is instantiated by internal forward models that are used to predict the proprioceptive and exteroceptive sensory consequences of one’s own motor commands. This prediction is compared with the actual movement as it unfolds, and any discrepancy between the two is used for online correction and learning of movements (Wolpert, Ghahramani, & Jordan, 1995). It has been argued that the same forward models can also be deployed to simulate others’ actions so as to predict how they will unfold (Wolpert, Doya, & Kawato, 2003; see also Friston, Mattout, & Kilner, 2011). By contrast, the prominent account of Pickering and Garrod (2013) suggests that prediction may not always use forward models, but may under some circumstances (such as during prediction of non-biological motion) rest upon a perceptual associative process. In the latter case, observers compare the current trajectory with past perceived trajectories and in this way come to predict how it will unfold (see also Stadler, Springer, Parkinson, & Prinz, 2012).
There is extensive behavioral evidence supporting the close link between action production and prediction (e.g., Aglioti, Cesari, Romani, & Urgesi, 2008; Balser et al., 2014; Brattan, Baker, & Tipper, 2015; Calvo-Merino, Glaser, Grèzes, Passingham, & Haggard, 2005; Colling, Thompson, & Sutton, 2014; Knoblich & Flach, 2001; Makris & Urgesi, 2013). For example, superior prediction performance has been reported for videos of self compared to other-generated actions, possibly because the used forward models are more accurate in the former case (Colling et al., 2014; Knoblich & Flach, 2001). On the other hand, there is evidence that subjects may use a visual matching strategy to predict actions, suggesting the possibility that the task does not necessarily require involvement of the motor system (Springer, Brandstädter, & Prinz, 2013; see also Springer & Prinz, 2010).
Several brain imaging studies suggest the existence of a fronto-parietal network that supports human action prediction. Typical activations include premotor cortex (PM), supplementary motor area (SMA), inferior frontal gyrus (IFG), supramarginal gyrus (SMG), superior parietal lobule (SPL), and the cerebellum, with increasing left-lateralization for cortical regions as a function of motor expertise and/or visual familiarity (see Yang, 2014 for a meta analysis). Whether prediction of non-biological motion trajectories is subserved by overlapping neural mechanisms is an issue of contention.
According to Schubotz (2007), while forward models in PM cortex may be ideally suited to produce and predict human actions, they are nevertheless also the best available neural resource for predicting many other events beyond (visually perceived) human actions. In line with this proposal, Coull, Vidal, Goulon, Nazarian, and Craig (2008) found activations in left pars opercularis (a portion of IFG), ventral PM, and SMG during collision judgments of inanimate objects. At variance with these data are studies that report differences between prediction of human and non-biological motion trajectories, with shared activity in left supplementary motor area (SMA) but additional activations in right extrastriate body area (EBA) in the former, and left lateral occipital cortex (LOC) in the latter case (Cross, Stadler, Parkinson, Schütz-Bosbach, & Prinz, 2013; see also Stadler, Springer, et al., 2012).
One important limitation of brain imaging studies is that they cannot provide information about the necessity of activated brain regions for a given task (cf. Caramazza, Anzellotti, Strnad, & Lingnau, 2014; Fellows et al., 2005). In this regard, the study of brain lesion patients is highly informative. In one of the few available patient studies addressing the neural substrate of prediction, Schubotz, Sakreida, Tittgemeyer, and von Cramon (2004) instructed right or left parietal- or premotor-lesioned patients, as well as right, left, and bilateral prefrontal-lesioned patients to predict sequences of geometrical shapes and spatial locations. They found impaired performance in both the parietal and premotor patients, whereas the prefrontal-lesioned patients performed normally, despite having larger lesion volumes. These data suggest that prediction of sequential information critically depends on parietal and premotor (but not prefrontal) cortices. Roth, Synofzik, and Lindner (2013) extended these findings to include an additional critical role for the cerebellum. Neither study assessed prediction of human action stimuli.
Patient studies are also useful for informing the question of whether the proposed relationship between action production and prediction is contingent on spatiomotor planning abilities, motor production abilities, or both. While these questions have not been addressed in patient populations, related research has focused on the ability of apraxic patients (known to have deficient spatiomotor planning) and hemiparetic patients (who exhibit impaired motor production) to simulate actions, which many theorists consider a precursor to or component of prediction (Barsalou, 2009; Mulligan, Lohse, & Hodges, 2015; Stapel, Hunnius, Meyer, & Bekkering, 2016; but see Vannuscorps & Caramazza, 2015).
For example, we (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005; Dawson, Buxbaum, & Duff, 2010; see also Coslett, Buxbaum, & Schwoebel, 2008) showed that patients with left parietal stroke and limb apraxia, a disorder of skilled action associated with prominent bimanual deficits in tool pantomime (but better performance with tools in hand), were unable to reliably indicate how they would grasp a dowel if they were to reach out and perform the action, whereas they performed normally in actual grasping. These data suggest that patients with limb apraxia may have deficient predictive models of action in the face of relatively intact feedback-driven motor control.
The evidence with regard to hemiparesis is inconclusive, with reports of intact simulation in hemiplegic stroke patients (Johnson, Sprehn, & Saykin, 2002; see also Sirigu et al., 1995), and conversely, of impairment in hemiparetic individuals born with cerebral palsy, especially for simulated actions of the affected hand (van Elk et al., 2010). In at least some circumstances, then, motor planning and production abilities may be associated with the ability to simulate one’s own actions. However, to our knowledge, the relationship between motor planning or production deficits and the ability to predict either the observed actions of others or non-biological motion trajectories has not been directly assessed.
An additional question of interest concerns the relationship between action recognition and understanding and prediction of others’ actions. On the one hand, if action perception relies upon direct matching through simulation processes in the motor system, as has been suggested (e.g., Rizzolatti, Fogassi, & Gallese, 2001), and simulation is, in turn, a component of prediction, then a strong relationship may be expected (Urgesi, Candidi, & Avenanti, 2014). That is, on such accounts, one should be impaired at recognizing actions that one cannot simulate/predict. However, according to Csibra (2008), action recognition and understanding is instantiated through top–down processing in temporal and inferior frontal association cortices resulting in a bias toward expecting certain outcomes, with the output feeding into the motor system to enhance its efficiency in simulating and hence predicting actions. Given that this account argues that action recognition is not dependent on simulation (but rather that simulation is influenced by recognition), it may assume a weaker relationship between recognition and prediction.
The aims of the present study were to identify critical neural substrates of prediction of observed human and non-biological motion trajectories and to assess the behavioral correlates of this ability. We used a video occlusion paradigm (e.g., Stadler et al., 2011) to examine motion prediction ability in chronic left-hemisphere stroke patients as well as in a matched sample of neurologically intact controls. Patients exhibited a full range of hemiparesis and limb apraxia.1 We also included our laboratory’s measure of patients’ action recognition impairment (Kalénine, Buxbaum, & Coslett, 2010; Tarhan, Watson, & Buxbaum, 2015). Video stimuli in the prediction task consisted of pantomimed and actual tool use actions, and non-biological motions of comparable complexity.
Based on prior neuroimaging studies that found activation of left SMA, PM, IFG, IPS, and SMG during prediction of either human or non-biological motion (Cross et al., 2013; Schubotz & von Cramon, 2002; Stadler et al., 2011; Wiener, Turkeltaub, & Coslett, 2010; Yang, 2014), we hypothesized that lesions in these regions would be associated with impairments in prediction of all three motion types. In terms of the behavioral correlates of action prediction ability, accounts that invoke forward models (Friston et al., 2011; Wolpert et al., 2003) predict an association between action planning and action prediction. Thus, given that apraxia is thought to be, in part, a disorder of action planning (Buxbaum, Johnson-Frey, et al., 2005; Eidenmuller, Randerath, Goldenberg, Li, & Hermsdorfer, 2014; Jax, Buxbaum, & Moll, 2006; Mutha, Sainburg, & Haaland, 2010; Ochipa et al., 1997; Wheaton, Fridman, Bohlhalter, Vorbach, & Hallett, 2009), such accounts would expect an association between praxis ability and motion prediction ability.
Under the assumption that forward models underlying action planning are used not only to predict human but also non-biological motions (Schubotz, 2007), this association should hold for all three movement types. By contrast, the perceptual associative account of prediction of Pickering and Garrod (2013) argues that forward models are unlikely to be used for non-biological motions, and thus would presumably expect praxis ability to be associated with human but not non-biological motion prediction ability. Finally, based on evidence that hemiplegic stroke patients may display intact action simulation abilities (Johnson et al., 2002), we hypothesized that there would not be an association between motor production ability (i.e., hemiparesis severity) and prediction ability for either human or non-biological motion trajectories.
METHODS
Participants
Forty chronic left-hemisphere stroke patients were recruited through a dedicated stroke patient research registry (Schwartz, Brecher, Whyte, & Klein, 2005). All patients were pre-morbidly right-handed according to self-report and at least 6 months post-stroke at the time of testing. To increase the likelihood of task instruction comprehension, patients with scores ≤4/10 (“severe” or “very severe” impairment) on the language comprehension subtest of the Western Aphasia Battery (WAB; Kertesz, 1982) were excluded pre-data acquisition, as is customary in our lab (e.g., Watson & Buxbaum, 2015). Data collection for two patients was aborted because it was discovered that their vision was insufficient to perform the tasks, while two other patients were excluded for demonstrably not following task instructions.
For nine patients, prediction performance was at chance for all three conditions (i.e., tool use, pantomimed tool use, non-biological motion) according to binomial tests (α = .05). Because it is unclear whether this was due to inability to understand the task instructions or inability to perform the task, these patients were also excluded. The final sample comprised 27 patients. Table 1 shows the patients’ demographic, neuropsychological, and lesion information.
Table 1.
Demographic, neuropsychological, and lesion information
| Patient | Age (years) | Gender | Education (years) | Pantomime production (0–1) | Grip strength (rh/lh) | Finger tapping freq (rh/lh) | Pantomime recognition (0–1) | WAB (comp) (0–10) | Lesion vol (cm3) |
|---|---|---|---|---|---|---|---|---|---|
| 1846 | 54 | F | 14 | .98 | .76 | .98 | .96 | 9.80 | 31.43 |
| 1687 | 76 | M | 27 | .92 | 1.10 | 1.06 | .88 | 8.85 | 40.95 |
| 0286* | 69 | M | 13 | .83 | .39 | .37 | .96 | 9.85 | 95.66 |
| 2464 | 64 | M | 20 | .98 | .95 | .98 | .83 | 9.60 | 51.40 |
| 1743 | 55 | M | 14 | .98 | .77 | 1.15 | .86 | 9.80 | 82.96 |
| 1857 | 76 | F | 12 | .83 | 1.36 | .81 | .65 | 9.80 | 17.86 |
| 2350 | 51 | M | 14 | .80 | .78 | 1.10 | .81 | 8.60 | 55.69 |
| 1371* | 76 | F | 12 | .63 | 1.02 | .82 | .86 | 9.25 | 21.50 |
| 2172 | 64 | F | 16 | .78 | .00 | .00 | .83 | 9.15 | 73.10 |
| 2221 | 35 | F | 19 | .86 | 1.24 | 1.39 | .96 | 8.85 | 63.92 |
| 2180* | 70 | M | 14 | .78 | 1.25 | 1.12 | .85 | 4.65 | 67.16 |
| 2079 | 58 | M | 12 | .88 | .83 | .90 | .82 | 8.90 | 57.64 |
| 0281 | 54 | F | 16 | .85 | 1.03 | 1.08 | 1.00 | 9.35 | 151.31 |
| 0865 | 63 | M | 12 | .78 | .00 | .00 | .91 | 8.50 | 71.75 |
| 0419 | 46 | F | 12 | .93 | .89 | 1.34 | .77 | 9.30 | 51.86 |
| 0206 | 61 | M | 13 | .73 | .00 | .00 | .83 | 8.55 | 103.94 |
| 2232 | 49 | F | 12 | .97 | 1.22 | .83 | .77 | 6.25 | 153.99 |
| 1088* | 51 | F | 16 | .90 | .89 | 2.08 | .84 | 9.70 | 89.07 |
| 2289* | 75 | F | 11 | .70 | 1.13 | n/a | .74 | 8.05 | 62.06 |
| 2481 | 69 | M | 19 | .65 | .86 | .67 | .81 | 7.90 | 73.68 |
| 2091 | 62 | M | 15 | .88 | .00 | .00 | .78 | 9.00 | 195.29 |
| 1238 | 57 | M | 13 | .95 | .94 | .30 | .91 | 9.95 | 172.21 |
| 2548 | 50 | M | 12 | .97 | .49 | 1.05 | .87 | 9.00 | 95.68 |
| 1392 | 71 | M | 17 | .88 | .67 | 1.09 | 1.00 | 8.70 | 72.42 |
| 2551* | 71 | F | 19 | .78 | .00 | .00 | .59 | 9.35 | 184.74 |
| 0583 | 68 | M | 19 | .88 | .00 | .00 | .86 | 7.65 | 356.21 |
| 2027 | 69 | M | 16 | .50 | .00 | .00 | .68 | 6.95 | 271.98 |
| 2328 | 50 | F | 13 | .83 | .00 | .00 | .65 | 9.50 | 140.55 |
| 2604* | 42 | F | 12 | .75 | .33 | .00 | .67 | 6.90 | 121.24 |
| 0083 | 54 | M | 11 | 1.00 | .00 | .55 | .96 | 10.00 | 50.98 |
| 1862 | 56 | M | 12 | 1.00 | .88 | 1.42 | .92 | 10.00 | 19.47 |
| 2508* | 68 | F | 12 | .35 | .00 | .00 | .74 | 6.45 | 51.90 |
| 2415* | 33 | F | 12 | .61 | .00 | .00 | .47 | 6.60 | 147.24 |
| 0042* | 61 | F | 16 | n/a | n/a | n/a | n/a | n/a | 224.41 |
| 1839* | 64 | M | 11 | n/a | n/a | n/a | n/a | n/a | 172.55 |
| 1591* | 56 | F | 12 | n/a | n/a | n/a | n/a | n/a | 173.60 |
| 2011 | 51 | F | 13 | .90 | .00 | .00 | .91 | 7.95 | 80.02 |
| 2667 | 64 | F | 13 | .73 | 1.00 | 1.11 | .76 | 8.85 | 34.16 |
| 2538* | 53 | M | 16 | .78 | .62 | 1.02 | .82 | 8.50 | 140.43 |
| 2378 | 57 | F | 12 | .88 | 1.22 | 1.18 | .83 | 10.00 | 128.90 |
Note. Patients excluded from the final sample are flagged with asterisks. Scores of .00 in the grip strength and finger tapping frequency columns indicate patients’ inability to perform the task with the right hand.
rh/lh = right hand divided by left hand; n/a = datapoint not available; freq = frequency; comp = comprehension; vol = volume.
In addition, 16 neurologically intact control participants were recruited from a control subject research registry. All control participants were right-handed according to self-report and had a score of ≥27 of 30 on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975). One control participant was excluded because her prediction performance was at chance in all three conditions. Two control participants were excluded for having an average score in one or more conditions of the prediction task more than two SDs below the group mean. There was no significant difference in age or years of education between the patients (41% female; mean age 58.7 years, range 35–76 years; mean education 14.7 years, range 11–27 years) and controls (38% female; mean age 60.2 years, range 38–80 years; mean education 15.9 years, range 12–22 years) included in the final sample (ps > .30).
Patient and control participants with a history of co-morbid or pre-morbid neurological disorders, alcohol or drug abuse, or psychosis were excluded from participating in the study. All participants gave informed consent for the behavioral portion of the experiment and were paid for participation and reimbursed for travel expenses. Thirty-eight patients additionally consented to participate in a structural magnetic resonance imaging (MRI) or computed tomography (CT) scanning protocol; brain scans of two patients were obtained from clinical records. The behavioral study was approved by the Institutional Review Board (IRB) of Einstein Healthcare Network and the scanning protocol was approved by the IRB of the Hospital of the University of Pennsylania.
Stimuli and Apparatus
Nine videos were constructed in which an actor at a desk performed left-handed everyday actions with a tool and its recipient object. In addition, nine videos were constructed showing the same actor performing pantomimed versions of the tool use actions. By flipping the videos 180° across the vertical axis, nine videos were created in which the same actions appeared to be performed with the right hand.2 Furthermore, six non-biological motion sequences were constructed using wind-up toys (Kikkerland Design Inc., New York, NY) and six using a programmable robotic ball (Sphero, Boulder, CO). The non-biological motion sequences were filmed on the same desk and were also flipped across the vertical axis to create 12 additional videos.
For the human action sequences, the camera was angled at an approximately 45° angle relative to the actor’s body midline which ensured the maximal amount of visible movement during each action. Non-biological videos were filmed using the same camera angle. Videos were presented in color on an Acer G215H monitor at a resolution of 640 × 480 pixels and a frame rate of 30 frames per second (see Figure 1 for static images taken from the tool use, pantomimed tool use, and non-biological motion videos).
Fig. 1.

Frames from tool use (A), pantomimed tool use (B), and non-biological (C) motion videos. The human actions in panels A and B consist of (pantomiming) the use of a fork.
To select videos for the experimental protocol that were of appropriate difficulty, preliminary data on the tool use, pantomimed tool use, and non-biological motion prediction tasks were collected from 10 young neurologically intact participants (age range: 21–26 years) who were recruited in accordance with guidelines of the IRB of Einstein Healthcare Network (for task procedure, see Section Motion Prediction Task below). Based on these data, four tool use videos and matching pantomimed tool use videos (i.e., using an iron, fork, razor, wrench) as well as four non-biological videos that featured the robotic ball were selected for which performance was on average 81.5% correct and did not differ significantly between the three motion types (p = .75). Given that performance on many cognitive tasks decreases with age, videos were selected with high but not ceiling performance in young controls to increase the likelihood that prediction performance for patients and matched older controls would be above floor but below ceiling.
Each video was preceded by a 1500 ms fixation cross, followed by the beginning of the motion sequence. After a mean duration of 4167 ms (range: 2300–5100 ms), the videos were fully covered by a black occluder for a duration of 800 ms. Occlusion onset always occurred during a large amplitude phase of the movement (e.g., during the fork lifting phase rather than while picking up the food; during a rolling phase, rather than while the ball made a slow turn) and, therefore, varied between videos. At occlusion offset, the videos continued to play in a way that was either congruent with the motion sequence pre-occlusion (i.e., the videos continued to play normally during occlusion) or incongruent (i.e., the videos were shifted 800 ms into the future; see Graf et al., 2007; Stadler et al., 2011, for similar paradigms).
Tool use, pantomimed tool use, and non-biological videos lasted ~ 6500 ms (range: 4600–8500 ms) and did not differ significantly in terms of total length, time from start of video until occlusion onset, and time from occlusion offset until end of video (all ps > .25). Each combination of stimulus factors, motion type, video orientation, and congruence, was presented three times, leading to a total of 12 (4 tool use, 4 pantomimed tool use, 4 non-biological) × 2 (flipped, non-flipped) × 2 (congruent, incongruent) × 3 repetitions = 144 experimental trials. The human (tool use and pantomimed tool use) and non-biological motion sequences were presented separately within two counterbalanced blocks of 96 and 48 randomly presented stimuli, respectively.
E-Prime 2.0 stimulus presentation software (Psychology Software Tools, Pittsburgh, PA) was used to control stimulus presentation and a yellow and a blue colored response key on an E-Prime serial response box were used to collect responses and associated reaction times (RT). A lever attached to a mechanical counter (Veeder-Root, Hartford, CT) was used to collect finger tapping frequency data, and a Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL) to collect grip strength data. Ten three-dimensional familiar tools (fork, razor, scissors, watch, toothbrush, comb, bottle opener, cigarette lighter, eraser, and nail clippers) were used to collect data on pantomimed tool use production impairment.
Procedure
Motion prediction task
Participants first learned which response key to press for stimuli that they perceived as congruent (“in time”) and which key to press for stimuli that they perceived as incongruent (“not in time”). Stimuli in this practice block consisted of the printed word “in time,” in response to which participants were required to press the key associated with congruent stimuli, or the printed word “not in time,” in response to which participants were required to press the key associated with incongruent stimuli. Stimulus-response mappings were counterbalanced between participants. This preliminary key-mapping practice was repeated if necessary until participants reached a criterion of ≥13 of 16 trials correct. All participants used their left hand to provide the responses, since the right hand is potentially paretic following left-hemisphere stroke.
In the second practice task, participants were presented with video stimuli, first in unoccluded form to familiarize participants with the appearance of the motion trajectories, followed by (occluded) congruent and incongruent versions of the same stimuli. Participants then performed a 32 trial video practice block in which they were randomly presented with the congruent and incongruent video stimuli and were instructed to indicate whether they perceived the stimulus to be “in time” (i.e., congruent) or “not in time” (i.e., incongruent). Participants were instructed to respond as quickly and accurately as possible and received performance feedback after each response. Trials timed out 5 s after video offset, at which time a prompt would appear asking the participant to attempt to respond faster.
Participants were encouraged to ask questions about the task requirements at any time during practice, in which case the trial was paused manually and the experimenter provided additional instruction. The stimuli used in the practice block were not used in the experimental block. At the end of practice, the experimental stimuli were played once in unoccluded form. Next, the first experimental block was administered. The task was the same as during video practice, except that performance feedback was not provided. Participants received a ~2 minute break after every 48 trials.
Additional Behavioral Tasks
Pantomimed tool use production task
Pantomime production was used as a measure of praxis ability. Participants were presented with one tool at a time. The instruction was to show how to correctly use the tool, without actually holding it, using the non-paretic left hand. Participants completed one practice trial with feedback from the experimenter. The 10 experimental trials were video recorded and scored offline for accuracy according to a detailed error taxonomy by a trained coder who obtained at least 85% agreement with previous coders (Buxbaum, Kyle, & Menon, 2005). Based on normative performance, scores <.895 are abnormal (Buxbaum, Kyle, et al., 2005; see Table 1).
Grip strength and finger tapping tasks
To obtain an index of motor production ability, grip strength and finger tapping frequency were measured in both hands. Three grip strength trials were performed for each hand and the ratio of mean grip force (kg) for the left versus right hand was obtained. For finger tapping frequency measurements, patients were instructed to tap as fast as they could on the counter’s lever for 10 s using their index finger. Two trials were performed for each index finger, and the ratio of mean tapping speed for the left versus right hand was obtained (see Table 1).
Pantomimed tool use recognition task
To assess participants’ ability to correctly identify pantomimed tool use actions, a 24-item pantomimed tool use recognition task was administered (Buxbaum, Kyle, et al., 2005; Kalénine et al., 2010). Participants heard an action verb-derived noun in the gerund form repeated twice, and simultaneously viewed the written noun (e.g., sawing). Next, they were presented with two videos of an actor performing pantomimed tool use actions. In one video, the action matched the noun (e.g., sawing), while the other did not because of a spatially incorrect hand posture, arm posture, or incorrect amplitude and timing. The task was to select the action that matched the noun. (A pre-test ensured that participants comprehended the verbs, see Buxbaum, Kyle, et al., 2005.) The order of the target and foil action within a trial was randomized. There were no time constraints for responding. Based on normative data, scores <.87 are abnormal (Buxbaum, Kyle, et al., 2005; see Table 1).
Imaging Methods
For 20 patients, research quality high-resolution whole-brain T1-weighted magnetic resonance (MR) images were collected on a 3T scanner (Siemens Trio, repetition time = 1620 ms, echo time = 3.87 ms, field of view = 192 × 256 mm, 1 × 1 × 1 mm voxels) or a 1.5 T scanner (Siemens Sonata, repetition time = 3000 ms, echo time = 3.54 ms, field of view = 24 cm, 1.25 × 1.25 × 1.2 mm voxels) using a Siemens eight-channel head coil. Seven patients were contraindicated for MR imaging and underwent whole-brain research quality computed tomography (CT) scans without contrast (60 axial slices, 3–5 mm slice thickness) on a 64-slice Siemens SOMATOM Sensation scanner.
For patients with research quality MR scans, lesions were segmented manually by trained research assistants on the patients’ T1-weighted MR images. Images were then registered to standardized space [the Montreal Neurological Institute (MNI) “Colin27” template brain; Holmes et al., 2015] using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann, & Gee, 2006; http://www.picsl.upenn.edu/ANTS). Subsequently, each lesion map was binarized so that lesioned voxels had a value of 1, and preserved voxels had a value of 0. To ensure that no errors had occurred during this process, lesion maps were inspected by the team neurologist who was naive to the behavioral data. For patients with CT scans, the team neurologist drew the lesions directly onto the Colin27 template brain using MRI-cron (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) after rotating its pitch to match the pitch of the patient’s scan. Schnur et al. (2009) have previously shown high intra and inter-rater reliability for this method.
Lesion Proportion Difference Analysis
To identify brain regions that are critically involved in motion prediction, a lesion proportion difference analysis was performed to identify voxels that are significantly more likely to be lesioned in low performing as compared to high performing patients. The 27 patients were divided into three groups of nine patients on the basis of their d’ score (a measure of participants’ sensitivity to stimuli that is unaffected by response bias) averaged across the three motion types (tool use, pantomimed tool use, non-biological). The nine lowest and nine highest scoring patients were included. Following Mirman and Graziano (2013), we excluded the middle third of patients to contrast severely impaired patients with mildly impaired/unimpaired patients, eliminating patients whose scores were ambiguous.3
We included voxels lesioned in at least 10% of these 18 patients (2 or more). Setting a lower limit for the inclusion of voxels in the analysis is a common procedure in lesion analysis, used to ensure the stability of results (Kemmerer, Rudrauf, Manzel, & Tranel, 2012). Figure 2 shows an overlap map on the Colin27 template of the lesions of the 18 patients included in the analysis. There is good coverage of the parietal and frontal lobes, the regions of greatest interest in this study.
Fig. 2.
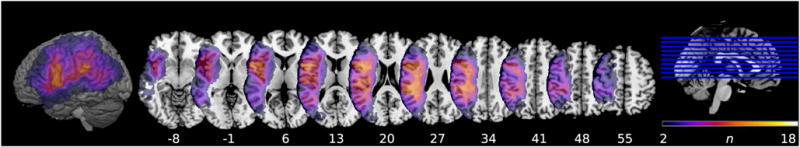
Overlap map of lesions included in the lesion proportion difference analysis (n = 18, i.e., the 9 highest and 9 lowest scoring patients on the prediction task). The map is displayed on the Colin27 template with z-coordinates of horizontal slices corresponding to MNI standardized space. The color bar represents the number of patients with lesions in a particular voxel (min = 2; max = 18). The cortical surface rendering is displayed at a search depth of 8 voxels.
For each of the included voxels, the proportion of patients with low prediction performance and a lesion in the voxel and the proportion of patients with high prediction performance and a lesion in the voxel was determined, and the observed distributions were compared to the expected distributions under the null hypothesis, resulting in a χ2 value for each voxel. The voxel-wise χ2 value map was then thresholded to 6.6349, which is the value corresponding to a significant χ2 test result at α = .01 and 1 degree of freedom (see Kemmerer et al., 2012; Mirman & Graziano, 2013 for more details of this approach). The Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) map in MRIcron was used to determine the neuroanatomic loci of voxels that surpassed the threshold. Significant clusters ≥ 100 voxels were subsequently used as predictors of behavioral performance in regression analyses (see below).
Multilevel Regression Analysis
Participants’ ability to distinguish between congruent and incongruent stimuli on the prediction task was indexed by d’. RT data for correct responses were also collected. Trials in which button presses occurred before occlusion offset or less than 250 ms after occlusion offset were removed from the analysis, as were trials with RTs > 2 SDs from the mean RT for that participant and condition. This led to elimination of approximately 5% of RTs of all correct responses for both patients and controls.
RT and d’ were analyzed using a multilevel regression approach in which prediction performance was modeled as a function of fixed behavioral and neuroanatomical factors of interest (to be described below), random intercepts for participants, and random slopes where possible. That is, because there is only one d’ value per cell of the design (one d’ per subject per condition), adding random slopes would in this case result in oversaturated models, precluding or leading to unreliable estimation of model parameters (e.g., Bates, Kliegl, Vasishth, & Baayen, 2015). Hence, we only included random slopes in the analysis of RTs, which was performed over unaggregated data.
The regressions were conducted with the “lme4” package in the R software environment (version 2.15.3; R Core Team, 2015). To detect and remove cases with undue influence on the multilevel model parameter estimates, the “influence. ME” package was used (Nieuwenhuis, te Grotenhuis, & Pelzer, 2012). Cases were regarded as too influential if their associated values of Cook’s distance were larger than 4/n, where n = number of participants. Model results presented below are based on data excluding any influential cases. To assess whether fixed factors significantly contributed to model fit, they were added individually and comparisons of models with and without the fixed factor were performed with likelihood ratio tests in which the statistic is −2 times the change in model log-likelihood, distributed as χ2 with degrees of freedom equal to the change in number of estimated parameters.
The p-values for the parameter estimates of the fixed factors were calculated using Satterthwaite’s approximation for degrees of freedom. We also report 95% confidence intervals (CI) around the parameter estimates. Marginal R2 (R2LMM(m)), which denotes the proportion of variance explained by the fixed factor(s) of a multilevel regression model (see Johnson, 2014; Nakagawa & Schielzeth, 2013), is reported as a measure of effect size.
In the first analysis, patients’ behavior was compared to controls with model comparisons that assessed the fixed factors Group (patient, control), Motion Type (tool use, pantomimed tool use, non-biological), and their interaction. A second analysis focused on the behavioral predictors of patient performance. Thus, we assessed the continuous behavioral predictors described in the Section Additional Behavioral Tasks (i.e., Praxis Ability, Grip Strength, Finger Tapping Frequency, and Pantomime Recognition) in separate model comparisons for each predictor. We also entered interaction terms for the behavioral predictors and the factor Motion Type to assess whether the strength of the behavioral predictors was conditioned by motion type.
A third regression analysis followed up on the results of the lesion proportion difference analysis performed on patients’ average d’ scores. First, we computed for each patient the % damage to each of the significant voxel clusters identified in that analysis. Subsequently, we performed separate model comparisons for each neuroanatomical cluster predictor to assess main effects of % Damage to Cluster and interactions with the factor Motion Type.
The fourth and final analysis assessed the behavioral and neuroanatomical cluster predictors simultaneously to determine whether behavior, lesion, or both influenced prediction performance when they were considered together. We first tested for the presence of a Pearson correlation between each of the behavioral and neuroanatomical cluster predictors. In case of a significant correlation, we assessed the predictive strength of the correlated predictors together in one multilevel regression model. All of the regressions in the second, third, and fourth analysis included the covariate TLV (see Table 1) to control for general effects of stroke severity on performance. The levels “control” and “non-biological” of the categorical fixed factors were treated as the baseline (reference) and model parameters were estimated for the levels “patient”, “tool use”, and “pantomimed tool use.”
RESULTS
Behavioral Results
Patients versus controls
In the model comparing d’ of patients and controls, including the predictor Group (patient, control) improved model fit relative to a reduced model that included only a random intercept for each participant. Patients were overall less accurate than controls. Adding the interaction term Group × Motion Type (tool use, pantomimed tool use, non-biological) relative to a reduced model that included only main effects for Group and Motion Type and random intercepts also improved model fit. Figure 3 shows the observed mean d’ scores for patients and controls separately for tool use, pantomimed tool use and non-biological motions. Table 2 shows the χ2 test results for each of the model comparisons, the marginal R2 of the full models, as well as the β estimates with associated SE, 95% CI and p value of each pairwise comparison of interest. The pairwise comparisons indicate that the interaction is driven by the fact that the difference between predicting non-biological motions versus pantomimed actions as well as between tool use versus pantomimed actions was significantly different between controls and patients, while the difference between non-biological motions versus tool use actions was not.
Fig. 3.
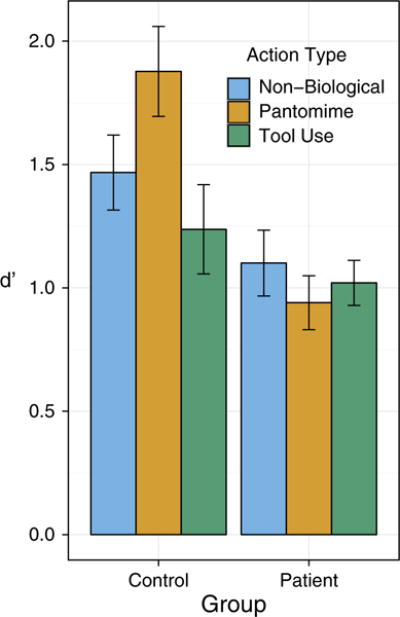
Observed mean prediction performance as indexed by d’ for Patients (right panel) and Controls (left panel) plotted separately for each level of Motion Type (tool use, pantomimed tool use, non-biological; see legend). Error bars represent ±1 SE.
Table 2.
χ2 test results for the analyses comparing patients’ prediction performance with that of controls (see body text for description of reduced models)
| Predictor | Pairwise comparisons | β | SE | 95% CI | pt |
|
χ2 | df |
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Patients vs. controls | −.582 | .198 | [−.980, − .183] | .006 | .092 | 7.76 | 1 | .005 | ||
| Group × Motion Type | Slope: pantomime vs. non-biological | −.571 | .227 | [− 1.019, − .123] | .013 | .106 | 10.88 | 2 | .004 | ||
| Slope: tool use vs. non-biological | .150 | .227 | [− .298, .598] | .511 | |||||||
| Slope: pantomime vs. tool use | −.721 | .227 | [− 1.169, − .273] | .002 |
Note. The marginal R2 of the full models, as well as β estimates with associated SE, 95% CI, and p value for the pairwise comparison(s) of interest are also displayed.
We also tested models with the same predictors, but with RT as the dependent variable. Adding the predictor Group improved model fit relative to a reduced model that included only random intercepts and random slopes for participants [χ2(1) = 11.07; p < .001]. Patients were slower overall than controls. Inclusion of the interaction Group × Motion Type did not significantly improve model fit relative to the reduced model without the interaction term (p = .707). Given the lack of effects on RT beyond the expected main effect of Group, the remaining analyses focus on d’.
Analysis of behavioral predictors
Using the data from patients only, we next examined the behavioral predictors Praxis Ability, Grip Strength, Finger Tapping Frequency, and Pantomime Recognition to evaluate their association with patients’ prediction d’ scores. A strong positive Pearson correlation was observed between grip strength and finger tapping frequency (r = .82; p < .001). We, therefore, used the average of these measures as a composite index of Motor Production Ability. As shown in Table 3, including the predictors Motor Production Ability and Praxis Ability (in separate models) significantly improved the fit of these models relative to reduced models that included TLV and random intercepts. This indicates that motion prediction performance is positively associated with both motor production ability and praxis ability, even when controlling for general non-specific effects of stroke severity (as indexed by TLV). By contrast, including the predictor Pantomime Recognition did not significantly improve model fit relative to a reduced model that included TLV and random intercepts.
Table 3.
χ2 test results for model comparisons assessing main effects of the behavioral predictors of patients’ prediction performance (see body text for description of reduced models)
| Predictors | β | SE | 95% CI | pt |
|
χ2 | df |
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| Motor production ability | .557 | .222 | [.103, 1.010] | .019 | .087 | 5.59 | 1 | .018 | ||
| Praxis ability | 2.924 | 1.054 | [.777, 5.071] | .010 | .103 | 6.72 | 1 | .010 | ||
| Pantomime recognition | 1.876 | 1.215 | [− .601, 4.352] | .135 | .040 | 2.28 | 1 | .131 |
Note. The marginal R2 of the full model, as well as β estimates with associated SE, 95% CI, and p value of the behavioral predictors are also displayed.
We next assessed (separately for each of the three behavioral predictors) whether adding the interaction term behavioral predictor × Motion Type improved model fit relative to reduced models that included only main effects for the behavioral predictor, Motion Type, TLV, and random intercepts. None of these model comparisons approached significance (all ps > .486). This indicates that reduced motor production ability and praxis ability were equivalently associated with deficits in all three prediction conditions.
Neuroanatomical Results
Lesion proportion difference analysis
As shown in Figure 4, the lesion proportion difference analysis identified three neuronatomical clusters ≥100 voxels that, when damaged, are associated with low mean d’ scores collapsed across tool use, pantomimed tool use and non-biological motion types. A dorsal cluster in frontal cortex included voxels in the precentral gyrus and middle frontal gyrus, extending slightly into the postcentral gyrus (81 voxels). A second cluster included voxels in the insula. Finally, a region in the parietal cortex included voxels in the inferior parietal lobe, superior parietal lobe and the postcentral gyrus. Table 4 lists the coordinates of the peak value of each of the three clusters.
Fig. 4.

Lesion proportion difference map for mean prediction performance (d’) displaying voxels associated with χ2 values surpassing a statistical threshold for significance (α = .01). Voxels are displayed on the Colin27 template; z-coordinates of horizontal slices correspond to MNI standardized space. The cortical surface rendering is displayed at a search depth of 8 voxels. Color bars represent χ2 value ranges.
Table 4.
Significant (α = .01) clusters from the lesion proportion difference analysis
| Cluster | No. of voxels | x | y | z | Location of peak value |
|---|---|---|---|---|---|
| 1 | 3942 | −25 | −3 | 37 | Left dorsal frontal cortex |
| 2 | 234 | −38 | 11 | −5 | Left insula |
| 3 | 140 | −49 | −40 | 53 | Left inferior parietal lobe |
Note. X, Y, and Z coordinates represent the location of the peak value within each cluster in MNI standardized space. Only clusters containing ≥100 voxels are reported.
Analysis of neuroanatomical predictors
We next assessed (in separate models) whether damage to these clusters selectively affected the ability to predict the three motion types. For each cluster, the comparison between a reduced model that only included random intercepts and TLV and a full model that additionally included a main effect of % Damage to Cluster was significant, corroborating (not surprisingly) the results of the lesion proportion difference analysis. Of greater interest, follow-up model comparisons assessed, for each cluster, whether damage was differentially associated with deficits in prediction of the different motion types by comparing reduced models that included only main effects of % Damage to Cluster, Motion Type, random intercepts and TLV to models that additionally included the interaction % Damage to Cluster × Motion Type. The interaction term did not approach significance for any of the clusters (all ps > .375). Thus, for each cluster, an increase in % damage was equivalently associated with deficits in the three prediction conditions.
Analysis of neuroanatomical and behavioral predictors
It is possible that behavioral predictors, rather than having a primary association with d’ scores, are merely subserved by brain regions having neuroanatomic proximity to or overlap with the regions critical for prediction. This hypothesis would predict that when neuroanatomic and behavioral predictors are included in the same model of prediction ability, only the former (or possibly neither) will remain significant. The final models we assessed included both neuroanatomic and behavioral predictors of d’ scores.
Given that we can assume independence of un-correlated predictors, we first tested for a correlation between % damage to each of our three neuroanatomic clusters and the behavioral predictors. Motor Production Ability correlated significantly with all of the neuroanatomical predictors (r ranging from −.54 to −.66; ps < .01). Praxis Ability correlated significantly only with % Damage to IPL Cluster (r = −.41; p = .034; other ps > .183).
Given these correlations, we performed model comparisons to assess changes in model fit when adding % Damage to Cluster to a reduced model that included random intercepts, TLV and Motor Production Ability, separately for each cluster. The addition of % Damage to Cluster resulted in a significant improvement in model fit for all clusters (ps < .05). Conversely, however, adding the predictor Motor Production Ability to a reduced model that included random intercepts, TLV and one of the cluster predictors (separately for each cluster) did not lead to a significant improvement in model fit for any of the clusters (ps > .166).
The same approach was followed for Praxis Ability and % Damage to IPL Cluster. Adding % Damage to Cluster to a reduced model that included random intercepts, TLV and Praxis Ability led to a significant improvement in model fit (p = .012) while adding Praxis Ability to a reduced model that included random intercepts, TLV and % Damage to IPL Cluster led to an improvement in model fit that just missed the significance threshold (p = .05). These analyses suggest that motor production ability is predictive of d’ only when damage to insula, IPL, or dorsal frontal cortex is not considered. Praxis ability, however, remains a significant predictor even when considering IPL damage.
DISCUSSION
This study identified neural substrates and behavioral correlates associated with the prediction of human and non-biological motion trajectories in patients with left-hemisphere stroke. Relative to the prediction performance of controls, patients’ performance was impaired. This impairment was associated with two distinct measures of action ability, limb apraxia and hemiparesis, but not with a measure of action recognition. Moreover, action impairment negatively affected prediction of not only human, but also non-biological motion. Furthermore, impaired prediction of both motion types was equivalently associated with voxel clusters in the left inferior parietal and dorsal frontal cortices as well as the left insula; motor production ability only predicted performance when lesions in these regions were not also taken into account. By contrast, praxis ability remained a significant predictor of performance even when IPL damage was considered. In all cases, these predictors remained significant even when we controlled for total lesion volume, a proxy for stroke severity.
The importance of the integrity of sensorimotor regions in left parietal and dorsal frontal cortex for motion prediction is consistent with previously observed neuroimaging activations in these regions in neurologically intact individuals engaged in prediction tasks (Balser et al., 2014; Cross et al., 2013; Schubotz & von Cramon, 2002; Stadler et al., 2011; van Elk, 2014; Wiener et al., 2010; Yang, 2014). Although bilateral activations are commonly reported, meta-analyses indicate that left hemisphere activations are most reliable (Wiener et al., 2010; Yang, 2014). Van Elk (2014), for example, found activations in left PM and IPL when participants observed an actor holding a tool (e.g., a wine opener), a target (wine bottle), and a distractor object (soda bottle) and were asked to predict the direction in which the tool would be moved.
Importantly, our lesion data strongly suggest that these regions in the left hemisphere play a critical (and not merely supportive or incidental) role in these tasks (see also Schubotz et al., 2004; Stadler, Ott, et al., 2012). We, furthermore, observed critical involvement of a cluster located in the insula. Although the left insula has not been implicated in prediction of perceptual stimuli, several recent neuroimaging studies have reported activations in the insula during motor planning (Bernardi et al., 2013; Di Russo et al., 2016) which, we will suggest below, is closely associated with prediction. The critical role of the insula cluster may also be related to the proximity of this cortical region to the superior longitudinal fasciculus, a prominent white matter pathway connecting frontal and parietal cortices (Makris et al., 2005; Schmahmann et al., 2007).
The present finding that motor production ability is associated with prediction task performance only when frontoparietal lesions are not considered is consistent with previous work focusing on the relationship between hemiparesis and the ability to simulate one’s own actions. Johnson (2000) asked hemiplegic patients to indicate how they would grasp a dowel if they were to reach out and perform the action. Patients were able to perform the simulation task unless their lesions involved frontoparietal cortex (see also Johnson, 2002). Given these reports, the present association between motor production ability and prediction ability may be explained by the co-occurrence of hemiparesis and lesions in the left premotor and parietal cortices in our sample. That is, motor production ability and prediction ability, rather than being directly causally related, may simply share similar or adjacent neuroanatomic substrates.
Unlike hemiparesis, the effect of limb apraxia (as indexed by performance on the pantomime production task) remained significant even when considering left IPL damage, suggesting a robust association between praxis ability and motion prediction ability. Apraxic patients have previously been shown to be impaired at planning and simulating their own actions, whereas motor tasks that can be performed adequately by relying on visual feedback (such as reaching to targets under visual guidance) are relatively intact (e.g., Buxbaum, Johnson-Frey, et al., 2005; Coslett et al., 2008; Dawson et al., 2010; Eidenmuller et al., 2014; Jax et al., 2006; Mutha et al., 2010; Ochipa et al., 1997; Sirigu et al., 1996; Wheaton et al., 2009).
For example, Buxbaum, Johnson-Frey et al. (2005) found that apraxics performed abnormally on Johnson’s (2000) grip simulation task, but executed the same grasps successfully. Similarly, Sirigu et al. (1996; see also Jeannerod, 1986) found that apraxics were impaired at simulating finger opposition sequences, with performance on the actual finger opposition task within the control range. Lastly, Dawson et al. (2010) and Eidenmuller et al. (2014) observed relatively impaired anticipatory grip force scaling in apraxics when asked to lift everyday objects (but see Li, Randerath, Goldenberg, & Hermsdörfer, 2011). Given that the anticipatory planning component of action production appears to be affected in apraxia, the present results are consistent with the proposal that action prediction relies on mechanisms that are shared with motor planning, which may include use of the same forward models (Friston et al., 2011; Wolpert et al., 2003).
Importantly, however, praxis ability was associated with deficits in prediction of both human action and non-biological motion, and there was no indication that these relationships were of different strengths. These data are broadly supportive of the prediction account of Schubotz (2007) in which forward models may be ideally suited to predict human actions, but also deployed to predict non-biological motion. By contrast, the observed robust association between praxis ability and the ability to predict non-biological motion is not well explained by the perceptual associative account of prediction by Pickering and Garrod (2013) in which forward models are used only to predict actions performed by agents that are “similar enough” to the observer, while trajectories lacking human characteristics are predicted based on past perceptual experience (see also Springer et al., 2013; Stadler, Springer, et al., 2012). This account would seem to suggest that non-biological motion should be predicted normally in at least some patients with apraxia.
Notably, we observed that neurologically intact controls were better at predicting pantomimed tool use actions than both actual tool use actions and non-biological motion. At present, we can only speculate about the cause of this pattern. One possibility is that controls paid closer attention to the pantomimed actions than to the other two motion types, perhaps because the former were more novel. However, this account predicts that patients, too, should benefit from the combination of novelty and increased attention, unless one posits an additional deficit in patients’ ability to allocate additional attention to the task. Further research is required to explain this aspect of the findings.
Finally, the absence of a significant association between action recognition and prediction enables us to hypothesize that prediction may rely on processes further “downstream” in the motor planning process than those tapped in action recognition tasks. Additionally, the weak association between action recognition and prediction is inconsistent with accounts suggesting that action recognition is critically dependent upon action simulation (Rizzolatti et al., 2001; Urgesi et al., 2014). Consistent with recent prior findings from our laboratory (Kalénine et al., 2010; Tarhan et al., 2015) and others (e.g., Negri et al., 2007) showing that brain regions beyond the human “mirror system” are critical for action recognition, the present data suggest that action recognition is not reducible to action simulation.
CONCLUSION
It has been argued that action production and prediction rely upon shared underlying mechanisms (e.g., Aglioti et al., 2008; Colling et al., 2014; Stapel et al., 2016). We report data from left-hemisphere stroke patients that enables us to refine this account. Consistent with the hypothesis that forward models are used both for action planning and prediction (Friston et al., 2011; Wolpert et al., 2003), our data suggest that the feed-forward components of action trajectory planning are critical for prediction of human as well as non-biological motion trajectories. Moreover, our results suggest that the left insula, inferior parietal, and dorsal frontal cortices are critical to the ability to predict both motion types. On first inspection, these data may appear to be inconsistent with widely cited prior findings that action expertise has a specific effect on the ability to predict the relevant action, but not other related skills (Aglioti et al., 2008). However, action planning capacities are presumably not all-or-none. Motor planning mechanisms may be used for prediction of all types of events that contain spatiotemporal trajectory information, with high levels of motor expertise serving to “tune” the precision with which exquisitely timed events can be predicted.
Acknowledgments
This research was supported by National Institutes of Health grants R01NS099061 (L.J.B.) and T32HD071844 (M.M.D.W., trainee), and the Moss Rehabilitation Research Institute Peer Review Committee. We thank Branch Coslett for help with lesion segmentation, Christine Watson for script writing and suggestions regarding neuroanatomical analyses, and several research assistants, in particular Leyla Tarhan, for pantomime coding and help with data collection.
Footnotes
Due to the dominance of the left hemisphere (in right-handers) for bimanual control of skilled actions, apraxia occurs bilaterally after left hemisphere lesions. Based on the limited reliability of testing for apraxia in patients who may have right hemiparesis, apraxia is typically tested only in the left hand, as we have done here.
Given a prior suggestion that simulation processes might be selectively affected for the paretic hand (van Elk et al., 2010), we initially assessed whether patients were selectively impaired at prediction of others’ actions performed with the hand corresponding to the contralesional (right) hand by comparing statistical models with and without an interaction that included both video orientation (i.e., flipped, non-flipped) and group. Inclusion of the video orientation factor did not improve model fit for either of our dependent variables (d’ and RT; ps > .339); consequently, the factor was excluded from the remaining analyses and will not be further discussed. See Section Multilevel Regression Analysis for details of our statistical approach.
To exclude the possibility that patients’ membership to the low and high scoring groups was due to general effects of stroke severity, total lesion volume (TLV) in cm3 of patients included in the two groups was submitted to a Mann-Whitney test. TLV was equivalent in low and high scoring patients (median = 103.94 cm3 vs. median = 73.68 cm3; p = .49).
The authors declare no conflict of interest.
References
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nature Neuroscience. 2008;11(9):1109–1116. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. (3 SPEC. ISS.) [DOI] [PubMed] [Google Scholar]
- Balser N, Lorey B, Pilgramm S, Stark R, Bischoff M, Zentgraf K, Munzert J. Prediction of human actions: Expertise and task-related effects on neural activation of the action observation network. Human Brain Mapping. 2014;35(8):4016–4034. doi: 10.1002/hbm.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. Simulation, situated conceptualization, and prediction. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364(1521):1281–1289. doi: 10.1098/rstb.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DM, Kliegl R, Vasishth S, Baayen H. Parsimonious mixed models. arXiv Preprint arXiv: 1506.04967. 2015:1–27. [Google Scholar]
- Bernardi G, Ricciardi E, Sani L, Gaglianese A, Papasogli A, Ceccarelli R, Pietrini P. How skill expertise shapes the brain functional architecture: An fMRI study of visuo-spatial and motor processing in professional racing-car and naive drivers. PLoS One. 2013;8(10):e77764. doi: 10.1371/journal.pone.0077764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattan VC, Baker DH, Tipper SP. Spatiotemporal judgments of observed actions: Contrasts between first- and third-person perspectives after motor priming. Journal of Experimental Psychology: Human Perception and Performance. 2015;41(5):1236–1246. doi: 10.1037/xhp0000079. [DOI] [PubMed] [Google Scholar]
- Bubic A, von Cramon DY, Schubotz RI. Prediction, cognition and the brain. Frontiers in Human Neuroscience. 2010;4:25. doi: 10.3389/fnhum.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43(6):917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cerebral Cortex. 2005;15(8):1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Anzellotti S, Strnad L, Lingnau A. Embodied cognition and mirror neurons: A critical assessment. Annual Review of Neuroscience. 2014;37:1–15. doi: 10.1146/annurev-neuro-071013-013950. [DOI] [PubMed] [Google Scholar]
- Clark A. Surfing uncertainty: Prediction, action, and the embodied mind. Oxford: Oxford University Press; 2015. [Google Scholar]
- Colling LJ, Thompson WF, Sutton J. The effect of movement kinematics on predicting the timing of observed actions. Experimental Brain Research. 2014;232(4):1193–1206. doi: 10.1007/s00221-014-3836-x. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Buxbaum LJ, Schwoebel J. Accurate reaching after active but not passive movements of the hand: Evidence for forward modeling. Behavioural Neurology. 2008;19(3):117–125. doi: 10.1155/2008/972542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Goulon C, Nazarian B, Craig C. Using time-to-contact information to assess potential collision modulates both visual and temporal prediction networks. Frontiers in Human Neuroscience. 2008;2:10. doi: 10.3389/neuro.09.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Stadler W, Parkinson J, Schütz-Bosbach S, Prinz W. The influence of visual training on predicting complex action sequences. Human Brain Mapping. 2013;34(2):467–486. doi: 10.1002/hbm.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G. Action mirroring and action understanding: An alternative account. In: Haggard P, Rosetti Y, Kawato M, editors. Sensorymotor foundations of higher cognition (Attention and Performance XXII) Oxford: Oxford University Press; 2008. pp. 435–459. [Google Scholar]
- Dawson AM, Buxbaum LJ, Duff SV. The impact of left hemisphere stroke on force control with familiar and novel objects: Neuroanatomic substrates and relationship to apraxia. Brain Research. 2010;1317:124–136. doi: 10.1016/j.brainres.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Lucci G, Sulpizio V, Berchicci M, Spinelli D, Pitzalis S, Galati G. Spatiotemporal brain mapping during preparation, perception, and action. Neuroimage. 2016;126:1–14. doi: 10.1016/j.neuroimage.2015.11.036. [DOI] [PubMed] [Google Scholar]
- Eidenmuller S, Randerath J, Goldenberg G, Li Y, Hermsdorfer J. The impact of unilateral brain damage on anticipatory grip force scaling when lifting everyday objects. Neuropsychologia. 2014;61(1):222–234. doi: 10.1016/j.neuropsychologia.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: An empirical study of impact in cognitive neuroscience. Journal of Cognitive Neuroscience. 2005;17(6):850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mattout J, Kilner J. Action understanding and active inference. Biological Cybernetics. 2011;104(1–2):137–160. doi: 10.1007/s00422-011-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Bressler SL. Past makes future: Role of pFC in prediction. Journal of Cognitive Neuroscience. 2015;27(4):639–654. doi: 10.1162/jocn_a_00746. [DOI] [PubMed] [Google Scholar]
- Graf M, Reitzner B, Corves C, Casile A, Giese M, Prinz W. Predicting point-light actions in real-time. NeuroImage. 2007;36(Suppl. 2):T22–T32. doi: 10.1016/j.neuroimage.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 2015;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ, Moll AD. Deficits in movement planning and intrinsic coordinate control in ideomotor apraxia. Journal of Cognitive Neuroscience. 2006;18(12):2063–2076. doi: 10.1162/jocn.2006.18.12.2063. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The formation of finger grip during prehension. A cortically mediated visuomotor pattern. Behavioural Brain Research. 1986;19(2):99–116. doi: 10.1016/0166-4328(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Johnson PCD. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods in Ecology and Evolution. 2014;5(9):944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SH. Imagining the impossible: Intact motor representations in hemiplegics. Neuroreport. 2000;11:729–732. doi: 10.1097/00001756-200003200-00015. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Sprehn G, Saykin AJ. Intact motor imagery in chronic upper limb hemiplegics: Evidence for activity-independent action representations. Journal of Cognitive Neuroscience. 2002;14(6):841–852. doi: 10.1162/089892902760191072. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex. 2012;48(7):826–848. doi: 10.1016/j.cortex.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. The western aphasia battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Knoblich G, Flach R. Predicting the effects of actions: Interactions of perception and action. Psychological Science. 2001;12(6):467–472. doi: 10.1111/1467-9280.00387. [DOI] [PubMed] [Google Scholar]
- Li Y, Randerath J, Goldenberg G, Hermsdörfer J. Size-weight illusion and anticipatory grip force scaling following unilateral cortical brain lesion. Neuropsychologia. 2011;49(5):914–923. doi: 10.1016/j.neuropsychologia.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Makris S, Urgesi C. Neural underpinnings of superior action prediction abilities in soccer players. Social Cognitive and Affective Neuroscience. 2013;10(3):342–351. doi: 10.1093/scan/nsu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Graziano KM. The neural basis of inhibitory effects of semantic and phonological neighbors in spoken word production. Journal of Cognitive Neuroscience. 2013;25(9):1504–1516. doi: 10.1162/jocn_a_00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan D, Lohse KR, Hodges NJ. An action-incongruent secondary task modulates prediction accuracy in experienced performers: Evidence for motor simulation. Psychological Research. 2015:1–14. doi: 10.1007/s00426-015-0672-y. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48(13):3855–3867. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4(2):133–142. [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology. 2007;24(8):795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis R, Te Grotenhuis M, Pelzer B. Influence. ME: Tools for detecting influential data in mixed effects models. R Journal. 2012;4(2):38–47. [Google Scholar]
- Ochipa C, Rapcsak SZ, Maher LM, Rothi LJ, Bowers D, Heilman KM. Selective deficit of praxis imagery in ideomotor apraxia. Neurology. 1997;49(2):474–480. doi: 10.1212/wnl.49.2.474. [DOI] [PubMed] [Google Scholar]
- Pickering MJ, Garrod S. An integrated theory of language production and comprehension. The Behavioral and Brain Sciences. 2013;36(4):329–347. doi: 10.1017/S0140525X12001495. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. Retrieved from https://www.R-project.org/ [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Roth MJ, Synofzik M, Lindner A. The cerebellum optimizes perceptual predictions about external sensory events. Current Biology. 2013;23(10):930–935. doi: 10.1016/j.cub.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, De Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: Towards a new framework. Trends in Cognitive Sciences. 2007;11(5):211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Sakreida K, Tittgemeyer M, von Cramon DY. Motor areas beyond motor performance: Deficits in serial prediction following ventrolateral premotor lesions. Neuropsychology. 2004;18(4):638–645. doi: 10.1037/0894-4105.18.4.638. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: An fMRI study. Neuroimage. 2002;15(4):787–796. doi: 10.1006/nimg.2001.1043. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y, Pierrot-Deseilligny C. Congruent unilateral impairments for real and imagined hand movements. Neuroreport. 1995;6(7):997–1001. doi: 10.1097/00001756-199505090-00012. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel J-R, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273(5281):1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Springer A, Brandstädter S, Prinz W. Dynamic simulation and static matching for action prediction: Evidence from body part priming. Cognitive Science. 2013;37(5):936–952. doi: 10.1111/cogs.12044. [DOI] [PubMed] [Google Scholar]
- Springer A, Prinz W. Action semantics modulate action prediction. Quarterly Journal of Experimental Psychology (2006) 2010;63(11):2141–2158. doi: 10.1080/17470211003721659. [DOI] [PubMed] [Google Scholar]
- Stadler W, Ott DVM, Springer A, Schubotz RI, Schütz-Bosbach S, Prinz W. Repetitive TMS suggests a role of the human dorsal premotor cortex in action prediction. Frontiers in Human Neuroscience. 2012;6:20. doi: 10.3389/fnhum.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler W, Schubotz RI, von Cramon DY, Springer A, Graf M, Prinz W. Predicting and memorizing observed action: Differential premotor cortex involvement. Human Brain Mapping. 2011;32(5):677–687. doi: 10.1002/hbm.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler W, Springer A, Parkinson J, Prinz W. Movement kinematics affect action prediction: Comparing human to non-human point-light actions. Psychological Research. 2012;76(4):395–406. doi: 10.1007/s00426-012-0431-2. [DOI] [PubMed] [Google Scholar]
- Stapel JC, Hunnius S, Meyer M, Bekkering H. Motor system contribution to action prediction: Temporal accuracy depends on motor experience. Cognition. 2016;148:71–78. doi: 10.1016/j.cognition.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Tarhan L, Watson C, Buxbaum L. Shared and distinct neuroanatomic regions critical for tool-related action production and recognition: Evidence from 131 left-hemisphere stroke patients. Journal of Cognitive Neuroscience. 2015;27(12):2491–2511. doi: 10.1162/jocn_a_00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Avenanti A. Neuroanatomical substrates of action perception and understanding: An anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Frontiers in Human Neuroscience. 2014;8:344. doi: 10.3389/fnhum.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M. The left inferior parietal lobe represents stored hand-postures for object use and action prediction. Frontiers in Psychology. 2014;5:1–12. doi: 10.3389/fpsyg.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, Crajé C, Beeren ME, Steenbergen B, van Schie HT, Bekkering H. Neural evidence for compromised motor imagery in right hemiparetic cerebral palsy. Frontiers in Neurology. 2010;1:1–7. doi: 10.3389/fneur.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannuscorps G, Caramazza A. Typical action perception and interpretation without motor simulation. Proceedings of the National Academy of Sciences of the United States of America. 2015;113(1):86–91. doi: 10.1073/pnas.1516978112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Buxbaum LJ. A distributed network critical for selecting among tool-directed actions. Cortex. 2015;65:65–82. doi: 10.1016/j.cortex.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton L, Fridman E, Bohlhalter S, Vorbach S, Hallett M. Left parietal activation related to planning, executing and suppressing praxis hand movements. Clinical Neurophysiology. 2009;120(5):980–986. doi: 10.1016/j.clinph.2009.02.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub PE, Coslett HB. Implicit timing activates the left inferior parietal cortex. Neuropsychologia. 2010;48(13):3967–3971. doi: 10.1016/j.neuropsychologia.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philosophical Transactions of the Royal Society of London. 2003;358(1431):593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Ghahramani Z, Jordan M. An internal model for sensorimotor integration. Science. 1995;269(5232):14–16. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Yang J. The influence of motor expertise on the brain activity of motor task performance: A meta-analysis of functional magnetic resonance imaging studies. Cognitive, Affective & Behavioral Neuroscience. 2014;15(2):381–394. doi: 10.3758/s13415-014-0329-0. [DOI] [PubMed] [Google Scholar]


