Abstract
Objectives
Prednisone is a widely used anti-inflammatory for a variety of conditions. While oral liquid formulations of prednisone enable weight-based dosing, children frequently find them to be objectionable due to bitter taste. This limitation of prednisone can adversely impact patient acceptance and may result in non-compliance. Efforts to mask flavours often result in poorly controlled, heterogeneous particle distributions and can provide ineffective taste masking. The present work utilized a novel drug delivery technology developed by Orbis Biosciences, Inc., to create an oral taste-masked formulation of prednisone.
Methods
The study examined the palatability of Orbis’ microsphere prednisone formulation in healthy young adults (n = 24). Four test articles were used in the study including a reference formulation (Roxanne Laboratories), a control and the test formulation (Orbis) prepared in two different ways. Study participants were randomized in a crossover design.
Key findings
Results indicated that the test prednisone formulation was indistinguishable from the control, and both were preferable to the reference formulation in every category of palatability assessed using a validated 9-point Hedonic Scale. The data also suggested that preparing the microsphere suspension immediately before administration results in the most ideal palatability properties.
Conclusions
In conclusion, the novel microsphere formulation technology was effective in taste-masking prednisone.
Keywords: immediate release, microsphere, palatability, prednisone, taste-masking
Introduction
In 2007, the World Health Assembly underscored the importance of providing safe, effective and proven medicines for children.[1] The widespread lack of paediatric-specific drug products, however, forces medical providers and pharmacists to use alternative solutions to treat their paediatric patients. These alternatives are not backed by bioavailability, stability and safety studies.[2,3] Extemporaneously prepared oral drug formulations can also be plagued by poor palatability characteristics, which can compromise patient adherence.[4] This lack of child-friendly formulations affects 40% of the global population, subjecting paediatric patients to avoidable adverse drug events, reduced compliance with medication regimens, limited access to new medications and prolonged treatable illnesses.[5] The pressing need for child-friendly, palatable medications suitable for administration to both infants and young children has been stressed (through regulatory guidance) by both the European Medicines Agency and the United States Food and Drug Administration.
Prednisone is a bitter-tasting corticosteroid used for its anti-inflammatory and immunosuppressant effects to treat a wide variety of conditions in both adults and children. For paediatric patients, an alternative form of administration can be produced by crushing solid oral prednisone formulations to avoid swallowing difficulties or achieve weight-based dosing. This approach, however, creates extremely poor taste and mouth feel properties which are objectionable to paediatric patients. While these limitations can be somewhat mitigated by the use of proprietary oral liquid formulations of prednisone, such products still retain their profoundly bitter taste characteristics. It is estimated that approximately half of children refuse to take even a liquid form of prednisone, with the large majority of those reporting bad taste as the single major reason for non-compliance.[3] Efforts to mask flavours using sweetening agents, coatings, agglomeration or microencapsulation often result in poorly controlled, heterogeneous particle size distributions that result in a gritty or granular mouth feel and can provide ineffective taste masking, characteristics that may also compromise patient acceptance.[6]
To address the challenge of palatable, paediatric-friendly drug formulations, Orbis Biosciences Inc. (Lenexa, KS, USA), has developed an innovative drug delivery platform that produces microspheres with specific physicochemical properties, which can be tailored to a wide variety of active pharmaceutical ingredients. This technology was used in the creation of a new taste-masked microsphere formulation containing prednisone. Herein, we report the results from the first human evaluation of this formulation, an effort to assess its palatability (both taste and mouth feel) and patient acceptance and provide ‘proof of concept’ regarding the potential utility of this formulation technology for children.
Materials and Methods
Ethical consideration
This study was approved by the Western IRB (WIRB) on 17 November 2015 (WIRB PRO NUM: 20152235) and was fully compliant with federal privacy regulations under the Health Insurance Portability and Accountability Act. All study-related procedures and data collection were performed after obtaining written informed consent from participants. All participants entering the study completed it without evidence of significant adverse events associated with the test articles or the study procedures. The lack of significant adverse effects was expected given that none of the test articles were swallowed.
Study design
This study used a single-blind, randomized, four-way crossover design. Participants in the study were blinded to identity (source) of the investigational articles they received. Additionally, investigators involved in data analysis and interpretation remained blinded to the identity of the test articles until all analyses were completed. The blind document was maintained by the research pharmacy service of the Arkansas Children's Research Institute.
Four test articles were used in this study. (1) Reference – prednisone oral solution USP 5 mg/5 ml (10 mg dose in 10 ml total volume) (Roxanne Laboratories, Columbus, OH, USA). (2) Test 24 h – prednisone-loaded microsphere suspension (10 mg dose in 10 ml total volume) prepared at least 24 hours in advance of taste test (Orbis Biosciences Inc.). (3) Test 5 min – prednisone-loaded microsphere suspension (10 mg dose in 10 ml total volume) prepared approximately 5 min before the taste test. (4) Control – prednisone-free microsphere suspension (10 ml total volume). The Reference article is a currently marketed proprietary formulation of premixed prednisone by Roxanne Laboratories, which contains prednisone and the following other excipients/carriers: alcohol (5%), citric acid, disodium edetate, fructose, hydrochloric acid, maltol, peppermint oil, polysorbate 80, propylene glycol, saccharin sodium, sodium benzoate, vanilla flavour and water. Orbis Biosciences’ investigational product was a suspension comprised of microspheres and a liquid component. The microspheres were formulated with prednisone, sorbitan monostearate, glyceryl monostearate and Eudragit E PO (amino methacrylate copolymer). The volume-based average diameter, D(4,3), was 216 μm. The liquid formulation included locust bean gum, xanthan gum, sucrose, flavour and sodium benzoate added to water, which had a viscosity of approximately 74 cP. The Control article was prednisone-free and acted as a negative control. Control microspheres were formulated with the same excipients as the investigational product using glyceryl monostearate as the balance in lieu of prednisone.
To randomize the four test articles, this study used a Latin square design for standard crossover studies with four periods and four treatments to minimize sequence and period effects. Patients were allocated to one of four sequences at random under a permuted block randomization scheme in a 1 : 1 allocation to eliminate bias associated with group assignment while producing groups of similar sizes.
Participants
This study was conducted in the Pediatric Clinical Research Unit of Arkansas Children's Hospital. All participants spoke English with a reading-level at or above grade 8 and were healthy adults between 18 and 40 years of age. Exclusion criteria included one or more of the following: history of smoking or using any tobacco products; previous history of taste disturbance; any condition or dietary habit known to interfere with the sense of smell and taste; any apparent abnormality of the oral cavity (including tongue and teeth) or recent dental surgery within 7 days of administration of the study articles; any structural or functional abnormality of the upper gastrointestinal tract; ingestion of any medication or nutritional supplement (with exception of paracetamol or hormonal oral contraceptives) in a 48-hour period before study; history of any illness within the 2 weeks before study; history of autonomic dysfunction, bronchospastic disease or atopic allergy; known hypersensitivity (i.e. allergic reaction) to any drug, food colouring agent or artificial sweetener; any history of participating in a clinical trial of a drug or device within a 30-day period from the time of study; and brushing of the teeth and or oral ingestion of any substance within one hour of the initial test article. Pregnancy (documented by urine pregnancy test on day of study) or lactation was also exclusion criteria. After providing written informed consent, all study participants demonstrated their ability to hold 10 ml of apple juice in their mouth for 5 s without swallowing before any other study-related procedures were initiated.
Data collection
Data collection took place in December 2015. Participants rated the overall palatability of each test article using a validated 9-Point Hedonic Scale (Figure S1). The 9-point Hedonic Scale is a balanced bipolar scale with four positive and four negative rankings and a central neutral response.[7] It is considered as the ‘gold standard’ method to assess palatability for drug products and is the most widely used scale for sensory evaluation.[8] Evaluation of both product acceptance and preference were assessed in each of the following palatability categories: smell, taste, texture/mouth feel and aftertaste. Participants were also asked to characterize the initial taste of each article using one of the following descriptors: salty, sweet, bitter, sour, savoury (i.e. umami) or tasteless. Each study questionnaire contained a section for comments to collect qualitative feedback. Willingness to take a given test product if prescribed in the future (a reflection of product acceptance or preference) was also assessed.
On the day of check-in and dosing, eligible and consented participants were given a breakfast comprised of dried cereal with 2 ounces of milk and 2 slices of whole wheat toast with butter (one pat) and jelly (15 g). Approximately one hour after the breakfast, they ingested a salt-free cracker followed by 120 ml of water to clear the palate and were then administered the baseline test questionnaire, after which a predetermined randomized sequence of investigational articles were given to each participant. Participants were instructed not to swallow the liquid, but instead to gently roll the liquid throughout their oral cavity for 5 s and then expectorate the contents of their mouth. Immediately thereafter, they were asked to complete the initial portion of the study questionnaire (Figure S1) to enable descriptive sensory assessment (taste, initial taste, texture/mouth feel, smell). Five minutes after completing the initial portion of the questionnaire, participants were asked to complete the remaining sections: (1) aftertaste, (2) willingness to take the test article again and (3) free-texted comment section. Following completion of one set of investigational articles and coupled questionnaire, subjects were given a salt-free cracker and 120 ml water to cleanse the palate. After a 30-minute washout period, each participant once again rinsed their oral cavity with water (as described above), and the remainder of the test articles were administered in an identical fashion. After completion of the assessment following the last test article, participants were discharged from the Pediatric Clinical Research Unit with instructions to telephone the clinical research coordinator over the following 24-hour period to report any adverse events.
De-identified data for analysis were entered into Research Electronic Data Capture (REDCap).[9] To maintain patient confidentiality and study data security, only key study personnel were granted access to this study's database upon the completion of participant enrolment in the study.
Statistical analysis
Using the Latin Square design for standard crossover studies with four periods and four products, participants were allocated to one of four sequences at random. The orders of randomization sequences I, II, III and IV are provided in Table 1.
Table 1.
Characteristics of the 24 participants by randomization sequence
| Randomization sequencea | |||||
|---|---|---|---|---|---|
| I (n = 5) | II (n = 7) | III (n = 6) | IV (n = 6) | P-valueTable-Fn 1 | |
| Gender, n (%) | |||||
| Male | 3 (60%) | 6 (85.7%) | 1 (16.7%) | 2 (33.3%) | 0.08 |
| Female | 2 (40%) | 1 (14.3%) | 5 (83.3%) | 4 (66.7%) | |
| Race, n (%) | |||||
| White | 5 (100%) | 7 (100%) | 5 (83.3%) | 6 (100%) | 0.71 |
| African American | 0 | 0 | 1 (16.7%) | 0 | |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 5 (100%) | 7 (100%) | 6 (100%) | 6 (100%) | – |
| Age (years), mean (SD) | 18.6 (0.9) | 19.9 (1.7) | 20 (3.5) | 20.8 (2.2) | 0.48 |
| Height (cm), mean (SD) | 178.9 (9) | 180.4 (7.1) | 166.4 (11.2) | 174.3 (7.4) | 0.05 |
| Weight (kg), mean (SD) | 77.5 (12.6) | 79.2 (14.8) | 62 (10.8) | 71.9 (11.7) | 0.11 |
aI: Reference, Control, Test 5 min, Test 24 h. II: Test 24 h, Test 5 min, Control, Reference. III: Test 5 min, Test 24 h, Reference, Control. IV: Control, Reference, Test 24 h, Test 5 min. Reference – prednisone solution USP 5 mg/5 ml; Test 24 h – prednisone-loaded microsphere suspension prepared at least 24 h in advance of taste test; Test 5 min – prednisone-loaded microsphere suspension prepared approximately 5 min before the taste test; Control – prednisone-free microsphere suspension. bP-values are from Fisher's exact test for categorical variables and one-way ANOVA for continuous variables.
Participant characteristics were summarized for each randomization sequence by mean and standard deviation for continuous variables and by frequency and percentage for categorical variables. For quantitative analysis of the 9-point Hedonic Scale, the rankings are converted to numerical values: ‘like extremely’ as ‘9’ and ‘dislike extremely’ as ‘1’. Raw scores of the numeric scale from the questionnaires were summarized and plotted for visualization. Linear mixed-effect models adjusting for gender and age were used to detect any significant difference between the four articles in terms of taste, texture/mouth feel, smell, aftertaste and willingness to take. Carryover effects between periods 1 and 2, 2 and 3, and 3 and 4 were evaluated by including the re-parameterized carryover effects in the linear mixed-effect model. Carryover effects between periods were tested for statistical significance using F-test. When significant carryover effects were detected, P-values of the F-test were reported, and both sets of results from models with and without adjustment for the carryover effect were presented. Pairwise comparison of mean Likert scores between any two articles were estimated and reported following the linear mixed-effect model using least squares means (LS-means) of fixed effects. All statistical analyses accepted a significance limit of α = 0.05 and were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA). Figure 1 was produced using Stata v14.0 (StataCorp LP, College Station, TX, USA).
Figure 1.
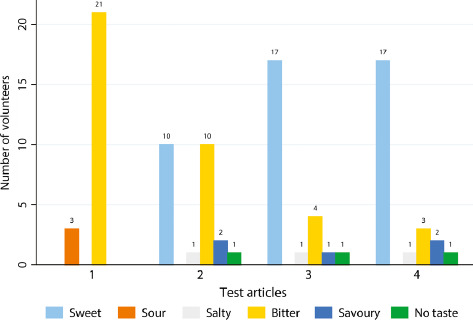
Summary of Taste Sensation after Administration of the Four Test articles. Test articles are (1) Reference – prednisone solution USP 5 mg/5 ml; (2) Test 24 h – prednisone-loaded microsphere suspension prepared at least 24 h in advance of taste test; (3) Test 5 min – prednisone-loaded microsphere suspension prepared approximately 5 min prior to the taste test; and (4) Control – prednisone-free microsphere suspension.
Results
There were a total of 24 eligible and consented participants, all of whom completed the study. Demographic characteristics for the study cohort are summarized in Table 1. Except for height, there were no significant differences among the four randomization sequences. The borderline significant difference for height was presumably due to more female participants in the Sequences III and IV.
LS-means estimates of the Likert scores from the six questions of palatability questionnaire are provided in Table 2. Pairwise comparisons of estimated Likert scores between any pairs of articles following the linear mixed-effect model are shown in Table 3.
Table 2.
Unadjusted and adjusted mean (SD) of Likert scores for the five palatability category
| Palatability category | Estimatea | Test articleb | |||
|---|---|---|---|---|---|
| Reference | Test 24 h | Test 5 min | Control | ||
| Taste score | Unadjusted | 2.21 (0.25) | 6.04 (0.25) | 7.04 (0.25) | 7.00 (0.25) |
| Adjusted | 1.78 (0.40) | 4.62 (0.42) | 6.43 (0.40) | 5.57 (0.42) | |
| Texture/mouth feel score | Unadjusted | 3.92 (0.30) | 5.17 (0.30) | 5.75 (0.30) | 6.33 (0.30) |
| Adjusted | 2.38 (0.54) | 3.69 (0.57) | 3.97 (0.55) | 5.00 (0.56) | |
| Smell | Unadjusted | 4.17 (0.26) | 5.83 (0.26) | 6.13 (0.26) | 6.21 (0.26) |
| Adjusted | 3.73 (0.48) | 4.83 (0.50) | 5.65 (0.48) | 5.18 (0.50) | |
| Aftertaste | Unadjusted | 2.25 (0.32) | 4.63 (0.32) | 5.17 (0.32) | 6.29 (0.32) |
| Adjusted | 2.80 (0.57) | 3.85 (0.60) | 5.55 (0.58) | 5.48 (0.60) | |
| Willingness to take | Unadjusted | 3.67 (0.32) | 6.54 (0.32) | 7.46 (0.32) | 7.79 (0.32) |
| Adjusted | 3.50 (0.53) | 4.90 (0.55) | 7.03 (0.53) | 6.09 (0.55) | |
aUnadjusted mean taste scores are controlled for age, gender, not adjusted for carryover effect. Adjusted mean taste scores are controlled for age, gender, as well as carryover effect. bReference – prednisone solution USP 5 mg/5 ml; Test 24 h – prednisone-loaded microsphere suspension prepared at least 24 h in advance of taste test; Test 5 min – prednisone-loaded microsphere suspension prepared approximately 5 min before the taste test; Control – prednisone-free microsphere suspension.
Table 3.
Score difference among four test articlesa, adjusted for age, gender and carryover effect
| Palatability category | Col minus row | Likert score difference, mean (95% CI) | ||
|---|---|---|---|---|
| Test 24 h | Test 5 min | Control | ||
| Taste | Reference | 2.85 (1.91, 3.78) | 4.66 (4.08, 5.23) | 3.79 (2.83, 4.75) |
| Test 24 h | – | 1.81 (0.90, 2.73) | 0.95 (0.40, 1.50) | |
| Test 5 min | – | – | −0.86 (−1.80, 0.07) | |
| Texture/mouth feel | Reference | 1.31 (0.02, 2.60) | 1.59 (0.75, 2.44) | 2.62 (1.31, 3.94) |
| Test 24 h | – | 0.28 (−0.98, 1.54) | 1.31 (0.51, 2.12) | |
| Test 5 min | – | – | 1.03 (−0.25, 2.32) | |
| Smell score difference | Reference | 1.11 (−0.03, 2.24) | 1.92 (1.21, 2.63) | 1.46 (0.30, 2.61) |
| Test 24 h | – | 0.82 (−0.29, 1.92) | 0.35 (−0.33, 1.03) | |
| Test 5 min | – | – | −0.47 (−1.59, 0.66) | |
| Aftertaste score | Reference | 1.05 (−0.32, 2.42) | 2.75 (1.88, 3.61) | 2.67 (1.28, 4.07) |
| Test 24 h | – | 1.70 (0.36, 3.03) | 1.63 (0.80, 2.45) | |
| Test 5 min | – | – | −0.07 (−1.43, 1.29) | |
| Willingness to take | Reference | 1.40 (0.18, 2.63) | 3.54 (2.79, 4.29) | 2.59 (1.35, 3.84) |
| Test 24 h | – | 2.14 (0.95, 3.33) | 1.19 (0.48, 1.90) | |
| Test 5 min | – | – | −0.94 (−2.16, 0.27) | |
Reference – prednisone solution USP 5 mg/5 ml; Test 24 h – prednisone-loaded microsphere suspension prepared at least 24 h in advance of taste test; Test 5 min – prednisone-loaded microsphere suspension prepared approximately 5 min before the taste test; Control – prednisone-free microsphere suspension.
Significant carryover effects between periods were indicated when the F-test for re-parameterized carryover effects had a P-value less than 0.05. Analysis of the taste scores revealed evidence of significant carryover effects for the Test 24 h (P = 0.003) and Control (P < 0.001) articles. After controlling for age and gender, the Test 5 min. article has the highest (most favourable) taste score (Table 2). The taste scores, whether adjusted or not for the carryover effect, differed significantly among the four articles (overall P < 0.0001). When comparing between pairs of the four test articles, the Test 5 min and Control scores were significantly higher than the Test 24 h and Reference scores (Table 3). The difference between the Test 5 min and Control scores was not statistically significant.
Of the 24 participants, 87.5% (n = 21) described the Reference article as bitter, 41.7% (n = 10) described the Test 24 h article as sweet, and another 41.7% (n = 10) described the Test 24 h article as bitter. The Test 5 min article was described as sweet by 70.8% (n = 17) of participants; the Control article was also described as sweet by 70.8% (n = 17) of participants (Figure 1).
For texture/mouth feel, carryover effects were detected for the Reference, Test 5 min and Control articles (P = 0.002, 0.02 and 0.02, respectively). After controlling for age and sex, the Control article had the highest score (Table 2). The texture/mouth feel scores, whether adjusted or not for the carryover effect, differed significantly among the four articles (overall P < 0.001). When comparing between pairs of the four test articles, the Test 24 h, 5 min and Control articles were preferable over reference (Table 3). The differences between the Test 24 h and the Test 5 min articles or the Test 5 min and Control articles were not statistically significant.
With respect to smell, significant carryover effect was detected for the Test 24 h article (P = 0.04). The Test 5 min article was the highest (most favourable) smell score after controlling for age and sex and adjusting for carryover effect (Table 2). The smell scores, whether adjusted or not for the carryover effect, differed significantly among the four articles (overall P < 0.001). Pairwise comparison showed that the Test 24 h, Test 5 min and Control articles were preferable over the Reference article (Table 3) while the differences between the Test 24 h, 5 min and Control articles were not statistically significant.
Significant carryover effect was found for the Control study article when analysing the aftertaste scores (P = 0.04). Overall, the Test 5 min article had the highest score in terms of aftertaste after controlling for age and sex and adjusting for carryover effect (Table 2). The aftertaste scores, whether adjusted or not for the carryover effect, differed significantly among the four test articles (overall P < 0.001). When comparing between any pairs of the study articles, the Test 5 min and Control articles were preferable over both the Reference and Test 24 h articles (Table 3). The differences between the Reference and Test 24 h articles or the Test 5 min and the Control articles were not statistically significant.
In terms of the participant's willingness to take the different study articles another time, significant carryover effects were detected for the Test 24 h (P = 0.005) and the Control articles (P < 0.001). The Test 5 min article had the highest score (i.e. most acceptable) after controlling for age and sex and adjusting for carryover effect (Table 2). The willing-to-take scores, whether adjusted or not for the carryover effect, differed significantly among the four test articles (overall P < 0.0001). Pairwise comparison revealed that the Test 5 min and Control articles scored significantly higher (i.e. more acceptable/preferable) than the Reference and Test 24 h articles (Table 3). The difference between the Test 24 h and Control articles was not statistically significant.
A summary of participant preference among the four investigational articles is summarized in Table 4. The Test 5 min (the Orbis microsphere formulation reconstituted immediately before administration) and the Control (prednisone-free microsphere suspension) articles were the most preferable in every category of the palatability assessed, and they were indistinguishable from each other. The Test 24 h article (the Orbis microsphere formulation reconstituted 24 h before administration) was preferred over the reference (proprietary prednisone formulation) article in terms of texture/mouth feel and smell. A ‘bitter’ taste was most commonly described for the reference study article whereas the Test 5 min and the Control articles were most commonly described as tasting ‘sweet’ by the participants.
Table 4.
Summary of participant preference among the four test articles
| Test articlea | Palatability/preference | |||||
|---|---|---|---|---|---|---|
| Taste | Initial taste | Texture/mouth feel | Smell | Aftertaste | Willingness to take | |
| Reference | Bitter | |||||
| Test 24 h | Bitter/sweet | + | ||||
| Test 5 min | + | Sweet | + | + | + | + |
| Control | + | Sweet | + | + | + | + |
Reference – prednisone solution USP 5 mg/5 ml; Test 24 h – prednisone-loaded microsphere suspension prepared at least 24 h in advance of taste test; Test 5 min – prednisone-loaded microsphere suspension prepared approximately 5 min before the taste test; Control – prednisone-free microsphere suspension.
Discussion
As described in a review by Matsui,[4] poor palatability of orally administered drugs can negatively impact paediatric medication adherence and especially creates a problem for medications which elicit a bitter taste perception. Of the taste sensations, bitterness is the most sensitive, and many individuals perceive it as unpleasant or disagreeable. A large number of naturally occurring bitter compounds are toxic. Consequently, the ability to detect bitter-tasting compounds at low thresholds is considered to convey a protective function in humans.[10] More recent studies concerning the biology of bitter taste perception have demonstrated that specific taste receptors such as TAS2R38, which are coupled to the G protein gustducin, are responsible for the human ability to taste bitter substances.[11] As demonstrated by Roudnitzky et al.,[12] there are genetic polymorphisms of functional significance in many of the bitter taste receptor (TAS2R) genes such that genomic structure influences the intensity of the bitter taste perception. Finally, there are also physicochemical determinants associated with the perceived intensity of bitter taste such as differences in chemical structure of a given molecule and concentration.[13]
Prednisone and prednisolone, corticosteroid anti-inflammatory agents approved for a variety of treatment indications in both children and adults, are both known to be inherently bitter. A variety of physical approaches have been used in an attempt to mask the taste of bitter-tasting drugs administered as both oral solid and liquid dosage forms.[14–16] In an attempt to overcome the limitations with current prednisone formulations (e.g. improved palatability, formulation flexibility to allow for weight-based dosing), the sponsor of this current study (Orbis Biosciences Inc.) developed a proprietary, free-flowing, drug-loaded microcapsule-based powder formulation of prednisone. The microsphere platform utilizes precision particle fabrication technology to mix a pH-responsive polymer with a poorly water-soluble drug in a water-free, single-step process. This initial evaluation of this new formulation was designed to determine if this novel formulation technology could effectively mask the taste of prednisone.
Despite its potential limitations associated with comparisons of taste perception between individuals or groups, a 9-point Hedonic Scale (Figure S1) was used to assess differences in taste perception within a given study participant between four different substances: a proprietary reference formulation of prednisone liquid, the microsphere prednisone formulation prepared in two different ways (prepared 24 h and immediately before administration) and a control microsphere formulation which contained no active pharmaceutical ingredients. This scale has been used for over 50 years to evaluate the degree of liking or disliking of foods or consumer products and has been adapted into a semi-quantitative tool designed to assess the relative degree of taste perception.[17] Specifically, the current assessment included not only the generation of a ‘taste score’, but also evaluated texture (mouth feel), smell, the perception of an aftertaste and the self-reported willingness of a participant to take one of the four test substances again (Table 2). The randomization sequence used for each of the four test substances was designed to minimize any bias associated with period or sequence effects. The microsphere formulation of prednisone prepared immediately before administration effectively masked the bitter taste of prednisone and was not different from the control (i.e. placebo) formulation with respect to solicitation of a sweet, sour, salty, bitter or savoury (umami) taste response. This similarity between these two formulations was also apparent for assessment of texture/mouth feel, smell, aftertaste and participant preference (i.e. willingness to take again) (Table 4).
In comparison to the assessment of taste characteristics of foods, there are greater experimental challenges in the sensory analysis of drugs.[15] Despite these challenges, it is increasingly recognized by drug regulatory agencies (both the US Food and Drug Administration and the European Medicines Agency) that formulations of drugs, especially liquids developed for paediatric or geriatric use, should be evaluated for palatability. While the 9-point Hedonic Scale used to assess the palatability of the new microsphere prednisone formulation in this study may have inherent limitations,[17] it continues to be used, along with more simplistic Likert-type scales, in the evaluation of palatability and swallowability for paediatric oral dosage forms.[8] For this reason, it was chosen over other more recently developed scales (e.g. a semantically labelled hedonic scale)[17] as the approach to conduct this first evaluation of the new prednisone microsphere formulation.
Conclusions
Palatability of oral drug formulations is one of the most important determinants of paediatric patient compliance. This study has demonstrated that a microsphere-based formulation of prednisone achieves near total taste masking of the drug. The results from this initial study support further investigation of this formulation including performance with regard to relative bioavailability characteristics.
Declarations
Conflict of interest
Dr Dormer, Ms. Shoults and Dr Meyer are employees of Orbis Biosciences, Inc., the company holding the patent for the formulation technology described. Drs Kearns, Bai and Neville, and Ms. Pierce have no conflicts to disclose of pertinence to this work.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health [grant number R44HD074326].
Supplementary Material
Figure S1. 9-Point Bipolar Hedonic Scale used in the palatability assessment questionnaire.
Acknowledgements
The authors would like to thank Julie Nick and Sunitha Kenchey at the Biostatistics Program, Department of Pediatrics, UAMS, for assistance with REDCap database, hosted by the Translational Research Institute at the University of Arkansas for Medical Sciences (NIH/NCRR and NCATS grant UL1TR000039). The assistance of Drs. Laura James, Jose Romero and Henry Farrar in the evaluation of our study participants is also gratefully acknowledged.
References
- Finney E. Children's Medicines: a situational analysis. http://www.who.int/childmedicines/progress/CM_analysis.pdf.
- Anonymous . Best Pharmaceuticals for Children Act. Washington, D.C.: Public Law 107–109, 107th Congress, 2002.
- Anonymous . Regulation (EC) No.1901/2006. Brussels: Official Journal of the European Union. [Google Scholar]
- Matsui D. Current issues in pediatric medication adherence. Paediatr Drugs 2007; 5: 283–288. [DOI] [PubMed] [Google Scholar]
- Milne CP, Bruss JB. The economics of pediatric formulation development for off-patent drugs. Clin Ther 2008; 11: 2133–2145. [DOI] [PubMed] [Google Scholar]
- Abdulla S et al. Early clinical development of artemether-lumefantrine dispersible tablet: palatability of three flavours and bioavailability in healthy subjects. Malar J 2010; 9: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. Hedonic scaling: a review of methods and theory. Food Qual Prefer 2011; 22(8): 733–747. [Google Scholar]
- Thompson CA et al. Industry survey on current practices in the assessment of palatability and swallowability in the development of pediatric oral dosage forms. Ther Innov Regul Sci 2013; 5: 542–549. [DOI] [PubMed] [Google Scholar]
- Harris PA et al. Research electronic data capture (Redcap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 2: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav 1994; 6: 1217–1227. [DOI] [PubMed] [Google Scholar]
- Maehashi K et al. Bitter peptides activate hTAS2Rs, the human bitter receptors. Biochem Biophys Res Commun 2008; 4: 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudnitzky N et al. Receptor polymorphism and genomic structure interact to shape bitter taste perception. PLoS Genet 2015; 9: e1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast RS, Roper J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem Senses 2007; 3: 245–253. [DOI] [PubMed] [Google Scholar]
- Anand V et al. Preparation and evaluation of taste-masked orally disintegrating tablets of prednisolone. Asian J Pharm Sci 2007; 6: 227–238. [Google Scholar]
- Coupland JN, Hayes JE. Physical approaches to masking bitter taste: lessons from food and pharmaceuticals. Pharm Res 2014; 11: 2921–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Chopra H. Role of taste and taste masking of bitter drugs in pharmaceutical industries – an overview. Int J Pharm Pharm Sci 2010; 2 (Suppl. 4): 14–18. [Google Scholar]
- Lim J et al. Derivation and evaluation of a labeled hedonic scale. Chem Senses 2009; 9: 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 9-Point Bipolar Hedonic Scale used in the palatability assessment questionnaire.


